Abstract
Background
The Institute of Medicine has listed the comparison of minimally invasive surgical techniques in its research agenda. This study seeks to evaluate a model for the comparison of minimally invasive procedures using patient reported outcomes.
Study Design
A double blinded randomized controlled trial (NCT01489436) was conducted. Baseline data were obtained, standardized anesthesia was induced and patients were randomized to single port (SP) or four port (FP) laparoscopic cholecystectomy. Perioperative care was standardized. Primary outcome was pain (VAS) on postoperative day 1; secondary outcomes were quality of life (PROMIS, LASA), serum cytokines, and heart rate variability. Analysis was intention to treat. Using identical occlusive dressings, patients and outcomes assessor remained blinded until postoperative day 2.
Results
Fifty-five patients were randomized to each arm. There was no difference in demographics. VAS pain score on postoperative day 1 was significantly different from baseline in each group (SP 1.6±1.9 to 4.2 ±2.4 versus FP 1.8±2.3 to 4.2 ±2.2) but not different from each other (p=0.83). Patients in the FP arm reported significantly less fatigue on postoperative day 7 than in the SP group (3.1±2.1 vs 4.2±2.2; p=0.009). Fewer patients in the FP group required postoperative oral narcotics prior to discharge (40% vs 60%, p=0.056). Cytokines levels and heart rate variability were similar between arms. In patients followed > 1 year, no difference in umbilical hernia rates was noted.
Conclusion
Early postoperative quality of life data captured differences in fatigue, indicating improved recovery after FP within a controlled trial. Physiologic measures were similar, suggesting the differences between SP and FP are minimal.
INTRODUCTION
Patients expect efficient medical care with minimal invasiveness and fast recovery.(1) The Institute of Medicine has listed the comparison of minimally invasive surgical techniques in its research agenda. This supports the assumption that comparative effectiveness investigation of minimally invasive surgical procedures may in the future serve as an important tool in the design of health care delivery. This study sought to evaluate a model for the comparison of minimally invasive procedures with each other using the example of single port (SP) and four port (FP) laparoscopic cholecystectomy.
Patient morbidity and mortality after minimally invasive outpatient procedures such as cholecystectomy is generally less than 7%. These traditional outcome measures thus have limited utility as procedure comparators. A number of studies have taken patient-reported outcomes (PRO) such as quality of life (QOL) into account. Patient-reported outcomes measures have generated considerable interest at National Institutes of Health, where a Patient-Reported Outcomes Measures Information System (PROMIS) has been developed and within this system, PROMIS-10, a short global assessment of QOL containing 10 questions.(2) The PROMIS system items are more sensitive to change compared to legacy instruments such as SF-36.(3) Recent research in PRO has also yielded the validated Linear Analog Self-Assessment (LASA) tool, a single item tool that can be used at the bedside. We have previously used both tools and found them responsive to perioperative changes in patients after laparoscopic surgery.(4–5) Several studies have compared SP and FP laparoscopic cholecystectomy previously, including using PRO. However, most studies were small, often underpowered, and did not account for confounders or did not collect preoperative baseline PRO data, making interpretation of the results difficult.(6–7)
To overcome the limitations of traditional outcome measures, some investigators have used biomarkers to compare surgical procedures. Each skin incision generates pain and a neutrophil-mediated immune response with systemic consequences. Leung et al(8) demonstrated significant differences in IL-1b and IL6 serum levels between patients undergoing laparoscopic versus open colectomy. The pro-inflammatory cytokine profile of patients in the laparoscopic group demonstrated significantly less increases than in the open group. Sarli et al(9) demonstrated that smaller laparoscopic trocar incisions led to significantly less pain and analgesic use within the first 24-hours postoperatively. Other studies have not been able to consistently confirm similar differences between groups. Contributing factors for the different reporting are variabilities in specimen procurement and the lack of attention to the influence of sex, age and circadian rhythms on circulating cytokine levels. A recent study with highly variable specimen procurement (± 24hrs) demonstrated differences in IL-6 serum levels between SP and FP cholecystectomy, although statistical significance was not reached with the small sample (n=35, p=0.06).(10)
A further tool to measure stress response in otherwise healthy individuals is heart rate variability.(11) Bickel et al(12) have used the ratio of high frequency (HF) bands and low frequency (LF) bands to compare the physiologic impact of variations in abdominal pressure of patients undergoing laparoscopic cholecystectomy under general anesthesia. His group was also able to show that the type of gas used for insufflation (helium versus CO2) changed the pattern of HF/LF ratio in an otherwise healthy patient cohort, clinically representing the increased peritoneal and systemic acidosis with CO2 pneumoperitoneum compared to helium.(13) Our randomized trial aimed to standardize perioperative care and control for age, gender, circadian rhythm, comorbidities and used a rigorous and validated set of early patient-reported, biologic and physiologic measures to compare two minimally invasive approaches to each other. We hypothesized that PRO would be able to detect clinically relevant differences between two minimally invasive surgical procedures.
METHODS
Informed consent was obtained for this Institutional Review Board approved study (NCT01489436) by a study coordinator blinded to the intervention.
Inclusion criteria were patients scheduled to undergo elective cholecystectomy for symptomatic gallbladder disease at a single center between August 2011 and February 2014. The center performs about 500 laparoscopic cholecystectomy procedures per year, two-thirds of them for elective indications. Exclusion criteria for the study were age <18 years, pregnancy, ASA-class >3, chronic narcotic pain medication, autoimmune disease or immune-modulating therapy, gallbladder cancer, and suspected acute cholecystitis. Patients who could not provide consent for the study or were not willing to participate in the study were also excluded.
After patient enrollment, baseline measurements were obtained, including demographics, Visual Analog Scale (VAS), LASA and PROMIS-10 data and cytokine levels (Table 1). Patients were scheduled for cholecystectomy as first or second case of the day to account for variations in circadian rhythm of the outcome measures. Anesthesia was induced using a standardized bodyweight-based protocol for all patients. Specifically, fentanyl infusion (2mcg/kg IBW), preoperative acetaminophen (1000 mg), intraoperative ketorolac (15 mg), saline irrigation (1 liter), and local anesthetic (0.25 bupivacaine, 30 cc) were utilized. Scheduled postoperative non-narcotic pain medication was used and oral narcotic pain medication as needed. Residents, nursing and anesthesia staff from all perioperative units were made aware of the trial, the individual study patient’s participation and blinding. Information regarding the trial was available on the intranet site, including information about the blinding.
Table 1.
Time Points for the Administration of the Assessment Tools
| Preoperative baseline | Intraoperative | PACU | 4 Hours | Day 1 | Day 7 | |
|---|---|---|---|---|---|---|
| PROMIS Global SF | X | X | X | X | ||
| LASA | X | X | X | X | ||
| VAS | X | X | X | X | X | |
| Blood draw | X | X | X | X | ||
| HRV† | X | |||||
| Morphine equivalents | X | X | X |
HRV, heart rate variability; LASA, Linear Analog Self-Assessment; PACU, Postanesthesia Care Unit; PROMIS Global SF, Patient-Reported Outcomes Measures Information System Global short form; VAS, Visual Analog Scale.
To allow for patient blinding, patient randomization was performed after the induction of anesthesia. Randomization was generated using a computerized randomization program developed by the Division of Biomedical Statistics and Informatics. The randomization was stratified by age, gender, body mass index (BMI) and insulin-dependent diabetes mellitus. After randomization results were displayed on a screen in the operating room, the surgical team opened the appropriate instrumentation.
After induction of anesthesia, the heart rate variability monitor was connected for the duration of the operative procedure. Repeat cytokine measures were obtained and the patient was prepped and draped in sterile fashion.
To decrease variability, a dedicated surgical team with a single surgeon was established to perform the SP and traditional laparoscopic cholecystectomy procedures in a standardized fashion. Prior to starting the trial, the team had performed more than 50 SP cholecystectomy procedures and overcome the described learning curve.(14) Described measures of intraoperative safety parameters, such as the “critical view of safety”, described by Strasberg et al(15) were applied. The technical details for each approach are outlined briefly below.
Single port cholecystectomy
A flexible, multi-sleeve 15-mm trocar (TriPort, Olympus/Advanced Surgical Concepts, Ireland) was inserted at the umbilicus via a 15-mm incision in Hasson technique. A 5-mm flexible-tip laparoscope and standard 5-mm instruments were used. As needed, an additional 2-mm grasping device was placed percutaneously for retraction of the gallbladder fundus. The gallbladder was removed through the wound protector portion of the umbilical port. Four occlusive sterile dressings were applied as for a standard laparoscopic cholecystectomy (Figure 1).
Figure 1.
Placement and image of identical occlusive dressing used for all patients.
Four port laparoscopic cholecystectomy
A 10-mm trocar was placed at the umbilicus via a 15-mm incision in Hasson technique. Three additional separate subcostal 5-mm trocars were placed via separate 5-mm incisions. A 10-mm, 30-degree camera was used for visualization and reusable 5-mm instruments. The gallbladder was removed through the umbilical trocar site using a specimen bag. To maintain patient blinding, identical occlusive dressings (Figure 1) were applied to all patients and removed 48 hours after surgery. Study and statistical staff were blinded to the procedure.
The patient was then transferred to the recovery room where pain assessment was performed on arrival and repeat cytokine measures were drawn one hour after the end of the procedure.
Before discharge, at 4 hours after the procedure, cytokine levels were drawn again and VAS, LASA and PROMIS-10 data obtained. On postoperative day 1, patients were asked to complete PRO assessment. On postoperative day 2, the patients were contacted and asked which procedure they believed they underwent, prior to taking off the band-aids. On postoperative day 7, PRO measures were repeated. The time points for research data collection are summarized in Table 1.
Thirty days after surgery, the patients received a phone call from the clinic nurse to assess for any postoperative complications, which were noted in the electronic medical record. At one year follow-up, records were reviewed to assess for the presence of an umbilical hernia.
For the physiologic data analysis, the blood collected for analysis of inflammatory cytokines was allowed to clot and within 30 minutes serum was separated by centrifugation, aliquoted into labeled microtubes, and frozen on dry ice for storage at −80°F. Once all specimen were collected, they were analyzed together using carbon-anode based ELISA techniques technique in the immune monitoring research unit.
For the heart rate variability data acquisition and analysis, we digitally captured electrocardiogram tracings and respiratory variation using real time data acquisition software developed by our Autonomics Research Group. Data were analyzed for frequency analysis. The ratio between the low and high frequency bands was calculated for each tracing at preset time points (baseline, after induction of anesthesia, during and after the procedure). Changes in the ratio from baseline within and between the groups were compared using repeated measures analysis
PROMIS and LASA
Questionnaires were filled out by the patients during the enrollment and before discharge and collected by the study coordinator. Questionnaires were returned by mail for postoperative day 1, 2 and 7. For statistical analysis, one-half standard deviation is considered the minimally important difference for an item in the PROMIS and LASA tools. For the PROMIS Global Health Short Form, the mean T-score (total score) for a large population (22,000 subjects, corresponding to the 2000 US census population) is set at 50 with the standard deviation of 10.(16)
Power calculation was conducted using pain as measured by the VAS as the primary endpoint. A 15-mm difference on a 100-mm VAS with a standard deviation of 25 mm at any time point is considered a clinically relevant difference.(17–18) To reach this difference with a power of 80% and an alpha of 5%, 45 patients in each arm were needed. Adapting to the slightly higher standard deviation that was seen for this study population after the initial 30 patients, to maintain 80% power and to account for drop-outs, 55 patients were enrolled in each arm.
Data Analysis
Summary statistics such as area under the curve were calculated for each patient using the assessed time points and applied to all PRO endpoints. The area under the curve scores were compared for SP and FP laparoscopic cholecystectomy using supplementary analyses of PRO scores. This approach involved t-tests and Wilcoxon procedures at each time point as well as repeated measures analysis and general estimating equations using SAS. Models included covariates of patient characteristics as well as the treatment arms adjusted for type of surgery to perform conditional analysis of treatment in the presence of potentially confounding variables. For cytokines and heart rate variability, the changes from baseline within and between the groups were compared using mixed-effect regression models along with t-test for specific time points.
RESULTS
From June 2011 to March 2014, patients from the practice of the single surgeon were screened for enrollment and 110 patients were randomized. The consort diagram depicts screened, enrolled, randomized and analyzed patients (Figure 2). A number of patients were excluded because they chose not to undergo cholecystectomy or underwent cholecystectomy along with another procedure (e.g. gynecologic procedure).
Figure 2.
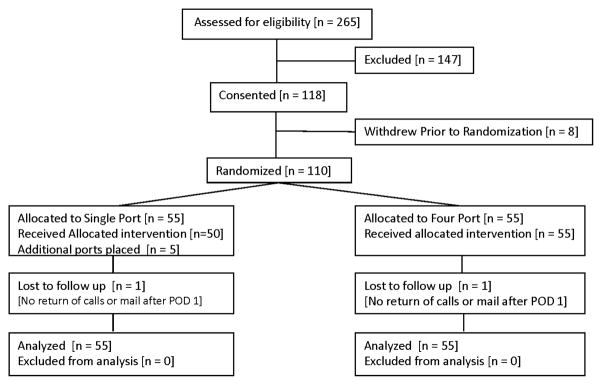
CONSORT chart delineating patient screening and enrollment.
Half of the patients were randomized to SP and half to standard FP cholecystectomy. Five patients of the SP group required additional port placement to complete the procedures. Intent-to-treat analysis was performed, maintaining these patients with their assigned group. Patients were mostly female (81%) with a median age of 47.5 years. Chronic cholecystitis (80%) was the main indication for the procedure. The demographic variables were well balanced between the two arms (Table 2). The mean operative time did not differ significantly between the two arms, 74 (+24) min for the SP group and 71 (± 25) min for the FP group. A total of 10 postoperative complications were noted: 6 (11%) in the FP group and 4 (7.1%) in the SP group. Three complications required additional intervention: 2 (3.6%) in the FP group and 1 (1.8%) in the SP group. In the FP group four patients experienced incision related problems (2 skin site bleeding/hematoma, 1 erythema, 1 wound dehiscence with small bowel incarceration), one patient required endoscopic retrograde cholangiopancreatography for retained common bile duct stones and one patient had abdominal pain for which no further cause was identified. In the SP group, one patient experienced abdominal pain for which no further cause was identified, one patient presented with shortness of breath and possible pneumonia versus atelectasis, one patient experienced postoperative nausea and vomiting and one patient required reoperation for wound dehiscence and bowel incarceration.
Table 2.
Demographics
| Characteristic | Single port (n=55) | 4 Port (n=55) | Total (n=110) | p Value |
|---|---|---|---|---|
| Mean age, y (SD) | 47.3 (16.5) | 49.5 (16.0) | 48.4 (16.2) | 0.4161 |
| Female sex, n (%) | 45 (81.8) | 44 (80.0) | 89 (80.9) | 0.8083 |
| Indication for procedure, n (%) | 0.7841 | |||
| Biliary colic | 53 (96.4) | 53 (96.4) | 106 (960) | |
| Gallbladder polyp | 1 (1.8) | 1 (1.8) | 2 (1.8) | |
| Other | 1 (1.8) | 1 (1.8) | 2 (1.8) | |
| 0.4424 | ||||
| Body mass index, kg/m2, mean (SD) | 32.1 (6.1) | 31.4 (6.1) | 31.7 (6.1) | |
| ASA, n (%) | 0.6988 | |||
| 1 | 9 (16.4) | 6 (10.9) | 15 (13.6) | |
| 2 | 39 (70.9) | 41 (74.5) | 80 (72.7) | |
| 3 | 7 (12.7) | 8 (14.5) | 15 (13.6) | |
| Diabetes, n (%) | 0.3607 | |||
| No | 52 (94.5) | 50 (90.9) | 102 (92.7) | |
| Yes | 0 (0.0) | 2 (3.6) | 2 (1.8) | |
| Insulin dependent | 3 (5.5) | 3 (5.5) | 6 (5.5) |
ASA, American Society of Anesthesiologists.
On postoperative day 2, 106 patients (96%) still had their occlusive bandages in place. Sixty-five patients (59%) believed that they had a SP surgery; whereas, 45 (40%) thought they had a FP cholecystectomy, specifically 47% of patients who underwent FP cholecystectomy believed that they had SP cholecystectomy.
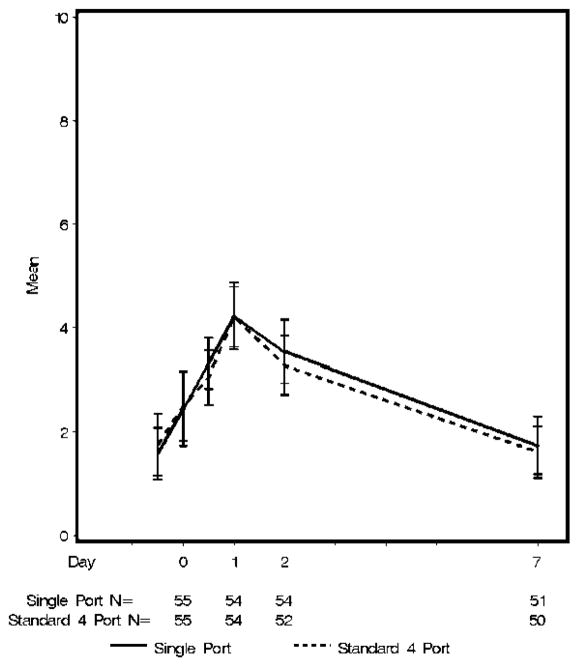
The primary outcome, VAS pain score on postoperative day 1 was significantly different from baseline in each group (SP 1.6±1.9 to 4.2 ±2.4 versus FP 1.8±2.3 to 4.2 ±2.2) but not different from each other (p=0.83) (Figure 3). The pain scores returned to baseline by postoperative day 7 (p=0.056). The mean (median) intravenous morphine-equivalents administered during anesthesia and recovery were similar between both groups: the SP group 39.8 (36) mg and 39.5 (36) mg in the FP group, p=0.97. Thirty-three of the 55 patients (60%) in the SP cholecystectomy group requested oral narcotic pain medication before discharge, as did 22 of 55 patients (40%) in the FP cholecystectomy group (p=0.056). The mean (median) dose of oral narcotic pain medication was similar between groups: 9.8 (10.5) mg morphine-equivalents in the SP group versus 8.5 (10.5) mg morphine-equivalents in the FP group (p= 0.35). There was no difference in the administration of non-narcotic pain medication between the groups; 95% received preoperative acetaminophen, 100% intraoperative local anesthetic, 85% intraoperative ketorolac, 75% postoperative acetaminophen before discharge.
Figure 3.
Visual Analog Scale pain scale by group over time.
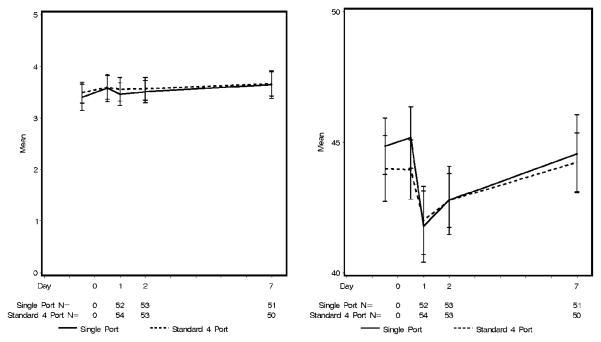
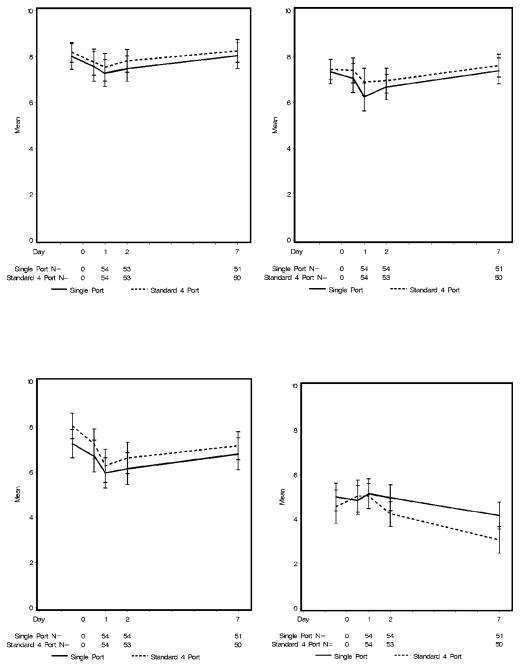
Patient overall QOL at baseline as measured by LASA was similar between both groups (SP 8.0 ±2.1 vs FP 8.2±1.6, p=0.97) as was PROMIS overall health (SP 3.4 ± 1 vs FP 3.5 ± 0.8, p=0.59), PROMIS physical T-score and PROMIS mental T-score. With the exception of lower LASA social wellbeing at baseline (mean 7.3 vs. 8.0, p=0.04) for patients in the SP group, all other baseline PRO scores were also similar between groups. Postoperatively, PROMIS and LASA overall health scores (Figure 4a, Figure 5a), PROMIS mental T-score and LASA mental, emotional, spiritual QOL and anxiety did not change significantly from baseline. Within the groups, PROMIS physical health T-score, LASA pain items, fatigue, physical and social QOL displayed clinically meaningful change (~1/2 SD) from baseline over time. The decrease in PROMIS physical health T-score from baseline to postoperative day 1 was larger in the SP group than in the FP group (−3.7 ±5.3 vs −1.9 ± 4.6, p= 0.11), however, it did not achieve clinical or statistical significance given the large standard deviation (Figure 4b). The same is true for the LASA physical QOL (Figure 5b).
Figure 4.
Patient-Reported Outcomes Measures Information System (A) overall quality of live, and (B) physical T-score by group over time.
Figure 5.
Linear Analog Self-Assessment (A) overall quality of life, (B) physical quality of life, (C) social quality of life, and (D) fatigue by group over time.
Both groups experienced similar clinically meaningful decreases in mean social activity scores from baseline to postoperative day 1. Social activity had almost returned to baseline by postoperative day 7 (Figure 5c).
Patients in the SP group reported significantly higher LASA fatigue scores on postoperative day 7 (4.2 vs. 3.1, p=0.0098), and significantly less decrease in fatigue from baseline to day 7 (−0.6 vs. −1.9, p=0.04) (Figure 5d). Significantly more patients in the SP group reported severe fatigue (score >5) compared to the FP group (49 vs 22 %; p=0.005).
For the heart rate variability analysis, data from 89 of the 110 patients were available. Due to an equipment malfunction or artifacts in the electrocardiogram readings data from 21 patients (19%) were lost, 9 in the SP group and 12 in the FP group. The distribution of demographic factors (age, gender, BMI, insulin dependent diabetes mellitus) for the remainder patients in both groups was similar. Electrocardiogram and respiratory signals were sampled at each QRS peak; digital filtering used required that the data was sampled at equally spaced intervals. Low (0.03–0.14) and high (0.14–0.30) frequency bands using a window size of 512 were compared after induction of anesthesia (base), after positioning in reverse Trendelenburg position (pTrend), after clipping of the cystic duct (Clip) and at the end of the procedure (End) (Table 3). As the low frequency values for the SP group appeared to be approaching statistical significance during the procedures [pTrend_low (medians of 6.1 vs. 4.2, p=0.08), Clip_low (medians of 5.5 vs 3.1, p=0.08], we re-analyzed the data after they were normalized to each patient’s baseline values and no significant differences were noted.
Table 3.
Heart Rate Variability Data Comparison during Cholecystectomy
| Single port (n=43) | 4 Port (n=40) | Total (n=83 | p Value | |
|---|---|---|---|---|
| Age>=65 y, n (%) | 9 (19.6) | 5 (11.6) | 14 (15.7) | 0.3041 |
| Female, n (%) | 38 (82.6) | 36 (83.7) | 74 (83.1) | 0.8886 |
| Insulin-dependent diabetes mellitus, n (%) | 3 (6.5) | 2 (4.7) | 5 (5.6) | 0.7017 |
| BMI >=30 kg/m2, n (%) | 27 (58.7) | 25 (58.1) | 52 (58.4) | 0.9576 |
| Base_low, mean (SD) | 12.8 (12.2) | 10.6 (10.0) | 11.7 (11.2) | 0.5887 |
| Base_high, mean (SD) | 37.3 (45.1) | 35.4 (36.2) | 36.4 (40.7) | 0.6342 |
| pTrend_low, mean (SD) | 14.6 (20.8) | 10.4 (23.0) | 12.6 (21.8) | 0.0802 |
| pTrend_high, mean (SD) | 30.5 (42.8) | 27.8 (54.8) | 29.2 (48.7) | 0.3386 |
| Clip, n | 39 | 36 | 75 | |
| Clip_low, mean (SD) | 10.1 (16.0) | 12.4 (40.5) | 11.2 (30.2) | 0.0858 |
| Clip_high, mean (SD) | 18.7 (18.0) | 22.9 (37.5) | 20.7 (28.9) | 0.6408 |
| End, n | 45 | 39 | 84 | |
| End_low, mean (SD) | 8.6 (11.6) | 8.3 (14.8) | 8.5 (13.1) | 0.2798 |
| End_high, mean (SD) | 45.2 (141.2) | 20.3 (28.4) | 33.7 (105.4) | 0.2778 |
Base_low, low frequency band at baseline; Base_high, high frequency band at baseline;; pTrend_low, low frequency band after achieving reverse Trendelenburg position; pTrend_high, high frequency band after achieving reverse Trendelenburg position; Clip_low, low frequency band after clipping of cystic duct; Clip_high, high frequency band after clipping of cystic duct; End_low, low frequency band at end of procedure; End_high, high frequency band at end of procedure.
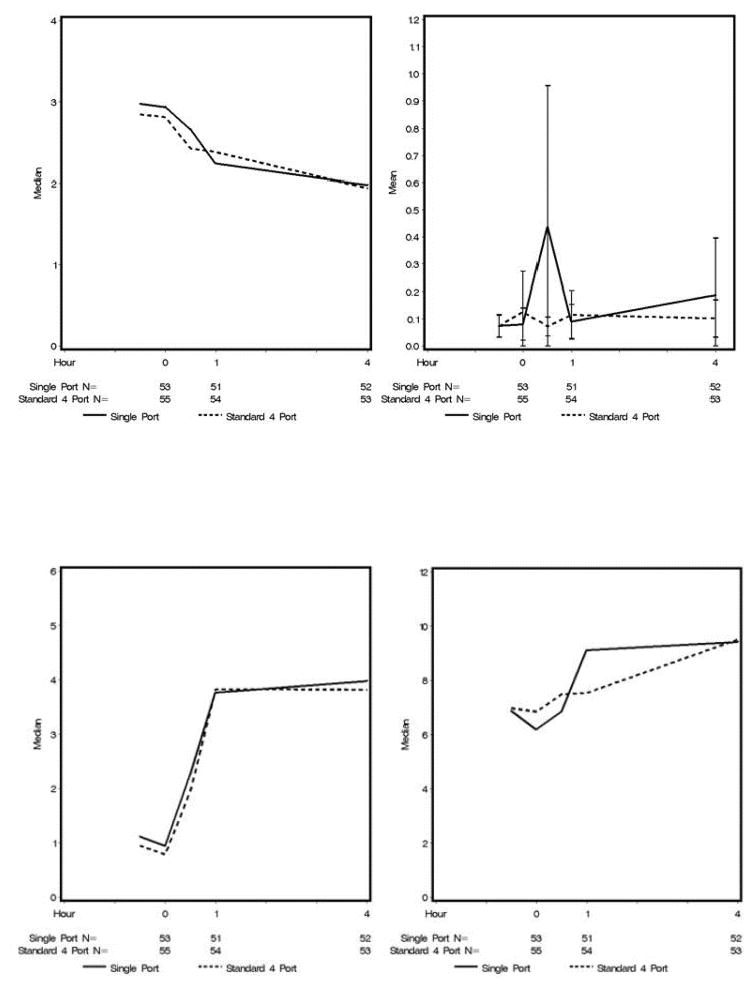
For the cytokines, there were no significant differences at any of the time points (Figure 6). Mean values were plotted for IL-1b values because the medians were all close to zero. Medians were plotted for the other cytokines because there were a few extreme outliers.
Figure 6.
Inflammatory cytokine levels by group over time.(A) TNFa, (B) IL-10, (C) IL-6, and (D) IL-8.
DISCUSSION
This study sought to test a model for the comparison of minimally invasive procedures. Given that gallbladder disease affects more than 750,000 patients per year in the United States and is a well-studied disease, we used the comparison of standard FP laparoscopy and SP cholecystectomy, a relatively new procedure to assess the invasiveness and patient benefit using PRO. In addition to the PRO, we analyzed inflammatory cytokines and heart rate variability as physiologic measures. To allow for comparison in the most controlled fashion, a single surgical team and standardized perioperative care was used, and the patient and study coordinator were blinded for the procedure type until the primary outcome was assessed. Standardization of a single surgical team to reduce variability was strongly favored by the funding agency. Concerns regarding the generalizability of the results are valid; however, the control procedure was performed in a standardized manner following evidence based guidelines accessible and used by the surgical community at large.(17) Thus, individual practitioners can assess how similar their cholecystectomy procedures are to the guidelines and expected outcomes and might estimate what the results of this study mean for their population.
Not surprisingly, there were not significant differences in traditional outcomes, including morbidity, mortality, length of stay and operative duration. In addition, no difference in heart rate variability data was noted. This suggests that the immediate effect of the procedure itself was fairly similar, given that Bickel et al (12–13) had been able to demonstrate statistically significant differences between laparoscopic cholecystectomy groups with high or low intra-abdominal pressure or different gas insufflation. Using highly sensitive analytic methods we also were not able to detect any difference in inflammatory serum cytokine levels although a previous trial appeared to be trending in that direction.(10) In comparison to this previous trial, we were able to enroll a larger number of patients and to control for circadian rhythm, gender, age, time to specimen preparation and other factors. Thus we postulate that there indeed may not be a difference in cytokine levels between procedures. Outlier results will have to be analyzed further.
Overall QOL measures were also similar between the groups and had largely recovered by one week postoperatively. The only postoperative PRO that revealed a clinically meaningful and statistically significant difference between the groups was postoperative fatigue. While this could be due to α-error, the fact that the group with the higher fatigue levels (SP) also seemed to be taking more oral narcotic pain medication before discharge (60% of patients compared to 40%, p=0.056) while maintaining similar pain scores in the postoperative period and otherwise having a similar distribution of factors affecting postoperative pain (e.g. gender, age, BMI, insulin dependent diabetes mellitus), suggests that the narcotic pain medication may have indeed had an influence on the fatigue scores. From a patient perspective, less fatigue may translate into faster recovery, a goal that is shared by many.
The results of our study should be compared with two other large randomized controlled trials on SP versus FP cholecystectomy and other smaller trials.(19–23)
Only one large Danish multicenter trial reported standardized perioperative pain medication a covariate of importance in a trial with pain as the primary or secondary outcome.(20) The Danish study did not find any difference in postoperative pain between patients undergoing SP or multiport cholecystectomy. In the Danish study, no other PRO were collected. A large international multicenter trial reported pain and QOL on SF 8 and 12 for up to one month as secondary outcomes postoperatively.(19) The study team encountered both clinically and statistically different physical QOL scores at two weeks (54.2 ± 6.7 versus 51.5 ± 8.3, p=0.03). They postulate that statistically significant difference in pain in the first postoperative week may contribute to this finding. However, the differences were likely not clinically meaningful (~0.7 cm on a 10 cm VAS) and the pain scores at two weeks were essentially identical for both groups. Thus we conclude that in these other trials and in our study postoperative pain is not clinically meaningfully different between the operative approaches, however the speed of the postoperative recovery (fatigue and physical well-being) is.
The data from this study may assist patients and surgeons in the discussion on how to choose a procedure. If fast postoperative recovery is the most important goal, FP cholecystectomy may be more advantageous than SP laparoscopic cholecystectomy. If cosmesis is the most important factor, SP entry may be chosen. Our study was not powered to evaluate a difference in complications, however, when standardized principles are used, such as providing the critical view of safety described by Strasberg (14), the SP procedure did not appear to have a markedly different safety profile than FP cholecystectomy. Additional factors that may influence the choice of approach are the ergonomic factors involved in SP laparoscopic cholecystectomy or cost. A companion study revealed that the small umbilical access site significantly increases physical surgeon workload.(24) While we did not perform a formal cost/charge analysis in this trial, we compared cost at the introduction of SP cholecystectomy at our institution. When using standard laparoscopic instruments for both procedures, the cost of the SP trocar was offset by eliminating the specimen bag, thus the cost for both procedures was comparable.
Acknowledgments
Support: Research reported in this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK93553 and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences.
ABBREVIATIONS
- SP
single port
- FP
four port
- QOL
quality of life
- PRO
patient-reported outcomes
- LASA
Linear Analog Self-Assessment
- PROMIS
Patient-Reported Outcomes Measures Information System
- HF
high frequency
- LF
low frequency
- VAS
Visual Analog Scale
- BMI
body mass index
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Bingener is on the board of Titan Medical, Inc., and received grants from Nestle and Stryker Endocsopy and meeting expenses from Intuitive Surgical.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Presented at the Western Surgical Association 122nd Scientific Session, Indian Wells, CA, November 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detsky A. What patients really want from health care. JAMA. 2011;306:2500–2501. doi: 10.1001/jama.2011.1819. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Health. Patient-Reported Outcomes Measurement Information System. 2004 [Google Scholar]
- 3.Fries J, Rose M, Krishnan The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011:38. doi: 10.3899/jrheum.110402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingener J, Sloan JA, Seisler DK, et al. PROMIS for Laparoscopy. J Gastrointest Surg Conditional Acceptance. 2015 doi: 10.1007/s11605-015-2789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antiel RM, Dupont S, Samaha M, et al. Patient Reported Outcomes Following Laparoscopic Ventral Hernia Repair Abstract. Oral presentation at Society of American Gastrointestinal and Endoscopic Surgeons; Salt Lake City, UT. 2014. [Google Scholar]
- 6.Bingener J, Ghahfarokhi LS, Skaran P, Sloan J. Responsiveness of quality of life instruments for the comparison of minimally invasive cholecystectomy procedures. Surg Endosc. 2013;27:2446–2453. doi: 10.1007/s00464-012-2756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efficace F, Bottomley A, Osoba D, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials--does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21:3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 8.Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: A prospective randomized trial. Ann Surg. 2000;231:506–511. doi: 10.1097/00000658-200004000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarli L, Iusco D, Gobbi S, et al. Randomized clinical trial of laparoscopic cholecystectomy performed with mini-instruments. Br J of Surg. 2003;90:1345–1348. doi: 10.1002/bjs.4315. [DOI] [PubMed] [Google Scholar]
- 10.McGregor CG, Sodergren MH, Aslanyan A, et al. Evaluating systemic stress response in single port vs. multi-port laparoscopic cholecystectomy. J Gastrointest Surg. 2011;15:614–622. doi: 10.1007/s11605-011-1432-y. [DOI] [PubMed] [Google Scholar]
- 11.Jan BU, Coyle SM, Oikawa LO, et al. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: a randomized controlled trial. Ann Surg. 2009;249:750–756. doi: 10.1097/SLA.0b013e3181a40193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bickel A, Yahalom M, Roguin N, et al. Power spectral analysis of heart rate variability during positive pressure pneumoperitoneum: the significance of increased cardiac sympathetic expression. Surg Endosc. 2002;16:1341–1344. doi: 10.1007/s00464-001-9211-6. [DOI] [PubMed] [Google Scholar]
- 13.Bickel A, Kukuev E, Popov O, et al. Power spectral analysis of heart rate variability during helium pneumoperitoneum: The mechanism of increased cardiac sympathetic activity and its clinical significance. Surg Endosc. 2005;19:71–76. doi: 10.1007/s00464-003-9304-5. [DOI] [PubMed] [Google Scholar]
- 14.Kunkala M, Bingener J, Park M, et al. Single-port and four-port laparoscopic cholecystectomy: difference in outcomes. Minerva Chir. 2013;68:155–162. [PubMed] [Google Scholar]
- 15.Strasberg S. Avoidance of biliary injury during laparoscopic cholecystectomy. Journal of Hepato-Biliary-Pancreatic Surgery. 2002;9:543–547. doi: 10.1007/s005340200071. [DOI] [PubMed] [Google Scholar]
- 16.Revicki D, Hays R, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H, Gray A, Bonnet F, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following laparoscopic cholecystectomy. Surg Endosc. 2005;19:1396–1415. doi: 10.1007/s00464-004-2173-8. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008;101:700–704. doi: 10.1093/bja/aen244. [DOI] [PubMed] [Google Scholar]
- 19.Phillips MS, Marks JM, Roberts K, et al. Intermediate results of a prospective randomized controlled trial of traditional four-port laparoscopic cholecystectomy versus single-incision laparoscopic cholecystectomy. Surg Endosc. 2012;26:1296–1303. doi: 10.1007/s00464-011-2028-z. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen LN, Rosenberg J, Al-Tayar H, et al. Randomized clinical trial of single-versus multi-incision laparoscopic cholecystectomy. Br J Surg. 2014;101:347–355. doi: 10.1002/bjs.9393. [DOI] [PubMed] [Google Scholar]
- 21.Emre Telciler K, Ilhan E, Yakan S, et al. Single-port laparoscopic cholecystectomy versus the classical four port laparoscopic cholecystectomy: a randomized prospective clinical trial. Minerva Chir. 2014;69:1–7. [PubMed] [Google Scholar]
- 22.Sodergren MH, Aslanyan A, McGregor CG, et al. Pain, well-being, body image and cosmesis: a comparison of single-port and four-port laparoscopic cholecystectomy. Minimally invasive therapy & allied technologies : MITAT. 2014;23:223–229. doi: 10.3109/13645706.2014.886594. [DOI] [PubMed] [Google Scholar]
- 23.Khorgami Z, Shoar S, Anbara T, et al. A randomized clinical trial comparing 4-port, 3-port, and single-incision laparoscopic cholecystectomy. J Invest Surg. 2014;27:147–154. doi: 10.3109/08941939.2013.856497. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed AO, Bingener J, Lowndes B, et al. Modified NASA Workload Tool Identifies Physical and Cognitive Surgeon Workload for Laparoscopic Procedures. Oral presentation at the American College of Surgeons Clinical Congress; 2014; San Francisco, CA. 2014. [Google Scholar]