Abstract
The human cytochrome P450 enzymes (P450s) catalyze oxidative reactions of a broad spectrum of substrates and play a critical role in the metabolism of xenobiotics, such as drugs and dietary compounds. CYP3A4 is known to be the main enzyme involved in the metabolism of drugs and most other xenobiotics. Dietary compounds, of which polyphenolics are the most studied, have been shown to interact with CYP3A4 and alter its expression and activity. Traditionally, the liver was considered the prime site of CYP3A-mediated first-pass metabolic extraction, but in vitro and in vivo studies now suggest that the small intestine can be of equal or even greater importance for the metabolism of polyphenolics and drugs. Recent studies have pointed to the role of gut microbiota in the metabolic fate of polyphenolics in human, suggesting their involvement in the complex interactions between dietary polyphenols and CYP3A4. Last but not least, all the above suggests that coadministration of drugs and foods that are rich in polyphenols is expected to stimulate undesirable clinical consequences. This review focuses on interactions between dietary polyphenols and CYP3A4 as they relate to structural considerations, food-drug interactions, and potential negative consequences of interactions between CYP3A4 and polyphenols.
1. Introduction
Cytochrome P450 enzymes (P450s) are responsible for the metabolism of a wide range of endogenous compounds (steroid hormones, lipids, and bile acids), as well as xenobiotics including drugs, environmental pollutants, and dietary products [1–4]. P450 enzymes are widely distributed among the phylogenetic trees [5] and considered as a significant player in the world around us, where life and the earth itself would be visibly different and diminished without cytochrome P450s [6]. A direct impact on humans is mediated especially through our own set of 57 P450s [7]. CYP is an abbreviation for cytochrome P450; the gene family is then indicated by a number following the letters “CYP.” Subfamilies are represented by a letter that is followed by yet another number to indicate the specific gene. For example, for the enzyme CYP3A4, “3” stands for the gene family, “A” for the subfamily, and “4” defines the gene that encodes a specific polypeptide [8].
Among this large family of oxidizing enzymes, CYP3A4 is recognized as the main enzyme involved in the metabolism of drugs in the liver and, no less importantly, in the gut. Hence, potential interactions between promising new drugs and CYP3A4 are assessed starting at the early stages of their development [9–11]. CYP3A4 is most abundant P450 in the human liver, accounting for 30% of the total P450 protein content but is also expressed in the prostate, breast, gut, colon, small intestine, and brain [12–17]. In the small intestine, CYP3A enzymes represent the principle drug-metabolizing system and account for approximately 80% of total P450 content [18–20]. Although the total amount of CYP3A expressed in the human small intestine represents approximately 1% of the amount expressed in the liver [21, 22], substantial drug extraction takes place during the absorption of orally administered drugs [23–26]. Orally administered substrates must pass through enterocytes while they can bypass hepatocytes by remaining in the sinusoidal blood before reaching the systemic circulation. The remarkably lower blood flow to the intestinal mucosa as compared to the liver allows for prolonged exposure to the intestinal metabolizing enzymes and lead to relatively high enterocytic drug concentrations. The predominance of CYP3A4 in human intestine and its high capacity enable it to can act several-fold more efficiently in the intestine than in the liver [20, 27, 28]. Furthermore, the intestine receives not only dietary compounds, but also phase I and II metabolites that have been excreted back into the intestine through the enterohepatic cycle [29, 30]. All these facts indicate the importance of intestinal CYP3A4 activity in the metabolism of dietary constituents. In rodents, the isofrom CYP3A is expressed predominantly in the liver, with only scant expression observed in the intestine [31–33]. The different isoforms and distinct expression levels and patterns for P450s in the intestine between humans and rodents limit the suitability of rodents as a model to predict drug metabolism or oral bioavailability in human [34]. This points the importance of studying the effects of ingested polyphenols and other dietary substrates on the metabolism of intestinal CYP3A4 in humans or in models other than rodents' intestine. The latter include cell cultures, microsomes, and microorganisms that express the specific P450 of interest or a whole array of P450s [35–39].
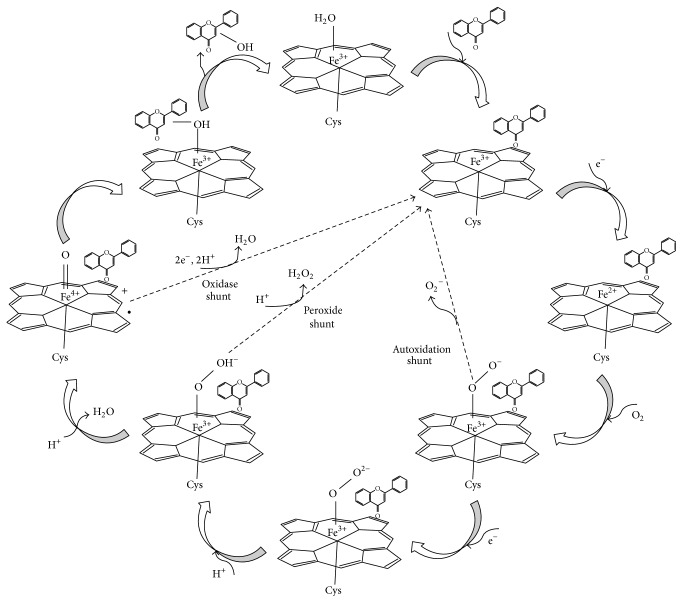
The active site of a substrate-free cytochrome P450 contains one-heme iron center anchored by the four bonds of the heme group, fifth proximal ligand of the conserved cysteine, and water molecule as the sixth distal ligand [1]. The catalytic mechanisms of P450 enzymes are thoroughly investigated in the literature, as demonstrated in a scheme based on previous publications (Figure 1) [1, 40–42]. Like most other P450 enzymes, CYP3A4 acts as a monooxygenase (e.g., it catalyzes the insertion of one atom of oxygen into an organic substrate while another oxygen atom is reduced to water) [43]. The substrate chemical characteristics and the preferred position of hydroxyl insertion change from one family of P450 to another [3, 44–46]. P450 enzymes play a major role in phase I metabolism of dietary xenobiotics, including polyphenols, whereby a hydroxyl group is introduced to the molecule. These metabolic products are more water-soluble and become available to phase II enzymes. The latter include UDP-glucuronosyl transferases and sulfotransferases that add to the increased water solubility of the hydroxylated polyphenols, producing glucuronides and sulfates, which are then eliminated from the body [29, 47, 48].
Figure 1.
The catalytic cycle of P450s: a flavonoid structure was selected to represent dietary polyphenols.
In recent studies, evidence has accumulated to indicate potent interactions between CYP3A4 and edible phytochemicals. These compounds, some of which are abundant in our diet, belong to the large and diverse family of polyphenolics, including flavonoids, phenolic acids, phenolic alcohol, stilbenoids, and lignans [49–53]. It is commonly accepted that the powerful antioxidant activity of polyphenolic compounds is due to their free-radical scavenging capacity and their iron-chelating activity [54–56]. Reviews of the health benefits of polyphenols demonstrate that these compounds have numerous therapeutic effects against several diseases (e.g., atherosclerosis, certain forms of carcinogenic processes, and cardiovascular and neurodegenerative diseases) [57–60]. Among the therapeutic implications of polyphenols on human health, the interactions between polyphenols and cytochrome P450 have been recently reviewed [56, 61–64]. These interactions were highlighted following the increased use of herbal medicines and supplements. As many of the herbs used in these preparations are known to be rich in polyphenolics, their interaction with the major enzyme of presystemic metabolism has attracted significant research attention [56, 65–67]. Since cytochrome P450 enzymes are responsible for the metabolism of a wide range of drugs and polyphenols, which might also change their antimicrobial potential and human toxicity, the simultaneous consumption of drugs, herbals, and plant foods raises concerns. The coadministration of active constituents derived from food or herbs and prescribed drugs may lead to undesirable clinical effects, which may include increased toxicity and/or treatment failure [67–69].
Here, we focus on the interactions of polyphenols with CYP3A4, the major enzyme in the gut and liver metabolism of drugs and xenobiotics. The effects of several subcategories of polyphenols on the expression and activity of CYP3A4 (inhibition or induction) are reviewed (Table 1). Structural and physicochemical considerations that define these interactions are also reviewed.
Table 1.
Potential interactions of polyphenols with CYP3A4.
| Category of polyphenols | Subcategory of polyphenols | Polyphenols in category | Interaction with CYP3A4 | References |
|---|---|---|---|---|
| Flavonoids | Flavonols | Kaempferol, galangin | Inhibition | [87, 148, 149] |
| Quercetin | Inhibition | [87, 148–150] | ||
| Induction of CYP3A4 mRNA expression in vivo and in prolonged-exposure assays | [87, 151, 153] | |||
| Flavones | Apigenin, chrysin, amentoflavone | Inhibition | [73] | |
| Luteolin, diosmetin | Inhibition | [156] | ||
| Flavone, tangeretin | Activation | [156] | ||
| α-Naphthoflavone | Activation | [11] | ||
| Flavonols | EGCG, ECG | Inhibition | [73, 121, 139, 162] | |
| Flavanones | Naringin, naringenin | Inhibition | [73, 165] | |
| Isoflavones | Genestein | Inhibition | [169–171] | |
| Activation (modest activation in clinical trials) | [172, 173] | |||
| Anthocyanins | Anthocyanins (and anthocyanins aglycones) | Inhibition | [174] | |
|
| ||||
| Nonflavonoids | Stilbenes | Resveratrol (and resveratrol derivatives) | Inhibition | [38, 175–179] |
| Lignans | Gomisins (B and C) | Inhibition | [182] | |
| Silymarin mixture | Inhibition (with slight activation at low concentrations) | [183, 184] | ||
| Tannins | Tannic acid | Inhibition | [185] | |
|
| ||||
| Phenolic acids | Hydroxycinnamic acid | Caffeic acid | Inhibition | [186] |
| Hydroxybenzoic acid | Gallic acid | Inhibition | [187, 188] | |
2. CYP3A4 and Food-Drug Interactions
Drug-metabolizing P450s such as CYP3A4 have relaxed selectivity and are able to bind and metabolize a large array of substrates of different size, shapes, and chemical properties, for example, many dietary polyphenols. Crystal structures, biophysical studies, and molecular dynamics have provided important insights into how drug-metabolizing P450s, especially CYP3A4, structurally adapt to a variety of inhibitors and substrates [70]. Indeed, CYP3A4 is involved in the metabolism of over 50% of marketed drugs that undergo metabolic elimination [71]. The high level of CYP3A4 expression in the intestine, as well as its broad substrate specificity may explain the accumulating data regarding its susceptibility to modulation by food constituents [38, 61, 72–75]. Examples of metabolic food-drug interactions involving the modulation of CYP3A4 activity by components from dietary and herbal sources are accumulating, including those of grapefruit with over 85 drugs, for example, cyclosporine and felodipine [27, 76–78], and those of St. John's wort [54, 79, 80], and red wine [38, 75, 81] with cyclosporine. In most of these cases, components in foods, drinks, food additives, and orally administered medicines were shown to inhibit CYP3A4 activity and, as a result, increase the actual dose of the drug that reaches the blood circulation in its active form, which often causes unfavorable and long-lasting interactions and probably fatal toxicity [82, 83]. Continuous exposure to these compounds, especially those that activate the xenobiotic nuclear receptor PXR (pregnane X receptor), may lead, in a feedback fashion, to increased expression of CYP3A4 in the intestine, making the food-drug interaction even more complex during extended periods of use [84–87]. Drug-drug, food-drug, and herb-drug interactions in the liver have been well documented in the literature [72, 88–90]. An intensive CYP3A4-dependent intestinal metabolism of low-absorbed compounds such as most polyphenols might be expected [29, 54, 91–93]. However, to the best of our knowledge the research in this area is limited and additional data are needed.
3. Polyphenols
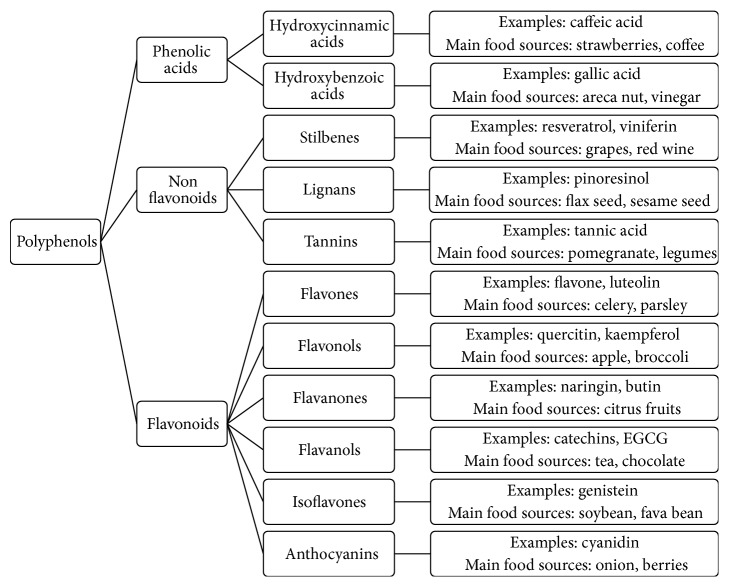
As reviewed in other works in this issue, polyphenols constitute a large and diverse family of compounds that is commonly divided into subfamilies that share similar chemistry: flavonoids, flavonols, phenolic acids, phenolic alcohols, stilbenoids, tannins, and lignans (Figure 2). Polyphenols are found in several foods, fruits, vegetables, and herbs [52, 94, 95]. In general, the total intake of polyphenols is approximated at 1 g/individual/day and polyphenols are considered by many to be the major source of antioxidants in our diet [51, 95–97]. However, this estimate varies depending on the type of diet. For example, total polyphenol intake in the Finnish diet is 817–919 mg/individual/day [98]. In the Vietnamese diet, it is 595 mg/individual/day [99], and in the Mediterranean diet, polyphenol intake ranges between 1800 and 3000 mg/individual/day [100]. Still, and due to their low absorption, it has been suggested that their major sites of antioxidant activity are the stomach [101] and the intestine [102]. Whether acting in the gastrointestinal tract or in the liver, the potent antioxidant effects of polyphenols are widely accepted as health promoting [103–105]. Antivira, antibacterial, anti-inflammatory, neuroprotective, and anticarcinogenic effects have also been attributed to polyphenols [106–109]. Medicinal herbs such as St. John's wort (Hypericum perforatum), ginseng (Panax ginseng), black cohosh (Actaea racemosa), echinacea (Echinacea purpurea), cranberry (Vaccinium macrocarpon), and ginger (Zingiber officinale) are rich sources of a vast array of polyphenolic compounds [74, 110–115]. The biochemical mechanisms underlying metabolic herb-drug interaction were well described in a recent review [72]. These herbal sources of polyphenols deserve special attention when the activity of P450s is discussed, due to the dramatic increase in the use of herbal medicines and supplements [65, 66]. Recent surveys suggest that one in three Americans use dietary supplements daily and among cancer patients the rate is much higher [54]. Moreover, medicinal herbs are not inspected by regulatory authorities such as the Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) [72]. Indeed, medical doctors as well as pharma professionals should be aware of the many interactions of polyphenolics with drugs and tools should be developed to assess the potential of individual polyphenolics to enter the active sites of P450 enzymes and become substrates, competitive inhibitors, or other types of inhibitors of these enzymes in the intestine and the liver. CYP3A4 should be a major point of focus in studies of the undesirable clinical consequences of the timed use of prescribed drugs and herbs [74].
Figure 2.
Classification of polyphenols.
4. Metabolism of Polyphenols by P450s
4.1. Metabolism of Polyphenols by P450 Enzymes
The metabolic fate of polyphenols is largely dictated by their chemical structure and depends on several parameters, including their functional groups (i.e., benzene or flavone derivatives), molecular weight, stereostructure, glycosylation, polymerization, and conjugation with other phenolics [97, 116, 117]. Flavonoids, which are the largest subgroup of polyphenols, have been identified as substrates of P450 enzymes [118, 119]. Flavonoids are hydroxylated and/or o-demethylated by various hepatic P450 enzymes prior to their elimination [67]. Jančová and coworkers showed that silybin, a flavono-lignan found in silymarin, is metabolized to o-demethylated product by CYP2C8 and CYP3A4 in vitro [120]. Meanwhile it has been reported that flavonoids rich with hydroxyl group such as green tea catechins are fairly water soluble and are not likely to be good substrates for P450 enzymes [121, 122]. This is consistent with findings that have demonstrated the importance of ligand hydrophobicity for interactions with these enzymes [38, 123, 124]. Paradoxically, inhibitory effects of green tea catechins on several P450 enzymes have been reported in in vivo trials [125, 126]. Another intensively studied polyphenol is the stilbene t-resveratrol (trans-3,4′,5-trihydroxystilbene), a polyphenol found in grape skins and red wine, peanuts, and a limited number of other plants, and its effects on CYP3A4 will be discussed later (Section 5.2.1). It exhibits a high level of membrane permeability and is categorized as a class-II compound in the Biopharmaceutical Classification System (BCS) [127]. t-Resveratrol has a low bioavailability (less than 1%) due to the low water solubility (a logP of 3.1), and the extensive first-pass metabolism by CYP3A4 in the intestine and in the liver, which extended by the enterohepatic recirculation. Further metabolism leads to the formation of the glucuronide and the sulfate metabolites of t-resveratrol [128, 129]. Recently, Singh and Pai reported the success of a systematically optimized nanoparticulate drug delivery system to increase the oral bioavailability of t-resveratrol in rats [130]. In a similar context in in vitro study, Seljak et al. developed a mixed lipid–mixed surfactant self-microemulsifying drug delivery system (SMEDDS) to improve the biopharmaceutical, pharmacokinetic, and toxicological characteristics of resveratrol, suggesting a way to lower the applied dose of resveratrol, to reduce toxicity while maintaining a sufficient pharmacological response [131].
4.2. Involvement of Microbiota in the Metabolism of Polyphenols
There is accumulating evidence to suggest that gut microbiota play a significant role in the metabolism, bioavailability, and bioactivity of dietary polyphenols [132–134]. The involvement of microbiota in the metabolism of these compounds generally starts with the hydrolysis of polymeric, glycosylated and/or esterified polyphenols by brush border and/or microbial enzymes, which is a prerequisite for the absorption and bioactivity of most compounds [134–136]. These biotransformations affect the structural characteristics of polyphenols and may generate metabolites with altered bioactivity profiles [30, 134]. Considering the water soluble green tea catechins, which should be very poor substrates for CYPs, their biotransformation by human gut microbiota could lead to the formation of better CYP substrates, as was demonstrated in vitro by Stoupi et al. [137]. Taken together, we suggest that gut microbiota may play a role in the formation of polyphenol-derived metabolites that are more likely to interact with P450 enzymes. The role of intestinal microbiota in the metabolism and bioavailability of dietary polyphenols has been examined [30, 132–136], but, unfortunately, data on the three-way interactions between polyphenols, microbiota and P450s are scarce.
5. Modulation of CYP3A4 Activity by Polyphenols
Interactions between polyphenols and CYP3A4 are important due to their potential implications for drug metabolism. These interactions can modulate the activity or expression of the enzyme. Kimura et al. demonstrated inhibitory effects of polyphenols on human CYP3A4 and CYP2C9 activity in vitro [73]. These inhibitory effects generally involve the formation of a covalent bond between the polyphenol and the CYP3A4 molecule, which leads to the inactivation of the enzyme, or reversible binding that causes reversible inhibition [138]. In some cases, the inhibition of P450 enzymes by polyphenols may have a chemopreventative effect, due to the potential activation of carcinogens by P450 enzymes within the course of their natural metabolic activity [81, 139–142]. The inhibition of xenobiotic-metabolizing phase I enzymes (i.e., P450 enzymes) could be one target of the chemopreventive effects of naturally occurring polyphenols. Alternatively, it could be the induction of phase II conjugation enzymes, such as UDP-glucuronosyl transferase and glutathione S-transferase, which are responsible for the detoxification of carcinogens [54].
5.1. Interactions between Flavonoids and CYP3A4
In large, flavonoids account for about two-thirds of the total intake of dietary polyphenols and phenolic acids account for the remaining one-third [33]. Flavonoids, which are found primarily in fruits, vegetables, and beverages such as tea and wine are bioactive compounds that carry several benefits for human health [142–144]. Flavonoids are known to modulate several P450 enzymes, including CYP1A1, CYP1A2, CYP1B1, CYP2C9, CYP3A4, and CYP3A5 [145, 146]. Hence, their interactions with CYP3A4 are studied in more systems than most other polyphenols and provide evidence for various interactions of polyphenols with this enzyme. There is accumulated evidence that within the family of polyphenols, flavonoids especially can modulate drug metabolism, and in several modes: by altering the expression and/or activity of P450 enzymes, by affecting the P-glycoprotein-mediated cellular efflux of drugs and/or by inhibiting the intestinal glucuronidation of the drug. This evidence indicates that the use of flavonoid-containing dietary supplements concurrent with conventional pharmacotherapeutic regimens should be considered in order to avoid drug-flavonoid interactions [54, 72, 143–146]. In this direction, studies are being conducted to develop methods for evaluating food-drug interactions. For example, Koe and coworkers recently developed a novel multiplex RT-qPCR in vitro assay to examine the P450 enzyme-induction properties of herb-derived compounds [147].
5.1.1. Flavonols
The flavonols kaempferol, quercetin, and galangin inhibit CYP3A4-mediated metabolism of xenobiotics in vitro [87, 148, 149]. Studies performed in vivo have shown conflicting modulation of CYP3A activity by quercetin. Choi et al. reported that oral administration of quercetin to rats led to inhibition of CYP3A, which caused a significant enhancement in the doxorubicin concentration in the plasma. On the contrary, Yu et al. reported an activation of the enzyme that resulted in a reduction in the plasma concentration of cyclosporine in a similar model. The latter observation suggests that this enzyme is not activated by the flavonols, but by their sulfated or glucorunidated products [150, 151]. No in vivo inhibition of CYP3A4-mediated metabolism of nifedipine was observed following the ingestion of a high dose of quercetin by others [152]. Interestingly, prolonged exposure to quercetin leads to a significant increase in CYP3A4 mRNA expression levels in cell cultures [87, 153]. We suggest that these findings might be related to the well-established induction of CYP3A4 in response to consumption of St. John's wort extract, which is a rich source of quercetin, in addition to another recognized inducer, the nonphenolic hyperforin [54, 85, 86, 154]. Kaempferol and quercetin have been found to inhibit intestinal UDP-glucuronyl transferase in vitro at clinically achievable concentrations, which may lead to an increase in the bioavailability of several drugs [146]. A recent study conducted on rats found that oral administration of morin, a flavonol found in many fruits and herbal medicines, increased the plasma half-life (t 1/2) of febuxostat, a drug used to treat gout 2.5-fold as compared with the control group, leading to significantly higher bioavailability. One suggested mode of action was that morin could be effective in inhibiting CYP1A1, CYP1A2 and CYP3A mediated metabolism of febuxostat [143].
5.1.2. Flavones
The flavones apigenin and chrysin have a marked inhibitory effect on CYP3A4 activity in vitro, with IC50 values of 0.4 μM and 0.9 μM, respectively. Amentoflavone (a dimer of apigenin) has even a stronger inhibitory effect, with an IC50 value of 0.07 μM [73]. Calculations of the lipophilicity of the two compounds provide support for previous suggestions that higher lipophilicity may contribute to stronger binding of the substrate. It is also possible that the larger stereodimensions of the dimer may lead to irreversible binding of the hydroxylation product to the enzyme, thereby achieving inhibition via a suicidal mode of action [12, 155]. A recent study in rats suggests that the coadministration of apigenin would be very useful for improving the bioavailability of paclitaxel in chemotherapeutic applications, due to the inhibitory effects of apigenin on CYP3A and P-glycoprotein, leading to higher concentration of paclitaxel in the plasma [144]. The ability of apigenin to inhibit intestinal UGT activity has also been investigated in vitro [146]. In a study designed to reveal structure-activity relationships, flavones possessing more than two hydroxyl groups (e.g., luteolin and diosmetin) were shown to inhibit the biotransformation of midazolam in vitro, whereas flavones that do not have hydroxyl groups in their A and B rings (e.g., flavone and tangeretin) stimulated midazolam metabolism [156]. These results may support the activation effect of α-naphthoflavone (a flavone with no hydroxyl groups) on CYP3A4 and two other CYP3A enzymes, CYP3A5 and CYP3A7 [11]. In addition, α-naphthoflavone represents an interesting case of heterotropic cooperativity in CYP3A4, as it interacts with a peripheral ligand binding site, located at the distal surface of the enzyme and surrounded by the F/F9 and G/G9 loops, resulting in allosteric mechanism [157–161].
5.1.3. Flavonols
Green tea flavonols epigallocatechingallate (EGCG) and epicatechingallate (ECG) inhibit the mutagenic action of aflatoxin B1 (AFB1) and 1′-hydroxylation of midazolam in vitro. Both actions are known to be mediated by CYP3A4 [139]. Inhibitory effects of catechins on CYP3A4 have been reported in several additional in vitro and in vivo studies, but no specific mode of action has been suggested [73, 121, 125, 126, 162].
5.1.4. Flavanones
The inhibition of CYP3A4 by grapefruit juice is probably the most well-known example of food-drug inhibition [76, 163]. It was suggested that the flavanone naringin, the predominant flavanone in grapefruit, might be responsible for the observed interaction effect [164]. However, naringin appears to be a weak inhibitor of CYP3A4, while its aglycone, naringenin, may be a more potent inhibitor. The IC50 value of naringin is 10-fold greater than that of naringenin in vitro and this difference is attributed to the lack of a hydroxyl group on ring A of naringin [73, 165]. This is in agreement with the finding of Shimada and coauthors regarding the importance of the hydroxylation of ring A flavones for the inhibition of CYP3A4 [148]. However, the most potent inhibitor of CYP3A4 in grapefruit has been suggested to be bergapten, a furanocoumarin derivative [165], that does not belong to the polyphenol family, but has a relatively similar structure. The inhibitory effects of other furanocoumarins on CYP3A4 activity in vitro are also well established [166–168].
5.1.5. Isoflavones
Isoflavones such as genistein and daidzein are found in soybean and hence are very abundant in many processed food products. Isoflavones differ from flavones in the location of their phenyl group. It has been suggested that isoflavones may act as phytoestrogens and they appear potential substrates or inhibitors of P450 enzymes. Conflicting data have been presented in several works describing in vitro and in vivo studies. For instance, soy isoflavones have been found to inhibit CYP3A4 metabolism [169–171], whereas the administration of genistein resulted in a modest induction of CYP3A enzymes among healthy participants [172, 173].
5.1.6. Anthocyanins
Dreiseitel et al. found that anthocyanins and their aglycones are weak inhibitors of CYP3A4 in vitro [174]. The IC50 values of anthocyanin derivatives ranged from 12.2 to 7,842 μM; whereas ketoconazole, a synthetic CYP3A4 inhibitor that is often used as a reference, has an IC50 value of 18.4 nM. Measurement of the IC50 values of the different aglycones revealed an inverse relationship between the number of sugar moieties per compound and the ability of anthocyanins to inhibit CYP3A4 [174]. This provides further support for the accumulating data pointing to the importance of lipophilicity for interaction with CYP3A4 [38, 123, 124]. We recently reached a similar conclusion using software to study docking of polyphenols, in which we observed a correlation between the logP values of ligands and their docking energies with CYP3A4 (CDOCKER energy expressed in Kcal/mole; Basheer and Kerem, unpublished data).
5.2. Interaction between Nonflavonoids and CYP3A4
5.2.1. Stilbenoids
The inhibitory effects of t-resveratrol on CYP3A4 in vitro and in vivo are well established, and it has been suggested that resveratrol might act as an irreversible, mechanism-based inactivator of this enzyme [38, 175–179]. This inhibition occurs when a CYP3A4 substrate/inhibitor forms a reactive intermediate at the CYP3A4 active site, leading to enzyme inactivation by modification to the heme or the apoprotein [180, 181]. Chan and Delucchi suggested that an electron-rich unsaturated molecule like resveratrol could be a substrate for CYP3A4 and might, in turn, inactivate CYP3A4 during the course of catalysis [175]. Clinical and rat trials have found that the administration of resveratrol increases the area under the plasma concentration-time curve (AUC) for several drugs [81, 177]. Thus, consuming large amounts of resveratrol could theoretically increase the bioavailability of and risk of toxicity from drugs that undergo extensive first-pass metabolism by CYP3A4 [179]. In vitro study of the effect of lipophilicity on the interactions of resveratrol derivatives with CYP3A4 revealed that methoxy-stilbenes have lower IC50 values and greater affinity for CYP3A4, as compared to the parent resveratrol and its glucosides [38]. CYP3A-mediated aromatic hydroxylation and epoxidation of resveratrol is possible and results in a reactive p-benzoquinone methide metabolite that is capable of binding covalently to CYP3A4, leading to inactivation and potential drug interactions [175].
5.2.2. Lignans
The lignans gomisins B and C, components of Schisandra fruit (Schisandra chinensis) extract, have been identified as potent inhibitors of CYP3A4 in vitro [182]. Other evidence for the inhibitory effects of plant lignans on CYP3A4 is provided by silymarin, a mixture of flavonolignans extracted from milk thistle (Silybum marianum). Silymarin (0.1 mM and 0.25 mM) significantly reduced the activity of CYP3A4 in human hepatocyte cultures by 50 and 100%, respectively, as determined by the formation of 6-β-hydroxy testosterone [183]. Studying the effects of selected lignans from silymarin (silybin, dehydrosilybin, silydianin and silycristin) on CYP3A4 activity as determined in vitro by nifedipine oxidation revealed that CYP3A4 activity is inhibited as the concentration of each flavonolignan increases. However, a slight increase in activity was also observed in the presence of low flavonolignan concentrations (0.1–1 μM) [184].
5.2.3. Tannins
Tannic acid, a type of hydrolysable tannin commonly found in plant foods, inhibited testosterone 6-β-hydroxylation (CYP3A4) in human- and rat-liver microsomes with IC50 values of 20.2 μM and 16.8 μM, respectively [185].
5.3. Interactions between Phenolic Acids and CYP3A4
Phenolic acids do not all belong to the polyphenols, but are commonly discussed together. The interaction of phenolic acids with CYP3A4 and their potential metabolism by the enzyme would be of high relevance as the research of the more multi-member interactions of CYP3A4, polyphenols and gut microbiota advances, due to the high antimicrobial activity of phenolic acids.
5.3.1. Hydroxycinnamic Acids
Caffeic acid (3,4-dihydrocinnamic acid), which do belong to the polyphenols, is one of the most common phenolic acids found in fruits, coffee, olive oil and dietary supplements. Caffeic acid has been shown to inhibit CYP3A4 activity in human liver microsomes by noncompetitive inhibition, with an IC50 of 0.72 μM. In addition, ester and amide analogues of caffeic acid have been found to act as competitive inhibitors, with IC50 values ranging from 0.31 μM to 0.82 μM [186].
5.3.2. Hydroxybenzoic Acids
Gallic acid (3,4,5-trihydroxybenzoic acid), also a member of the polyphenols and is abundant in many beverages, for example, wine, tea, pomegranate juice and olive oil, has an inhibitory effect on androstenedione 6-β-hydroxylase activity in vitro (apparent K i value 70 μM), which is regarded as a marker for CYP3A enzyme activity [187]. In another study, Stupans and coworkers provided additional evidence for the inhibition of CYP3A activity by gallic acid. In that study, they showed that pre-incubation of human liver microsomes with 100 μM gallic acid before the assay of androstenedione 6-β-hydroxylase activity significantly increased the inhibitory effects of the gallic acid. In addition, they reported that the removal of gallic acid-derived products from the incubation mixture completely restored CYP3A activity [188].
6. Structure-Activity Relationships
Various interactions have been demonstrated between compounds belonging to the large family of polyphenols and P450 enzymes. While members of this family share many structural and functional features, existing reports do not provide sufficient information to allow us to fully understand the rules that determine the nature of these interactions. The number of hydroxyl groups, stereostructure, molecular weight and lipophilicity all seem to have some sort of effect on individual results. Up to date, the protein data bank (PDB) contains 18 crystal structure of human CYP3A4. One of the most prominent characteristics reported was the large, highly ordered hydrophobic core of phenylalanine residues above the active site [189, 190]. A recent review concluded that the CYP3A4 active site is considerably larger than the active site of any other P450 isoform [191].
CYP3A4 substrates form hydrogen bonds with the Asn74 residue of CYP3A4. Structural requirements of CYP3A4 substrates have been suggested to include a hydrogen-bond acceptor atom located 5.5–7.8 Å from the site of metabolism and 3 Å from the oxygen molecule associated with the heme [192]. A three-dimensional pharmacophore based on 38 substrates of CYP3A4 possessed two hydrogen bond acceptors, one hydrogen bond donor, and one hydrophobic region [193]. Inhibitor pharmacophores include three hydrophobes at distances of 5.2 to 8.8 Å from a hydrogen-bond acceptor, three hydrophobes at distances of 4.2 to 7.1 Å from a hydrogen-bond acceptor and at an additional 5.2 Å from another hydrogen-bond acceptor, or one hydrophobe at a distance of 8.1 to 16.3 Å from the two furthest of three hydrogen-bond acceptors [194].
Substrates or inhibitors can bind to CYP3A4 at multiple sites due to the flexible structure of this enzyme's active site [195–197]. For example, a study of the crystal structures of human CYP3A4 in complex with two well characterized drugs, ketoconazole and erythromycin, revealed that the enzyme undergoes dramatic conformational changes upon ligand binding, with an increase in the volume of the active site of more than 80%. These structures represent two distinct open conformations of CYP3A4 because ketoconazole and erythromycin induce different types of coordinate shifts [198]. CYP3A4, like many of P450 enzymes, have large and flexible substrate binding pockets capable of accommodating large substrates or alternatively two or three smaller molecules [199]. Examples on CYP3A4 cooperativity and its non-Mechaelis-Menten kinetics are found in several studies [195, 200, 201]. However, recent studies demonstrate a very complex allosteric mechanism of P450's including overlay of a multiple substrate-binding space-filling mechanism, enzyme conformational changes induced by ligands and modulation of protein-protein interactions in the enzyme oligomers [158, 202]. Allosteric behavior includes homotropic and heterotropic activation and inhibition effects depending on thermodynamic factors as demonstrated by Denisov and Sligar. The latter suggest that “functional cooperativity” best describes P450s fold that includes remote binding sites which may serve for the allosteric regulation of equilibrium and/or kinetic functional properties, including substrate binding and product dissociation, stability of oxy-complex and autoxidation [203].
6.1. Quantitative Structure-Activity Relationship (QSAR)
Didziapetris and coworkers developed a structure-activity relationship model to predict the probability that a compound can inhibit human CYP3A4, based on data for more than 800 compounds from various literature sources. Their model is based on GALAS methodology, which involves QSAR (quantitative structure-activity relationship) and local similarity-based corrections. The findings of the GALAS model revealed that increasing the size of the molecule via the incorporation of hydrophobic aliphatic or aromatic residues enhances the ability of the compound to inhibit CYP3A4, while a strong acidic or basic group in the molecule reduces its inhibition potential. This model emphasizes the importance of lipophilicity and the presence of hydrophobic groups on the inhibition potency of compounds, which is consistent with the phenylalanine residues already seen at the active site [123]. An additional QSAR study based on five statistical tools identified a strong correlation between the n-octanol/water partition coefficient (logP) and the binding affinity of compounds for CYP3A4 [124]. In line with these findings, a study on the influence of lipophilicity on the interactions of hydroxystilbenes with CYP3A4 revealed that methoxy-stilbenes had lower IC50 values and greater affinity for CYP3A4, as compared to the parent resveratrol and its glucosides. These results support the hypothesized role of lipophilicity in the interaction of polyphenols with CYP3A4 [38]. Other QSAR analyses conducted by Lewis and coworkers rationalized the lipophilicity relationships in CYP3A4 inhibitors in terms of typical active-site interactions such as hydrogen bonding and π–π stacking, whereas the multiple binding sites in the heme environment could lead to variation in gradients [204, 205].
Mao et al. showed that the traditional QSAR model applied to one data set does not lead to predictive models that would be useful for in silico filtering of chemical libraries and presents a multiple pharmacophore hypothesis (MPH) that is a conceptual extension of the conventional QSAR approach. Their study was based on 2,400 marketed drugs and made use of pair-wise comparisons of IC50 activity values for different substrates of CYP3A4. The substrates were then characterized according to the proximal and distal binding relative. MPH provides us with structural insight into how multiple substrates of CYP3A4 may interact with the enzyme (e.g., the extent to which their binding sites may lie in close proximity to one another or even overlap) [206].
7. Concluding Remarks
A number of studies in recent years have highlighted the potential risk inherent in the uncontrolled use of herbal medicines concurrent with conventional therapeutic regimens and emphasized the need for regulation in this field based on a set of evaluation criteria [207–211]. We propose here that it is the polyphenols in the herbal preparations that interact with CYP3A4, modify the metabolism of xenobiotics and drugs, and consequently change the active doses of prescribed medicines and the nature of the prescribed compounds. The abundance of polyphenols in many food products, the abundance of CYP3A4 in the intestine, its broad ranges of substrates/inhibitors and cooperativity, the potential involvement of gut microbiota in polyphenol-CYP3A4 interactions and vice versa, the extended exposure of the intestinal enzyme to polyphenol metabolites through the enterohepatic cycle and the short-term inhibition, and long-term induction of CYP3A4 by some phenolic compounds all contribute to the interest in the polyphenol-CYP3A4 interactions and their outcomes and underscore the need for further research in this area.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Denisov I. G., Makris T. M., Sligar S. G., Schlichting I. Structure and chemistry of cytochrome P450. Chemical Reviews. 2005;105(6):2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 2.Domanski T. L., He Y. A., Khan K. K., Roussel F., Wang Q., Halpert J. R. Phenylalanine and tryptophan scanning mutagenesis of CYP3A4 substrate recognition site residues and effect on substrate oxidation and cooperativity. Biochemistry. 2001;40(34):10150–10160. doi: 10.1021/bi010758a. [DOI] [PubMed] [Google Scholar]
- 3.Nebert D. W., Russell D. W. Clinical importance of the cytochromes P450. The Lancet. 2002;360(9340):1155–1162. doi: 10.1016/s0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 4.de Montellano P. R. O. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd. Springer; 2005. [Google Scholar]
- 5.Nelson D. R. The cytochrome P450 homepage. Human Genomics. 2009;4(1):59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson D. R. A world of cytochrome P450s. Philosophical transactions of the Royal Society of London, Series B: Biological Sciences. 2013;368(1612) doi: 10.1098/rstb.2012.0430.20120430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nebert D. W., Wikvall K., Miller W. L. Human cytochromes P450 in health and disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368(1612) doi: 10.1098/rstb.2012.0431.20120431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nebert D. W., Nelson D. R., Coon M. J., et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA and Cell Biology. 1991;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 9.Zanger U. M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology & Therapeutics. 2013;138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich F. P. Cytochrome P450 and chemical toxicology. Chemical Research in Toxicology. 2008;21(1):70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 11.Ekins S., Stresser D. M., Williams J. A. In vitro and pharmacophore insights into CYP3A enzymes. Trends in Pharmacological Sciences. 2003;24(4):161–166. doi: 10.1016/s0165-6147(03)00049-x. [DOI] [PubMed] [Google Scholar]
- 12.Lown K. S., Bailey D. G., Fontana R. J., et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. The Journal of Clinical Investigation. 1997;99(10):2545–2553. doi: 10.1172/jci119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolars J. C., Lown K. S., Schmiedlin-Ren P., et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics and Genomics. 1994;4(5):247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson C. S., Tyndale R. F. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends in Pharmacological Sciences. 2011;32(12):708–714. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh C., Marchi N., Desai N. K., et al. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52(3):562–571. doi: 10.1111/j.1528-1167.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guengerich F. P. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annual Review of Pharmacology and Toxicology. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z., Fasco M. J., Figge H. L., Keyomarsi K., Kaminsky L. S. Expression of cytochromes P450 in human breast tissue and tumors. Drug Metabolism and Disposition. 1996;24(8):899–905. [PubMed] [Google Scholar]
- 18.Lin J. H., Chiba M., Baillie T. A. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacological Reviews. 1999;51(2):135–158. [PubMed] [Google Scholar]
- 19.Paine M. F., Hart H. L., Ludington S. S., Haining R. L., Rettie A. E., Zeldin D. C. The human intestinal cytochrome P450 ‘pie’. Drug Metabolism & Disposition. 2006;34(5):880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galetin A., Gertz M., Houston J. B. Contribution of intestinal cytochrome P450-mediated metabolism to drug-drug inhibition and induction interactions. Drug Metabolism and Pharmacokinetics. 2010;25(1):28–47. doi: 10.2133/dmpk.25.28. [DOI] [PubMed] [Google Scholar]
- 21.Yang J., Tucker G. T., Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clinical Pharmacology and Therapeutics. 2004;76(4, article 391) doi: 10.1016/j.clpt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Paine M. F., Khalighi M., Fisher J. M., et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. Journal of Pharmacology and Experimental Therapeutics. 1997;283(3):1552–1562. [PubMed] [Google Scholar]
- 23.Karlsson F. H., Bouchene S., Hilgendorf C., Dolgos H., Peters S. A. Utility of in vitro systems and preclinical data for the prediction of human intestinal first-pass metabolism during drug discovery and preclinical development. Drug Metabolism and Disposition. 2013;41(12):2033–2046. doi: 10.1124/dmd.113.051664. [DOI] [PubMed] [Google Scholar]
- 24.Siissalo S., Heikkinen A. T. In vitro methods to study the interplay of drug metabolism and efflux in the intestine. Current Drug Metabolism. 2013;14(1):102–111. doi: 10.2174/138920013804545241. [DOI] [PubMed] [Google Scholar]
- 25.Gertz M., Davis J. D., Harrison A., Houston J. B., Galetin A. Grapefruit juice-drug interaction studies as a method to assess the extent of intestinal availability: utility and limitations. Current Drug Metabolism. 2008;9(8):785–795. doi: 10.2174/138920008786049276. [DOI] [PubMed] [Google Scholar]
- 26.Hall S. D., Thummel K. E., Watkins P. B., et al. Molecular and physical mechanisms of first-pass extraction. Drug Metabolism and Disposition. 1999;27(2):161–166. [PubMed] [Google Scholar]
- 27.Bailey D. G., Dresser G., Arnold J. M. O. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? Canadian Medical Association Journal. 2013;185(4):309–316. doi: 10.1503/cmaj.120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gertz M., Harrison A., Houston J. B., Galetin A. Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metabolism and Disposition. 2010;38(7):1147–1158. doi: 10.1124/dmd.110.032649. [DOI] [PubMed] [Google Scholar]
- 29.van Duynhoven J., Vaughan E. E., Jacobs D. M., et al. Metabolic fate of polyphenols in the human superorganism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):4531–4538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson G., Clifford M. N. Colonic metabolites of berry polyphenols: the missing link to biological activity? British Journal of Nutrition. 2010;104(supplement 3):S48–S66. doi: 10.1017/s0007114510003946. [DOI] [PubMed] [Google Scholar]
- 31.Kaminsky L. S., Zhang Q.-Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metabolism and Disposition. 2003;31(12):1520–1525. doi: 10.1124/dmd.31.12.1520. [DOI] [PubMed] [Google Scholar]
- 32.Martignoni M., Groothuis G., de Kanter R. Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metabolism and Disposition. 2006;34(6):1047–1054. doi: 10.1124/dmd.105.009035. [DOI] [PubMed] [Google Scholar]
- 33.Lindell M., Lang M., Lennernäs H. Expression of genes encoding for drug metabolising cytochrome P450 enzymes and P-glycoprotein in the rat small intestine; comparison to the liver. European Journal of Drug Metabolism and Pharmacokinetics. 2003;28(1):41–48. doi: 10.1007/BF03190865. [DOI] [PubMed] [Google Scholar]
- 34.Cao X., Gibbs S. T., Fang L., et al. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharmaceutical Research. 2006;23(8):1675–1686. doi: 10.1007/s11095-006-9041-2. [DOI] [PubMed] [Google Scholar]
- 35.Awortwe C., Fasinu P. S., Rosenkranz B. Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. Journal of Pharmacy and Pharmaceutical Sciences. 2014;17(1):1–19. doi: 10.18433/j30k63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmstock N., Gonzalez F. J., Baes M., Annaert P., Augustijns P. PXR/CYP3A4-humanized mice for studying drug-drug interactions involving intestinal P-glycoprotein. Molecular Pharmaceutics. 2013;10(3):1056–1062. doi: 10.1021/mp300512r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroer K., Kittelmann M., Lütz S. Recombinant human cytochrome P450 monooxygenases for drug metabolite synthesis. Biotechnology and Bioengineering. 2010;106(5):699–706. doi: 10.1002/bit.22775. [DOI] [PubMed] [Google Scholar]
- 38.Regev-Shoshani G., Shoseyov O., Kerem Z. Influence of lipophilicity on the interactions of hydroxy stilbenes with cytochrome P450 3A4. Biochemical and Biophysical Research Communications. 2004;323(2):668–673. doi: 10.1016/j.bbrc.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 39.Tang L., Ye L., Lv C., Zheng Z., Gong Y., Liu Z. Involvement of CYP3A4/5 and CYP2D6 in the metabolism of aconitine using human liver microsomes and recombinant CYP450 enzymes. Toxicology Letters. 2011;202(1):47–54. doi: 10.1016/j.toxlet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Grinkova Y. V., Denisov I. G., McLean M. A., Sligar S. G. Oxidase uncoupling in heme monooxygenases: human cytochrome P450 CYP3A4 in Nanodiscs. Biochemical and Biophysical Research Communications. 2013;430(4):1223–1227. doi: 10.1016/j.bbrc.2012.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamdane D., Zhang H., Hollenberg P. Oxygen activation by cytochrome P450 monooxygenase. Photosynthesis Research. 2008;98(1–3):657–666. doi: 10.1007/s11120-008-9322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meunier B., de Visser S. P., Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chemical Reviews. 2004;104(9):3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 43.de Montellano P. R. O., de Voss J. J. Cytochrome P450. 3rd. New York, NY, USA: Springer; 2005. Substrate oxidation by cytochrome P450 enzymes; pp. 183–245. [DOI] [Google Scholar]
- 44.Zhou S.-F., Liu J.-P., Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metabolism Reviews. 2009;41(2):89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 45.Choi M. H., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Characterization of testosterone 11β-hydroxylation catalyzed by human liver microsomal cytochromes P450. Drug Metabolism and Disposition. 2005;33(6):714–718. doi: 10.1124/dmd.104.003327. [DOI] [PubMed] [Google Scholar]
- 46.Yasui-Furukori N., Hidestrand M., Spina E., Facciolá G., Scordo M. G., Tybring G. Different enantioselective 9-hydroxylation of risperidone by the two human CYP2D6 and CYP3A4 enzymes. Drug Metabolism & Disposition. 2001;29(10):1263–1268. [PubMed] [Google Scholar]
- 47.Liu Z., Hu M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opinion on Drug Metabolism and Toxicology. 2007;3(3):389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manach C., Hubert J., Llorach R., Scalbert A. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Molecular Nutrition and Food Research. 2009;53(10):1303–1315. doi: 10.1002/mnfr.200800516. [DOI] [PubMed] [Google Scholar]
- 49.Corcoran M. P., McKay D. L., Blumberg J. B. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. Journal of Nutrition in Gerontology and Geriatrics. 2012;31(3):176–189. doi: 10.1080/21551197.2012.698219. [DOI] [PubMed] [Google Scholar]
- 50.Ferrazzano G. F., Amato I., Ingenito A., Zarrelli A., Pinto G., Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules. 2011;16(2):1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 52.Lambert J. D., Sang S., Yang C. S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Molecular Pharmaceutics. 2007;4(6):819–825. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 53.Lambert J. D., Sang S., Yang C. S. Possible controversy over dietary polyphenols: benefits vs risks. Chemical Research in Toxicology. 2007;20(4):583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- 54.Wanwimolruk S., Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1) EXCLI Journal. 2014;13:347–391. [PMC free article] [PubMed] [Google Scholar]
- 55.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 56.Rodeiro I., Donato M. T., Lahoz A., Garrido G., Delgado R., Gómez-Lechón M. J. Interactions of polyphenols with the P450 system: possible implications on human therapeutics. Mini-Reviews in Medicinal Chemistry. 2008;8(2):97–106. doi: 10.2174/138955708783498131. [DOI] [PubMed] [Google Scholar]
- 57.Blanco-Colio L. M., Valderrama M., Alvarez-Sala L. A., et al. Red wine intake prevents nuclear factor-κB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia. Circulation. 2000;102(9):1020–1026. doi: 10.1161/01.cir.102.9.1020. [DOI] [PubMed] [Google Scholar]
- 58.Hooper L., Kroon P. A., Rimm E. B., et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition. 2008;88(1):38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 59.Spencer J. P. E. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes and Nutrition. 2009;4(4):243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surh Y. J. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Fragoso L., Martínez-Arismendi J. L., Orozco-Bustos D., Reyes-Esparza J., Torres E., Burchiel S. W. Potential risks resulting from fruit/vegetable-drug interactions: effects on drug-metabolizing enzymes and drug transporters. Journal of Food Science. 2011;76(4):R112–R124. doi: 10.1111/j.1750-3841.2011.02155.x. [DOI] [PubMed] [Google Scholar]
- 62.Korkina L., De Luca C., Pastore S. Plant polyphenols and human skin: friends or foes. Annals of the New York Academy of Sciences. 2012;1259(1):77–86. doi: 10.1111/j.1749-6632.2012.06510.x. [DOI] [PubMed] [Google Scholar]
- 63.Muthiah Y. D., Ong C. E., Sulaiman S. A., Tan S. C., Ismail R. In-vitro inhibitory effect of Tualang honey on cytochrome P450 2C8 activity. Journal of Pharmacy and Pharmacology. 2012;64(12):1761–1769. doi: 10.1111/j.2042-7158.2012.01551.x. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz D., Kisselev P., Schunck W.-H., Roots I. Inhibition of 17β-estradiol activation by CYP1A1: genotype- and regioselective inhibition by St. John's Wort and several natural polyphenols. Biochimica et Biophysica Acta—Proteins and Proteomics. 2011;1814(1):168–174. doi: 10.1016/j.bbapap.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 65.Lillywhite J. M., Simonsen J. E., Wilson V. Growing Chinese medicinal herbs in the United States: understanding practitioner preferences. Agriculture and Human Values. 2012;29(2):151–159. doi: 10.1007/s10460-011-9332-z. [DOI] [Google Scholar]
- 66.Jain A., Sharma R., Gahalain N., Chaudary J., Gupta G. K. Herbal plants used in diabetic complications: an overview. Journal of Pharmacy Research. 2011;4(4):986–988. [Google Scholar]
- 67.Zhou S., Gao Y., Jiang W., Huang M., Xu A., Paxton J. W. Interactions of herbs with cytochrome P450. Drug Metabolism Reviews. 2003;35(1):35–98. doi: 10.1081/DMR-120018248. [DOI] [PubMed] [Google Scholar]
- 68.Izzo A. A. Herb-drug interactions: an overview of the clinical evidence. Fundamental and Clinical Pharmacology. 2005;19(1):1–16. doi: 10.1111/j.1472-8206.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 69.Marathe P. H., Rodrigues A. D. In vivo animal models for investigating potential CYP3A- and Pgp-mediated drug-drug interactions. Current Drug Metabolism. 2006;7(7):687–704. doi: 10.2174/138920006778520598. [DOI] [PubMed] [Google Scholar]
- 70.Poulos T. L. Fifty Years of Cytochrome P450 Research. Tokyo, Japan: Springer; 2014. Cytochrome P450 dynamics; pp. 75–94. [DOI] [Google Scholar]
- 71.Topletz A. R., Dennison J. B., Barbuch R. J., Hadden C. E., Hall S. D., Renbarger J. L. The relative contributions of CYP3A4 and CYP3A5 to the metabolism of vinorelbine. Drug Metabolism & Disposition. 2013;41(9):1651–1661. doi: 10.1124/dmd.113.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brantley S. J., Argikar A. A., Lin Y. S., Nagar S., Paine M. F. Herb-drug interactions: challenges and opportunities for improved predictions. Drug Metabolism and Disposition. 2014;42(3):301–317. doi: 10.1124/dmd.113.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura Y., Ito H., Ohnishi R., Hatano T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food and Chemical Toxicology. 2010;48(1):429–435. doi: 10.1016/j.fct.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Rodeiro I., Donato M. T., Jimenez N., et al. Inhibition of human P450 enzymes by natural extracts used in traditional medicine. Phytotherapy Research. 2009;23(2):279–282. doi: 10.1002/ptr.2613. [DOI] [PubMed] [Google Scholar]
- 75.Harris R. Z., Jang G. R., Tsunoda S. Dietary effects on drug metabolism and transport. Clinical Pharmacokinetics. 2003;42(13):1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 76.Bailey D. G., Malcolm J., Arnold O., Spence J. D. Grapefruit juice-drug interactions. British Journal of Clinical Pharmacology. 1998;46(2):101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ku Y.-M., Min D. I., Flanigan M. Effect of grapefruit juice on the pharmacokinetics of microemulsion cyclosporine and its metabolite in healthy volunteers: does the formulation difference matter? Journal of Clinical Pharmacology. 1998;38(10):959–965. doi: 10.1002/j.1552-4604.1998.tb04393.x. [DOI] [PubMed] [Google Scholar]
- 78.Min D. I., Ku Y.-M., Perry P. J., et al. Effect of grapefruit juice on cyclosporine pharmacokinetics in renal transplant patients1. Transplantation. 1996;62(1):123–125. doi: 10.1097/00007890-199607150-00023. [DOI] [PubMed] [Google Scholar]
- 79.Markowitz J. S., Donovan J. L., deVane C. L., et al. Effect of St. John's Wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. Journal of the American Medical Association. 2003;290(11):1500–1504. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 80.Barone G. W., Gurley B. J., Ketel B. L., Lightfoot M. L., Abul-Ezz S. R. Drug interaction between St. John's wort and cyclosporine. Annals of Pharmacotherapy. 2000;34(9):1013–1016. doi: 10.1345/aph.10088. [DOI] [PubMed] [Google Scholar]
- 81.Chow H.-H. S., Garland L. L., Hsu C.-H., et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prevention Research. 2010;3(9):1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou S. F., Xue C. C., Yu X. Q., Li C., Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Therapeutic Drug Monitoring. 2007;29(6):687–710. doi: 10.1097/ftd.0b013e31815c16f5. [DOI] [PubMed] [Google Scholar]
- 83.Chen X.-W., Sneed K. B., Pan S.-Y., et al. Herb-drug interactions and mechanistic and clinical considerations. Current Drug Metabolism. 2012;13(5):640–651. doi: 10.2174/1389200211209050640. [DOI] [PubMed] [Google Scholar]
- 84.Qiu F., Jiang J., Ma Y., et al. Opposite effects of single-dose and multidose administration of the ethanol extract of danshen on CYP3A in healthy volunteers. Evidence-Based Complementary and Alternative Medicine. 2013;2013:8. doi: 10.1155/2013/730734.730734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahimi R., Abdollahi M. An update on the ability of St. John's wort to affect the metabolism of other drugs. Expert Opinion on Drug Metabolism and Toxicology. 2012;8(6):691–708. doi: 10.1517/17425255.2012.680886. [DOI] [PubMed] [Google Scholar]
- 86.Xie H. G., Kim R. B. St. John’s wort-associated drug interactions: Short-term inhibition and long-term induction? Clinical Pharmacology & Therapeutics. 2005;78(1):19–24. doi: 10.1016/j.clpt.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Patel J., Buddha B., Dey S., Pal D., Mitra A. K. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity. The American Journal of Therapeutics. 2004;11(4):262–277. doi: 10.1097/01.mjt.0000101827.94820.22. [DOI] [PubMed] [Google Scholar]
- 88.Vijayakumar T. M., Kumar R. M., Agrawal A., Dubey G. P., Ilango K. Comparative inhibitory potential of selected dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6 with fluorometric high-throughput screening. Journal of Food Science and Technology. 2014 doi: 10.1007/s13197-014-1472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brantley S. J., Graf T. N., Oberlies N. H., Paine M. F. A systematic approach to evaluate herb-drug interaction mechanisms: investigation of milk thistle extracts and eight isolated constituents as CYP3A inhibitors. Drug Metabolism and Disposition. 2013;41(9):1662–1670. doi: 10.1124/dmd.113.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodeiro I., Gómez-Lechón M. J., Perez G., et al. Mangifera indica L. extract and mangiferin modulate cytochrome P450 and UDP-glucuronosyltransferase enzymes in primary cultures of human hepatocytes. Phytotherapy Research. 2013;27(5):745–752. doi: 10.1002/ptr.4782. [DOI] [PubMed] [Google Scholar]
- 91.Boullata J. I. Drug and nutrition interactions: not just food for thought. Journal of Clinical Pharmacy and Therapeutics. 2013;38(4):269–271. doi: 10.1111/jcpt.12075. [DOI] [PubMed] [Google Scholar]
- 92.Fahmi O. A., Maurer T. S., Kish M., Cardenas E., Boldt S., Nettleton D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3a4 inhibition, inactivation, and induction determined in vitro . Drug Metabolism and Disposition. 2008;36(8):1698–1708. doi: 10.1124/dmd.107.018663. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y.-H., Jones D. R., Hall S. D. Prediction of cytochrome P450 3A inhibition by verapamil enantiomers and their metabolites. Drug Metabolism and Disposition. 2004;32(2):259–266. doi: 10.1124/dmd.32.2.259. [DOI] [PubMed] [Google Scholar]
- 94.Ross J. A., Kasum C. M. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annual Review of Nutrition. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 95.Scalbert A., Johnson I. T., Saltmarsh M. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 2005;81(1):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 96.Chabert P., Auger C., Pincemail J., Schini-Kerth V. B. Systems Biology of Free Radicals and Antioxidants. Berlin, Germany: Springer; 2014. Overview of plant-derived antioxidants; pp. 4005–4022. [DOI] [Google Scholar]
- 97.Pérez-Jiménez J., Díaz-Rubio M. E., Saura-Calixto F. Non-extractable polyphenols, a major dietary antioxidant: occurrence, metabolic fate and health effects. Nutrition Research Reviews. 2013;26(2):118–129. doi: 10.1017/s0954422413000097. [DOI] [PubMed] [Google Scholar]
- 98.Ovaskainen M.-L., Törrönen R., Koponen J. M., et al. Dietary intake and major food sources of polyphenols in Finnish adults. Journal of Nutrition. 2008;138(3):562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 99.Thu N. N., Sakurai C., Uto H., et al. The polyphenol content and antioxidant activities of the main edible vegetables in Northern Vietnam. Journal of Nutritional Science and Vitaminology. 2004;50(3):203–210. doi: 10.3177/jnsv.50.203. [DOI] [PubMed] [Google Scholar]
- 100.Saura-Calixto F., Serrano J., Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chemistry. 2007;101(2):492–501. doi: 10.1016/j.foodchem.2006.02.006. [DOI] [Google Scholar]
- 101.Kanner J., Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radical Biology & Medicine. 2001;31(11):1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 102.Kerem Z., Chetrit D., Shoseyov O., Regev-Shoshani G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. Journal of Agricultural and Food Chemistry. 2006;54(26):10288–10293. doi: 10.1021/jf0621828. [DOI] [PubMed] [Google Scholar]
- 103.Dufresne C. J., Farnworth E. R. A review of latest research findings on the health promotion properties of tea. Journal of Nutritional Biochemistry. 2001;12(7):404–421. doi: 10.1016/S0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 104.Shahidi F., Zhong Y. Novel antioxidants in food quality preservation and health promotion. European Journal of Lipid Science and Technology. 2010;112(9):930–940. doi: 10.1002/ejlt.201000044. [DOI] [Google Scholar]
- 105.Khan N., Mukhtar H. Cancer and metastasis: prevention and treatment by green tea. Cancer and Metastasis Reviews. 2010;29(3):435–445. doi: 10.1007/s10555-010-9236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aggarwal B. B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochemical Pharmacology. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Garrido G., González D., Lemus Y., et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacological Research. 2004;50(2):143–149. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Nichenametla S. N., Taruscio T. G., Barney D. L., Exon J. H. A review of the effects and mechanisms of polyphenolics in cancer. Critical Reviews in Food Science and Nutrition. 2006;46(2):161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 109.Zaveri N. T. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sciences. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Kalogeropoulos N., Yannakopoulou K., Gioxari A., Chiou A., Makris D. P. Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John's wort) extract. LWT—Food Science and Technology. 2010;43(6):882–889. doi: 10.1016/j.lwt.2010.01.016. [DOI] [Google Scholar]
- 111.Lee H. J., Lee H.-S., Cho H. J., Kim S. Y., Suh H. J. Utilization of hydrolytic enzymes for the extraction of ginsenosides from Korean ginseng leaves. Process Biochemistry. 2012;47(3):538–543. doi: 10.1016/j.procbio.2011.12.004. [DOI] [Google Scholar]
- 112.Maizura M., Aminah A., Aida W. M. W. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. International Food Research Journal. 2011;18(2):529–534. [Google Scholar]
- 113.Jiang B., Kronenberg F., Balick M. J., Kennelly E. J. Stability of black cohosh triterpene glycosides and polyphenols: potential clinical relevance. Phytomedicine. 2013;20(6):564–569. doi: 10.1016/j.phymed.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 114.Georgieva S. S., Christova-Bagdassarian V. L., Atanassova M. S. Comparative evaluation of the polyphenol composition and antioxidant capacity of propolis and Echinacea purpurea . Journal of Experimental and Integrative Medicine. 2014;4(1):51–56. doi: 10.5455/jeim.050913.or.089. [DOI] [Google Scholar]
- 115.Anhê F. F., Roy D., Pilon G., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2014 doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 116.Rechner A. R., Kuhnle G., Bremner P., Hubbard G. P., Moore K. P., Rice-Evans C. A. The metabolic fate of dietary polyphenols in humans. Free Radical Biology and Medicine. 2002;33(2):220–235. doi: 10.1016/S0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 117.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews. 1998;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 118.Doostdar H., Burke M. D., Mayer R. T. Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology. 2000;144(1–3):31–38. doi: 10.1016/s0300-483x(99)00215-2. [DOI] [PubMed] [Google Scholar]
- 119.Rice-Evans C. Flavonoid antioxidants. Current Medicinal Chemistry. 2001;8(7):797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 120.Jančová P., Anzenbacherová E., Papoušková B., et al. Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metabolism & Disposition. 2007;35(11):2035–2039. doi: 10.1124/dmd.107.016410. [DOI] [PubMed] [Google Scholar]
- 121.Yang C. S., Pan E. The effects of green tea polyphenols on drug metabolism. Expert Opinion on Drug Metabolism and Toxicology. 2012;8(6):677–689. doi: 10.1517/17425255.2012.681375. [DOI] [PubMed] [Google Scholar]
- 122.Sang S., Lambert J. D., Ho C.-T., Yang C. S. The chemistry and biotransformation of tea constituents. Pharmacological Research. 2011;64(2):87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 123.Didziapetris R., Dapkunas J., Sazonovas A., Japertas P. Trainable structure-activity relationship model for virtual screening of CYP3A4 inhibition. Journal of Computer-Aided Molecular Design. 2010;24(11):891–906. doi: 10.1007/s10822-010-9381-1. [DOI] [PubMed] [Google Scholar]
- 124.Roy K., Roy P. P. QSAR of cytochrome inhibitors. Expert Opinion on Drug Metabolism and Toxicology. 2009;5(10):1245–1266. doi: 10.1517/17425250903158940. [DOI] [PubMed] [Google Scholar]
- 125.Misaka S., Kawabe K., Onoue S., et al. Green tea extract affects the cytochrome P450 3A activity and pharmacokinetics of simvastatin in rats. Drug Metabolism and Pharmacokinetics. 2013;28(6):514–518. doi: 10.2133/dmpk.DMPK-13-NT-006. [DOI] [PubMed] [Google Scholar]
- 126.Nishikawa M., Ariyoshi N., Kotani A., et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metabolism and Pharmacokinetics. 2004;19(4):280–289. doi: 10.2133/dmpk.19.280. [DOI] [PubMed] [Google Scholar]
- 127.Amri A., Chaumeil J. C., Sfar S., Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? Journal of Controlled Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 128.Walle T. Bioavailability of resveratrol. Annals of the New York Academy of Sciences. 2011;1215(1):9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 129.Marier J.-F., Vachon P., Gritsas A., Zhang J., Moreau J.-P., Ducharme M. P. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. Journal of Pharmacology and Experimental Therapeutics. 2002;302(1):369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 130.Singh G., Pai R. S. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. Journal of Pharmacy and Pharmacology. 2014;66(8):1062–1076. doi: 10.1111/jphp.12232. [DOI] [PubMed] [Google Scholar]
- 131.Seljak K. B., Berginc K., Trontelj J., Zvonar A., Kristl A., Gašperlin M. A self-microemulsifying drug delivery system to overcome intestinal resveratrol toxicity and presystemic metabolism. Journal of Pharmaceutical Sciences. 2014;103(11):3491–3500. doi: 10.1002/jps.24114. [DOI] [PubMed] [Google Scholar]
- 132.Parkar S. G., Trower T. M., Stevenson D. E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12–19. doi: 10.1016/j.anaerobe.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 133.Parkar S. G., Stevenson D. E., Skinner M. A. The potential influence of fruit polyphenols on colonic microflora and human gut health. International Journal of Food Microbiology. 2008;124(3):295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 134.Bolca S., van de Wiele T., Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Current Opinion in Biotechnology. 2013;24(2):220–225. doi: 10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 135.Morand C., Dubray C., Milenkovic D., et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. The American Journal of Clinical Nutrition. 2011;93(1):73–80. doi: 10.3945/ajcn.110.004945. [DOI] [PubMed] [Google Scholar]
- 136.Lampe J. W. Interindividual differences in response to plant-based diets: implications for cancer risk. The American Journal of Clinical Nutrition. 2009;89(5):1553S–1557S. doi: 10.3945/ajcn.2009.26736d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stoupi S., Williamson G., Drynan J. W., Barron D., Clifford M. N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin B2 by human faecal microbiota. Molecular Nutrition and Food Research. 2010;54(6):747–759. doi: 10.1002/mnfr.200900123. [DOI] [PubMed] [Google Scholar]
- 138.Dresser G. K., Spence J. D., Bailey D. G. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clinical Pharmacokinetics. 2000;38(1):41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 139.Muto S., Fujita K.-I., Yamazaki Y., Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis. 2001;479(1-2):197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 140.Wolf C. R. Chemoprevention: increased potential to bear fruit. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):2941–2943. doi: 10.1073/pnas.071042698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parys S., Kehraus S., Krick A., et al. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry. 2010;71(2-3):221–229. doi: 10.1016/j.phytochem.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 142.Athar M., Back J. H., Tang X., et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicology and Applied Pharmacology. 2007;224(3):274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sahu K., Siddiqui A. A., Shaharyar M., Malik S. Pharmacokinetic interaction between febuxostat and morin in rats. Expert Opinion on Drug Metabolism and Toxicology. 2014;10(3):307–312. doi: 10.1517/17425255.2014.885017. [DOI] [PubMed] [Google Scholar]
- 144.Kumar K. K., Priyanka L., Gnananath K., Babu P. R., Sujatha S. Pharmacokinetic drug interactions between apigenin, rutin and paclitaxel mediated by P-glycoprotein in rats. European Journal of Drug Metabolism and Pharmacokinetics. 2014 doi: 10.1007/s13318-014-0203-z. [DOI] [PubMed] [Google Scholar]
- 145.Ferreira A., Pousinho S., Fortuna A., Falcão A., Alves G. Flavonoid compounds as reversal agents of the P-glycoprotein-mediated multidrug resistance: biology, chemistry and pharmacology. Phytochemistry Reviews. 2014 doi: 10.1007/s11101-014-9358-0. [DOI] [Google Scholar]
- 146.Gufford B. T., Chen G., Lazarus P., Graf T. N., Oberlies N. H., Paine M. F. Identification of diet-derived constituents as potent inhibitors of intestinal glucuronidation. Drug Metabolism & Disposition. 2014;42(10):1675–1683. doi: 10.1124/dmd.114.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koe X. F., Muhammad T. S. T., Chong A. S. C., Abdul Wahab H., Tan M. L. Cytochrome P450 induction properties of food and herbal-derived compounds using a novel multiplex RT-qPCR in vitro assay, a drug-food interaction prediction tool. Food Science & Nutrition. 2014;2(5):500–520. doi: 10.1002/fsn3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shimada T., Tanaka K., Takenaka S., et al. Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chemical Research in Toxicology. 2010;23(12):1921–1935. doi: 10.1021/tx100286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sarkar M. A., Scambia G., Ranelletti F. O., et al. Quercetin not only inhibits P-glycoprotein efflux activity but also inhibits CYP3A isozymes. Cancer Chemotherapy and Pharmacology. 1995;36(5):448–449. doi: 10.1007/bf00686195. [DOI] [PubMed] [Google Scholar]
- 150.Choi J.-S., Piao Y.-J., Kang K. W. Effects of quercetin on the bioavailability of doxorubicin in rats: role of CYP3A4 and P-gp inhibition by quercetin. Archives of Pharmacal Research. 2011;34(4):607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- 151.Yu C.-P., Wu P.-P., Hou Y.-C., et al. Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. Journal of Agricultural and Food Chemistry. 2011;59(9):4644–4648. doi: 10.1021/jf104786t. [DOI] [PubMed] [Google Scholar]
- 152.Rashid J., McKinstry C., Renwick A. G., Dirnhuber M., Waller D. G., George C. F. Quercetin, an in vitro inhibitor of CYP3A, does not contribute to the interaction between nifedipine and grapefruit juice. British Journal of Clinical Pharmacology. 1993;36(5):460–463. doi: 10.1111/j.1365-2125.1993.tb00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raucy J. L. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metabolism and Disposition. 2003;31(5):533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 154.Lau W. C., Welch T. D., Shields T., Rubenfire M., Tantry U. S., Gurbel P. A. The effect of St John's Wort on the pharmacodynamic response of clopidogrel in hyporesponsive volunteers and patients: increased platelet inhibition by enhancement of CYP3A4 metabolic activity. Journal of Cardiovascular Pharmacology. 2011;57(1):86–93. doi: 10.1097/fjc.0b013e3181ffe8d0. [DOI] [PubMed] [Google Scholar]
- 155.Kamel A., Harriman S. Inhibition of cytochrome P450 enzymes and biochemical aspects of mechanism-based inactivation (MBI) Drug Discovery Today: Technologies. 2013;10(1):e177–e189. doi: 10.1016/j.ddtec.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 156.Quintieri L., Palatini P., Nassi A., Ruzza P., Floreani M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochemical Pharmacology. 2008;75(6):1426–1437. doi: 10.1016/j.bcp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 157.Sineva E. V., Rumfeldt J. A. O., Halpert J. R., Davydov D. R. A large-scale allosteric transition in cytochrome P450 3A4 revealed by luminescence resonance energy transfer (LRET) PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0083898.e83898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davydov D. R., Davydova N. Y., Sineva E. V., Kufareva I., Halpert J. R. Pivotal role of P450-P450 interactions in CYP3A4 allostery: the case of α-naphthoflavone. Biochemical Journal. 2013;453(2):219–230. doi: 10.1042/bj20130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davydov D. R., Rumfeldt J. A. O., Sineva E. V., Fernando H., Davydova N. Y., Halpert J. R. Peripheral ligand-binding site in cytochrome P450 3A4 located with fluorescence resonance energy transfer (FRET) Journal of Biological Chemistry. 2012;287(9):6797–6809. doi: 10.1074/jbc.M111.325654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frank D. J., Denisov I. G., Sligar S. G. Analysis of heterotropic cooperativity in cytochrome P450 3A4 using α-naphthoflavone and testosterone. Journal of Biological Chemistry. 2011;286(7):5540–5545. doi: 10.1074/jbc.m110.182055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roberts A. G., Atkins W. M. Energetics of heterotropic cooperativity between α-naphthoflavone and testosterone binding to CYP3A4. Archives of Biochemistry and Biophysics. 2007;463(1):89–101. doi: 10.1016/j.abb.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Netsch M. I., Gutmann H., Schmidlin C. B., Aydogan C., Drewe J. Induction of CYP1A by green tea extract in human intestinal cell lines. Planta Medica. 2006;72(6):514–520. doi: 10.1055/s-2006-931537. [DOI] [PubMed] [Google Scholar]
- 163.Bailey D. G., Arnold J. M. O., Spence J. D. Grapefruit juice and drugs. How significant is the interaction? Clinical Pharmacokinetics. 1994;26(2):91–98. doi: 10.2165/00003088-199426020-00002. [DOI] [PubMed] [Google Scholar]
- 164.Fuhr U., Kummert A. L. The fata of naringin in humans: a key to grapefruit juice-drug interactions? Clinical Pharmacology and Therapeutics. 1995;58(4):365–373. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 165.Ho P.-C., Saville D. J., Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. Journal of Pharmacy & Pharmaceutical Sciences. 2001;4(3):217–227. [PubMed] [Google Scholar]
- 166.Iwanaga K., Hayashi M., Hamahata Y., et al. Furanocoumarin derivatives in Kampo extract medicines inhibit cytochrome P450 3A4 and P-glycoprotein. Drug Metabolism and Disposition. 2010;38(8):1286–1294. doi: 10.1124/dmd.110.032847. [DOI] [PubMed] [Google Scholar]
- 167.VanderMolen K. M., Ainslie G. R., Paine M. F., Oberlies N. H. Labeled content of two furanocoumarins in dietary supplements correlates with neither actual content nor CYP3A inhibitory activity. Journal of Pharmaceutical and Biomedical Analysis. 2014;98:260–265. doi: 10.1016/j.jpba.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ueng Y.-F., Chen C.-C., Yamazaki H., et al. Mechanism-based inhibition of CYP1A1 and CYP3A4 by the furanocoumarin chalepensin. Drug Metabolism and Pharmacokinetics. 2013;28(3):229–238. doi: 10.2133/dmpk.dmpk-12-rg-113. [DOI] [PubMed] [Google Scholar]
- 169.Foster B. C., Vandenhoek S., Hana J., et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10(4):334–342. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 170.Roberts-Kirchhoff E. S., Crowley J. R., Hollenberg P. F., Kim H. Metabolism of genistein by rat and human cytochrome P450s. Chemical Research in Toxicology. 1999;12(7):610–616. doi: 10.1021/tx9802320. [DOI] [PubMed] [Google Scholar]
- 171.Scott L. M., Durant P., Leone-Kabler S., et al. Effects of prior oral contraceptive use and soy isoflavonoids on estrogen-metabolizing cytochrome P450 enzymes. Journal of Steroid Biochemistry and Molecular Biology. 2008;112(4-5):179–185. doi: 10.1016/j.jsbmb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xiao C.-Q., Chen R., Lin J., et al. Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica. 2012;42(2):173–178. doi: 10.3109/00498254.2011.615954. [DOI] [PubMed] [Google Scholar]
- 173.Chen Y., Xiao C.-Q., He Y.-J., et al. Genistein alters caffeine exposure in healthy female volunteers. European Journal of Clinical Pharmacology. 2011;67(4):347–353. doi: 10.1007/s00228-010-0964-5. [DOI] [PubMed] [Google Scholar]
- 174.Dreiseitel A., Schreier P., Oehme A., Locher S., Hajak G., Sand P. G. Anthocyanins and their metabolites are weak inhibitors of cytochrome P450 3A4. Molecular Nutrition and Food Research. 2008;52(12):1428–1433. doi: 10.1002/mnfr.200800043. [DOI] [PubMed] [Google Scholar]
- 175.Chan W. K., Delucchi A. B. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sciences. 2000;67(25):3103–3112. doi: 10.1016/S0024-3205(00)00888-2. [DOI] [PubMed] [Google Scholar]
- 176.Piver B., Berthou F., Dreano Y., Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicology Letters. 2001;125(1–3):83–91. doi: 10.1016/s0378-4274(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 177.Hong S.-P., Choi D.-H., Choi J.-S. Effects of resveratrol on the pharmacokinetics of diltiazem and its major metabolite, desacetyldiltiazem, in rats. Cardiovascular Therapeutics. 2008;26(4):269–275. doi: 10.1111/j.1755-5922.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 178.Chi Y. C., Lin S. P., Hou Y. C. A new herb-drug interaction of Polygonum cuspidatum, a resveratrol-rich nutraceutical, with carbamazepine in rats. Toxicology and Applied Pharmacology. 2012;263(3):315–322. doi: 10.1016/j.taap.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 179.Detampel P., Beck M., Krähenbühl S., Huwyler J. Drug interaction potential of resveratrol. Drug Metabolism Reviews. 2012;44(3):253–265. doi: 10.3109/03602532.2012.700715. [DOI] [PubMed] [Google Scholar]
- 180.Correia M. A., de Montellano P. R. O. Cytochrome P450. 3rd. Berlin, Germany: Springer; 2005. Inhibition of cytochrome P450 enzymes; pp. 247–322. [Google Scholar]
- 181.Sahali-Sahly Y., Balani S. K., Lin J. H., Baillie T. A. In vitro studies on the metabolic activation of the furanopyridine L- 754,394, a highly potent and selective mechanism-based inhibitor of cytochrome P450 3A4. Chemical Research in Toxicology. 1996;9(6):1007–1012. doi: 10.1021/tx960060b. [DOI] [PubMed] [Google Scholar]
- 182.Iwata H., Tezuka Y., Kadota S., Hiratsuka A., Watabe T. Identification and characterization of potent CYP3A4 inhibitors in Schisandra fruit extract. Drug Metabolism and Disposition. 2004;32(12):1351–1358. doi: 10.1124/dmd.104.000646. [DOI] [PubMed] [Google Scholar]
- 183.Venkataramanan R., Ramachandran V., Komoroski B. J., Zhang S., Schiff P. L., Strom S. C. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metabolism and Disposition. 2000;28(11):1270–1273. [PubMed] [Google Scholar]
- 184.Zuber R., Modrianský M., Dvořák Z., et al. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytotherapy Research. 2002;16(7):632–638. doi: 10.1002/ptr.1000. [DOI] [PubMed] [Google Scholar]
- 185.Yao H.-T., Chang Y.-W., Lan S.-J., Yeh T.-K. The inhibitory effect of tannic acid on cytochrome P450 enzymes and NADPH-CYP reductase in rat and human liver microsomes. Food and Chemical Toxicology. 2008;46(2):645–653. doi: 10.1016/j.fct.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 186.Jaikang C., Niwatananun K., Narongchai P., Narongchai S., Chaiyasut C. Inhibitory effect of caffeic acid and its derivatives on human liver cytochrome P450 3A4 activity. Journal of Medicinal Plants Research. 2011;5(15):3530–3536. [Google Scholar]
- 187.Stupans I., Stretch G., Hayball P. Olive oil phenols inhibit human hepatic microsomal activity. Journal of Nutrition. 2000;130(9):2367–2370. doi: 10.1093/jn/130.9.2367. [DOI] [PubMed] [Google Scholar]
- 188.Stupans I., Tan H.-W., Kirlich A., Tuck K., Hayball P., Murray M. Inhibition of CYP3A-mediated oxidation in human hepatic microsomes by the dietary derived complex phenol, gallic acid. Journal of Pharmacy and Pharmacology. 2002;54(2):269–275. doi: 10.1211/0022357021778303. [DOI] [PubMed] [Google Scholar]
- 189.Yano J. K., Wester M. R., Schoch G. A., Griffin K. J., Stout C. D., Johnson E. F. The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-Å resolution. The Journal of Biological Chemistry. 2004;279(37):38091–38094. doi: 10.1074/jbc.c400293200. [DOI] [PubMed] [Google Scholar]
- 190.Williams P. A., Cosme J., Vinković D. M., et al. Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science. 2004;305(5684):683–686. doi: 10.1126/science.1099736. [DOI] [PubMed] [Google Scholar]
- 191.Sridhar J., Liu J., Foroozesh M., Stevens C. L. K. Insights on cytochrome P450 enzymes and inhibitors obtained through QSAR studies. Molecules. 2012;17(8):9283–9305. doi: 10.3390/molecules17089283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lewis D. F. V., Lake B. G. Molecular modelling and quantitative structure-activity relationship studies on the interaction of omeprazole with cytochrome P450 isozymes. Toxicology. 1998;125(1):31–44. doi: 10.1016/S0300-483X(97)00159-5. [DOI] [PubMed] [Google Scholar]
- 193.Ekins S., Bravi G., Wikel J. H., Wrighton S. A. Three-dimensional-quantitative structure activity relationship analysis of cytochrome P-450 3A4 substrates. Journal of Pharmacology and Experimental Therapeutics. 1999;291(1):424–433. [PubMed] [Google Scholar]
- 194.Ekins S., de Groot M. J., Jones J. P. Pharmacophore and three-dimensional quantitative structure activity relationship methods for modeling cytochrome p450 active sites. Drug Metabolism and Disposition. 2001;29(7):936–944. [PubMed] [Google Scholar]
- 195.Galetin A., Clarke S. E., Houston J. B. Multisite kinetic analysis of interactions between prototypical CYP3A4 subgroup substrates: midazolam, testosterone, and nifedipine. Drug Metabolism and Disposition. 2003;31(9):1108–1116. doi: 10.1124/dmd.31.9.1108. [DOI] [PubMed] [Google Scholar]
- 196.Ohkura K., Kawaguchi Y., Watanabe Y., Masubuchi Y., Shinohara Y., Hori H. Flexible structure of cytochrome P450: promiscuity of ligand binding in the CYP3A4 heme pocket. Anticancer Research. 2009;29(3):935–942. [PubMed] [Google Scholar]
- 197.Kenworthy K. E., Clarke S. E., Andrews J., Houston J. B. Multisite kinetic models for CYP3A4: simultaneous activation and inhibition of diazepam and testosterone metabolism. Drug Metabolism and Disposition. 2001;29(12):1644–1651. [PubMed] [Google Scholar]
- 198.Ekroos M., Sjögren T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13682–13687. doi: 10.1073/pnas.0603236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Denisov I. G., Frank D. J., Sligar S. G. Cooperative properties of cytochromes P450. Pharmacology & Therapeutics. 2009;124(2):151–167. doi: 10.1016/j.pharmthera.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Atkins W. M. Non-Michaelis-Menten kinetics in cytochrome P450-catalyzed reactions. Annual Review of Pharmacology and Toxicology. 2005;45:291–310. doi: 10.1146/annurev.pharmtox.45.120403.100004. [DOI] [PubMed] [Google Scholar]
- 201.Hutzler J. M., Tracy T. S. Atypical kinetic profiles in drug metabolism reactions. Drug Metabolism and Disposition. 2002;30(4):355–362. doi: 10.1124/dmd.30.4.355. [DOI] [PubMed] [Google Scholar]
- 202.Davydov D. R., Halpert J. R. Allosteric P450 mechanisms: multiple binding sites, multiple conformers of both? Expert Opinion on Drug Metabolism and Toxicology. 2008;4(12):1523–1535. doi: 10.1517/17425250802500028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Denisov I. G., Sligar S. G. A novel type of allosteric regulation: functional cooperativity in monomeric proteins. Archives of Biochemistry and Biophysics. 2012;519(2):91–102. doi: 10.1016/j.abb.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lewis D. F., Lake B. G., Dickins M. Quantitative structure-activity relationships (QSARs) in inhibitors of various cytochromes P450: the importance of compound lipophilicity. Journal of Enzyme Inhibition and Medicinal Chemistry. 2007;22(1):1–6. doi: 10.1080/14756360600952183. [DOI] [PubMed] [Google Scholar]
- 205.Lewis D. F. V., Lake B. G., Dickins M. Quantitative structure-activity relationships (QSARs) in CYP3A4 inhibitors: the importance of lipophilic character and hydrogen bonding. Journal of Enzyme Inhibition and Medicinal Chemistry. 2006;21(2):127–132. doi: 10.1080/14756360500532747. [DOI] [PubMed] [Google Scholar]
- 206.Mao B., Gozalbes R., Barbosa F., et al. QSAR modeling of in vitro inhibition of cytochrome P450 3A4. Journal of Chemical Information and Modeling. 2006;46(5):2125–2134. doi: 10.1021/ci0600915. [DOI] [PubMed] [Google Scholar]
- 207.Matoušková P., Bártíková H., Boušová I., et al. Effect of defined green tea extract in various dosage schemes on drug-metabolizing enzymes in mice in vivo . Journal of Functional Foods. 2014;10:327–335. doi: 10.1016/j.jff.2014.06.026. [DOI] [Google Scholar]
- 208.Ezuruike U. F., Prieto J. M. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. Journal of Ethnopharmacology. 2014;155(2):857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 209.Bunchorntavakul C., Reddy K. R. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacology and Therapeutics. 2013;37(1):3–17. doi: 10.1111/apt.12109. [DOI] [PubMed] [Google Scholar]
- 210.Kim E. J. Y., Chen Y., Huang J. Q., et al. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. Journal of Ethnopharmacology. 2013;146(1):40–61. doi: 10.1016/j.jep.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 211.Neergheen-Bhujun V. S. Underestimating the toxicological challenges associated with the use of herbal medicinal products in developing countries. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/804086.804086 [DOI] [PMC free article] [PubMed] [Google Scholar]