Abstract
The large reservoir of human latent tuberculosis (TB) contributes to the global success of the pathogen, Mycobacterium tuberculosis (Mtb). We sought to test whether aerosol infection of rabbits with Mtb H37Rv could model paucibacillary human latent TB. The lung burden of infection peaked at 5 weeks after aerosol infection followed by host containment of infection that was achieved in all rabbits. One-third of rabbits had at least one caseous granuloma with culturable bacilli at 36 weeks after infection suggesting persistent paucibacillary infection. Corticosteroid-induced immunosuppression initiated after disease containment resulted in reactivation of disease. Seventy-two percent of rabbits had culturable bacilli in the right upper lung lobe homogenates compared to none of the untreated controls. Discontinuation of dexamethasone led to predictable lymphoid recovery, with a proportion of rabbits developing multicentric large caseous granuloma. The development and severity of the immune reconstitution inflammatory syndrome (IRIS) was dependent on the antigen load at the time of immunosuppression and subsequent bacillary replication during corticosteroid-induced immunosuppression. Clinically, many aspects were similar to IRIS in severely immunosuppressed HIV-infected patients who have functional restoration of T cells in response to effective (highly active) antiretroviral therapy. This corticosteroid model is the only animal model of the IRIS. Further study of the rabbit model of TB latency, reactivation and IRIS may be important in understanding the immunopathogenesis of these poorly modeled states as well as for improved diagnostics for specific stages of disease.
Keywords: Tuberculosis, rabbit, animal models, latency, reactivation, IRIS, immune reconstitution
Introduction
Mycobacterium tuberculosis (Mtb) is a successful pathogen because it can persist for years in humans who manifest no clinical signs of infection. The tenacity of the tubercle bacillus was demonstrated in old published data where it was shown that inactive tuberculous lesions from clinically well patients, who died from other causes, contained enough bacilli to cause disease in the susceptible guinea pig 1. Other reports showed that pulmonary lesions from treated and untreated patients with inactive disease still contained visible acid-fast bacilli, that were non-cultivatable 2, 3. Evidently, human latent tuberculosis is a paucibacillary disease in which the persistent bacillus varies in its ability to be histologically visible, cultivatable, or capable of causing disease in a guinea pig.
Various animals and human pathologic specimens have been used as models of latency 4. In mice, the number of bacteria in the organs plateau with acquisition of specific immunity. During chronic infection, many culturable bacilli remain, but, if untreated, the mice would ultimately die of chronic infection and progressive inflammation. In the Cornell model of latent TB, mice were treated with isoniazid and pyrazinamide to achieve culture-negative disease. This paucibacillary latent state could be reactivated with corticosteroids 5, 6. Hence, this model does not reproduce human latent TB because culture-negative persistence was attained with chemotherapy, and not by host immunity. Non-human primates have also been employed to reproduce latent TB. After a very low-dose intratracheal infection, approximately 40% of cynomolgus monkeys developed clinically quiescent disease with little gross pathologic evidence of infection 7. This latent state is metastable, however, and can reactivate easily when the animals are stressed, and can occur without exogenous immunosuppression.
In 1928, Lurie first showed the resistance of outbred market rabbits to intravenous infection with Mtb. In his model, lung CFU peaked at 4 weeks, but by 4 months after infection, all animals tested had culture-negative whole lung homogenates. Interestingly, at 6 months, 2 of 3 animals had a few culturable colonies obtained from homogenized lungs, suggesting that bacilli were persisting at a very low level 8. Rabbits are the most resistant animal model to Mtb infection and occupy an important niche, because humans are also relatively resistant; the majority of exposed infected individuals contain infection with only a minority of infected people who progress to active tuberculosis infection (~10%). We have previously shown the rabbit's ability to differentiate Mtb on a virulence spectrum 9 as well as to model the effects of malnutrition on the course of tuberculosis 10. We present, herein, the results of 2 long-term aerosol infections with Mtb H37RV with confirmation of initial replication of bacilli, clearance of visible granulomas over time with approximately one-third of rabbits with persistent caseous granulomas that are culture-positive. The majority of rabbits developed culturable disease with the intramuscular administration of corticosteroids. Discontinuation of corticosteroids resulted in immune recovery and the development of immune reconstitution inflammatory syndrome (IRIS) in some of the rabbits that was dependent on the burden of Mtb antigens in the lung.
Materials & Methods
Microorganisms
Mtb H37Rv strain (kind gift of Dr. David Sherman) was passaged twice in mice and then used in this study. Mycobacteria were cultivated in 7H9 Middlebrook liquid media supplemented with oleic acid albumin, dextrose and catalase (OADC, Becton Dickenson, Inc., Sparks, MD), 0.2% glycerol, and 0.05% Tween 80 9.
Animals and Infection
Pathogen-free New Zealand White rabbits were purchased from Covance Research Products, Inc. (Denver, PA.), housed either at George Washington University Medical Center (GWUMC) or Johns Hopkins University (JHU), aerosol-infected with Mtb at United States Army Research Institute of Infectious Diseases (USAMRIID) by previously published methods 9, transported back to GWUMC or JHU, and housed under Biosafety Level-3 (BSL-3) conditions according to established and approved protocols at GWUMC, Johns Hopkins University, and USAMRIID. In the first experiment, 3 animals were necropsied on the first day after aerosol exposure, and then 6 rabbits every 5 weeks until 20 weeks as previously published 9, 11. In the second experiment, 3 animals were necropsied at 5, 10, and 15 weeks. At weeks 25 and 36, six infected rabbits (that were not treated with corticosteroids) were necropsied at each time point. The right upper lobes of the lungs were homogenized in 5 ml of phosphate-buffered saline (PBS), and colony forming units were enumerated. In addition, granulomas noted in other lobes at weeks 25 and 36 were individually excised, homogenized in 500μl of PBS and the entire homogenate was plated on 7H10 agar. Impinger concentrations of organism during aerosol infection and each rabbit's minute ventilation measured with plethysmography were used to calculate the number of bacilli inhaled, as previously described 9. Twelve rabbits (higher-dose experiment) and 21 rabbits (lower-dose experiment) were treated with intramuscular dexamethasone from week 10 to week 15 as previously published 10. Four weeks after aerosol infection, all animals were skin tested with Old Tuberculin (Wyeth Lederle, Pearl River, NY). Delayed type hypersensitivity (DTH) reactions were read at 48 hours by previously published methods 9.
Four Hartley guinea pigs were each injected intraperitoneally with 1 ml of defrosted frozen rabbit lung homogenate (which was culture-negative on 7H10 Middlebrook agar). The guinea pigs were maintained in BSL-3 containment for 8 weeks and monitored clinically (weight and appearance). The animals were skin tested with 100 TU PPD after 8 weeks. At the conclusion of 8 weeks, the animals were all euthanized and necropsied according to previously established protocols 12.
Flow cytometry
In the first, higher-dose experiment granulomas were excised, weighed, cut into small pieces, treated with 50ug/ml Liberase RI (Roche Diagnostic, Alameda, CA), and incubated at 37°C for 1 hour. Granuloma pieces and whole, hilar lymph nodes were then individually sieved to obtain single cell suspensions, washed three times with HBSS containing 1% fetal bovine serum, and then suspended in complete RPMI 1640 containing 10% fetal bovine serum, glutamine, HEPES, penicillin, streptomycin, and Fungizone. The suspensions were centrifuged, washed, fixed with 4% paraformaldehyde and incubated with fluorescence-conjugated antibodies to CD4, CD8, CD11b, CD11c (Antigenix America, Huntington Station, NY), Class I MHC, Class II MHC, or IgM (Serotec, Raleigh, NC) to compare the relative proportions of different cell populations by flow cytometry.
Peripheral blood was collected at the time of necropsy and placed immediately in tubes containing sodium citrate. In brief, 1 ml of blood was fixed with fixative/lysis solution (10 g/L paraformaldehyde; 10.2 g/L cacodylic acid; 6.65 g/L sodium chloride; pH 7.2); unlysed cells were washed twice with PBS-BSA buffer (PBS supplemented with 0.5% BSA and 0.1% of sodium azide). Cells were stained in a 96-well round bottomed microplate (Nalge Nunc Intl., NY, USA) with CD4, CD8, CD11b, CD11c, B cell, MHC Class I, MHC Class II antibodies for 30 minutes on ice. The data on fluorescently labeled cells were acquired in a FACScalibur flow cytometer (Becton Dickinson, CA, USA). Fifty thousand events were counted.
Statistical analysis
All comparisons of non-normally distributed continuous data were analyzed with the Mann-Whitney U test using Statview 5.0.1 (SAS, Cary, N.C.) or Prism (GraphPad Software, CA). P value≤ 0.05 was considered statistically significant.
Results
Long-term infection with Mtb H37Rv uniformly results in paucibacillary disease
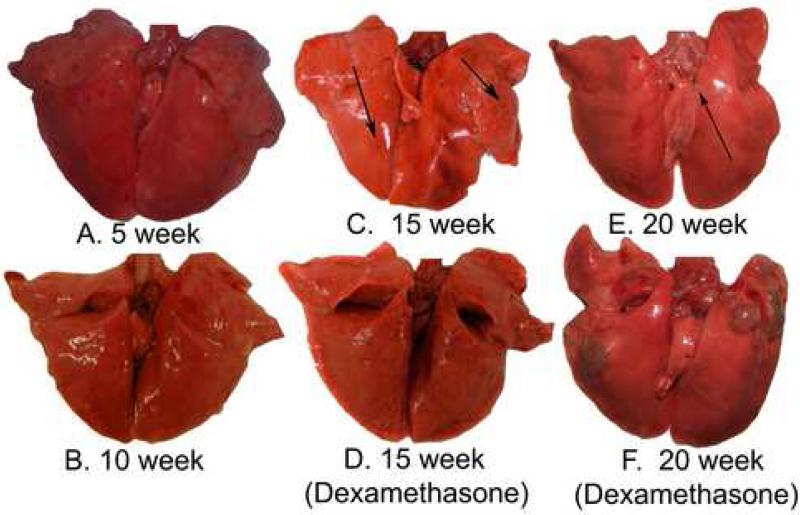
In the first higher-dose aerosol experiment, 42 rabbits were aerosol-infected with Mtb H37Rv (106 CFU original suspension in nebulizer) with an average inhaled dose of 3.57 ± 0.05 logCFU calculated from the plethysmography. All rabbits (except 3 necropsied on day 1 after infection) had a skin test response to tuberculin (mean ± SE, 352 mm3 ± 106) 4 weeks after aerosol infection. Bacillary numbers were increased at 5 weeks compared to day 1 after aerosol infection consistent with disease establishment and replication (Table 1, Figure 1). In the second lower-dose experiment, 42 rabbits were aerosol-infected with an average inhaled dose of 3.17 ± 0.21 logCFU. Again, all rabbits had a skin test response to tuberculin (mean ± SE, 114 mm3 ± 85) 4 weeks after infection. In both experiments, both the number of tubercles and the bacillary burden in the lungs decreased significantly over time (Table I, Figure 1). Interestingly in both experiments, at the latest time points, the number of tubercles was small, but the remaining tubercles had relatively large diameters, suggesting that the remaining tubercles at this time point were not healing (Table 1, Figure 2E).
Table I.
Disease parameters associated with Mtb H37Rv aerosol infection of New Zealand White rabbits.
| No. Weeks after aerosol | No. of rabbits | No. of tubercles | Tubercle diameter (mm) | Lung weight (g) | Right upper Lobe CFU (median [range]) | No. rabbits culture-negative at any site |
|---|---|---|---|---|---|---|
| Higher-Dose Aerosol | ||||||
| Day 1 | 3 | 0 | 0 | 9.7 ± 0.4 | 80 [70-95] | 0/3 |
| Week 5 | 6 | 187 ± 18 | 1.5 ± 0.1 | 13.8± 1.3 | 4688 [2800-26,750] | 0/6 |
| Week 10 | 6 | 105 ± 49 | 1.0 ± 0.1 | 14.0 ± 0.5 | 1138 [125-20,250] | 0/6 |
| P=0.09 | P=0.009 | |||||
| Week 15 | 6 | 9 ± 5 | 1.0 ± 0.3 | 14.0 ± 0.9 | 0 [0-2597] | 4/6 |
| P=0.002 | P=0.06 | P=0.01 | ||||
| Week 20 | 6 | 4 ± 1 | 1.3 ± 0.3 | 13.0 ± 0.5 | 75 [0-325] | 3/6 |
| P=0.002 | P=0.31 | P=0.004 | ||||
| Lower-Dose Aerosol | ||||||
| Week 5 | 3 | 47 ± 9 | 2.0 ± 0.2 | 13.7 ± 0.7 | 5800 [2075-24,500] | 0/3 |
| Week 10 | 3 | 7 ± 5 | 1.2 ± 0.2 | 14.6 ± 0.8 | 0 [0-75] | 2/3 |
| P=0.05 | ||||||
| Week 15 | 3 | 17 ± 8 | 0.8 ± 0.1 | 12.2 ± 1.0 | 125 [100-750] | 0/3 |
| P=0.05 | ||||||
| Week 25 | 6 | 1 ± 1 | 0.8 ± 0.3 | 12.6 ± 1.2 | 0 | 4/6* |
| Week 36 | 6 | 1 (1 rabbit) | 2.0 | 11.7 ± 0.2 | 0 [0-150] | 4/6** |
| P=0.02 | ||||||
P values compared to 5 week values except for those denoted with an asterisk which are compared to the untreated control at the same time point
No rabbits had culture (+) right upper lung lobe, but 2 rabbits had a single granuloma that was culture (+)
One rabbit had culture (+) right upper lung lobe, and one other rabbit had culture (+) hilar lymph node
Figure 1.
Lung TB burden at time intervals after aerosol infection. A) Number of grossly visible granuloma in the lungs of rabbits sacrificed at different time intervals (shown on the X axis). Higher-dose aerosol experiment is shown in black circles. Lower-dose aerosol experiment is shown in grey squares. B) Mean number of CFU (± SE) in the entire right upper lobe of the rabbits sacrificed at different time intervals. The solid line shows the mean log10CFU in Mtb H37Rv-infected untreated rabbits at each time point in the higher-dose experiment. The dotted line shows the results from the lower-dose aerosol experiment. P-values are compared to the 5 week log10CFUs of the same experiment. In the lower-dose experiment, there were fewer granulomas established and the clearance of both granulomas and bacilli was more rapid.
Figure 2.
Gross pathology of rabbit lungs at A) 5 weeks after aerosol infection with Mtb H37Rv. Note the multiple caseous lesions in all lung lobes. B) At 10 weeks after infection, a few active lesions with caseous necrosis are seen. C) At 15 weeks after infection, a few abortive tubercles are observed noted by arrows. D) At 15 weeks after infection, and following 5 weeks of high dose dexamethasone treatment, no lesions are seen due to lymphoid depletion. E) At 20 weeks after aerosol infection, 1 active caseous lesion is seen at arrowhead. F) At 20 weeks after infection, and following treatment with dexamethasone (from weeks 10 to15) and immune reconstitution (from weeks 15 to 20), large tubercles with multiple caseous foci are seen with high bacillary numbers consistent with IRIS.
In summary, after aerosol infection, Mtb establishes disease in rabbits with containment of the infection to a paucibacillary state. A lower inhaled dose results in fewer established granulomas at 5 weeks with more rapid containment of disease. At both doses, larger caseous tubercles remained in a proportion of the rabbits at all time points after 15 weeks (up to 36 weeks,) after aerosol infection.
Bacillary persistence up to 36 weeks after aerosol infection in rabbits
We observed that, in the higher-dose experiment, 4 of the right upper lobe lung homogenates were culture-negative by agar dilution 15 weeks after aerosol infection (in rabbits that were not treated with corticosteroids). In these rabbits, there were no grossly visible granulomas in the lobe that was homogenized. Injection of 1ml of each of two of the 4 rabbit lung homogenates (4 ml out of 10 ml total) intraperitoneally into four guinea pigs did not result in conversion of the tuberculin skin test or in any grossly detectable disease in any of these animals when necropsied at 8 weeks. These data suggest that most bacillary CFU are contained within granulomas in the lung and cannot be found outside the lesions, since none of these animals had any grossly visible granulomas in the right upper lung lobe, were culture-negative on agar, and lung homogenate did not cause disease when inoculated into guinea pigs. At 20 weeks, 3 of 6 rabbits analyzed had a culture-negative right upper lung lobe homogenate and the 3 culture-positive rabbits had a further decline in CFU compared to 15 weeks after infection (Table 1).
In the lower-dose experiment, 2 of 3 rabbits became culture-negative in the right upper lung lobe by week 10 with all rabbit lung homogenates culture-positive at week 15, but at very low levels. Again, because bacilli appear to persist in lung granulomas only, culture positivity was dependent on the number of granuloma in the right upper lobe. This cohort of rabbits infected with a lower-dose aerosol was followed for 36 weeks. Lung granulomas were culture-positive in 2 of 6 rabbits at 25 weeks. At 36 weeks after infection, 1 rabbit had a culture-positive granuloma, the other rabbit had RUL homogenate that was culture-positive and a draining hilar lymph node with culturable bacilli, again suggesting bacillary persistence in this model. The proportion of rabbits with culturable bacilli may have been higher if the entire lung had been homogenized for each rabbit in the experiment since other lobes may have had smaller granuloma that were not grossly appreciated at the time of necropsy.
In summary, we have shown for the first time, and in 2 separate experiments, that Mtb persists in a proportion of the rabbits (3/6 and 2/6 in the higher- and lower-dose, respectively) up to 36 weeks after low-dose aerosol infection.
Flow cytometric analysis of cells from peripheral blood, draining hilar lymph nodes, and lung granulomas of rabbits infected with Mtb H37Rv strain in the higher-dose experiment
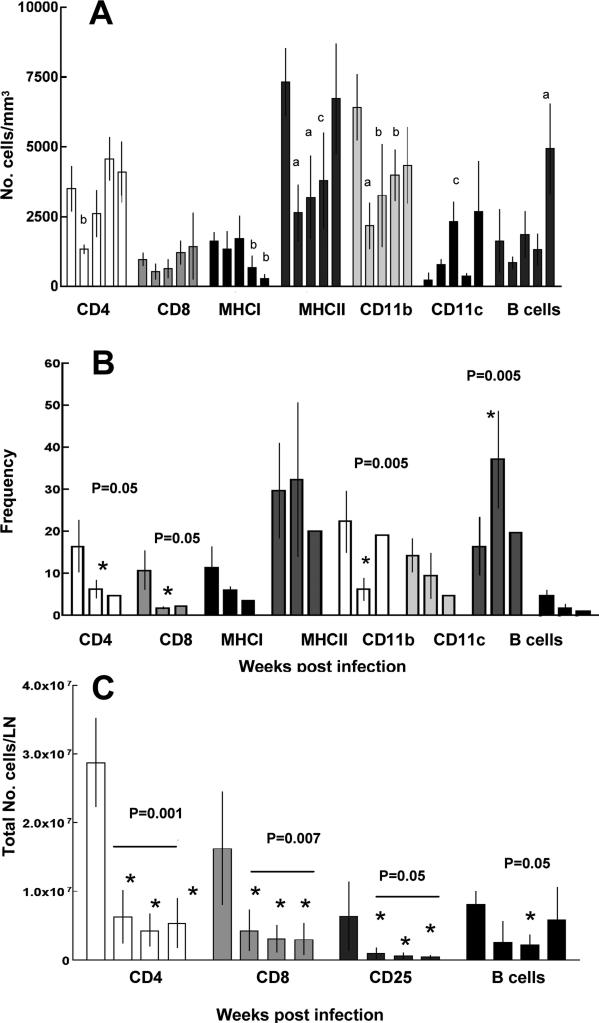
To characterize the cells that were present in peripheral blood, hilar lymph nodes, and lung granulomas at the various time points after aerosol infection, single cell suspensions were analyzed by flow cytometry. In peripheral blood, the absolute number of CD4+ T cells and MHC Class II-positive cells significantly decreased 5 weeks after infection compared to blood from uninfected rabbits (P=0.05, Figure 3A). As infection progressed, the numbers of CD4+ T cells/ml in the peripheral blood returned to baseline by 20 weeks of infection. Interestingly, the absolute number of B cells decreased slightly 5 weeks after infection, but markedly increased to a significantly higher level at 20 weeks (P= 0.001). In contrast, the converse was observed in granulomas collected from infected lungs at 5, 10 and 15 weeks (not enough granulomas were present at 20 weeks for analysis). The proportion of CD4+ T cells was highest early in infection (5 weeks, P=0.05), and then decreased at both 10 weeks and decreased further at 15 weeks (Figure 3B). The patterns of absolute cell numbers in the draining hilar lymph node (as well as the frequencies of cells expressing various cell surface markers [data not shown]) paralleled those in the lung granuloma (Figure 3C). Because so few granuloma were present after week 5 in the lower-dose experiment, this flow cytometric comparison of cell types was not possible.
Figure 3.
Flow cytometric analysis of A) Number of cells/mm3 expressing each cell surface marker shown on X-axis in peripheral blood pre-treatment and at 5, 10, 15 and 20 weeks (left to right) after infection. Statistically significant differences are denoted with the letter “a” for P=0.001, “b” for P=0.07 and “c” for P=0.05 when compared to the values of the uninfected rabbits (left bar). B) Frequency (percentage) of cells expressing particular cell surface markers in single cell suspensions of granulomas from infected lungs at weeks 5, 10 and 15 (left to right) and C) Total number of cells expressing particular cell surface markers in a draining hilar lymph node from weeks 5, 10, 15 and 20 (left to right). Asterisk denotes statistically significant differences compared to week 5. Each time point shows the mean ± SE of 6 rabbits.
Immune reconstitution after dexamethasone treatment is associated with increased bacillary burden in lungs
To observe the effect of steroid treatment on the course of infection, we treated rabbits with daily intramuscular dexamethasone injections from week 10 to week 15 following aerosol infection with H37Rv. A total of 12 rabbits in the higher-dose experiments and 21 rabbits in the lower-dose aerosol experiment were subjected to the steroid regimen. At 10 weeks, the average number of tubercles was already lower compared to 5 weeks after infection (higher-dose experiment, lower-dose experiment; P=0.09, P=0.05), suggesting that the acquired immune response elicited by the infection was controlling disease progression (Figure 1, Table I).
After the dexamethasone treatment that induced lymphopenia in the rabbits, corticosteroids were discontinued and the rabbits were allowed to “immune reconstitute” for the next 5 weeks in the first higher-dose aerosol experiment. The remaining 6 dexamethasone-treated rabbits were culture-positive 5 weeks after the withdrawal of steroids, suggesting that viable bacilli likely existed in all rabbits when the steroid treatment was stopped (which may not have been reflected in homogenization of a single lung lobe at week 15 when 4 of 6 rabbits were culture-negative). The bacillary burdens in these dexamethasone-treated rabbits were significantly higher than that in the untreated controls (median [range]; 1356 [375-71,000] vs. 75 [0-325]; P=0.002) (Tables I and II). Two of the 6 rabbits developed large tubercles consistent with immune reconstitution and worsening of TB that were not seen in any of the control rabbits (Figure 2F). In particular, in the two rabbits that had the largest tubercles, the CD4+ T cell count had increased from a mean of 639 ± 79 cells/ml (mean ± SE) at the conclusion of dexamethasone therapy (significantly decreased compared to untreated controls at the same time point, P=0.002) to 2925 and 2604 cells/ml respectively (Figure 4). A third rabbit had intermediate-sized tubercles in the left upper lobe, but had not yet had significant rebound in CD4+ T cell counts. The rabbits without abnormally large tubercles had only 1260 ± 42 cells/ml. Therefore, rabbits with the most rapid and robust reconstitution of CD4+ T cells were most likely to develop more severe disease both by gross appearance and by enumeration of viable bacilli.
Table II.
Disease parameters associated to Mtb H37Rv aerosol infection of rabbits after intramuscular dexamethasone treatment from weeks 10 to 15.
| No. Weeks after aerosol | No. of rabbits | No. of tubercles | Tubercle diameter (mm) | Lung weight (g) | Right upper lobe CFU (median [range]) | No. rabbits culture-negative at any site |
|---|---|---|---|---|---|---|
| Higher-Dose Aerosol | ||||||
| Week 15 | 6 | 9 ± 4 | 0.7 ± 0.3 | 12.0 ± 0.5 | 4050 [0-23,000] | 2/6 |
| P=0.13* | ||||||
| Week 20 | 6 | 14 ± 5 | 1.7 ± 0.7 | 13.0 ± 0.7 | 1356 [375-71,000] | 0/6 |
| P=0.002* | ||||||
| Lower-Dose Aerosol | ||||||
| Week 15 | 3 | 30 ± 23 | 1.2 ± 0.3 | 12.2 ± 0.3 | 0 [0-8850] | 2/3 |
| Week 25 | 18 | 7 ± 5 | 0.7 ± 0.3 | 12.2 ± 0.4 | 50 [0-6000]** | 3/18 |
P values compared to 5 week values except for those denoted with an asterisk which are compared to the untreated control at the same time point
No P value could be calculated since all RUL homogenates in the control group were culture-negative
Figure 4.
Absolute number of CD4+ T cells/ml in peripheral blood for individual rabbits at 5, 10, 15 and 20 weeks after infection in steroid-treated (open diamonds) and untreated infected controls (closed triangles) in the higher dose aerosol experiment.
To further examine the role of immune reconstitution on TB disease, 18 rabbits were similarly treated with dexamethasone from 10 to 15 weeks after aerosol infection and then allowed to immune reconstitute. In this experiment, the aerosol dose was lower, resulting in more uniform containment to paucibacillary disease at the time of dexamethasone initiation (Table I). In contrast to the higher-dose experiment, 10 weeks were allowed to elapse after the conclusion of dexamethasone therapy in the lower-dose experiment. All rabbits recovered T and B cell counts during this time interval (data not shown). Fifteen of 18 rabbits (83%) were culture-positive in right upper lung lobe homogenates (13 of 18) (Table II) and/or in lung granuloma homogenates (7 of 18) after immunosuppression. Only 3 rabbits had large, multicentric, caseous lesions suggestive of IRIS. In the first higher-dose experiment, 50% of the rabbits developed IRIS, whereas, in the lower-dose experiment only 16% of the rabbits developed IRIS. The burden of Mtb organisms was lower in the second experiment at the time of dexamethasone immunosuppression and suggests an important role for Mtb organisms and/or antigens in the pathogenesis of IRIS.
Histologic Examination of Lung Tissue Sections from H37Rv Infection
At 5 weeks after aerosol infection with higher-dose H37Rv, many of the lung granulomas had a pinpoint area of central caseous necrosis with nearby polymorphonuclear leukocytes. The rest of the granuloma was comprised of mature and immature epithelioid macrophages surrounded by a mantle of lymphocytes and plasma cells (Figure 5A). At 10 weeks after infection, only two rabbits still had significant numbers of tubercles, some of which had larger areas of caseous necrosis (Figure 5B). In the untreated rabbits at 15 weeks after infection, the lesions were smaller and no longer showed appreciable areas of caseation (Figure 5C). In the rabbits treated for 5 weeks with dexamethasone, few grossly visible granulomas were discernible. Histologically, their lesions consisted of thickened alveolar walls of tuberculous granulation tissue with plugs of epithelioid macrophages in the air spaces (Figure 5D). In these rabbits, the bronchial-associated lymphoid tissue (BALT) was more prominent (Figure 5E). While the lymphocytes were recovering for 5 weeks after the discontinuation of dexamethasone, prominent granulomas formed with multiple, large caseous centers in rabbits that developed IRIS (Figures 2F & 5F). These lesions showed pockets of mature and immature epithelioid macrophages with patchy areas of lymphocytes scattered throughout (Figure 5F). Some of the epithelioid macrophages entered the airways.
Figure 5.
Histopathology of infected rabbit lungs. Lung granuloma at A) 5 weeks after infection with Mtb H37Rv (100x), B) 10 weeks; 2 rabbits had increased areas of caseation surrounded by epithelioid macrophages and then by a lymphoid mantle (100x), while the other 4 rabbits had healing lesions, C) 15 weeks, the caseous center has been replaced by epithelioid macrophages in this healing lesion (200x), D) 15 weeks after infection and at the conclusion of intramuscular dexamethasone treatment showing intra-alveolar plug of epithelioid macrophages (200x), E) prominent bronchial-associated lymphoid tissue and no organized granulomas in same lung specimen as (D) (100x), and F) Large lung granuloma with multiple epithelioid macrophage centers with interspersed lymphocytes in immune reconstituted rabbit lung (20x).
Discussion
In our rabbit aerosol infection model, the strain Mtb H37Rv established infection in the lung and replicated during the first 5 weeks of infection, after which both the number of culturable bacilli and grossly visible lung tubercles gradually decreased over the course of 36 weeks. In contrast to other models, none of the rabbits spontaneously reactivated. From the data presented in this manuscript, we can conclude that among common laboratory animals, the rabbit is the most resistant to Mtb aerosol infection. The rabbits are tuberculin skin test positive, but they are clinically asymptomatic with infection, which is controlled to a paucibacillary latent state by the host immune response as in the majority of infected humans. Dexamethasone-induced immunosuppression initiated when infection was paucibacillary resulted in a reversal of host containment of the infection consistent with reactivation.
In rabbits, culturable bacilli are contained within caseous granulomas that persist despite an effective adaptive immune response. At the last time point 36 weeks after aerosol infection, the few remaining granulomas were relatively large with grossly visible caseous centers that were invariably culture-positive. In both experiments, this occurred in one-third of rabbits infected with the Mtb H37Rv strain. Conversely, our data also suggest that a proportion of productively infected rabbits cure disease despite skin test conversion. Three of 18 (12%) rabbits did not develop culture-positive infection despite exogenous corticosteroids. What remains unexplained is the ~50% of rabbits that did not have visible caseous granuloma and had culture-negative right upper lung lobes or hilar lymph nodes, but were still able to reactivate disease. To investigate the possibility that there were viable bacilli outside of granulomas, guinea pigs were intraperitoneally inoculated with culture-negative lung homogenates (with no caseous granulomas) from the rabbits. After 8 weeks, the guinea pigs showed no evidence of productive infection (tuberculin skin test negative, no clinical signs e.g. weight loss, and organ homogenates were culture-negative at necropsy) suggesting that there are few, if any, bacilli outside of these granulomas. Another possible explanation is that small lesions in the lower lobes were missed since we did not exhaustively analyze the histology of these lung lobes microscopically or that the bacilli are not culturable on conventional Middlebrook agar.
The proportion of latent to cured rabbits is determined by the relative virulence of the Mtb strain used and its ability to establish disease, replicate and cause caseous necrosis in lung lesions at the time of peak infection. For example, after aerosol infection with Mtb CDC1551 at a dose comparable to that of Mtb H37Rv infection above, there was poor establishment of infection, no caseous necrosis in pulmonary granulomas at 5 weeks, and uniform cure of disease in these rabbits despite tuberculin skin test positivity 4 weeks after infection and subsequent immunosuppressive therapy (data not shown, submitted Mendez et al.). In contrast, both Mtb H37Rv and Erdman established disease well with increasing CFU at 5 weeks compared to day 1 after infection. Lung pathology demonstrated caseous pulmonary necrosis (Erdman more than H37Rv) that led to disease that was controlled over time, but could be reactivated with corticosteroids. The more virulent strain for rabbits, Mtb Erdman, was more likely to lead to chronic progressive disease, even in those rabbits not treated with corticosteroids 9.
Withdrawal of dexamethasone at the conclusion of 5 weeks of immunosuppressive treatment led to recovery of lymphocyte numbers. This immune reconstitution was accompanied by an increase in bacillary multiplication. The pathology of immune reconstitution corroborated that seen in published experiments where rabbits were pre-treated with steroids and then infected with Mtb 13. These rabbits developed uniformly more severe disease than did untreated rabbits. Discontinuation of steroids was followed by enhanced DTH skin responses, subsequent cavitary disease, and exuberant increases in bacillary numbers.
This finding of enlarging lesions despite reversal of steroid-induced lymphopenia has interesting parallels with immune reconstitution inflammatory syndromes (IRIS) in human tuberculosis. This phenomenon has been described in patients who initially improve with anti-tuberculous chemotherapy, then have immune-mediated enlargement of existing lesions, but remain culture-negative 14, 15. It has also been described in acquired immunodeficiency syndrome (AIDS) patients with subclinical tuberculosis (normal chest radiographs) that were treated with highly active anti-retroviral therapy (HAART) in whom CD4+ T cells rebound and culture-positive tuberculosis is unmasked and progresses rapidly after treatment initiation 15-17. With the introduction of HAART in areas where tuberculosis in endemic, reactivation of undiagnosed or latent tuberculosis may prove to be a problem, and would result in enhanced transmission of the disease. Clinically, similar immunorestitution disease syndromes have been described in HIV-negative patients receiving immunosuppressive therapy18, further validating our corticosteroid immunosuppression model as potentially relevant to TB-IRIS in HIV-tuberculosis co-infected patients who may be receiving HAART. The rabbit model may be useful in developing early immunologic and/or diagnostic criteria to identify patients, who have an increased risk for developing IRIS.
The proportion of rabbits that developed IRIS was much higher in the first, higher-dose experiment than in the subsequent lower-dose experiment, suggesting that the number of organisms plays an important role in the pathogenesis of IRIS. In the higher-dose experiment, corticosteroids were initiated at a time point when the mean bacillary CFU was still relatively high, thus allowing more organisms to accumulate during immunosuppression. Data in humans has indirectly supported the role of tuberculosis antigen and/or organism burden in the pathogenesis of IRIS. Increased risk has been noted with disseminated disease. In addition, the risk of IRIS decreases with increasing interval between the initiation of effective TB treatment and the initiation of HAART 19.
Finally, this model offers an opportunity to study the immunopathogenesis of a resistant host response to tuberculosis. Five weeks after aerosol infection with H37Rv, our data suggests that lymphocytes from the peripheral blood may be recruited to both the lungs and the draining hilar lymph nodes, resulting in a decreased number of lymphocytes in the peripheral blood. The blood lymphocytes then recovered during the course of the 20 week infection. Similarly, in patients with active tuberculosis compared to those in patients with latent disease, the number of peripheral blood CD4+ T cells was decreased 20. In the same study, patients with latent disease were also more likely to have more peripheral B lymphocytes than healthy controls, again similar to our 20 week rabbits in which the absolute number of B cells in the peripheral blood was significantly elevated compared to 5 weeks after infection. Several recent publications have pointed to a role for B cells in host containment of tuberculosis both at the level of the granuloma and in clinically asymptomatic disease 21, 22. In addition, the protective effect of intravenous immunoglobulin in tuberculous mice provides other supportive data for the importance of B cells 23. The relative resistance of rabbits offers more opportunities to measure possible immune correlates of protection.
In summary, after aerosol infection of rabbits with Mtb H37Rv there was initial bacillary replication followed by decreasing disease burden (tubercle number and CFU) over 20 weeks. Treatment with exogenous corticosteroids resulted in lymphopenia and bacillary reactivation with culturable bacilli in lungs in the majority of rabbits. With immune reconstitution after dexamethasone discontinuation, the CD4+ T cells rebounded, bacillary replication was accelerated, and enlarged, necrotic pulmonary lesions occurred. Clearly the propensity to develop IRIS is dependent on both the resistant host response of the rabbits and the number of organisms or bacillary antigen present in the lungs. These findings have implications for HIV-tuberculosis co-infected individuals in the era of expanding access to HAART.
Acknowledgements
The authors would like to thank Raghunand Tirumalai and John Chan for critical review of the manuscript and invaluable scientific input, Sandeep Tyagi for his assistance with the rabbits, and Mike Manion, Dr. Doris Hughes, and Robert Bergman for veterinary care. The authors have no conflicting financial interests. This work was supported by funding from the National Institutes of Health (1R01 HL71554 & DMID-NIAID Contract 03-09).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Opie EL, Aronson JD. Tubercle bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesions. Arch Pathol Lab Med. 1927;4:1–21. [Google Scholar]
- 2.Medlar EM, Bernstein S, Steward DM. A bacteriologic study of resected tuberculous lesions. Amer Rev Tuberc. 1952;66:36–43. doi: 10.1164/art.1952.66.1.36. [DOI] [PubMed] [Google Scholar]
- 3.Wayne LG. The bacteriology of resected tuberculous pumonary lesions. II. Observations on bacilli which are stainable but which cannot be cultured. Amer Rev Respir Disease. 1960;82 doi: 10.1164/arrd.1960.82.3.370. [DOI] [PubMed] [Google Scholar]
- 4.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 5.McCune RM, M. FF, Lambert HP, McDermott W. Microbial Persistence II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123:469–86. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCune RM, M. FF, Lambert HP, McDermott W. Microbial Persistence I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–68. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurie MB. The fate of human and bovine tubercle bacilli in various organs of the rabbit. J Exp Med. 1928;48:155–82. doi: 10.1084/jem.48.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manabe YC, Dannenberg AM, Jr., Tyagi SK, Hatem CL, Yoder M, Woolwine SC, Zook BC, Pitt ML, Bishai WR. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect Immun. 2003;71:6004–11. doi: 10.1128/IAI.71.10.6004-6011.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesavan AK, Mendez SE, Hatem CL, Lopez-Molina J, Aird K, Pitt ML, Dannenberg AM, Jr., Manabe YC. Effects of dexamethasone and transient malnutrition on rabbits infected with aerosolized Mycobacterium tuberculosis CDC1551. Infect Immun. 2005;73:7056–60. doi: 10.1128/IAI.73.10.7056-7060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman SE, Hatem CL, Tyagi S, Aird K, Lopez-Molina J, Pitt ML, Zook BC, Dannenberg AM, Jr., Bishai WR, Manabe YC. Susceptibility to tuberculosis: clues from studies with inbred and outbred New Zealand White rabbits. Infect Immun. 2004;72:1700–5. doi: 10.1128/IAI.72.3.1700-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ly LH, Russell MI, McMurray DN. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol. 2007;9:1127–36. doi: 10.1111/j.1462-5822.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 13.Lurie MB. Resistance to Tuberculosis. Harvard University Press; Cambridge: 1964. [Google Scholar]
- 14.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 15.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest. 2001;120:193–7. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- 16.Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in abidjan. Am J Respir Crit Care Med. 2005;172:123–7. doi: 10.1164/rccm.200410-1342OC. [DOI] [PubMed] [Google Scholar]
- 17.Breen RA, Smith CJ, Cropley I, Johnson MA, Lipman MC. Does immune reconstitution syndrome promote active tuberculosis in patients receiving highly active antiretroviral therapy? AIDS. 2005;19:1201–6. doi: 10.1097/01.aids.0000176221.33237.67. [DOI] [PubMed] [Google Scholar]
- 18.Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882–92. doi: 10.1086/313809. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. Aids. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 20.Corominas M, Cardona V, Gonzalez L, Cayla JA, Rufi G, Mestre M, Buendia E. B-lymphocytes and co-stimulatory molecules in Mycobacterium tuberculosis infection. Int J Tuberc Lung Dis. 2004;8:98–105. [PubMed] [Google Scholar]
- 21.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–32. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 22.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–28. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 23.Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, Dagg B, Tascon RE, Lowrie DB, Colston MJ, Jolles S. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–9. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]