Abstract
This study was aimed at investigating the cardiovascular effects of an Olea europea L. leaf extract (OEE), of a Hibiscus sabdariffa L. flower extract (HSE), and of their 13 : 2 w/w mixture in order to assess their cardiac and vascular activity. Both extracts were fully characterized in their bioactive compounds by HPLC-MS/MS analysis. The study was performed using primary vascular endothelial cells (HUVECs) to investigate the antioxidant and cytoprotective effect of the extracts and their mixture and isolated guinea-pig left and right atria and aorta to evaluate the inotropic and chronotropic activities and vasorelaxant properties. In cultured HUVECs, OEE and HSE reduced intracellular reactive oxygen species formation and improved cell viability, following oxidative stress in dose-dependent manner. OEE and HSE exerted negative inotropic and vasorelaxant effects without any chronotropic property. Interestingly, the mixture exerted higher cytoprotective effects and antioxidant activities. Moreover, the mixture exerted an inotropic effect similar to each single extract, while it revealed an intrinsic negative chronotropic activity different from the single extract; its relaxant activity was higher than that of each single extract. In conclusion OEE and HSE mixture has a good potential for pharmaceutical and nutraceutical application, thanks to the synergistic effects of the single phytochemicals.
1. Introduction
Hypertension is a chronical medical condition which represents a major risk factor for myocardial infarction, heart failure, stroke, peripheral arterial disease, and aortic aneurysm and is a cause of chronic kidney disease. This pathology often occurs along with metabolic syndrome and it is associated with a decreased life expectancy [1–3].
The central role in the pathogenesis belongs to the vascular endothelium. Vascular biology assumes a pivotal role in the initiation and perpetuation of hypertension and cardiovascular organ damage. Oxidative stress (ROS and RNS), inflammation, increased expression of redox-sensitive proinflammatory genes, cell adhesion molecules, and recruitment migration vascular dysfunction (T cells and B cells) are the primary pathophysiologic and functional mechanisms that induce vascular disease [4]. All these are closely interrelated and establish a deadly combination that leads to endothelial dysfunction (ED), vascular smooth muscle and cardiac dysfunction, hypertension, vascular disease, atherosclerosis, and cardiovascular diseases (CVD) [5]. Conventional pharmacological treatment for hypertension includes diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blocker, β-receptors blockers, L-Type calcium channel blockers, and central α-receptors agonists.
International medical societies have long since introduced nonpharmacological recommendations in their treatment guidelines [6], due to the several limitation of the pharmacological treatments: possible side effects, poor adherence to therapy (half of the patients stop taking their medication at one year from starting) [7], or also the very slow actual development of new drugs for hypertension.
The use of nonpharmacological treatments, including, among others, the administration of nutraceutical supplements based on botanicals, has been growing in the recent years. Several plants, such as Eucommia ulmoides Oliv. [8], Allium sativum L. [9], and Nigella sativa L. [10], exert antihypertensive effects through different mechanisms and have been investigated for their clinical efficacy [11–13]. The main class of natural compounds responsible for the vascular effects is represented by polyphenols. Some grape juices and grape skin extracts rich in polyphenols exert endothelium-dependent relaxations in aortic rings [14]. Furthermore, polyphenol-rich sources, including extracts from red wines and green and black tea, determine endothelium-dependent relaxations in large arteries, arterioles, and veins that are prevented by competitive inhibitors of eNOS and guanylyl cyclase [14, 15]. In addition, red wine polyphenols have been shown to induce endothelium-dependent relaxation in porcine coronary artery rings [16]. Other studies demonstrated that polyphenol-rich red wine extracts suppress the angiotensin II-stimulated upregulation of several NADPH oxidase subunits including NOX1 and p22phox and the associated oxidative stress and hypertension [17]. Kane et al. [18] demonstrated that red wine polyphenols decrease angiotensin II-induced vascular expression of COX and the increased endothelium-derived contracting factors.
In addition, a chestnut wood extract, rich in ellagitannins, has been shown to exhibit an antioxidant activity and to produce a cardioprotective effect [19]. Polyphenols from terrestrial and marine plants exert several biological activities which result not only in a reduction of the blood pressure, but also in a protection from a wild variety of chronic diseases, including those affecting cardiovascular system [20, 21].
In this study we focused our attention on Olea europaea L. leaf extract (OEE) and on Hibiscus sabdariffa L. calyces extract (HSE). Phenolic compounds found in olive plant (Olea europaea L.), including hydroxytyrosol, oleuropein, flavonoids, chalcones, and tannins have been shown to exert many beneficial effects towards cardiovascular system [22]. Several studies have demonstrated that olive leaves exert antihypertensive, antiatherogenic, anti-inflammatory, hypoglycemic, and hypocholesterolemic effects [23]. In addition, olive leaf extract, or its main component oleuropein, shows protective effects in atherosclerosis [24], diabetes [25], hypertension [26], cardiotoxicity [27, 28], neurotoxicity [29], gastric lesions [30], and cancers [31].
Olive leaf extract exerts the hypotensive action through a direct activity on vascular smooth muscle where it determines a calcium antagonistic effect [32] and on endothelium [33]. A clinical trial confirmed the efficacy and safety of an olive leaf extract, named EFLA 943, in lowering systolic and diastolic blood pressures in subjects with stage-1 hypertension [34].
Hibiscus sabdariffa L. calices extracts have been reported to contain several polyphenols including flavonoids such as cyanidin 3-rutinoside, delphinidin 3-sambubioside, cyanidin 3-sambubioside, cyaniding-3-glucoside, delphinidin 3-glucoside, and hibiscus acid [35]. All these compounds of Hibiscus sabdariffa L. extract have been shown to attenuate atherosclerosis through several mechanisms such as the antioxidative activity [36], inhibition of LDL oxidation [37], and smooth muscle cell proliferation [38]. The hypotensive action of Hibiscus sabdariffa L. extract occurs, at least in part, through a diuretic activity, due to the modulation of the aldosterone action [39]. The latter action is mainly exerted by anthocyanins such as delphinidin-3-sambubioside and cyanidin-3-sambubioside, by phenylpropanoids such as chlorogenic acid, and, to a lesser extent, by flavonoids such as quercetin and rutin [39]. In addition, the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa L. are ACE competitive inhibitors [40]. HSE relaxes, in a concentration-dependent manner, aortic rings precontracted with KCl high concentration (80 mM) and phenylephrine (PE), thus it probably exerts a vasorelaxant activity through a mechanism involving voltage and receptor operated Ca2+ calcium channels (VOCC and ROCC, resp.) [41]. In addition, HSE exerts a direct activity towards vascular endothelium, producing a vasorelaxant effect via the activation of endothelium-derived nitric oxide/cGMP-relaxant pathway and the increased synthesis/release of endothelium-derived nitric oxide [41]. Several clinical trials confirmed hypotensive and antihypertensive effectiveness and safety of Hibiscus sabdariffa L. extract [42–44].
This study is aimed at investigating the cardiovascular effects of an Olea europea L. leaf extract (EFLA 943), of an Hibiscus sabdariffa L. calyces extract, and of a mixture of the two extracts, containing 86.67% of EFLA 943 and 13.33% of Hibiscus sabdariffa L. flower powder extract (13 : 2 w/w) in order to assess their cardiac and vascular synergistic or antagonistic activity. This EFLA 943/HSE ratio is commercially available as an adjuvant food supplement in the treatment of hypertension.
In this study, we have fully characterized both EFLA 943 and HSE extracts in their polyphenol composition, and we have evaluated the antioxidant and cytoprotective activity of both single extracts and their mixture in human umbilical vein endothelial cells (HUVECs). Moreover, the effect on cardiovascular system has been investigated by the evaluation of inotropic and chronotropic effects on guinea pig isolated left and right atria and of vasorelaxant effect in guinea pig isolated aortic strips. Comparison with ileum longitudinal smooth muscle was performed to discriminate the potential relaxant effect in vascular and nonvascular smooth muscle tissue.
2. Materials and Methods
2.1. Materials
Olea europea L. leaves extract (Benolea (EFLA 943)) (OEE) was supplied by Frutarom (Switzerland Ltd.). The extract, manufactured from the dried leaves of Olea europaea L., was obtained applying an ethanol (80% m/m) extraction procedure as previously described [45] and the product was purified by a patented procedure (US Patent 6024998) to remove undesired contaminants and residues. The solvent was subsequently removed resulting in a free flowing powder, containing 18−26% w/w oleuropein by HPLC analysis.
The Hibiscus sabdariffa L. flower powder extract (HSE) was supplied by Nutraceutica S.r.l. (via Idice 270/1 40050 Monterenzio, Bologna, Italy). Briefly dried calyces of Hibiscus sabdariffa L. were subjected to extraction with distilled water for 48 hrs. The extract was filtered and concentrated under reduced pressure and completely evaporated in a vacuum oven at a temperature not exceeding 40°C. The aqueous extract was dried using Freeze Dryer system. For deep information please visit Nutraceutica website (http://www.nutraceutica.it/).
The mixture of two extracts, containing 86.67% of EFLA 943 and 13.33% of Hibiscus sabdariffa L. flower powder extract, represents the active ingredient of a food supplement proposed as coadjuvant in the treatment of hypertension.
The extracts and the mixture were dissolved in water shortly before use.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile (HPLC grade) was from VWR (Milano, Italy), and formic acid (98%–100%) was from Merck (Darmstadt, Germany). Deionized water was obtained from an Elix 10 water purification system from Millipore (Bedford, MA, USA).
2.2. Phytochemical Analysis
2.2.1. Extraction of Phenolic Compounds
OEE and HSE (0.1 g) were subjected to extraction using 2 mL of a methanol/water solution (50 : 50, v/v) in a 15-mL centrifuge tube. The mixture was blended (Ultra-Turrax, IKA, Staufen, Germany) for 5 min and then centrifuged for 5 min at 2500 g. The hydroalcoholic extract was collected and the powders were reextracted with 2 mL of methanol/water solution. Finally, the hydroalcoholic fractions were combined, diluted up to 5 mL and filtered through 0.2 μm regenerated cellulose filters (Schleicher & Schuell, Dassel, Germany).
2.2.2. Liquid Chromatography Analysis of OEE and HSE
The analysis of OEE and HSE to detect phytochemicals was performed by a 1290 Infinity series liquid chromatography instrument (HPLC) equipped with a quaternary pump (Agilent Technologies, Waldbronn, Germany) coupled online with a UV-Vis detector. The separation of phenolic compounds was carried out on a reverse phase C18 100 Å Kinetex column (2.6 μm, 100 × 3.00 mm I.D., Phenomenex, Torrance, CA, USA). Gradient elution was carried out with a solvent system of water/formic acid (100 : 0.5 v/v) as mobile phase A and acetonitrile as mobile phase B; the total run-time was 30 minutes and the gradient elution was as follows: from 0 to 5 min solvent B increased from 5% to 15%, at 10 min solvent B reached 25%, at 23 min solvent B reached 50%, and finally at 28 min solvent B was 100%; at 30 min 5% solvent B was restored. The column was thermostated at 30°C and equilibrated for 5 min prior to each analysis. An injection volume of 2.5 μL and a flow rate of 0.7 mL min−1 were used. The chromatograms were monitored at three wavelengths (280, 320, and 345 nm) characteristic for a high number for aromatic compounds. Each wavelength was suitable for each group of compounds: 280 nm was used for secoiridoids, 320 nm for hydroxycinnamic acids, and 345 nm for flavones and flavonols.
For the structural elucidation and the detection of other compounds such as hibiscus acid, a typical compound present in HSE, the HPLC system was coupled online to a triple quadrupole mass spectrometer detector (QqQ, 6420 Triple Quad/LC MS, Agilent Technologies) equipped with a TurboIonSpray source operating in negative-ion mode. The declustering potential (DP), collision energy (CE), and focusing potential (FP) were optimized for oleuropein and vanillic acid. TurboIonSpray source of QqQ setting was as follows: capillary voltage −3500 V; nebulizer gas (N2) 50 (arbitrary units); curtain gas (N2) 12 (arbitrary units); collision gas (N2) 4 (arbitrary units); focusing potential −200 V; entrance potential 10 V; drying gas (N2) heated to 250°C and introduced to a flow rate of 12 mL min−1. Full-scan data acquisition was performed scanning from m/z 100 to 800 in profile mode and using a cycle time of 2 s with a step size of 0.1 μm and a pause between each scan of 0.002 s; dwell time was set at 0.016 s.
In order to quantify through UV-Vis detector the amount of phenolic compounds in the hydroalcoholic OE extract, calibration curves were prepared with the available standards: oleuropein, verbascoside, quercetin-3-O-rhamnosyl-glucoside (rutin), and apigenin. The other compounds, for which no commercially standards were available, were tentatively quantified on basis of the other compounds bearing similar structures.
A calibration curve (r 2 > 0.99) of vanillic acid was also obtained to quantify hibiscus acid by ESI-QqQ-MS detector.
2.3. Animals
Guinea-pigs (males and females, 300–400 g) obtained from Charles River (Calco, Como, Italy) were housed in a controlled environment with a 12:12-h light-dark cycle at 22°C and provided with chow diet and water ad libitum. All animals used in this study were housed and treated according to the directives on the protection of animals used for scientific purposes (Directive 2010/63/EU of the European Parliament and of the Council) and the WMA Statement on Animal Use in Biomedical Research. All procedures followed the guidelines of animal care and were approved by the Ethics Committee of the University of Bologna (Bologna, Italy). The animals were sacrificed by cervical dislocation; the organs were immediately removed and used as below described.
2.3.1. Atrial Preparations
The removed heart was washed by perfusion through the aorta with oxygenated Tyrode solution containing (mM): NaCl 136.9; KCl 5.4; CaCl2 2.5; MgCl2 1.0; NaH2PO4 xH2O 0.4; NaHCO3 11.9; and glucose 5.5. The physiological salt solution (PSS) was buffered at pH 7.4 by saturation with 95% O2–5% CO2 gas, and the temperature was maintained at 35°C. The following isolated guinea-pig heart preparations were used: spontaneously beating right atria and left atria driven at 1 Hz. For each preparation, the entire left and right atria were dissected from the ventricles, cleaned of excess tissue, and hung vertically in a 15 mL organ bath containing PSS continuously bubbled with 95% O2–5% CO2 at 35°C, pH 7.4. The contractile activity was recorded isometrically by means of force transducer (FT 0.3, Grass Instruments Corporation, Quincy, MA, USA) using Power Lab software (ADInstruments Pty Ltd., Castle Hill, Australia). The left atria were stimulated by rectangular pulses of 0.6–0.8 ms duration and about 50% threshold voltage through two platinum contact electrodes in the lower holding clamp (Grass S88 Stimulator). The right atria were in spontaneous activity. After the tissues were beating for several min, a length-tension curve was determined, and the muscle length was maintained at the value eliciting 90% of maximum contractile force observed at the optimal length. A stabilization period of 45–60 min was allowed before the atria were challenged by various agents. During the equilibration period, the bathing solution was changed every 15 min and the threshold voltage was ascertained for the left atria. Atrial muscle preparations were used to examine the inotropic and chronotropic activity of the extracts and mixture (0.01–10 mg/mL), dissolved in PSS. During the generation of cumulative concentration-response curves, the next higher concentration of different extracts or mixture was added only after the preparation reached a steady state. All data are reported as means ± SEM. The EC50 values were calculated from concentration-response curves [46].
2.3.2. Aortic Strips and Ileum Longitudinal Smooth Muscle (GPLSM) Preparations
The thoracic aorta and ileum were placed in Tyrode solution containing (mM): NaCl, 118; KCl 4.75; CaCl2 2.54; MgSO4 1.20; KH2PO4 1.19; NaHCO3 25; and glucose 11, equilibrated with 95% O2–5% CO2 at pH 7.4. The vessel was cleaned of extraneous connective tissue. Two helicoidal strips (10 mm × 1 mm) were cut from each aorta beginning from the end most proximal to the heart. Vascular strips were then tied with surgical thread (6–0) and suspended in a jacketed tissue bath (15 mL) containing aerated PSS at 35°C. Aortic strips were secured at one end to plexiglass hooks and connected via the surgical thread to a force displacement transducer (FT 0.3, Grass Instruments Corporation) for monitoring changes in isometric contraction. Aortic strips were subjected to a resting force of 1 g. The intestine was removed above the ileocaecal junction. GPILSM segments of 2 cm length were mounted under a resting tension of 300–400 mg. Strips were secured at one end to a force displacement transducer (FT 0.3, Grass Instruments Corporation) for monitoring changes in isometric contraction and washed every 20 min with fresh PSS for 1 h. After the equilibration period, guinea-pig aortic and GPLSM strips were contracted by washing in PSS containing 80 mM KCl (equimolar substitution of K+ for Na+). When the contraction reached a plateau (about 45 min) different concentrations of the extracts and mixture (0.01–10 mg/mL) were added cumulatively to the bath allowing for any relaxation to obtain an equilibrated level of force. All data are reported as means ± S.E.M. The IC50 were calculated from concentration-response curves [46].
2.4. Antioxidant and Cytoprotective Activities
2.4.1. Cell Culture and Treatments
HUVECs were cultured as previously reported [47]. Briefly, cells were plated on gelatin-coated multiwell plates and maintained in complete medium M200 containing 10% FBS and growth factors at 37°C with 5% CO2. Cells from passages 3 to 6 were used. Cells at 80% confluence were treated for 24 h with different concentrations (0.05–100 μg/mL) of OEE and HSE or a 13 : 2 w/w mix of the two and used for further analysis.
2.4.2. Determination of Cell Viability
Viability of control and treated cells was measured using the MTT assay as previously reported [48]. For the flow cytometry analysis the cells were double labelled with Annexin V conjugated to Phycoerythrin (Annexin V-PE) and 7-amino-actinomycin D (7 AAD) and immediately analysed on a GuavaEasyCyte flow cytometer (Guava Technologies, Hayward, CA) in accordance with the manufacturer's instructions as reported in [49]. The percentage of viable cells was reported with respect to the total number of cells.
2.4.3. Detection of Intracellular Reactive Oxygen Species
The formation of reactive oxygen species (ROS) was evaluated using a fluorescent probe, DCFH-DA, as previously reported [50]. Briefly, controls and treated cells were washed with PBS and then incubated with 5 μM DCFH-DA in PBS for 30 min. After DCFH-DA removal, the cells were incubated with 100 μM H2O2 for 30 min. Cell fluorescence from each well was measured using a microplate spectrofluorometer (λ ex = 485 nm and λ em = 535 nm). Intracellular antioxidant activity was expressed as the percentage of inhibition of intracellular ROS produced by H2O2 exposure.
2.4.4. Determination of Cytoprotective Effect
Cytoprotection against H2O2 induced cell damage was assessed using the MTT assay as previously reported [19]. Control and treated cells were exposed to 150 μM H2O2 in PBS for 3 h after which cells were changed to a fresh culture medium. MTT was added to the medium at the final concentration of 0.5 mg/mL and incubated for 4 h at 37°C. DMSO was added to dissolve the formazan crystals and the absorbance was measured at 595 nm using a microplate reader VICTOR3 V Multilabel Counter. Data were expressed as percentage of viable cells with respect to controls times. For the flow cytometry analysis the cells were double labelled with Annexin V conjugated to Annexin V-PE and 7 AAD and analysed on a GuavaEasyCyte flow cytometer in accordance with the manufacturer's instruction.
2.5. Statistical Analysis
Data on atria and on vascular and nonvascular smooth muscle were analyzed by the Student's t-test and presented as means ± S.E.M. [46]. P value less than 0.05 has been considered significant. The potency of different extract and its mixture, defined as EC50, EC30, and IC50 were calculated from concentration-response curves (Probit analysis using Litchfield and Wilcoxon [46] or GraphPad Prism [51, 52] software).
Data from HUVEC cultures are means ± S.D. and were analyzed by one-way analysis of variance
(ANOVA) followed by Dunnett's test, and P < 0.05 has been considered significant.
3. Results
3.1. Characterization of Olea europea L. Leaf and Hibiscus sabdariffa L. Flower Extracts
A complete characterization of OEE and HSE was realized by HPLC coupled to a UV-Vis and QqQ-Ms detector. When reference standard was not available, a tentative identification of compounds was made on the basis of spectroscopic properties, molecular weight, and the search of the main [M-H]-ion together with the interpretation of its fragments (Table 1).
Table 1.
UV absorption bands, mass spectrometry, and quantitative analysis of hibiscus acid and phenolic compounds in HSE and OEE.
| Compound | λ max (nm) | Mass spectrometry data | Quantitative data (mg g−1) | |||
|---|---|---|---|---|---|---|
| MW | [M-H]− | Main fragments | OEE | HSE | ||
| Hibiscus acid1 | — | 190 | 189 | — | 139.2 ± 0.47 | |
| Secoiridoids | ||||||
| Hydroxyoleuropein2 | 280 | 556 | 555 | 539, 377, 197 | 8.96 ± 0.42 | — |
| Elenolic acid glucoside2 | 240 | 404 | — | — | 23.65 ± 0.79 | — |
| Oleuropein2 | 280 | 540 | 539 | 377, 197 | 215.1 ± 1.64 | — |
| Oleuropein isomer2 | 280 | 540 | 523 | 377, 197 | 51.09 ± 0.35 | — |
| Ligstroside2 | 280 | 524 | 523 | 361, 191 | 11.03 ± 0.04 | — |
| Hydroxycinnamic acids | ||||||
| Verbascoside3 | 320 | 624 | 623 | 461, 315, 135 | 4.67 ± 0.44 | — |
| Flavonols | ||||||
| Rutin4 | 345 | 610 | 609 | 301, 179 | 0.69 ± 0.06 | — |
| Flavones | ||||||
| Luteolin-7-O-rutinoside5 | 345 | 594 | 593 | 447, 285 | 0.74 ± 0.04 | — |
| Luteolin-7-O-glucoside5 | 345 | 448 | 447 | 285 | 5.83 ± 0.24 | — |
| Luteolin-4-O-glucoside5 | 345 | 448 | 447 | 285 | 4.20 ± 0.12 | — |
Hibiscus acid and phenolic compounds quantified as: 1vanillic acid mg/g; 2oleuropein mg/g; 3verbascoside mg/g; 4rutin mg/g; 5apigenin mg/g. Values are means ± S.D. (n = 3).
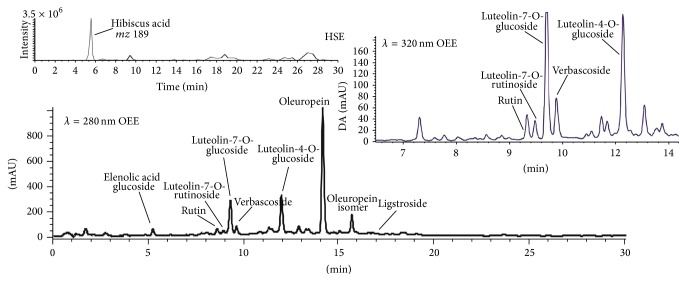
Hibiscus acid was detected using mass spectrometry by matching the information of molecular ion at m/z 189 in negative mode (Figure 1). Elenolic acid glucoside was tentatively identified by UV spectra at 240 nm but not corroborated by mass spectra. The identification of hydroxy oleuropein and oleuropein isomer was corroborated by detection of the molecular ion (at m/z 555 and 539, resp.) and their aglycone fragment at m/z 377. Verbascoside was identified by molecular ion at m/z 623 and various fragments (m/z 461, 315) that are in accordance with the fragmentation pathway. Luteolin-4-O-rutinoside, luteolin-7-O-glucoside, and luteolin-4-O-glucoside were detected by an intense molecular ion at m/z 447 and the diagnostic fragment at m/z 285 of luteolin derivatives.
Figure 1.
Phenolic profile of OEE at λ = 280 and 320 nm and extracted ion chromatography of hibiscus acid. The analysis of phenolic compounds was performed by liquid chromatography coupled online with a UV-Vis detection and a triple quadrupole mass spectrometer as reported in Methods section.
To quantify phenolic compounds, calibration curves were obtained for each standard with high linearity (r 2 = 0.99) by plotting the standard concentration as a function of the peak area obtained from HPLC-UV. Hibiscus acid was expressed as equivalents of vanillic acid, not present in the extract, but used as standard for the quantification of this compound. Only hibiscus acid was identified and quantified in HSE, probably due to the extraction process used by the producer that washed or degraded the phenolic acids and flavonoids commonly reported in other hibiscus extracts. Phenolic compounds characterized in OEE include oleuropein and its isomers, luteolin-4-O- and luteolin-7-O-glucoside, luteolin-4-O-rutinoside, ligstroside, elenolic acid glucoside, verbascoside, and rutin (Figure 1).
The four major compounds found in olive leaf powder extract were oleuropein and oleuropein isomer and two glucoside isomers of luteolin (215, 51, and 5.8 and 4.2 mg g−1, resp.). Other minor compounds, detected in less quantity were hydroxy oleuropein, ligstroside, verbascoside, and rutin as reported in Table 1. All the compounds reported are typically present in olive leaf as previously reported [53, 54].
3.2. Effects of OEE, HSE, and Their Mixture on Guinea-Pig Isolated Cardiac and Smooth Muscle Tissues
The effects of single extracts and mixture were derived on guinea-pig isolated left and right atria to evaluate their inotropic and chronotropic effects, respectively, and on K+-depolarized (80 mM) guinea-pig vascular (aorta) and nonvascular ileum longitudinal smooth muscle (GPILSM) strips to assess calcium antagonist activity. Tested samples were checked at increasing doses to evaluate the percent decrease of developed tension on isolated left atrium driven at 1 Hz (negative inotropic activity), the percent decrease in atrial rate on spontaneously beating right atrium (negative chronotropic activity), and the percent inhibition of calcium-induced contraction on K+-depolarized aortic strips and GPILSM (vascular and nonvascular relaxant activity, resp.).
3.2.1. Guinea-Pig Left and Right Atria
Data relative to inotropic and chronotropic activities of OEE, HSE, and their mixture were reported in Table 2. Both extracts (1 mg/mL) produced negative inotropic effect in left atria driven at 1 Hz (68 ± 2.4% and 76 ± 0.9%, resp.). On the contrary, OEE potency was 1.9 times higher than that of HSE extract (EC50 = 0.14 mg/mL (c.l. 0.10–0.18) and EC50 = 0.27 mg/mL (c.l. 0.21–0.35), resp.). The mixture exerts an intrinsic activity, slightly lower than that exerted by the single extracts; however its potency was not significantly different from the potency of OEE (EC50 = 0.16 mg/mL (c.l. 0.12–0.020) and EC50 = 0.14 mg/mL (c.l. 0.10–0.18), resp.) but it was 1.7 times higher than that of HSE (EC50 = 0.16 mg/mL (c.l. 0.12–0.20) and EC50 = 0.27 mg/mL (c.l. 0.21–0.35) resp.). Both extracts did not show negative chronotropic activity. As shown in Table 2 intrinsic activity was less than 50% (37 ± 2.4% and 46 ± 0.7%, resp.) at the maximal dose tested (10 mg/mL for OEE and 1 mg/mL for HSE, resp.). On the contrary, the mixture (1 mg/mL) revealed a negative chronotropic intrinsic activity (84 ± 2.0% at 10 mg/mL) with a potency 7.6 time higher than the inotropic negative potency (EC30 = 1.21 mg/mL (c.l. 1.10–1.33) and EC50 = 0.16 mg/mL (c.l. 0.12–0.20), resp.).
Table 2.
Inotropic and chronotropic effects of OEE, HSE, and their mixture.
| Extract | Left atrium | Right atrium | ||||
|---|---|---|---|---|---|---|
| Negative inotropy | Negative chronotropy | |||||
| Activitya (M ± SEM) | EC50 b (mg/mL) | 95% conf. lim. | Activityc (M ± SEM) | EC30 b (mg/mL) | 95% conf. lim. | |
| OEE | 68 ± 2.4 | 0.14 | 0.10–0.18 | 37 ± 2.4 | ||
| HSE | 76 ± 0.9 | 0.27 | 0.21–0.35 | 46 ± 0.7d | ||
| Mixture | 60 ± 1.4 | 0.16 | 0.12–0.20 | 84 ± 2.0 | 1.21 | 1.10–1.33 |
aDecrease in developed tension on isolated guinea-pig left atrium at 1 mg/mL concentration, expressed as percent changes from the control (n = 5-6). The left atria were driven at 1 Hz. The 1 mg/mL concentration gave the maximum effect for most compounds. bCalculated from concentration-response curves (Probit analysis by Litchfield and Wilcoxon [46] with n = 6-7). When the maximum effect was <50%, the EC50 ino., EC30 chrono., values were not calculated. cDecrease in atrial rate on guinea-pig spontaneously beating isolated right atrium 10 mg/mL concentration, expressed as percent changes from the control (n = 7-8). The 10 mg/mL concentration gave the maximum effect for most compounds. Pretreatment heart rate ranged from 165 to 190 beats/min. dAt 1 mg/mL concentration.
3.2.2. Guinea Pig Smooth Muscle
Both extracts reduced in a dose-dependent manner the contraction induced by 80 mM K+ on the vascular smooth muscle (Table 3). Both OEE and HSE had similar intrinsic activity at the same maximal concentration (10 mg/mL) analyzed. On the contrary, the vasorelaxant potency of OEE was 6.7 times higher than that of HSE (IC50 = 5.15 mg/mL (c.l. 4.68–5.59) and IC50 = 6.63 mg/mL (c.l. 6.34–6.92), resp.). The mixture revealed an intrinsic activity lower than that of the single extracts at the same maximal concentration tested. In agreement with data on negative inotropic effect, the potency of the mixture was not significantly different from those of OEE (IC50 = 5.89 mg/mL (c.l. 5.56–6.25) and IC50 = 5.15 mg/mL (c.l. 4.68–5.59), resp.). Moreover, the vasorelaxant effect of mixture is about 1.1 times higher than that of the HSE (IC50 = 5.89 mg/mL (c.l. 5.56–6.25) and IC50 = 6.63 mg/mL (c.l. 6.34–6.92), resp.). On the contrary, only OEE demonstrated intrinsic activity in nonvascular smooth muscle (Table 3). The OEE was 6.7 times more potent in the nonvascular (GPLSM) than in the vascular smooth muscle (aorta) (IC50 = 0.77 mg/mL (c.l. 0.34–1.01) and IC50 = 5.15 mg/mL (c.l. 4.68–5.59), resp.) evidencing its selectively. In GPILSM, the mixture revealed an intrinsic activity not significantly different from these of OEE (Table 2) with a potency 1.8 times lower than that of OEE alone (IC50 = 1.39 mg/mL (c.l. 1.13–1.54) and IC50 = 0.77 mg/mL (c.l. 0.34–1.01), resp.) suggesting that the addition of a little amount of HSE to OEE is able to reduce the selectivity toward the nonvascular tissue.
Table 3.
Relaxant activity of OEE, HSE, and their mixture on K+-depolarized guinea pig smooth muscle.
| Extract | Aorta | GPILSM | ||||
|---|---|---|---|---|---|---|
| Activitya (M ± SEM) | IC50 b (mg/mL) | 95% conf. lim. | Activitya (M ± SEM) | IC50 b (mg/mL) | 95% conf. lim. | |
| OEE | 90 ± 1.1 | 5.15 | 4.68–5.59 | 90 ± 1.7 | 0.77 | 0.34–1.01 |
| HSE | 93 ± 1.4 | 6.63 | 6.34–6.92 | 21 ± 0.7c | ||
| Mixture | 70 ± 3.6 | 5.89 | 5.56–6.25 | 90 ± 1.2 | 1.39 | 1.13–1.54 |
aPercent inhibition of calcium-induced contraction on K+-depolarized (80 mM) guinea-pig aortic strips at 10 mg/mL concentration. The 10 mg/mL concentration gave the maximum effect for most compounds, respectively. bCalculated from concentration-response curves (Probit analysis by Litchfield and Wilcoxon [46] with n = 6-7). When the maximum effect was <50%, the IC50 values were not calculated. cAt 5 mg/mL concentration.
3.3. Antioxidant and Cytoprotective Effects in Cultured HUVECs
Since olive leaves and hibiscus flower extracts are rich in phenolic compounds, we have investigated the ability of the extracts to protect cultured HUVECs from oxidative stress.
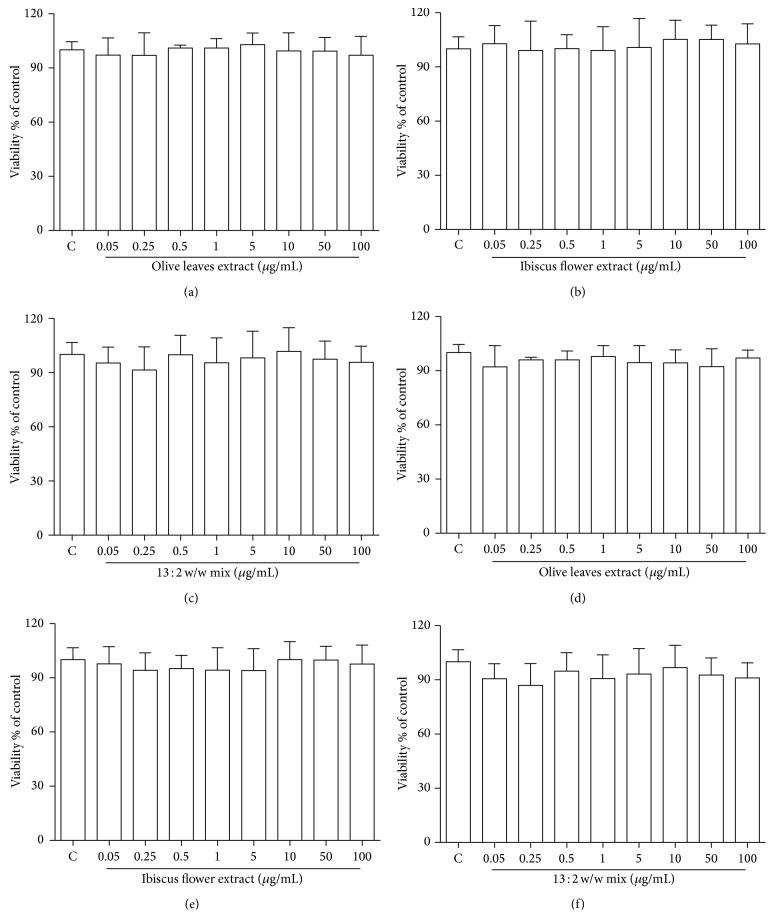
Figure 2 shows, by both MTT (Figures 2(a), 2(b), and 2(c)) and flow cytometry analysis (Figures 2(d), 2(e), and 2(f)), that OEE, HSE, and their mixture did not exert any toxic effect on cultured HUVECs on a wide range of concentrations (0.05–100 μg/mL).
Figure 2.
Cell viability of HUVECs treated with OEE, HSE, and their mixture. HUVECs were treated as described in Methods section. Cell viability was analysed by the MTT test as reported in the Methods section ((a), (b), and (c)). Cell viability was analysed by flow cytometry. Cells were double-labelled with Annexin V-PE 7 AAD and analyzed by a Guava EasyCyte flow cytometer ((d), (e), and (f)). Data are reported as means ± S.D. of four independent experiments.
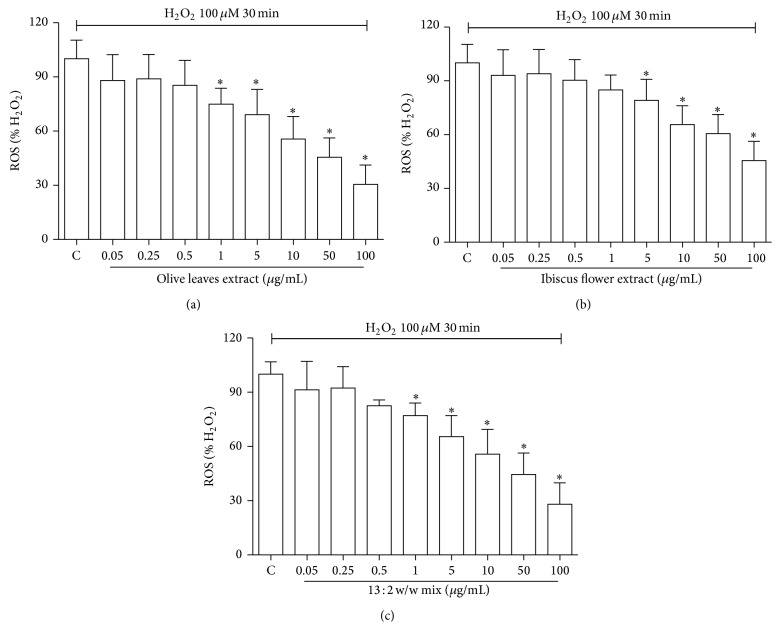
ROS level measurement revealed that ROS production was significantly reduced in extract treated cells after 24 h in a dose-dependent manner. Figure 3(a) shows significant decrease in ROS production, as detected by DCFH-DA assay that was observed in both OEE and HSE treated HUVECs following exposure to H2O2 (Figures 3(a) and 3(b)), with OEE exhibiting the highest effect, with a significant decrease in ROS production at 1 μg/mL, while HSE evidenced a significant effect only at 5 μg/mL.
Figure 3.
Effect of OEE, HSE, and their mixture on intracellular ROS production. HUVECs were treated with OEE (a), HSE (b), and their mixture (c) (0.05–100 μg/mL) for 24 h; oxidative damage was then induced exposing the cells to 100 μM H2O2 for 30 min and intracellular ROS were determined using the peroxide-sensitive fluorescent probe DCFH-DA as described in Methods section. Data are expressed as percent of control cells treated with H2O2. Values represent means ± S.D. of four independent experiments. * P < 0.05 with respect to H2O2-treated cells.
The mix revealed a significant antioxidant activity at 1 μg/mL (Figure 3(c)). The calculated IC50 values were 17.34 μg/mL and 25.04 μg/mL for OEE and HSE, respectively. Interestingly, the IC50 value of the mixture (17.00 μg/mL) was lower, although not significantly, than the OEE one.
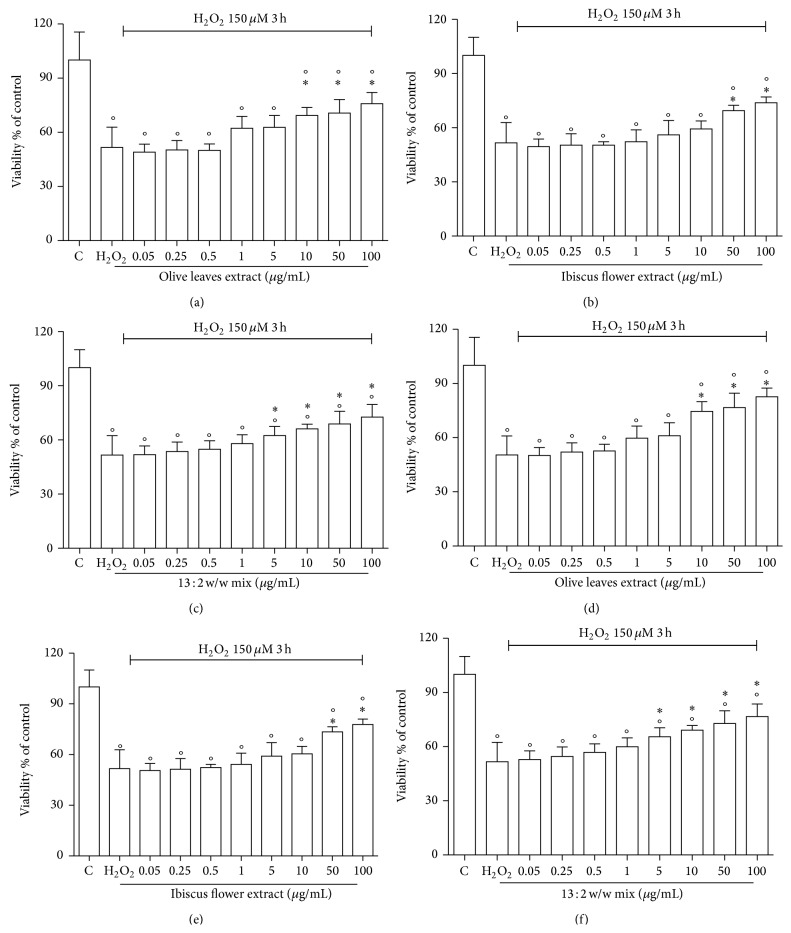
Incubation of HUVECs with 150 μM H2O2 for 3 hours caused a significant decrease in cell viability (Figure 4), as detected by MTT reduction assay. OEE revealed a significant cytoprotective effect at 10 μg/mL (Figure 4(a)) while HSE at 50 μg/mL (Figure 4(b)). The mixture was able to significantly protect cells against H2O2 induced damage at 5 μg/mL (Figure 4(c)), suggesting a synergistic effect in cytoprotection. Flow cytometry analysis confirmed MTT data (Figures 4(d), 4(e), and 4(f)).
Figure 4.
Effect of OEE, HSE, and their mixture on cell viability in HUVECs exposed to H2O2. HUVECs were treated with OEE, HSE, and their mixture (0.05–100 μg/mL) for 24 h; oxidative damage was then induced exposing the cells to 150 μM H2O2 for 3 h and cellular damage was assessed by both MTT assay ((a), (b), and (c)) and flow-cytometry analysis ((d), (e), and (f)). Data are reported as percent cell viability in comparison to control cells. Each bar represents the mean ± S.D. of four independent experiments. * P < 0.05 with respect to H2O2-treated cells, °P < 0.05 with respect to control cells.
4. Discussion
OEE and HSE are rich in phytochemicals, the effects of which have been widely investigated. They have been used in the human diet as an extract, a herbal tea, and a powder. Their bioactive compounds have been demonstrated to have antioxidant, antihypertensive, antiatherogenic, anti-inflammatory, hypoglycemic, and hypocholesterolemic properties [23, 55].
In the present paper, we have focused the attention on the potential application of OEE and HSE in the prevention/counteraction of hypertension, a pathological condition affecting a large number of populations. Hypertension management is a primary factor in the prevention of pathological events as heart attacks, cerebrovascular diseases, and renal failure.
Conventional approach relies on drugs with different biological targets. Oral antihypertensive drugs associated to exercise, lifestyle, and dietary modification are essential approaches for hypertension therapy [1]. However, hypertension control is not always satisfactory [56], and nowadays some patients have turned to natural substances [57], which, as complementary therapy, appear to be promising in reducing blood pressure and relieving signs and symptoms in hypertensive patients [58].
Olea europea L. and Hibiscus sabdariffa L. extracts are known to exert antihypertensive effects [23, 55]. In this paper using both cultured HUVECs and guinea-pig left and right atria and vascular smooth muscle we have demonstrated their antioxidant and cytoprotective effects and their ability to modulate cardiac inotropy and chronotropy together with their relaxant activity on vascular smooth muscle.
Olea europea L. is an evergreen tree typical of the Mediterranean region. Not only olive oil, but also olive leaves revealed antihypertensive properties mainly attributed to the presence of polyphenols (as oleuropein and analogues), due to their L-type calcium channels blocking ability [26, 32]. Oleuropein is therefore a functional analogue of well-known L-Type calcium channel entry blockers such as nifedipine.
Interesting studies on oleuropein and olive polyphenols metabolism and bioavailability have been recently conducted. Suárez et al. identified the presence of oleuropein metabolites in a simulated in vitro model of gastrointestinal digestion [59]. Data on oleuropein bioavailability have been reported by García-Villalba et al., who demonstrated that many phenolic metabolites are detectable in urine sample of women supplemented with 250 mg of an oleuropein-rich olive leaves extract. Moreover the authors showed that some metabolites such as hydroxytyrosol glucuronide are present in urine at micromolar concentration [60]. Absorption of biologically active compounds has been demonstrated after chronic administration of olive leaves extract to rats and mice [61]. de Bock et al. published data showing that oleuropein is bioavailable in the parental form after oral ingestion of an olive leaves extract with a peak plasma concentration in the order of ng/mL [62]. Possible mechanisms have been proposed for oleuropein and oleuropein metabolites absorption; Manna et al. proposed that oleuropein-glycoside may diffuse through the lipid bilayer of the epithelial cell membrane and be absorbed via a glucose transporter. Moreover additional mechanisms for oleuropein-glycoside absorption are potential via the paracellular or transcellular passive diffusion [63].
OEE concentrations used in “in vitro” assays on HUVEC cells are representative of oleuropein and oleuropein metabolite concentrations found in plasma after ingestion of olive leaf extracts.
Data obtained on cardiac guinea pig isolated tissues show that OEE exerted negative inotropic effect on left atrium driven at 1 Hz, without negative chronotropic effect on spontaneous beating right atrium in contrast with nifedipine, which present both negative inotropic and chronotropic effects [64]. Differently from nifedipine, OEE is presumably selective for the Cav1.2 subunit, known as cardiac isoform, widely expressed in the cardiovascular system where it regulates the vascular tone and cardiac inotropy, without effect on Cav1.3 subunit predominantly expressed in neurons and in cardiac pacemaker cells responsible for the chronotropic effect [65]. Similarly to nifedipine, OEE reduced the potassium (80 mM) induced contraction on guinea-pig aorta strips. As far as the vascular and not vascular smooth muscle relaxant activity, OEE is similar to nifedipine. Nifedipine selectivity for vascular smooth muscle over the cardiac parameters has so far allowed its use and that of subsequent 1,4-DHP's generations in the treatment of hypertension [66, 67]. Differently from nifedipine, in OEE, the inotropic effect prevails over the effects on smooth muscle.
Olive (Olea europaea) leaf extract, at the dosage regimen of 500 mg twice daily, was similarly effective in lowering systolic and diastolic blood pressures in subjects with stage-1 hypertension as Captopril, given at its effective dose of 12.5–25 mg twice daily [34].
The OEE concentrations used in “ex vivo” assays on isolated tissue are in line with doses reported in human intervention studies with oleuropein enriched nutritional supplements [34, 68]
Hibiscus sabdariffa L. is a native shrub of tropical Africa. Dried flowers are used to prepare an infusion with soothing and refreshing properties [69]. The hypotensive activity is partly due to its vascular smooth muscle vasorelaxant properties, as shown in Table 3. HSE share common inotropic and chronotropic effects with OEE, but with less potency. HSE, similarly to OEE, shows relaxant effects on the vascular but not on the nonvascular smooth muscle. It has been recently reported that diuresis and inhibition of the angiotensin I-converting enzyme are less important mechanisms to explain the beneficial actions than those related to the antioxidant, anti-inflammatory, and endothelium-dependent effects [70].
These effects could be ascribed to hibiscus acid, the main phytochemical present in hibiscus calyces [71]; moreover hibiscus flowers are rich in anthocyanosides [72], known to increase microvessels resistance and reduce the permeability and damage [73, 74].
Recently Fernández-Arroyo et al. published data regarding bioavailability and metabolism of Hibiscus sabdariffa L. organic acids and polyphenols after oral ingestion in rats. Through HPLC-ESI-TOF-MS analysis, the authors demonstrated that phenolic acids were detectable in plasma without any structural modification; most flavonols were found as quercetin or kaempferol glucuronide conjugates. After oral administration, hibiscus acid, hibiscus acid hydroxyethyl ester, and the metabolite hydroxycitric acid reached high concentrations in plasma, contributing to micromole amounts of organic acids in plasma [75].
To demonstrate that a multifaceted and likely synergistic mechanism accounts for the hypotensive action of OEE and HSE mixture, in vitro study on HUVECs has been performed to evaluate the antioxidant and cytoprotective effects of the two extracts and mixture towards endothelial cells which exert a central role in the regulation of blood pressure.
OEE and HSE revealed cytoprotective and antioxidant properties and OEE extract showed the highest effect. Several authors demonstrated the synergistic effect of many plant extracts administered in polyherbal formulations [75–78]. In this study we have demonstrated that the mixture of the two extracts exerts higher cytoprotective and antioxidant activities than each single extract. Both of these functions are critically involved in cardiovascular protection and hypertension control as clearly reported in the literature. Oxidative stress is also markedly increased in hypertensive patients. If oxidative stress is indeed a cause of hypertension, then antioxidants should have beneficial effects on hypertension control and reduction of oxidative damage should result in a reduction in blood pressure [79].
Another key factor in evaluating the effect of OEE and HSE on the endothelium is their capacity to react to different injuries [80]. Tissue damage at the vascular wall and inflammation leads to stress in the endothelium, so studies on the cytoprotective effect at the endothelium level are of great importance. Some factors have been identified which impair vascular endothelium function, both by their direct effects on the vascular vasomotor capacity, or by influencing cellular regulators, such as inflammatory mediators (ICAM, VCAM). What we currently know about the etiopathogeny of atherosclerosis is that it is a chronic oxidative stress-related inflammatory disease. This study performed on the antioxidant and cytoprotective effects of OEE and HSE throws light on this important point, delineating the cellular mechanism in vascular health protection.
The mixture showed an intrinsic inotropic activity (left atrium) lower than each single extracts, while it exerted negative chronotropic effect; globally the inotropic effect is lower than chronotropic effect, in contrast to nifedipine, characterized by a higher chronotropic than inotropic effect [64]. Despite the low percentage in the mixture, HSE was able to strengthen the effect of OEE, revealing a synergistic cardiac and vascular protective effect. However, even if the amounts of the other phytochemicals are very low, the percentage of HSE is sufficient to reduce the effects of OEE on nonvascular tissue, directing the mixture selectivity towards the vascular tissue.
On the whole, these findings show that the combined treatment of cultured cells, atria, and vascular tissue with the mixture revealed high cardiac and vascular protective activities that, in our specific experimental conditions, were higher than that observed for the most powerful component (OEE).
In conclusion OEE and HSE mixture has a huge potential for pharmaceutical and nutraceutical application, thanks to the synergistic effects of the single phytochemicals.
Acknowledgments
The authors are grateful to Alessandro Casoni for animal care. They are also grateful to University of Bologna and Fondazione del Monte di Bologna e Ravenna for their financial support.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Matteo Micucci and Marco Malaguti contributed equally to the paper.
References
- 1.Chobanian A. V., Bakris G. L., Black H. R., et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.hyp.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Behradmanesh S., Nasri P. Serum cholesterol and LDL-C in association with level of diastolic blood pressure in type 2 diabetic patients. Journal of Renal Injury Prevention. 2012;1:23–26. doi: 10.12861/jrip.2012.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behradmanesh S., Nasri H. Association of serum calcium with level of blood pressure in type 2 diabetic patients. Journal of Nephropathology. 2013;2:254–257. doi: 10.12860/JNP.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Y., Pastrana J. L., Li X., et al. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Frontiers in Bioscience. 2013;18(2):638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World Journal of Cardiology. 2014;6(2):38–66. doi: 10.4330/wjc.v6.i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Ansary L. A., Tricco A. C., Adi Y., et al. A systematic review of recent clinical practice guidelines on the diagnosis, assessment and management of hypertension. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0053744.e53744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrijens B., Vincze G., Kristanto P., Urquhart J., Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. British Medical Journal. 2008;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo L. F., Wu W. H., Zhou Y. J., Yan J., Yang G. P., Ouyang D. S. Antihypertensive effect of Eucommia ulmoides Oliv. extracts in spontaneously hypertensive rats. Journal of Ethnopharmacology. 2010;129(2):238–243. doi: 10.1016/j.jep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Ried K., Frank O. R., Stocks N. P., Fakler P., Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovascular Disorders. 2008;8, article 13 doi: 10.1186/1471-2261-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehkordi F. R., Kamkhah A. F. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundamental and Clinical Pharmacology. 2008;22(4):447–452. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 11.Greenway F., Liu Z., Yu Y., Gupta A. A clinical trial testing the safety and efficacy of a standardized Eucommia ulmoides oliver bark extract to treat hypertension. Alternative Medicine Review. 2011;16(4):338–347. [PubMed] [Google Scholar]
- 12.Ried K., Frank O. R., Stocks N. P. Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. European Journal of Clinical Nutrition. 2013;67(1):64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huseini H. F., Amini M., Mohtashami R., et al. Blood pressure lowering effect of Nigella sativa l. seed oil in healthy Volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytotherapy Research. 2013;27(12):1849–1853. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick D. F., Hirschfield S. L., Coffey R. G. Endothelium-dependent vasorelaxing activity of wine and other grape products. The American Journal of Physiology—Heart and Circulatory Physiology. 1993;265(2):H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 15.Schini-Kerth V. B., Auger C., Kim J.-H., Étienne-Selloum N., Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Archiv. 2010;459(6):853–862. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- 16.Ndiaye M., Chataigneau M., Lobysheva I., Chataigneau T., Schini-Kerth V. B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. The FASEB Journal. 2005;19(3):455–457. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 17.Sarr M., Chataigneau M., Martins S., et al. Red wine polyphenols prevent angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase. Cardiovascular Research. 2006;71(4):794–802. doi: 10.1016/j.cardiores.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Kane M. O., Etienne-Selloum N., Madeira S. V. F., et al. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: preventive effect of red wine polyphenols. Pflugers Archiv. 2010;459(5):671–679. doi: 10.1007/s00424-009-0759-7. [DOI] [PubMed] [Google Scholar]
- 19.Chiarini A., Micucci M., Malaguti M., et al. Sweet chestnut (Castanea sativa Mill.) bark extract: cardiovascular activity and myocyte protection against oxidative damage. Oxidative Medicine and Cellular Longevity. 2013;2013:10. doi: 10.1155/2013/471790.471790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpéné C., Gomez-Zorita S., Deleruyelle S., Carpéné M. A. Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Current Medicinal Chemistry. 2014;22(1):150–164. doi: 10.2174/0929867321666140815124052. [DOI] [PubMed] [Google Scholar]
- 21.Kim T. H., Ku S. K., Lee T., Bae J.-S. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food and Chemical Toxicology. 2012;50(6):2188–2195. doi: 10.1016/j.fct.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 22.Fistonić I., Šitum M., Bulat V., Harapin M., Fistonić N., Verbanac D. Olive oil biophenols and women's health. Medicinski Glasnik (Zenica) 2012;9(1):1–9. [PubMed] [Google Scholar]
- 23.El S. N., Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutrition Reviews. 2009;67(11):632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Geng C., Jiang L., et al. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. European Journal of Nutrition. 2008;47(5):235–243. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]
- 25.Al-Azzawie H. F., Alhamdani M.-S. S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sciences. 2006;78(12):1371–1377. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Somova L. I., Shode F. O., Ramnanan P., Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. Journal of Ethnopharmacology. 2003;84(2-3):299–305. doi: 10.1016/S0378-8741(02)00332-X. [DOI] [PubMed] [Google Scholar]
- 27.Andreadou I., Iliodromitis E. K., Mikros E., et al. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. Journal of Nutrition. 2006;136(8):2213–2219. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- 28.Andreadou I., Sigala F., Iliodromitis E. K., et al. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. Journal of Molecular and Cellular Cardiology. 2007;42(3):549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Seddik L., Bah T. M., Aoues A., Slimani M., Benderdour M. Elucidation of mechanisms underlying the protective effects of olive leaf extract against lead-induced neurotoxicity in Wistar rats. Journal of Toxicological Sciences. 2011;36(6):797–809. doi: 10.2131/jts.36.797. [DOI] [PubMed] [Google Scholar]
- 30.Dekanski D., Janićijević-Hudomal S., Ristić S., et al. Attenuation of cold restraint stress-induced gastric lesions by an olive leaf extract. General Physiology and Biophysics. 2009;28:135–142. [PubMed] [Google Scholar]
- 31.Hamdi H. K., Castellon R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochemical and Biophysical Research Communications. 2005;334(3):769–778. doi: 10.1016/j.bbrc.2005.06.161. [DOI] [PubMed] [Google Scholar]
- 32.Scheffler A., Rauwald H. W., Kampa B., Mann U., Mohr F. W., Dhein S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. Journal of Ethnopharmacology. 2008;120(2):233–240. doi: 10.1016/j.jep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Khayyal M. T., El-Ghazaly M. A., Abdallah D. M., Nassar N. N., Okpanyi S. N., Kreuter M.-H. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittel-Forschung/Drug Research. 2002;52(11):797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- 34.Susalit E., Agus N., Effendi I., et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18(4):251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Sindi H. A., Marshall L. J., Morgan M. R. A. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa . Food Chemistry. 2014;164:23–29. doi: 10.1016/j.foodchem.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 36.Tseng T.-H., Kao E.-S., Chu C.-Y., Chou F.-P., Wu H.-W. L., Wang C.-J. Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food and Chemical Toxicology. 1997;35(12):1159–1164. doi: 10.1016/s0278-6915(97)85468-3. [DOI] [PubMed] [Google Scholar]
- 37.Chen J.-H., Wang C.-J., Wang C.-P., Sheu J.-Y., Lin C.-L., Lin H.-H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chemistry. 2013;141(1):397–406. doi: 10.1016/j.foodchem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Huang C.-N., Chan K.-C., Lin W.-T., Su S.-L., Wang C.-J., Peng C.-H. Hibiscus sabdariffa inhibits vascular smooth muscle cell proliferation and migration induced by high glucoses—a mechanism involves connective tissue growth factor signals. Journal of Agricultural and Food Chemistry. 2009;57(8):3073–3079. doi: 10.1021/jf803911n. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez-Ferrer E., Alarcón-Alonso J., Aguilar-Rojas A., et al. Diuretic effect of compounds from hibiscus sabdariffa by modulation of the aldosterone activity. Planta Medica. 2012;78(18):1893–1898. doi: 10.1055/s-0032-1327864. [DOI] [PubMed] [Google Scholar]
- 40.Ojeda D., Jiménez-Ferrer E., Zamilpa A., Herrera-Arellano A., Tortoriello J., Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa . Journal of Ethnopharmacology. 2010;127(1):7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 41.Ajay M., Chai H. J., Mustafa A. M., Gilani A. H., Mustafa M. R. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. Journal of Ethnopharmacology. 2007;109(3):388–393. doi: 10.1016/j.jep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Herrera-Arellano A., Flores-Romero S., Chávez-Soto M. A., Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11(5):375–382. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Herrera-Arellano A., Miranda-Sánchez J., Ávila-Castro P., et al. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Medica. 2007;73(1):6–12. doi: 10.1055/s-2006-957065. [DOI] [PubMed] [Google Scholar]
- 44.McKay D. L., Chen C.-Y. O., Saltzman E., Blumberg J. B. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. Journal of Nutrition. 2010;140(2):298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 45.Perrinjaquet-Moccetti T., Busjahn A., Schmidlin C., Schmidt A., Bradl B., Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytotherapy Research. 2008;22(9):1239–1242. doi: 10.1002/ptr.2455. [DOI] [PubMed] [Google Scholar]
- 46.Tallarida R. J., Murray R. B. Manual of Pharmacologic Calculations with Computer Programs. 2nd. New York, NY, USA: Springer; 1987. [Google Scholar]
- 47.Caliceti C., Aquila G., Pannella M., et al. 17β-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-notch1 axis in human endothelial cells. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0071440.e71440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angeloni C., Leoncini E., Malaguti M., Angelini S., Hrelia P., Hrelia S. Role of quercetin in modulating rat cardiomyocyte gene expression profile. American Journal of Physiology—Heart and Circulatory Physiology. 2008;294(3):H1233–H1243. doi: 10.1152/ajpheart.01091.2007. [DOI] [PubMed] [Google Scholar]
- 49.Angeloni C., Motori E., Fabbri D., et al. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3K/Akt activation. American Journal of Physiology—Heart and Circulatory Physiology. 2011;300(6):H2196–H2205. doi: 10.1152/ajpheart.00934.2010. [DOI] [PubMed] [Google Scholar]
- 50.Angeloni C., Spencer J. P. E., Leoncini E., Biagi P. L., Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89(1):73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Motulsky H., Christopoulos A. Fitting Models to Biological Data Using Linear and Non Linear Regression. 2003. http://www.graphpad.com/ [Google Scholar]
- 52.Motulsky H. J. Prism 5 Statistics Guide. San Diego, Calif, USA: GraphPad Software Inc.; 2007. http://www.graphpad.com. [Google Scholar]
- 53.Silva S., Gomes L., Leitão F., Coelho A. V., Boas L. V. Phenolic compounds and antioxidant activity of Olea europaea L. Fruits and leaves. Food Science and Technology International. 2006;12(5):385–396. doi: 10.1177/1082013206070166. [DOI] [Google Scholar]
- 54.Quirantes-Piné R., Lozano-Sánchez J., Herrero M., Ibáñez E., Segura-Carretero A., Fernández-Gutiérrez A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochemical Analysis. 2013;24(3):213–223. doi: 10.1002/pca.2401. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins A. L., Lamm M. G., Funk J. L., Ritenbaugh C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia. 2013;85(1):84–94. doi: 10.1016/j.fitote.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redwood H. Hypertension, society, and public policy. European Heart Journal. 2007;9(Supplement B):B13–B18. doi: 10.1093/eurheartj/sum003. [DOI] [Google Scholar]
- 57.Ernst E. Complementary/alternative medicine for hypertension: a mini-review. Wiener Medizinische Wochenschrift. 2005;155(17-18):386–391. doi: 10.1007/s10354-005-0205-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Xiong X. Current situation and perspectives of clinical study in integrative medicine in China. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/268542.268542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suárez M., Romero M.-P., Motilva M.-J. Development of a phenol-enriched olive oil with phenolic compounds from olive cake. Journal of Agricultural and Food Chemistry. 2010;58(19):10396–10403. doi: 10.1021/jf102203x. [DOI] [PubMed] [Google Scholar]
- 60.García-Villalba R., Larrosa M., Possemiers S., Tomás-Barberán F. A., Espín J. C. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: comparison between pre- and postmenopausal women. European Journal of Nutrition. 2014;53:1015–1027. doi: 10.1007/s00394-013-0604-9. [DOI] [PubMed] [Google Scholar]
- 61.Huang P. L., Huang P. L., Lee-Huang S. Oleuropein and related compounds reduce atherosclerosis. The Open Conference Proceedings Journal. 2010;1(1):81–86. doi: 10.2174/22102892010010100081. [DOI] [Google Scholar]
- 62.de Bock M., Thorstensen E. B., Derraik J. G. B., Henderson H. V., Hofman P. L., Cutfield W. S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Molecular Nutrition & Food Research. 2013;57(11):2079–2085. doi: 10.1002/mnfr.201200795. [DOI] [PubMed] [Google Scholar]
- 63.Manna C., Galletti P., Maisto G., Cucciolla V., D'Angelo S., Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Letters. 2000;470(3):341–344. doi: 10.1016/S0014-5793(00)01350-8. [DOI] [PubMed] [Google Scholar]
- 64.Locatelli A., Cosconati S., Micucci M., et al. Ligand based approach to L-type calcium channel by imidazo[2,1-b]thiazole-1,4-dihydropyridines: from heart activity to brain affinity. Journal of Medicinal Chemistry. 2013;56(10):3866–3877. doi: 10.1021/jm301839q. [DOI] [PubMed] [Google Scholar]
- 65.Zuccotti A., Clementi S., Reinbothe T., Torrente A., Vandael D. H., Pirone A. Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends in Pharmacological Sciences. 2011;32(6):366–375. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Ioan P., Carosati E., Micucci M., et al. 1,4-dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (Part 1): action in ion channels and GPCRs. Current Medicinal Chemistry. 2011;18(32):4901–4922. doi: 10.2174/092986711797535173. [DOI] [PubMed] [Google Scholar]
- 67.Carosati E., Ioan P., Micucci M., et al. 1,4-Dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (part 2): action in other targets and antitargets. Current Medicinal Chemistry. 2012;19(25):4306–4323. doi: 10.2174/092986712802884204. [DOI] [PubMed] [Google Scholar]
- 68.Perrinjaquet-Moccetti T., Busjahn A., Schmidlin C., Schmidt A., Bradl B., Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytotherapy Research. 2008;22(9):1239–1242. doi: 10.1002/ptr.2455. [DOI] [PubMed] [Google Scholar]
- 69.Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L.—a phytochemical and pharmacological review. Food Chemistry. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Joven J., March I., Espinel E., et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Molecular Nutrition & Food Research. 2014;58(6):1374–1378. doi: 10.1002/mnfr.201300774. [DOI] [PubMed] [Google Scholar]
- 71.Ramirez-Rodrigues M. M., Plaza M. L., Azeredo A., Balaban M. O., Marshall M. R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa . Journal of Food Science. 2011;76(3):C428–C435. doi: 10.1111/j.1750-3841.2011.02091.x. [DOI] [PubMed] [Google Scholar]
- 72.Camelo-Méndez G. A., Ragazzo-Sánchez J. A., Jiménez-Aparicio A. R., Vanegas-Espinoza P. E., Paredes-López O., del Villar-Martínez A. A. Comparative study of anthocyanin and volatile compounds content of four varieties of Mexican roselle (Hibiscus sabdariffa L.) by multivariable analysis. Plant Foods for Human Nutrition. 2013;68(3):229–234. doi: 10.1007/s11130-013-0360-2. [DOI] [PubMed] [Google Scholar]
- 73.Mian E., Curri S. B., Lietti A., Bombardelli E. Anthocyanosides and microvessels wall: new findings on the mechanism of action of their protective effect in syndromes due to abnormal capillary fragility. Minerva Medica. 1977;68(52):3565–3581. [PubMed] [Google Scholar]
- 74.Ghiringhelli C., Gregoratti L., Marastoni F. Capillarotropic action of anthocyanosides in high dosage in phlebopathic statis. Minerva Cardioangiologica. 1978;26:255–276. [PubMed] [Google Scholar]
- 75.Fernández-Arroyo S., Herranz-López M., Beltrán-Debón R., et al. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Molecular Nutrition and Food Research. 2012;56(10):1590–1595. doi: 10.1002/mnfr.201200091. [DOI] [PubMed] [Google Scholar]
- 76.Petchi R. R., Vijaya C., Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin—nicotinamide induced diabetic wistar rats. Journal of Traditional and Complementary Medicine. 2014;4(2):108–117. doi: 10.4103/2225-4110.126174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aziz N., Mehmood M. H., Mandukhal S. R., Bashir S., Raoof S., Gilani A. H. Antihypertensive, antioxidant, antidyslipidemic and endothelial modulating activities of a polyherbal formulation (POL-10) Vascular Pharmacology. 2009;50(1-2):57–64. doi: 10.1016/j.vph.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Gao J. L., He T. C., Li Y. B., Wang Y. T. A traditional chinese medicine formulation consisting of Rhizoma corydalis and Rhizoma curcumae exerts synergistic anti-tumor activity. Oncology Reports. 2009;22(5):1077–1083. doi: 10.3892/or_00000539. [DOI] [PubMed] [Google Scholar]
- 79.Baradaran A., Nasri H., Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. Journal of Research in Medical Sciences. 2014;19(4):358–367. [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado-Lista J., Perez-Martinez P., Garcia-Rios A., Perez-Caballero A. I., Perez-Jimenez F., Lopez-Miranda J. Mediterranean diet and cardiovascular risk: beyond traditional risk factors. Critical Reviews in Food Science and Nutrition. 2014 doi: 10.1080/10408398.2012.726660. [DOI] [PubMed] [Google Scholar]