Abstract
The composite human gut microbiomes of Western populations have changed over the past century, brought on by new environmental triggers that often have a negative impact on human health. Diets high in saturated fats, refined sugars and low in fiber are leading candidates for these events and for triggering the increased prevalence of immune-mediated diseases like inflammatory bowel diseases (IBD). Our studies have shown that consumption of a “western” diet high in saturated (milk derived)-fat (MF) or n-6 polyunsaturated (safflower oil)-fat (SF) have similar effects on the structure of the colonic microbiome of wild type and IL-10−/− mice, characterized by increased Bacteroidetes and decreased Firmicutes. However, the MF diet uniquely promotes the expansion of an immunogenic sulfite-reducing pathobiont, Bilophila wadsworthia, a member of the Deltaproteobacteria and minor component of the gut microbiome. This bacterial bloom results from a MF diet-induced shift in hepatic conjugation of bile acids, from glycocholic (GC) to taurocholic (TC) acid, which is important for solubilizing the more hydrophobic MF diet. However, it is also responsible for delivery of taurine-derived sulfur to the distal bowel, promoting the assemblage of bile-tolerant microbes such as B. wadsworthia. The bloom of this species promotes a Th1-mediated immune response and the development of colitis in IL-10−/− mice. A similar bloom of B. wadsworthia is seen when IL-10−/− mice are fed a low-fat (LF) diet supplemented with TC. B. wadsworthia colonization of monoassociated germ-free IL-10−/− mice was dependent on the host consuming either a high saturated MF diet or the gavage with TC. Together these data provide a plausible explanation for the link between diseases such as IBD and dietary-mediated selection of gut microbial pathobionts in genetically susceptible hosts. With this knowledge, it may be possible to mitigate the bloom of these types of pathobionts by modifying the conjugation states of bile acids.
Keywords: Diet, IBD, Microbiome, Pathobiont, Bile acids
IBD is a general term for a group of diseases affecting the intestine of which Crohn’s disease (CD) and ulcerative colitis (UC) are the two major clinical phenotypes. The onset of IBD typically occurs in adolescence and a majority of those affected progress to a relapsing and chronic disease [1–2]. Hallmarks of UC include diffuse mucosal inflammation that extends proximally from the rectum to a varying degree. Along with severe inflammation and the production of a complex mixture of inflammatory mediators, extensive superficial mucosal ulceration develops. Histopathological features include crypt abscesses the presence of a significant number of neutrophils within the lamina propria and depletion of goblet cell mucin. CD, on the other hand, can involve any site along the gastrointestinal tract and the inflammation is often patchy, segmental, and transmural, however, involvement of the terminal ileum is common with early lesions often appearing over Peyer’s patches [3]. The underlying bases for these events most likely stem from an individual’s genetic predisposition and development of a gut dysbiosis that compromises intestinal epithelial barrier function, mucosal immune responses, and tissue protective and wound healing responses.
The identification of genetic variations through genome-wide association studies (GWAS) gave great hope for predicting who is at increased risk for IBD. Although the clinical utility of this information has yet to be realized, important insights into the etiopathogenesis of IBD have been gained. They clearly provide strong support for a genetic basis for IBD, but they also pointed out that the development of these diseases involves other factors. For example, NOD2 mutations, only account for 25% of CD cases in Caucasians, and is not implicated in the pathogenesis of UC [4]. Furthermore, these genetic discoveries do not explain the variation in incidence between different populations, nor the rising incidence of disease in industrialized countries over the past 50 years [4]. These observations suggest that rapid shifts in environmental and perhaps even epigenetic factors are playing an increasing role in promoting the increased incidence of IBD. Together, this points to a “multi-hit” hypothesis in which a genetically susceptible individual encounters a single or series of environmental triggers that tip the scale of a compensated immune response in favor of dysregulated inflammation.
While environmental triggers such as use of antibiotics, non-steroidal anti-inflammatory drugs, smoking, and diet are widely cited in the pathogenesis of IBD, shifts in diet in Western countries and recently other industrialized nations have to be considered among these factors, as these changes are highly associated with a myriad of “new-age” complex immune diseases [5]. Specifically, modern conveniences, larger choices of low-cost, calorie-dense foods, and sedentary lifestyle in industrialized countries have led to a culture of overindulgence and excess. Before the development of agriculture and animal husbandry, dietary choices were limited to minimally-processed, wild plant and animal foods. With domestication of plants and animals, dietary nutrient content subtly, but progressively shifted toward more processed foods for which the human genome had little evolutionary experience [6]. Refined grains, sugars, vegetable oils, alcohol, salt, and fatty domesticated meats that were not present in the pre-agricultural diet now make up the primary constituents of the post-agricultural, typical Western diet that are consumed both out of balance and in caloric excess [6]. Thus, relatively rapid changes in Western lifestyle and diet have subjected the human condition to new stresses that challenge evolutionarily-determined relationships between host and environment. In the context of the gut, dietary antigens are the most common type of luminal antigen alongside bacterial antigens, therefore it is reasonable to propose that they may play a significant role in IBD etiology. Possible mechanisms include the direct effect of dietary antigens, alteration of gene expression, changes in gastrointestinal permeability, and alteration of the composition and functional impact of the enteric flora.
In our studies, we investigated whether one major macronutrient of Western diets that has changed considerably over a short period of time, fat, had the ability to precipitate a compositional change in the gut microbiota depending on the type of fat consumed, and whether this change could differentially affect the onset and severity of experimental colitis. Appreciating the complex interplay of genetics, environment, immune response, and microbiota in the etiopathogenesis of IBD, we utilized the genetically pre-disposed mouse model for spontaneous colitis, the IL-10−/− mouse, in both conventionalized and germ-free states, and created three high fat diets that closely modeled the macronutrient composition of the standard American diet as reported by NHANES in 2011 [7]. The only variation between these diets was the type of fat used (safflower-SF, milk-fat-MF, or lard- LD). With the exception of the low-fat (LF) purified mouse diet, the high fat diets were isocaloric with fat composition held constant at 37% of total calories.
Twenty-one-day exposure to the three study diets in genetically non-susceptible C57BL/6j mice resulted in significant differences in the structure of the enteric microbiota as assessed by both Sanger-based and 454-based DNA sequencing of 16S rRNA libraries from cecal contents and stool. The dramatic impact of these diets on the community structure of the microbiota was detectable within the first week of dietary intake. Each of the high fat diets reduced the richness of the microbiota compared with the LF diet. Furthermore, the LF and LD diets promoted Firmicutes, but decreased most other phyla, whereas PUFA and MF diets promoted Bacteroidetes and decreased Firmicutes [8]. While MF and PUFA diets had similar effects on Bacteroidetes and Firmicutes, a significant bloom of a member of the Deltaproteobacteria, Bilophila wadsworthia, was consistently observed only with the highly-saturated MF diet. Blooms of this rare microorganism most likely reflect changes in microbial assembly rules thereby permitting its growth. In fact, B. wadsworthia is often detected as a highly abundant microbe in human pathological conditions such as appendicitis and other intestinal inflammatory disorders where it has been reported to be genotoxic as well as pro-inflammatory, owing to its immune-activating and sulfite-reducing properties. The sulfite-reducing properties, specifically, leads to abundant hydrogen sulfide production in conditions that favor blooms of this bacterium. [9–10].
The unusual bloom of B. wadsworthia from nearly undetectable on the LF diet to 5% abundance on MF, prompted the investigation into whether this diet-induced “pathobiont” may precipitate disease in a genetically susceptible model. Indeed, the MF diet increased the onset and incidence of colitis in IL-10−/− mice, driving it from a spontaneous rate of 25–30% (on LF) to 60% over a 6-month period [8]. In contrast, the incidence of colitis in IL-10−/− mice fed the SF diet was no different than those fed the LF diet. The colitis seen in mice fed MF was also more severe and extensive, paralleling differences in histological colitis scores. Mucosal cytokine levels from the distal colon were significantly elevated compared to LF and was correlated to MF-induced blooms of B. wadsworthia as measured by qPCR. Collectively, these observations suggest that the bloom of sulfite-reducing Deltaproteobacteria, particularly B. wadsworthia, can promote colitis in hosts that are genetically susceptible or have compromised barrier function.
Studies in germ-free (GF) IL-10−/− mice monoassociated with B. wadsworthia that were consuming either the LF, SF, or MF diet answered whether B. wadsworthia’s growth was truly MF-dependent. Five-weeks post-monoassociation, colonization of the colon could only be established in mice fed the MF diet, whereas on the LF or SF diet, B. wadsworthia was undetectable. These colonized mice went on to develop colitis, characterized by large lymphoid aggregates (under-developed in uncolonized GF mice), crypt hyperplasia, and immune cell infiltration [8]. This colitis was not as severe as when B. wadsworthia blooms in the presence of other microbes suggesting that the composite microbial community has the ability to keep certain pathobionts from growing uncontrollably. Furthermore, increased levels of TH1 cytokines, as well as low or undetectable levels of TH17 cytokines in the colonic mucosa of these mice, were consistent with the induction of a distinct TH1 immune response which was further confirmed in the mesenteric lymph nodes (MLN) of mice colonized on the MF diet [8]. None of these immune responses were observed in mice consuming the MF diet in the absence of B. wadsworthia, proving that the diet itself is not immunogenic. The response in IL-10−/− MLNs was much greater than that in C57BL/6 MLNs, most likely due to the absence of IL-10 modulating signals. This suggested a genetic or microbially-induced defect in mucosal barrier integrity could lead to increased uptake of luminal antigens and/or bacterial adjuvants that overwhelm the net suppressive tone of the mucosal immune system. Alternatively, a defect in epithelial repair could potentiate damage incurred by MF diet that causes only transient damage in genetically normal or healthy hosts. The net effect of either pathway renders the gut mucosa to constant stimulation by innate and acquired mucosal immune responses by luminal adjuvants and antigens, respectively.
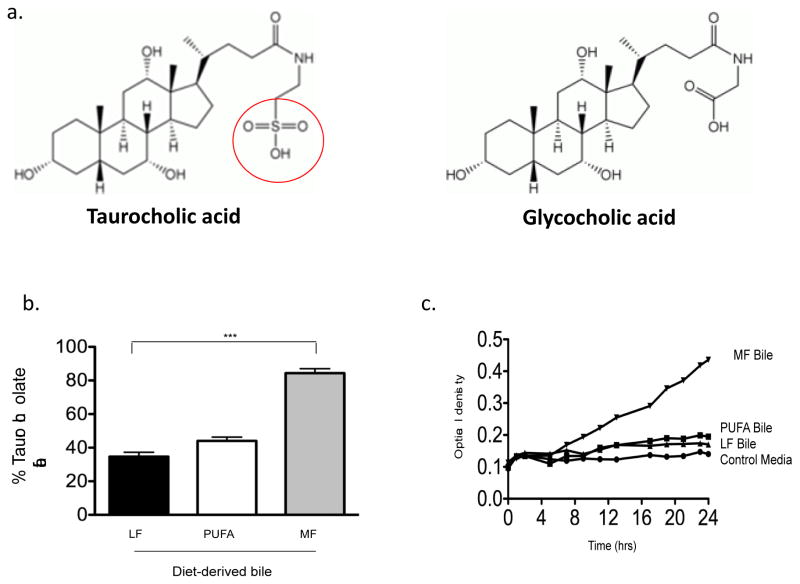
B. wadsworthia is a well-known sulfite-reducer that uses sulfite as the terminal electron acceptor of the electron transport chain, resulting in the formation of H2S as a byproduct [11]. Thus, increased availability of organic sulfur promotes its growth under anaerobic conditions. One source for this is the sulfur moiety on the taurine-conjugated (TC) bile acids that are preferentially formed when exposed to MF diets, due to TC’s ability to increase micellar formation and solubilization of milk fats [12–14]. This was confirmed by mass spectrometry measurements of gall bladder aspirates from mice fed LF, SF, and MF diets. Given that the literal meaning of Bilophilia is “bile-loving,” it has been well established that B. wadsworthia flourishes in the presence of bile in vitro, more specifically TC. Knowing this, we determined the ability of gall bladder aspirates obtained from mice on the 3 study diets to promote B. wadsworthia growth in vitro. First, mass spectrometry revealed that in-fact, the bile composition in the gall bladder aspirates of mice consuming MF diet consisted primarily of TC, while LF- and SF-fed mice had roughly equal representation of TC and GC. These aspirates were then used to spike the liquid growth media of freshly inoculated cultures. Remarkably, B. wadsworthia growth was selectively and robustly stimulated by bile from MF-fed mice only. To determine whether MF effect on B. wadsworthia was indeed mediated by TC in vivo, conventional IL-10−/− mice were fed the LF diet only and gavaged with either TC or GC daily for one week. This resulted in a bloom of B. wadsworthia with TC, nearly identical to the bloom observed with consumption of the MF diet. In contrast, GC and PBS had little effect and B. wadsworthia remained undetectable. The bloom of B. wadsworthia observed in TC-gavaged mice was associated with increased incidence and severity of colitis and TH1 cytokines were increased in both the mucosa and MLN. Furthermore, monoassociation with B. wadsworthia can only be established in GF IL-10−/− mice when accompanied by TC administration, and not by GC or PBS, as demonstrated by the re-isolation of B. wadsworthia from the cecal contents of TC-fed mice. These mice developed colitis, and again exhibited elevated TH1 mucosal and MLN intracellular cytokines. Thus, the effects of MF on the microbiota, mucosal immunity, and colitis can be reproduced by TC.
This study supports the notion that composition of dietary fats results in differential effects on the enteric microbiota leading to shifts in the host inflammatory response. The bloom of sulfite-reducing bacteria such as B. wadsworthia occurs through a unique mechanism that involves MF-promoted taurine conjugation of hepatic bile acids, which, through repeated cycles of the enterohepatic circulation, increases sulfhydryl bioavailability that promotes the growth of colonic microbiota capable of sulfite reduction. These types of bacteria are of great clinical interest as they are often recovered from biopsies and stool of IBD patients [15–18] and in our studies appear to have a strong correlation to severity of colitis. This, in turn, begs the question of how to reverse such dysbiosis. From a dietary standpoint, great promise exists for nutritional therapy in re-shaping the microbiome. Many individuals with IBD search for complementary and alternative treatments to help ease their symptoms. While as many as half of all Crohn’s patients may turn to these types of treatments, few have been rigorously studied for efficacy, safety, and mechanism of action. For years, fish oil supplements have been attempted for treatment of IBD with hopeful yet varied results [19–20]. The mechanism of their action in IBD is unknown, but has been attributed to their anti-inflammatory effects, primarily mediated by docosohexanoic (DHA) and eicosopentanoic (EPA) ω-3 fatty acids [21]. However, equally plausible is the possibility that ω-3 fatty acids change the enteric microbiome in a way that may be less immunogenic and disease-promoting in individuals genetically prone to IBD.
In a follow-up set of unpublished studies, we asked whether there might be a beneficial way to manipulate fats to suppress growth of pathobionts through ω-3 supplementation. Findings from these studies reveal that a 5% supplementation of our established pro-inflammatory MF diet with ω-3 fish oil completely inhibited blooms of B. wadsworthia that resulted in lower incidence and severity of colitis similar to mice consuming LF. Furthermore, cytokines at both the mucosal and MLN levels were reduced in the ω-3 supplemented group compared to MF alone, and not statistically different from LF control. The above effects appear to be mediated through a shift in bile acid composition by the ω-3 supplementation.
They dynamic interplay of diets, host physiology, and the microbiome triangulate in such a way that it is never unidirectional. Our microbiome can be modulated, groomed, and re-shaped by the foods we eat and our underlying host chemistry. In these studies, we show one possible pathway in which the fats we choose to consume on a regular basis can indirectly influence the gut microbiota through altered bile acid composition, and depending on your fat of choice, could lead to disease or prevent it. Further studies in humans are necessary to understand the long-term consequences of diets on the microbiome and disease susceptibility, but the existing evidence to-date makes clear that our intestinal microbes are exquisitely sensitive to our dietary choices. ((CE gilt das als Disclosure Statement?))
Figure 1. MF-based saturated fat exerts differential effects on phyla and sulfite-reducing bacteria compared to other fats, and increases severity of colitis.
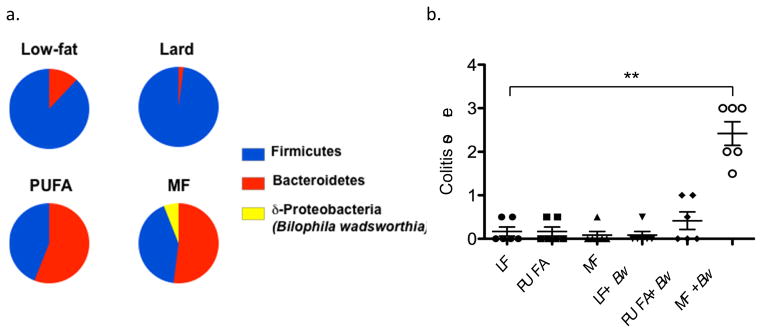
a, C57BL/6 mice (n=12) were placed on either a low-fat (LF), saturated lard-based fat, saturated milkfat-based (MF), or polyunsaturated safflower oil-based (PUFA) diet for 24 days. Total fat content of the three high-fat diets was equal at 37% kcal but the bloom of B. wadsworthia was only observed in the MF diet. b Successful monocolonization of germ-free IL10−/− mice with B. wadsworthia only occurred in the presence of MF. Attempts to determine which fatty acid component of MF and Lard might account for the differences in phyla distribution were unsuccessful because of technical challenges in solubilizing stearic acid- the major saturated fatty acid component of MF.
Figure 2. MF consumption shifts bile composition toward greater proportion of taurocholate and promotes growth of B. wadsworthia (Bw) in vitro.
A. Chemical structures of taurocholic and glycocholic acids, illustrating the distinctive sulfur moiety with taurine conjugation. b. Gall bladder aspirates from SPF IL10−/− mice consuming LF, PUFA or MF for 5 weeks were analyzed by HPLC and mass spectrometry to determine bile composition. c. The gallbladder aspirates were then added to the growth media of Bw at time=0. Normal Bw growth media contains taurine, however added taurine in this media was removed. Bile was added at 1% of media and optical density was recorded every hour for 24 hrs. At 4 hours post-innoculation, Bw growth began to rapidly increased in the media containing bile from MF-fed mice.
Figure 3.
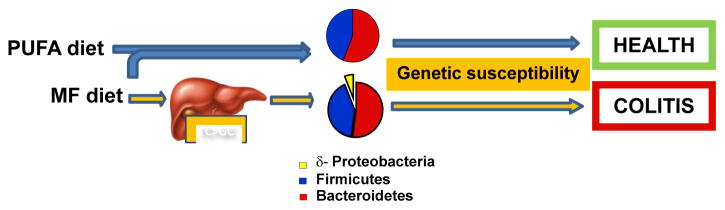
Figure of proposed model by which different fats can differentially affect inflammatory outcomes by altering the gut microbiome.
Acknowledgments
The authors received funding from the National Institutes of Health grants P30 DK42086, R37 DK47722, R01 DK097268, T32 DK07074 to E.B.C and F32 DK09025 to S.R.D., the Leona M. and Harry B. Helmsley Charitable Trust, and the Gastro-intestinal Research Foundation of Chicago.
References
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel diseases. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer S. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. 2006;12(5):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Frassetto L, Morris RC, Jr, Sellmeyer DE, Todd K, Sebastian A. Diet, evolution and aging-the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr. 2001;40:200–213. doi: 10.1007/s394-001-8347-4. [DOI] [PubMed] [Google Scholar]
- 6.Cordain L, Watkins BA, Florant GL, Kelher M, Rogers L, Li Y. Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease. Eur J Clin Nutr. 2002;56:181–191. doi: 10.1038/sj.ejcn.1601307. [DOI] [PubMed] [Google Scholar]
- 7.National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes.htm.
- 8.Devkota S, Wang Y, Musch M, Leone V, Fehlner-Peach H, Nadimpalli A, Anontonpoulos D, Jabri B, Chang EB. Dietary fat-induced Taurocholic acid promotes pathobiont expansion and colitis in IL10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron EJ, Summanen P, Downes J, Roberts M, Wexler H, Finegold S. Bilophila wadsworthia, gen. nov. and sp. nov. a unique gram-negative anaerobic rod recovered from appendicitis specimens and human faeces. J Gen Microbiol. 1989;135:3405–3411. doi: 10.1099/00221287-135-12-3405. [DOI] [PubMed] [Google Scholar]
- 10.Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 11.Laue H, Denger K, Cook AM. Taurine reduction in anaerobic respiration of Bilophila wadsworthia RZATAU. Appl Environ Microbiol. 1997;6:2016–2021. doi: 10.1128/aem.63.5.2016-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham TO, Van Thiel DH, Little JM, Lester R. Synthesis of taurocholate by rat fetal liver in organ culture: Effects of cortisol in vitro. Am J Physiol. 1979;237:E177–184. doi: 10.1152/ajpendo.1979.237.2.E177. [DOI] [PubMed] [Google Scholar]
- 13.Lindstedt S, Avigan J, Goodman DS, Sjovall J, Steinberg D. The effects of dietary fat on the turnover of cholic acid and on the composition of the biliary bile acids in man. J Clin Invest. 1965;44:1754–1765. doi: 10.1172/JCI105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rueda A, Manas M, Valverde A, Fernandez JI, Naranjo JA, Martinez-Victoria-E Conjugated bile acids and intestinal flora during the pre-ruminant stage in goat: Influence of a lamb milk replacer. Arch Physiol Biochem. 1996;104:246–251. doi: 10.1076/apab.104.2.246.12884. [DOI] [PubMed] [Google Scholar]
- 15.Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;86:103–112. [Google Scholar]
- 16.Zinkevich V, Beech IW. Screening of sulfate-reducing bacteria in colonoscopy samples from healthy and colitic human gut mucosa. FEMS Micro Ecol. 2000;34(2):147–155. doi: 10.1111/j.1574-6941.2000.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 17.Loubinoux J, Mory F, Pereira IA, Le Faou AE. Bacteremia caused by a strain of Desulfovibrio related to the provisionally named Desulfovibrio fairfieldensis. J Clin Microbiol. 2000;38:931–934. doi: 10.1128/jcm.38.2.931-934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowan FE, Docherty NG, Coffey JC, O’Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Brit J Surg. 2009;96(2):151–158. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- 19.Salomon P, Kornbluth AA, Janowitz HD. Treatment of ulcerative colitis with fish oil n-3-[omega]-fatty acid: an open trial. J Clin Gast. 1990;12:2. doi: 10.1097/00004836-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Belluzzi A, Corrado B, Massimo C, Angelo P, Stefano B, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. New Engl J Med. 1996;334:1557–60. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- 21.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]