Abstract
The oxidative damages induced by a redox imbalance cause age-related changes in cells and tissues. Superoxide dismutase (SOD) enzymes play a pivotal role in the antioxidant system and they also catalyze superoxide radicals. Since the loss of cytoplasmic SOD (SOD1) resulted in aging-like phenotypes in several types of murine tissue, SOD1 is essential for the maintenance of tissue homeostasis. Melinjo (Gnetum gnemon Linn) seed extract (MSE) contains trans-resveratrol (RSV) and resveratrol derivatives, including gnetin C, gnemonoside A, and gnemonoside D. MSE intake also exerts no adverse events in human study. In the present studies, we investigated protective effects of MSE on age-related skin pathologies in mice. Orally MSE and RSV treatment reversed the skin thinning associated with increased oxidative damage in the Sod1 −/− mice. Furthermore, MSE and RSV normalized gene expression of Col1a1 and p53 and upregulated gene expression of Sirt1 in skin tissues. In vitro experiments revealed that RSV significantly promoted the viability of Sod1 −/− fibroblasts. These finding demonstrated that RSV in MSE stably suppressed an intrinsic superoxide generation in vivo and in vitro leading to protecting skin damages. RSV derivative-rich MSE may be a powerful food of treatment for age-related skin diseases caused by oxidative damages.
1. Introduction
Intrinsic skin aging induced by chronological or intrinsic factors leads to skin atrophy [1]. Skin collagen components show age-dependent reductions in both male and female subjects, resulting in age-related skin thinning in older individuals [2]. Accumulated evidence suggests that oxidatively modified proteins, DNA, and lipids in the skin and other organs during aging are progressively accumulated [3], indicating that reactive oxygen species (ROS) are strongly associated with skin aging. To attenuate oxidative damages, multiple antioxidative and repair systems exert in cells. Superoxide dismutase (SOD) plays a central role in the antioxidative systems due to its ability to catalyze cellular superoxide radicals (O2 •−) to H2O2. H2O2 is further degraded to O2 and H2O by catalase, glutathione peroxidases, and peroxiredoxins. Copper/zinc superoxide dismutase (SOD1) is localized to react intracellular O2 •− in the cytoplasm. Our previous studies demonstrated that Sod1-deficient (Sod1 −/−) mice showed enhancement of intracellular O2 •− and various aging-like organ phenotypes, suggesting that cytoplasmic O2 •−-mediated oxidative damages primarily cause aging-like changes in various tissues [4]. Particularly, Sod1 insufficiency resulted in both epidermal and dermal atrophies associated with downregulation of extracellular matrix-related genes including Col1a1 and with upregulation of age-related genes including p53 [5, 6]. Therefore, Sod1 −/− mouse is a suitable model for studying skin aging in older people.
Melinjo (Indonesian name; Gnetum gnemon Linn) is an arboreal dioecious plant that is widely cultivated in Southeast Asia. Its fruits and seeds are used as an ordinary vegetable in Indonesia. Melinjo seeds contain various stilbenoids including trans-resveratrol (3,5,4′-trihydroxy-trans-stilbene), its glucoside, resveratrol dimer (gnetin C), and resveratrol dimer glucoside (gnetin L, gnemonoside A, gnemonoside C, and gnemonoside D) [7]. Melinjo seed extract (MSE) revealed DPPH radical scavenging [7], lipase and α-amylase inhibitory [7], antimicrobial, immunostimulatory [7], angiogenesis inhibitory [8], tyrosinase inhibitory activities [9], promotion of melanin biosynthesis [9], and prevention of endothelial senescence [10]. Recently, Tatefuji et al. also reported that acute and subchronic MSE administration showed no adverse effect in rat [11]. In human study, MSE administration decreases the serum uric acid levels by inhibiting the reabsorption of uric acid in the renal tubular epithelia as well as by increasing the HDL cholesterol levels by PPAR agonistic activity with no cause of the damage to health [12]. Furthermore, Tani et al. reported that single and repeated administration of MSE demonstrated no clinical noteworthy abnormalities [13]. MSE contains 1.2 mg/g (5.26 μmol/g) of RSV [13], while average content of RSV was 1.04 mg/L in red wine [14]. Therefore, MSE becomes a nutrient source of RSV with harmless long-term intake. RSV has been identified as a Sirt1 activator that has been shown to protect various organs against aging [15, 16]. Furthermore, RSV possesses antioxidative activity and protective effect of ROS- and ultraviolet-induced cell death [17, 18]. In addition, Sirt1 is a key modulator of cellular pathways involved in inherited dermatologic diseases and skin cancers [19], suggesting that Sirt1 activation is a molecular target for dermatological therapy. In the present study, we investigated antiatrophic effects of MSE and RSV on age-related skin pathologies in Sod1 −/− mice.
2. Materials and Methods
2.1. Reagents
MSE (Lot number YMP-M-110115) was provided by the Institute for Bee Products & Health Science, Yamada Bee Company, Inc. (Okayama, Japan). The MSE contains trans-resveratrol (RSV, 0.10% w/w), gnetin C (2.03% w/w), gnemonoside A (16.35% w/w), and gnemonoside D (3.97% w/w). Resveratrol (RSV) was obtained from Tokyo Chemical Industry Co. Ltd. (CAS 501-36-0, Tokyo, Japan). The purity of RSV is more than 98%.
2.2. Mice and Diets
Sod1 −/− mice were purchased from the Jackson Laboratory (Bar Hrbor, ME, USA). The genotyping of Sod1 −/− allele was performed using genomic PCR with genomic DNA isolated from the tail tip, as previously reported [20]. The animals were housed in a room temperature of 24 ± 1°C, a relative humidity of 55 ± 10%, and a 12 h light/dark cycle and were fed ad libitum. Experimental procedures were approved by the Animal Care and Use Committee of Chiba University. At 4 weeks of age, mice were randomly divided into four groups and fed respective experimental diets for 12 weeks: control MF diet (composition: 7.9% water, 21.1% protein, 5.1% lipid, 5.8% ash, 2.8% fiber, and 55.3% soluble nitrogen free extract, 359 kcal/100 g, Oriental Yeast Co., Ltd., Tokyo, Japan), control MF diet containing 0.04% (w/w) RSV as previously described [15], and control MF diets containing 0.1% or 0.5% (w/w) MSE (Lot number YMP-M-110115) according to the previous study [8].
2.3. Histology
For histological morphology, skin specimens from back tissues were dissected and fixed in a 20% formalin neutral buffer solution (Wako, Osaka, Japan) overnight. After dehydration and penetration, skin specimens were embedded in paraffin and sectioned on a microtome (ROM-380, Yamato Koki Kogyo Co. Ltd., Saitama, Japan) at 4 μm thickness by standard techniques. Hematoxylin and eosin staining for skin morphology and Sirius red staining for total collagen deposition were performed as described previously [21–23]. The thickness of the skin tissue was measured using Leica QWin V3 image software (Leica, Germany).
2.4. Measurement of Oxidative Stress Markers
In order to measure the 8-isoprostane content, blood was collected from the left ventricular space and centrifuged at 12,000 rpm for 5 min at room temperature. Plasma was separated from the clotted blood and added 100 μM indomethacin and 0.005% dibutylhydroxytoluene. The 8-isoprostane level was measured using the 8-isoprostane EIA Kit (Cayman Chemical Company) according to the manufacturer's instructions. The plasma was also assayed for the protein concentration using the DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA), and 8-isoprostane levels were normalized to the protein level.
For intracellular ROS measurement, bone marrow cells (5–10 × 105 cells/two tibias of a mouse) were collected by flushing tibias with phosphate-buffered saline using 26-G needles and stained with 10 μM of CM-H2DCFDA (DCF, Life Technologies Corporation) for 30 min at 37°C. Primary dermal fibroblasts were incubated with 10 μM DCF for 30 min at 37°C. After incubation, cells were trypsinized and resuspended in PBS. Their fluorescence intensities were assessed using a flow cytometer (BD FACSCanto II, BD Biosciences).
2.5. Cell Culture
Skin tissues were dissected from Sod1 −/− neonates at 5 days of age. The primary dermal fibroblasts were isolated by dissociation in 0.2% collagenase type 2 (Worthington Biochemical Corporation Lakewood, NJ, USA) at 37°C for 60 min. Cells were cultured in α-MEM (Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS), 100 unit/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in a humidified incubator with 5% CO2 and 1% O2. Cells were treated with 10 μM RSV at 72 h. We determined the concentration and duration of RSV treatment in this study according to our previous paper [6].
2.6. Outgrowth Assay
The back skin was sterilized with 70% ethanol, rinsed with PBS (Takara Bio Inc., Shiga, Japan), and punched out into discs measuring 5 mm in diameter using dermal punch (Nipro, Tokyo, Japan). The punched skin discs were placed into a 12-well culture plate (Falcon BD, Franklin Lakes, NJ) and cultured with or without 10 μM RSV in α-MEM containing 20% FBS, 100 units/mL of penicillin, and 0.1 mg/mL of streptomycin at 37°C in a humidified incubator with 5% CO2 and 1% O2. The number of outgrowth fibroblasts originating from the mouse skin disc was directly counted at 72 h after culture. The method of this experiment was performed as described previously [5].
2.7. Quantitative PCR
Total RNA was extracted from back skin using the Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA using reverse transcriptase (ReverTra Ace qPCR RT Master Mix, Toyobo, Osaka, Japan). Real-time PCR was performed on a MiniOpticon (Bio-Rad) with the SYBR Green PCR Master Mix (Bio-Rad) according to the manufacturer's instructions. All data were normalized to the level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The following primers were used for the analysis: Gapdh, forward, 5′-ATGTGTCCGTCGTGGATCTGA-3′, and reverse, 5′-TGCCTGCTTCACCACCTTCT-3′; Col1a1, forward, 5′-CATGTTCAGCTTTGTGGACCT-3′, and reverse, 5′-GCAGCTGACTTCAGGGATGT-3′; p53, forward, 5′-ACGCTTCTCCGAAGACTGG-3′, and reverse, 5′-AGGGAGCTCGAGGCTGATA-3′; Sirt1, forward, 5′-CAGTGAGAAAATGCTGGCCTA-3′, and reverse, 5′-TTGGTGGTACAAACAGGTATTGA-3′.
2.8. Statics
The statistical analyses were performed using Student's t-test for comparisons between two groups and Tukey's test for comparisons among three groups. Differences between the data were considered significant when the P values were less than 0.05. All data are expressed as the mean ± standard deviation (SD).
3. Results
3.1. MSE and RSV Attenuate the Skin Atrophy of Sod1 −/− Mice
SOD1, one of the cellular antioxidant enzymes, plays a pivotal role in regulating oxidative and reductive balance. Sod1 −/− mice showed age-related atrophic morphology in their skin accompanied by the degeneration of collagen [5]. Therefore, we have used Sod1 −/− mice for skin aging research and for screening of antiatrophic compounds in skin thickness [24–26]. In this context, we investigated antiatrophic effects of MSE and RSV on the skin thickness of Sod1 −/− mice.
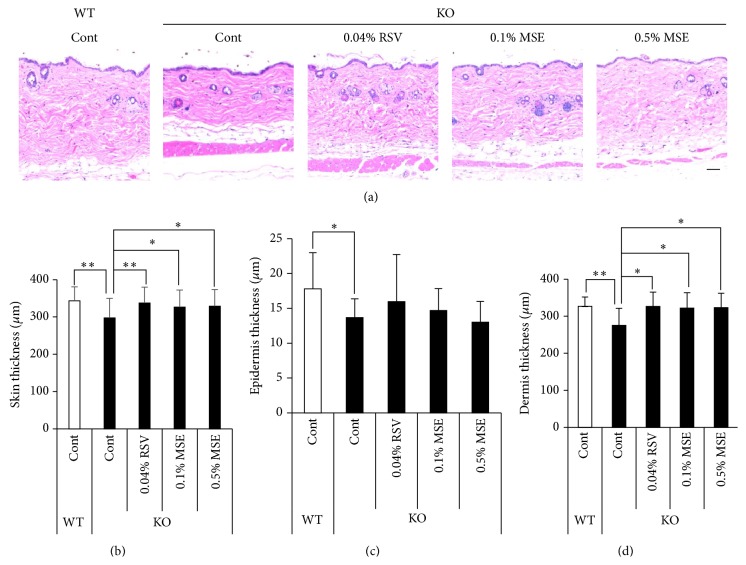
In a preliminary experiment, MF diets containing 5% or 0.5% MSE were orally administrated to the Sod1 +/+ and Sod1 −/− mice daily for 12 weeks beginning at 4 weeks of age. The results showed that both MSE diets improved the skin thickness of Sod1 −/− mice and there were no adverse effects of skin pathologies of Sod1 +/+ mice (data not shown). Therefore, we selected the control diet containing 0.5% MSE to confirm antiatrophic effect on Sod1 −/− skin. MSE and RSV were orally administered to the Sod1 +/+ and Sod1 −/− mice under the same conditions. As shown in Figure 1(a), the skin of Sod1 −/− mice was significantly thinner compared to that of Sod1 +/+ mice, confirming skin atrophy in Sod1 −/− mice. The back skin of Sod1 −/− that had been administrated with the MSE diets was significantly thicker compared to that of Sod1 −/− mice treated with the control diet (Figures 1(b)–1(d)). RSV diet also improved skin atrophy of Sod1 −/− mice compared to Sod1 −/− mice treated with the control diet (Figures 1(b)–1(d)). To investigate the adverse effect of MSE and RSV diets, we similarly administered MSE and RSV to the Sod1 +/+ mice. No significant difference in skin thickness and morphology was observed in Sod1 +/+ mice treated with MSE and RSV (data not shown), indicating that MSE and RSV were safety food factors in skin during short-time treatment. In addition, Sirius red staining revealed that the skin of Sod1 −/− mice was decreased in staining intensity compared to that observed in Sod1 +/+ mice (Figure 2(a)), confirming dermal collagen decline in Sod1 −/− mice. Notably, both MSE and RSV diets increased the Sirius red intensity in Sod1 −/− dermis (Figure 2(a)), implying enhancement of collagen level in Sod1 −/− skin.
Figure 1.
MSE and RSV attenuate skin atrophy in the Sod1 −/− mice. (a) Hematoxylin and eosin staining of the back skin of Sod1 −/− (KO) and Sod1 +/+ (WT) mice treated with the MSE or RSV. MSE and RSV containing diets were administrated for 12 weeks. The thickness of (b) total, (c) epidermis, and (d) dermis of the back skin of the Sod1 −/− and Sod1 +/+ mice treated with MSE or RSV (n = 10–12). The statistical evaluations were performed using the Tukey's test. These data indicate the mean ± SD; ∗ P < 0.05, ∗∗ P < 0.01. The scale bar represents 100 μm.
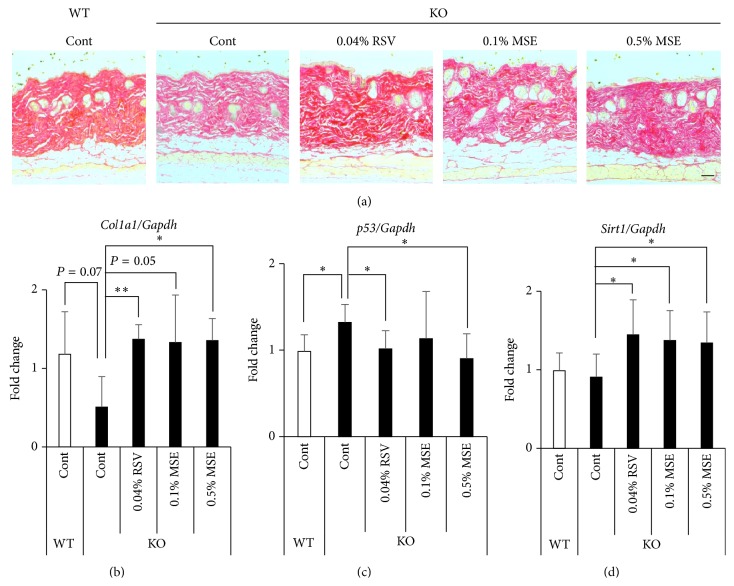
Figure 2.
MSE and RSV attenuate collagen decline in skin tissues of Sod1 −/− mice. (a) Sirius red staining of the back skin of Sod1 −/− and Sod1 +/+ mice treated with the MSE or RSV. Relative mRNA expression of (b) Sirt1, (c) Col1a1, and (d) p53. Each of the mRNA expressions was determined by qRT-PCR (n = 8–12). The statistical evaluations were performed using the two-tailed Student's t-test for unpaired values. These data indicate the mean ± SD; ∗ P < 0.05, ∗∗ P < 0.01. The scale bar represents 100 μm.
3.2. MSE and RSV Alter Gene Expression in Sod1 −/− Skin
To investigate skin atrophy-preventing mechanism of MSE and RSV on skin atrophy in Sod1 −/−, we analyzed expression patterns of type I collagen and age-related genes in skin. In Sod1 −/− skin, mRNA level of Col1a1 was significantly downregulated compared to those of Sod1 +/+, indicating reduced collagen biosynthesis (Figure 2(b)). Moreover, p53, one of the major age-related genes, also significantly upregulated in Sod1 −/− skin (Figure 2(c)). MSE and RSV treatment significantly normalized mRNA levels of Col1a1 and p53 in Sod1 −/− skin (Figures 2(b) and 2(c)). Interestingly, we revealed that MSE and RSV treatment also significantly upregulated Sirt1 expression, suggesting the molecular link between Sirt1 expression and skin thinning in Sod1 −/− mice (Figure 2(d)). These findings demonstrated that application of MSE and RSV diets improved the skin atrophy accompanied by normalization and activation of age-related genes in Sod1 −/− mice.
3.3. MSE and RSV Significantly Attenuate Oxidative Damage in Sod1 −/− Mice
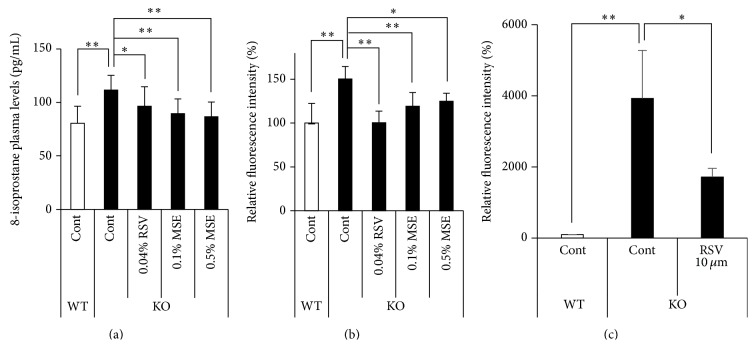
Sod1 −/− mice showed significant increase of several oxidative damage markers, including lipid peroxidation, in tissues [20, 24, 27, 28]. In order to evaluate oxidative damage, we measured the lipid peroxidation levels in the plasma. Regarding the 8-isoprostane levels, MSE and RSV containing diets significantly reduced the 8-isoprostane content in the plasma (Figure 3(a)). Furthermore, MSE and RSV containing diets decreased intracellular ROS level in cells from bone marrow (Figure 3(b)). These data indicate that MSE and RSV treatment mitigated oxidative damage in Sod1 −/− mice.
Figure 3.
MSE and RSV decrease oxidative damage and ROS production. (a) 8-isoprostane content in plasma obtained from Sod1 −/− and Sod1 +/+ mice treated with MSE and RSV (n = 10–12). (b) The intracellular ROS levels of bone marrow cells of Sod1 −/− and Sod1 +/+ mice were measured using a DCF dye (n = 5-6). (c) The relative intracellular ROS level in Sod1 −/− fibroblasts treated with 10 μM RSV for 72 h was measured by a DCF dye (n = 3). The statistical evaluations were performed using the Tukey's test. These data indicate the mean ± SD; ∗ P < 0.05, ∗∗ P < 0.01.
3.4. MSE and RSV Significantly Restore Viability in Sod1 −/− Fibroblasts
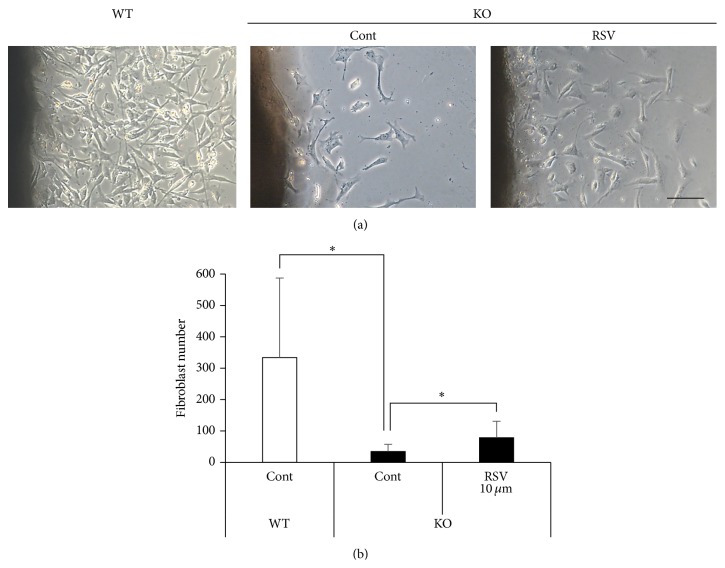
We investigated whether the RSV treatment attenuated intracellular ROS production and promoted the proliferation of Sod1 −/− fibroblasts in vitro. Preliminary experiments revealed that RSV treatment for 24 h with various concentrations of 30 to 100 μM slightly suppressed cell viability of Sod1 +/+ fibroblasts. In contrast, 10 μM RSV treatment for 72 h showed no adverse effect of cell viability in Sod1 +/+ fibroblasts. Therefore, we determined dose and duration of the RSV experiment in vitro. Flow cytometer analysis indicated that RSV treatment significantly decreased intracellular ROS generation in Sod1 −/− fibroblasts (Figure 3(c)). Moreover, the organ culture experiments using skin discs revealed that the Sod1 −/− fibroblasts showed marked suppression of their outgrowth capacity compared to that observed in the Sod1 +/+ mice (Figure 4(a)). Treatment with 10 μM RSV significantly enhanced the fibroblasts outgrowth activity from the Sod1 −/− skin discs (Figure 4(b)). These findings collectively suggested that the RSV promoted the migration and proliferation of Sod1 −/− fibroblasts in vitro.
Figure 4.
RSV promotes fibroblasts outgrowth from Sod1 −/− skin. (a and b) Number of outgrowth fibroblasts of Sod1 +/+ and Sod1 −/− mice in the skin disc culture treated with 10 μM RSV for 72 h (n = 8). Fibroblast number was counted on day 3. The statistical evaluations were performed using the two-tailed Student's t-test for unpaired values. These data indicate the mean ± SD; ∗ P < 0.05. The scale bar represents 100 μM.
4. Discussion
In the present study, we demonstrated that MSE and RSV significantly reversed skin thinning via reduction of oxidative damages in Sod1 −/− mice (Figure 1). Recently, we have reported that Sod1 −/− fibroblasts showed excessive ROS accumulation associated with mitochondrial dysfunction [6]. In vitro study also revealed that RSV treatment significantly reduced intracellular ROS generation and restored cell viability in Sod1 −/− fibroblasts (Figures 3(c) and 4). Accumulating evidence revealed that RSV activates mitochondrial function and antioxidant defense leading to suppressing ROS generation [29]. Furthermore, SIRT1 also increases mitochondrial function and biogenesis and promotes cell proliferation and migration [15, 30, 31]. In a human study, treatment with a nutraceutical supplement containing resveratrol, procyanidin, and ellagic acid induced reduction of skin wrinkling, as well as reducing systemic and skin oxidative stress in a clinical setting [32]. These findings suggested that the Sirt1-mediated antioxidant activities of RSV contribute to attenuate skin damages in mammals. To rescue age-related changes in tissues of Sod1 −/− mice, we have evaluated beneficial effects of several antioxidants in vivo. Ascorbic acid administration significantly attenuated bone loss and fragility of Sod1 −/− mice [28]. Transdermal administration of ascorbic acid derivatives also normalized skin thinning of Sod1 −/− mice [25, 26]. Furthermore, Iuchi et al. reported that oral N-acetylcysteine treatment mitigated hemolytic anemia of Sod1 −/− mice by suppressed ROS generation in red blood cells [27]. Recently, Shibuya et al. showed that an SOD/catalase mimetic, PAPLAL, treatment attenuated skin atrophy [33]. These reports strongly supported that antioxidants, such as RSV, ascorbic acid, N-acetylcysteine, and PAPLAL, positively improved oxidative damage-induced organ pathologies.
As shown in Figure 2(c), Sod1 deficiency showed upregulation of p53 gene expression, which regulates cellular senescence and death, in skin (Figure 2(c)). We previously reported that Sod1 loss induced O2 •− generation and upregulated p53 protein level in skin fibroblasts [6]. Ascorbic acid derivatives significantly downregulated p53 expression and improved cell viability in Sod1 −/− fibroblasts [6, 25]. In a genetically modified model, p53 activation induced accelerated aging-like phenotypes, including skin atrophy, in p53 mutant mice [34]. Gannon et al. also reported that p53 activation by Mdm2-specific loss in keratinocytes induced epidermal stem cell senescence and atrophy in mice, suggesting that p53 activation in skin accelerated aging-like skin thinning in mice [35]. MSE and RSV treatment significantly downregulated mRNA level of p53 in Sod1 −/− skin (Figure 2(c)). These data indicated that MSE and RSV treatment may delay skin aging via reducing the p53 upregulation in skin.
RSV promotes the activity and expression of Sirt1 [15]. MSE and RSV also normalized the gene expression of Col1a1 and upregulated the gene expression of Sirt1 in skin of Sod1 −/− mice (Figures 2(b) and 2(d)). Sirt1 upregulation by RSV may protect skin aging from oxidative damage in Sod1 −/− mice. Actually, Lee et al. reported that RSV treatment or Sirt1 overexpression significantly inhibited matrix metalloprotease-9 expression and appeared to protect collagen from degradation after ultraviolet radiation in human dermal fibroblasts and skin tissues [36]. Serravallo et al. reported that Sirt1 plays a pivotal role in modulating skin diseases including psoriasis, autoimmune disease, cutaneous fungal infection, inherited dermatological diseases, and cancer [19]. These findings indicated that upregulation of Sirt1 expression protected skin damages in vivo.
Recently, Konno et al. reported that RSV and MSE showed the agonistic activity for PPARα and PPARγ in vitro [12]. It is reported that a PPARα/γ dual agonist, MHY966, treatment significantly suppressed UVB-induced collagen digestion, lipid peroxidation, and inflammatory response via activating PPARα and PPARγ in mouse skin during photoaging [37]. Moreover, Mastrofrancesco et al. reported that PPARγ activation in skin normalized inflammatory response in IL-21-induced epithelial hyperplasia in mice [38]. These reports suggested that RSV and MSE may activate PPARα and PPARγ leading to attenuating the skin atrophy in Sod1 −/− mice.
Finally, we, here, focused on RSV in MSE and antiatrophic effects of RSV in Sod1 −/− skin. Since MSE also contains several RSV-derivatives such as gnetin C, gnemonoside A, and gnemonoside D, we cannot rule out antiatrophic effects of the RSV derivatives in MSE. Further analysis should be needed to clarify the beneficial effect of other RSV derivatives in MSE on skin atrophy in Sod1 −/− mice.
5. Conclusion
In the present study, we demonstrated that MSE and RSV treatment effectively attenuated aging-like skin pathologies accompanied by upregulation of Sirt1 expression in Sod1 −/− skin. MSE and RSV also exhibited less adverse effect on skin morphology. Consistent with our results, many interventions reported safety of MSE and RSV treatment in human. Therefore, MSE is useful for nutrient source of RSV as well as safety antioxidant for delaying skin aging in humans.
Acknowledgments
The authors thank Tomoki Ikuta, Keiko Kobayashi, and Tomoki Tatefuji (Yamada Bee Company) for providing MSE and helpful discussions. They also thank Dr. Keiji Kobayashi, Dr. Masato Koike, and Toshihiko Toda, from Chiba University, for their valuable technical assistance.
Conflict of Interests
This research was supported by the institute for Bee Products & Health Science, Yamada Bee Company, Inc.
References
- 1.Poljsak B., Dahmane R. G., Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Panonica Adriat. 2012;21(2):33–36. [PubMed] [Google Scholar]
- 2.Shuster S., Black M. M., McVitie E. The influence of age and sex on skin thickness, skin collagen and density. British Journal of Dermatology. 1975;93(6):639–643. doi: 10.1111/j.1365-2133.1975.tb05113.x. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T., Nojiri H., Kawakami S., Uchiyama S., Shirasawa T. Model mice for tissue-specific deletion of the manganese superoxide dismutase gene. Geriatrics & Gerontology International. 2010;10(supplement 1):S70–S79. doi: 10.1111/j.1447-0594.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya S., Ozawa Y., Toda T., et al. Collagen peptide and vitamin C additively attenuate age-related skin atrophy in Sod1-deficient mice. Bioscience, Biotechnology, and Biochemistry. 2014;78(7):1212–1220. doi: 10.1080/09168451.2014.915728. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K., Shibuya S., Koyama H., et al. Sod1 loss induces intrinsic superoxide accumulation leading to p53-mediated growth arrest and apoptosis. International Journal of Molecular Sciences. 2013;14(6):10998–11010. doi: 10.3390/ijms140610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato E., Tokunaga Y., Sakan F. Stilbenoids isolated from the seeds of melinjo (Gnetum gnemon L.) and their biological activity. Journal of Agricultural and Food Chemistry. 2009;57(6):2544–2549. doi: 10.1021/jf803077p. [DOI] [PubMed] [Google Scholar]
- 8.Kunimasa K., Ohta T., Tani H., et al. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract suppresses multiple angiogenesis-related endothelial cell functions and tumor angiogenesis. Molecular Nutrition and Food Research. 2011;55(11):1730–1734. doi: 10.1002/mnfr.201100098. [DOI] [PubMed] [Google Scholar]
- 9.Yanagihara M., Yoshimatsu M., Inoue A., Kanno T., Tatefuji T., Hashimoto K. Inhibitory effect of gnetin C, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biological and Pharmaceutical Bulletin. 2012;35(6):993–996. doi: 10.1248/bpb.35.993. [DOI] [PubMed] [Google Scholar]
- 10.Ota H., Akishita M., Tani H., et al. trans-resveratrol in Gnetum gnemon protects against oxidative-stress-induced endothelial senescence. Journal of Natural Products. 2013;76(7):1242–1247. doi: 10.1021/np300841v. [DOI] [PubMed] [Google Scholar]
- 11.Tatefuji T., Yanagihara M., Fukushima S., Hashimoto K. Safety assessment of melinjo (Gnetum gnemon L.) seed extract: acute and subchronic toxicity studies. Food and Chemical Toxicology. 2014;67:230–235. doi: 10.1016/j.fct.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Konno H., Kanai Y., Katagiri M., et al. Melinjo (Gnetum gnemon L.) seed extract decreases serum uric acid levels in nonobese Japanese males: a randomized controlled study. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/589169.589169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tani H., Hikami S., Iizuna S., et al. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. Journal of Agricultural and Food Chemistry. 2014;62(8):1999–2007. doi: 10.1021/jf4048435. [DOI] [PubMed] [Google Scholar]
- 14.Sato M., Suzuki Y., Okuda T., Yokotsuka K. Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Bioscience, Biotechnology and Biochemistry. 1997;61(11):1800–1805. doi: 10.1271/bbb.61.1800. [DOI] [PubMed] [Google Scholar]
- 15.Baur J. A., Sinclair D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews Drug Discovery. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard B. P., Sinclair D. A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends in Pharmacological Sciences. 2014;35(3):146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang J.-H., Surh Y.-J. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutation Research: Genetic Toxicology and Environmental Mutagenesis. 2001;496(1-2):181–190. doi: 10.1016/S1383-5718(01)00233-9. [DOI] [PubMed] [Google Scholar]
- 18.Adhami V. M., Afaq F., Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5(1):74–82. doi: 10.1016/S1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serravallo M., Jagdeo J., Glick S. A., Siegel D. M., Brody N. I. Sirtuins in dermatology: applications for future research and therapeutics. Archives of Dermatological Research. 2013;305(4):269–282. doi: 10.1007/s00403-013-1320-2. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa D., Nojiri H., Saita Y., et al. Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. Journal of Bone and Mineral Research. 2013;28(11):2368–2380. doi: 10.1002/jbmr.1981. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama S., Shimizu T., Shirasawa T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. The Journal of Biological Chemistry. 2006;281(42):31713–31719. doi: 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- 22.Nojiri H., Shimizu T., Funakoshi M., et al. Oxidative stress causes heart failure with impaired mitochondrial respiration. Journal of Biological Chemistry. 2006;281(44):33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 23.Nauta A. C., Grova M., Montoro D. T., et al. Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059167.e59167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami K., Inagaki J., Saito M., et al. Skin atrophy in cytoplasmic SOD-deficient mice and its complete recovery using a vitamin C derivative. Biochemical and Biophysical Research Communications. 2009;382(2):457–461. doi: 10.1016/j.bbrc.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya S., Kinoshita K., Shimizu T. Protective effects of vitamin C derivatives on skin atrophy caused by Sod1 deficiency. In: Preedy V. R., editor. Handbook of Diet, Nutrition and the Skin. Wageningen Academic; 2012. pp. 351–364. [Google Scholar]
- 26.Shibuya S., Nojiri H., Morikawa D., Koyama H., Shimizu T. Protective effects of vitamin C on age-related bone and skin phenotypes caused by intracellular reactive oxygen species. In: Preedy V. R., editor. Oxidative Stress and Dietary Antioxidants. Academic Press; 2013. pp. 137–155. [Google Scholar]
- 27.Iuchi Y., Okada F., Onuma K., et al. Elevated oxidative stress in erythrocytes due to a SOD1 deficiency causes anaemia and triggers autoantibody production. Biochemical Journal. 2007;402(2):219–227. doi: 10.1042/BJ20061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nojiri H., Saita Y., Morikawa D., et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. Journal of Bone and Mineral Research. 2011;26(11):2682–2694. doi: 10.1002/jbmr.489. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z., Sonntag W. E., de Cabo R., Baur J. A., Csiszar A. Mitochondrial protection by resveratrol. Exercise and Sport Sciences Reviews. 2011;39(3):128–132. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Zhang M., Dong H., et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28(3):445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 31.Price N. L., Gomes A. P., Ling A. J. Y., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buonocore D., Lazzeretti A., Tocabens P., et al. Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebo-controlled, double-blind study. Clinical, Cosmetic and Investigational Dermatology. 2012;5:159–165. doi: 10.2147/CCID.S36102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibuya S., Ozawa Y., Watanabe K., et al. Palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in mice. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109288.e109288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyner S. D., Venkatachalam S., Choi J., et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 35.Gannon H. S., Donehower L. A., Lyle S., Jones S. N. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Developmental Biology. 2011;353(1):1–9. doi: 10.1016/j.ydbio.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J. S., Park K. Y., Min H. G., et al. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Experimental Dermatology. 2010;19(12):1060–1066. doi: 10.1111/j.1600-0625.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 37.Park M. H., Park J. Y., Lee H. J., et al. The novel PPAR α/γ dual agonist MHY 966 modulates UVB-induced skin inflammation by inhibiting NF-κB activity. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076820.e76820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastrofrancesco A., Kovacs D., Sarra M., et al. Preclinical studies of a specific PPARγ modulator in the control of skin inflammation. Journal of Investigative Dermatology. 2014;134(4):1001–1011. doi: 10.1038/jid.2013.448. [DOI] [PubMed] [Google Scholar]