Abstract
Purpose
The Adolescent Medicine Trials Network Protocol 113 (ATN113) is an open-label, multi-site demonstration project and phase II safety study of HIV pre-exposure prophylaxis with 15-17 year old young men who have sex with men that requires adolescent consent for participation. The purpose of this study was to examine factors related to the process by which Institutional Review Boards (IRBs) and researchers made decisions regarding whether to approve and implement ATN113, so as to inform future biomedical HIV prevention research with high-risk adolescent populations.
Methods
Participants included seventeen researchers at thirteen sites in twelve states considering ATN113 implementation. Qualitative descriptive methods were used. Data sources included interviews and documents generated during the initiation process.
Results
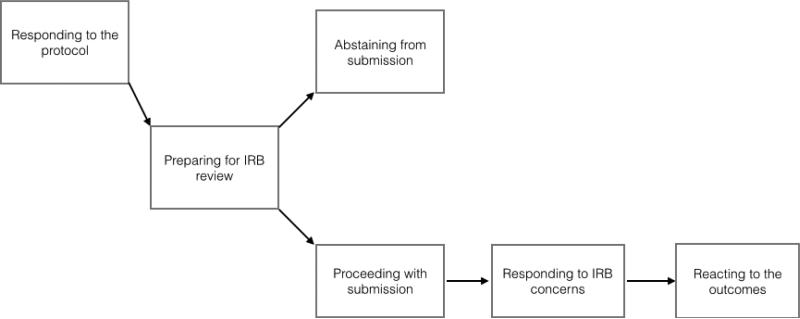
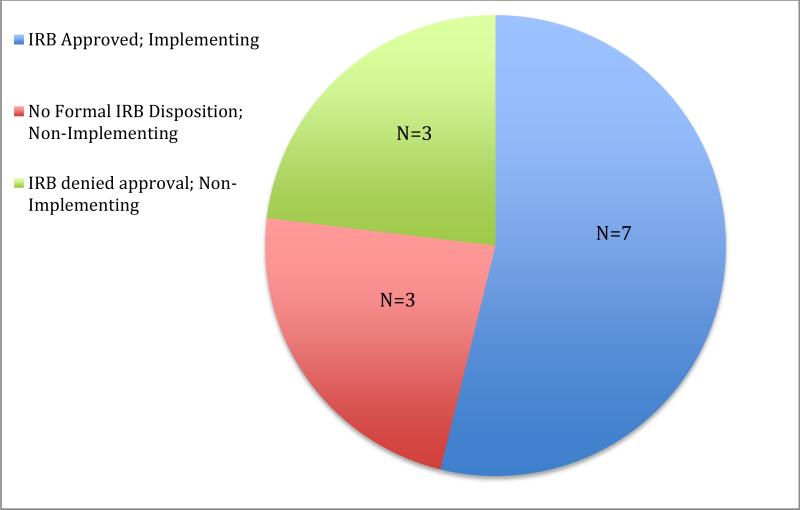
A common process for initiating ATN113 emerged, and informants described how they identified and addressed practical, ethical and legal challenges that arose. Informants described the process as responding to the protocol, preparing for IRB submission, abstaining from or proceeding with submission, responding to IRB concerns and reacting to the outcomes. A complex array of factors impacting approval and implementation were identified; and ATN113 was ultimately implemented in 7 of 13 sites. Informants also reflected on lessons learned that may help inform future biomedical HIV prevention research with high-risk adolescent populations.
Conclusions
The results illustrate factors for consideration in determining whether to implement such trials, demonstrate that such protocols have the potential to be approved, and highlight a need for clearer standards regarding biomedical HIV prevention research with high-risk adolescent populations.
Keywords: adolescent medicine, informed consent by minors, HIV, pre-exposure prophylaxis, ethics committee, research, jurisprudence
An emerging approach to the prevention of Human Immunodeficiency Virus (HIV) transmission is Pre-Exposure Chemoprophylaxis (PrEP) using daily oral doses of the antiretroviral drug combination Emtricitabine/Tenofovir (FTC/TDF). FTC/TDF PrEP has been approved by the U.S. Food and Drug Administration (FDA) for high-risk adult populations.1,2 Although young men who have sex with men (YMSM) are disproportionately affected by HIV,3,4 minor youth were excluded from clinical trials informing this FDA indication for use.
Minor adolescents are frequently excluded from biomedical HIV prevention research due to the legal and ethical complexity of including them. Researchers often have ethical concerns about adolescent vulnerability and capacity for research-related decision-making, and legal concerns about navigating laws that may require parental consent.5-8 Mandates for parental consent pose even greater barriers to recruitment in studies that address sensitive issues such as sexuality and sexual practices.7-9 In the case of YMSM, for example, youth may be unwilling to participate in a study in which the informed consent process is likely to result in their sexual status and/or sexual activity being revealed to their family, potentially resulting in rejection or violence.10,11 In certain circumstances such as these, ethical considerations supporting adolescent inclusion in research (e.g., the critical importance of clinical trials data on PrEP safety for YMSM or the high vulnerability of YMSM to HIV) may overshadow those requiring parental consent.5,8,12
Federal regulations governing research conducted with FDA oversight stipulate parental consent for research with minors; and waiver of parental consent is therefore not permitted.13,14 However, in limited circumstances where adolescents meet criteria for emancipation, are considered “mature minors,” or are otherwise allowed to consent on their own behalf to the treatment or care being studied under state law, they may legally be permitted to consent to the research on their own behalf without parental consent .8,15 Local Institutional Review Boards (IRBs) routinely determine whether the consent procedures proposed in a study are both ethically justified and compliant with state and federal law.16 No biomedical HIV prevention trial has previously been conducted in the United States among adolescents aged 15 to 17 years without parental consent; and the process by which researchers and IRBs undertake the difficult task of reviewing and implementing such protocols with high-risk minor populations has never been examined.
The ATN and Protocol 113
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN), funded primarily by the National Institute of Child Health and Human Development, conducts HIV prevention and treatment research among youth aged 12-24 years in 14 clinical sites and surrounding communities. The challenge of balancing the legal and ethical justifications for and against minor self-consent was addressed by the ATN during a Phase II PrEP safety study (ATN Protocol 113, subsequently identified as ATN113) for 15-17 year old YMSM. Motivated by the reality that FTC/TDF is likely to be used off-label for PrEP purposes among YMSM, the ATN sought to obtain safety data for this indication, including assessments of patterns of use, adherence, and changes in sexual risk and protective behaviors. The protocol also included an efficacious behavioral risk reduction intervention to address potential risk compensation associated with PrEP.
ATN113 was open to all sites, based on a common protocol that was approved by experts within the ATN and the National Institutes of Health. Additional opinions regarding adolescent self-consent were provided by the ATN Ethics Advisory Panel, the Office of Human Research Protection and the FDA. These bodies concluded that it was legally and ethically appropriate for minor adolescents to consent to ATN113 participation on their own behalf when permitted under state law as interpreted by their local IRB; and the protocol was written to require adolescent self-consent. As part of routine network protocol implementation procedures, an IRB submission packet was provided to all sites that included an IRB submission cover letter summarizing the protocol, an IRB submission template and the ATN113 protocol itself. Materials specific to the external consultations described above were also provided.
A summary of the ATN113 recruitment and consent process, as described in the protocol, follows. Recruitment is to be conducted using venue-based methods and/or online-based methods. For venue-based recruitment, potential participants are to be approached in coffee houses, gay youth centers, book clubs, House Ball community gatherings, parent groups and clinics caring for the target population. ATN study sites have the option of also using social networking sites and geo-social networking mobile applications to approach potential participants. For venue-based recruitment, verbal consent is to be obtained before screening potential participants for preliminary eligibility using a handheld device, and for online-based recruitment a web-based screener is to be used. If a participant is deemed eligible based on preliminary criteria, he is to be offered an in-person screening appointment at the study site. On the day of the in-person screening, prior to determining final eligibility, the purpose, procedures, requirements, risks and benefits of the study are to be thoroughly discussed with the potential participant and written consent obtained. Prior to screening for final eligibility, an “assessment of understanding” questionnaire must also to be administered to ensure understanding.
Study Purpose and Aims
The specific aims of the ATN113 sub-study described in this article were to examine: (1) the initiation process by which ATN investigators and other study personnel, in collaboration with their local IRBs, evaluated the issue of adolescent self-consent and reached decisions regarding whether or not to approve and implement the ATN113 protocol; and (2) reflections on valuable lessons learned. Understanding this initiation process is critically important as new biomedical HIV prevention technologies, including microbicides and vaccines, continue to emerge. Lessons learned during this process may help guide researchers, IRB members, and policy makers in the responsible conduct of future biomedical HIV prevention research with minor participants who are at substantial risk for HIV infection.
METHODS
Qualitative Descriptive (QD) methods, which provide an in-depth description of experiences shared by a group facing a common challenge,17 were used to meet the sub-study's aims. The QD approach is particularly useful for generating straightforward summaries of information to guide future intervention. It relies on purposive sampling, moderately structured interviews with key informants, and low-inference content analysis. This study was exempted from full review by the Indiana University IRB – Human Subjects.
Study Population, Setting and Dates
The study population included ATN investigators and study personnel at all sites contemplating implementation of ATN113. Research activities took place via phone and email between May and November 2013. Verbal consent was obtained, and all informants received written information explaining the study's purpose and aims.
Data Collection Procedures
Sources of data included: (1) moderately structured interviews with informants; (2) informal documented correspondence between informants and their respective IRBs related to ATN113 (e.g., emails and verbal communication logs); and (3) formal IRB memoranda related to ATN113 (e.g., letters of approval). Digitally recorded telephone interviews were conducted by the first author addressing the following topics: (1) personal experience with research requiring minor consent, (2) perception of IRB experience with research requiring minor consent, (3) issues informants found to be troubling, (4) issues the IRB found to be problematic, (5) processes by which identified issues were addressed, (6) final IRB disposition, (7) opinions regarding the IRB disposition and implementation decision, and (8) lessons learned that may inform future studies.
Data Management and Analysis
Interviews were transcribed verbatim by a professional transcription service. Two authors (ALG and ASK) compared the transcripts to the audio-recordings, making corrections as needed. Data were uploaded to a cloud-based qualitative data analysis program (Dedoose, Hermosa Beach, CA, USA). Authors ALG and ASK then divided the sites into two groups and reviewed all data associated with each site, excerpting all text units addressing the study's aims. Each text unit was coded to reflect its content, and a case-ordered meta-matrix18 was used to organize and summarize the data into categories and sub-categories. ALG and ASK then reconvened to select exemplar segments of text illustrating each category. To protect privacy, each site was randomly assigned a letter to represent it in publications and presentations.
RESULTS
Seventeen informants from 13 ATN sites in 12 states participated in the study. The remaining ATN site did not participate for administrative reasons not related to the study. A majority of informants were female, White and Non-Hispanic (Table 1). The data reflect a process (Figure 1) by which study teams initiated ATN113, and describe the practical, ethical and legal challenges that arose during this process. Below, we discuss each step in the process, the challenges and solutions that emerged, and lessons learned along the way. Exemplary quotes are contained in Tables 2 and 3.
Table 1.
Key Informant Demographics (N=17)
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 5 (29.4) |
| Female | 12 (70.6) |
| Mean Age in Years | 48 |
| Race | |
| Black/African American | 1 (5.9) |
| White/Caucasian | 14 (82.3) |
| Missing | 2 (11.8) |
| Ethnicity | |
| Hispanic/Latino | 1 (5.9) |
| Non-Hispanic/Latino | 14 (82.3) |
| Missing | 2 (11.8) |
| Professional Role at ATN Site | |
| Investigator | 12 (70.6) |
| Study Coordinator/Other | 5 (29.4) |
Figure 1.
ATN113 Initiation Process
Table 2.
Exemplar Quotes: The IRB Protocol Initiation Process1
| Responding to the Protocol |
| • Responding as a parent: “I have really struggled a bit ... my litmus test is ‘Would I let my kids participate in this?’” (Site M; IRB Denied Approval; Non-Implementing) |
| • Responding emotionally as a professional: “[T]he population we're dealing with has the potential for having unstable housing and financial support ... it's not the best population to have on a medication that potentially could have side effects because you ... have to be worried about your ability to contact the subject in the future and make sure that they're coming in, so that you can monitor." (Site L; No Formal IRB Disposition; Non-Implementing) |
|
Preparing for IRB Review |
| • Giving notice to the IRB: “You know, we took a really, really, really proactive approach. So, long before 113 would be released we met ... our IRB holds, I guess for lack of a better term they call it ‘office hours’ ... and the four heads of the pediatric panel met with myself and the study coordinator.” (Site R; IRB Approved; Implementing) |
| • Educating the IRB: “I think part of the leg work we did was educating the community. A lot of the community members are on the IRB Board, so part of our strategy was making sure that those community members and the organizations that they belong to were educated about PrEP and why we were doing it.” (Site V; IRB Approved; Implementing) |
| • Exploring State Law: “I think the key sentence for us is: ‘Any physician may examine, diagnose, and treat minors infected with sexually transmitted diseases without the knowledge or consent of the parents.’ So, the key things in our state language are...one, it does not specifically [refer to] prophylaxis...and then, it specifically says ‘infected with STD’ and doesn't leave much room in there to say ‘suspected of’ or ‘at risk of.’ So what room we had was to ask, both at our hospital level legal counsel as well as the contact at the state legal counsel ... ‘Does this cover prophylaxis?’” (Site N; No Formal Disposition; Non-Implementing) |
|
Abstaining from or Proceeding with IRB Submission |
| • Abstaining: “[The IRB] declined to review the protocol altogether because of the interpretation of [State] law.” (Site L; No Formal IRB Disposition; Non-Implementing) |
| • Proceeding: “[I]t was a huge submission. There were 50 attachments but I pulled everything that...possibly could come to support this.” (Site W; IRB Approved; Implementing) |
|
Responding to IRB Concerns |
| • Considering informed consent: “So, first round was convincing them that ... the law was permissive for treating people at risk for HIV infection. The State allows you to seek your own care for STIs and HIV infection ... but the glitch here was that they're not really infected. This is prevention. But there is case law ... the law is silent on prevention, but there's case law that supports it, applying the same rule to prevention.” (Site L; No Formal Protocol Disposition; Non-Implementing) |
| • Weighing risk versus benefit: “The committee discussed that Truvada is FDA approved in adults and has been shown to be very safe and effective for the same; the only major issue in this study is the age of subjects, and ensuring their compliance with the study regimen (which the committee noted is an issue in general with this population). The committee noted that since these subjects are at high risk for getting HIV, the potential benefit of this study is very apparent, especially given the incorporation of behavioral interventions.” (Site D; IRB Approved; Implementing)2 |
| • Considering the need for protectors: “[T]hey said, ‘It would be good to see if there could be a parent spokesperson who could also be there [at a meeting of the IRB].’ So we said we might bring some youth to give the youth perspective of why parents’ consent can be difficult or not difficult. And they said, ‘We would also like to hear from a parent.’” (Site N; No Formal IRB Disposition; Non-implementing) |
| • Avoiding liability: ““What they wanted was an assurance that they would not be legally liable ...” (Site H, IRB denied; Non-implementing) |
|
Reflecting on the Outcomes |
| • Reflecting on Approval and Implementation: “One thing ... that came up a lot in the [IRB] meeting ... was ... OK, are we abiding by state statute or not? And honestly, that's a grey area. You know, we feel comfortable with our decision, but just like the attorney has told us, someone could come in here and interpret it a different way. But notwithstanding that, ... we feel really comfortable with our interpretation.” (Site B, IRB Approved; Implementing) |
| • Reflecting on Denial of Approval and Non-Implementation: “So, I think that as the research PI ... wanting to make sure our site ... is able to implement the protocol from ATN, I'm disappointed. And, the personal side of me that was on the fence about how I would explain to the mother in the ER about her kid ... is a little bit relieved that is was taken out of my hands today.” (Site I, IRB denied; Non-implementing) |
Data sources are informant interviews unless otherwise noted. Study site, IRB decision, and protocol status are noted in parentheses after each text segment.
Table 3.
Exemplar Quotes: Reflections on Lessons Learned1
| Be Persistent |
| • “And as circumstances change, you know, reconsider or have others reconsider their previous decisions.” (Site W; Protocol Approved; Implementing) |
|
Strive for Collaborative Relationships with IRB Members |
| • “Rather than just putting it in and making the IRB go through the motions, I think I would try again to ... query the [IRB] administrator and try to maybe speak to the lawyer and see, behind the scenes, if there's a way to word certain segments of the submission or ... try to strategize a way to submit it that could be acceptable.” (Site K; No Formal Protocol Disposition; Non-Implementing) |
|
Engage Experts Early and Often |
| • “What I should have done initially was get lawyers involved, which I didn't. The IRB initially got the lawyer involved. So, in the future, if I'm doing something that there's a question ... how law may impact a consent process, then I'll get lawyers involved from the beginning because we went back and forth, I believe it was four times, and that takes a lot of time.” (Site L; No Formal Protocol Disposition; Non-Implementing) |
Data sources are informant interviews. Study site, IRB decision, and protocol status are noted in parentheses after each text segment.
ATN Initiation Process
Responding to the Protocol
Informants reflected on personal responses to ATN113, describing feelings such as worry or enthusiasm. Most reported initial concerns that stemmed from the sense of responsibility they felt as providers or fear they felt as parents. From a professional perspective, informants reported feeling accountable to participants’ parents, and worrying about how parents might react if a participant experienced serious study-related side effects. Many were concerned about the study's legality, and expressed anxiety about implications for their respective institutions. Others discussed concerns about the vulnerability of prospective participants. They worried that low comprehension and economic deprivation would make it difficult to ensure that participants were fully aware of the risks of participation and competent to follow through with protocol requirements such as attending appointments, taking medications as prescribed, and reporting side effects. Informants also spoke about responding emotionally as parents, and several expressed tension between their professional and parental perspectives. A large majority of informants, however, reported that they were reassured as the process progressed.
Preparing for IRB Submission
Many informants doubted that their IRB would approve ATN113, and all anticipated challenges during the review process. Informants characterized existing relationships with local IRBs as well established, positive and valuable; with several specifically noting IRB willingness to consider controversial or cutting edge research. Most reported beginning the process by giving their IRB advance notice by phone or email, leveraging existing relationships to initiate informal review prior to formal protocol submission. During these exchanges, they reported that IRB members often specified which aspects of the protocol would be problematic and indicated the type of documentation that would help mitigate concerns. Informants also attended meetings with IRB members to discuss the protocol in advance of submission, and educated IRB members about PrEP by collating journal articles, sharing previous experience with the study drug, and encouraging people to attend talks about its use.
Informants identified the legality of minor self-consent for research participation as the issue they anticipated as having the most difficulty with, and conversations with IRB members supported this concern. Reviewing state laws relevant to minor consent was therefore a common activity. Informants reported researching state statutes, interpreting them independently or with the help of a consultant, and then providing this information to the IRB, either in advance of submission or as part of the submission package.
Abstaining from or Proceeding with Submission
Informants gauged IRB responses in the preparatory phase, and then decided whether to proceed. Eleven sites proceeded with submission. Of note, one submitted the protocol despite IRB forewarning that it would be denied and another moved forward with submission but later withdrew the protocol upon the ATN's request for administrative purposes. Two sites abstained from submission entirely, one because informants were advised by the IRB that it would not approve the protocol with adolescent self-consent and the other because informants were advised by an attorney that it could not be approved under state law.
Responding to IRB Concerns
Informants at all sites revealed IRB concerns about the informed consent process. These concerns included issues with the form itself (length, language, level of specificity); objective measurement of participant understanding; and the legality of minor self-consent for research participation. In response, informants described editing the proposed consent form (in one case, with the help of a consultant to make the form more age-appropriate) and engaging attorney consultants to draft legal memoranda regarding legality. Informants also reported that IRB members believed additional protections should be provided to minor participants, regardless of regulatory necessity, by parents, surrogate adult decision-makers and/or the IRB. Researchers were responsive to these requests when legally and logistically practicable.
Informants also described IRB member concerns about the balance of the risks (e.g., coercion, privacy issues related to the use of social media for recruitment, and concerns about drug safety and drug resistance) versus the benefits of participation (e.g., behavioral counseling and prevention of infection). Many of these concerns were raised and addressed as completely as possible in the preparatory phase, but when they arose after submission, informants responded by engaging in discussions with IRB members and modifying the protocol when possible. Some IRB members expressed concern that they personally, or their institutions, might be held legally liable for adverse participant outcomes and sought reassurance that this would not happen. The legal advice solicited and provided by researchers was not always sufficient to assuage IRB concerns.
Reacting to the Outcomes
Seven sites were ultimately granted IRB approval, three were denied approval and three did not receive a formal IRB disposition (Figure 2). Although various issues were addressed by IRBs in their formal approval and non-approval memoranda, all IRBs that issued formal dispositions specifically addressed the legality of minor consent with interpretations of state law that varied widely. For example, very similarly worded statutes in two states with no common law precedent for how the statutes should be interpreted came to opposite conclusions about the legality of adolescent consent for preventive research.
Figure 2.
Outcomes of the ATN113 Protocol Initiation Process
Informants at all seven sites that received approval and implemented the protocol expressed confidence in the decisions reached by their respective IRBs, and their own decisions to implement. Reactions were mixed among the three sites that were denied approval; informants at two sites reported feeling simultaneously disappointed and relieved, while an informant at the third was disappointed with the decision and ultimately decided to open a PrEP clinic for at-risk minors. Informants from two of three sites that did not receive a formal disposition expressed disappointment that they could not be involved in what they perceived to be an important trial.
Reflections on Lessons Learned
Informants reflected on lessons learned during the ATN113 initiation process and shared advice they believed could help other researchers with the initiation process in future studies of this nature. They emphasized the importance of persistence, searching for solutions to problems and returning to the IRB with new information. Informants also spoke of striving for collaborative relationships with IRB members by empathizing with their work load and responsibilities, being transparent in all exchanges, providing as much information as possible up front, and communicating in person. They advocated for identifying and engaging experts early and often. For example, they spoke of seeking formal or informal ethical and legal consultation, working closely with compliance officers, and approaching States’ Attorneys General for non-binding interpretations of state statutes. See Table 3.
DISCUSSION
This study examines the process by which researchers and IRBs across the nation undertook the difficult task of initiating a biomedical HIV prevention protocol that required high-risk minor youth to consent to research participation on their own behalf. The results illustrate the complex legal and ethical factors researchers and IRBs must consider in determining whether to conduct biomedical trials with this population. To place the results in context, clinical trials data from adult studies provide strong evidence of FTC/TDF efficacy, safety, and an acceptable range of side effects; and the drug combination is already widely used for HIV treatment with adolescents. These realities, combined with (1) disproportionately high HIV infections rates among YMSM,3 (2) the fact that HIV infection itself is an incurable disease, (3) the critical need for safety data among youth who may well be prescribed FTC/TDF for PrEP use off-label even in the absence of such data,5 (4) the familial rejection and potential violence experienced by many YMSM,10,11 and (5) justifiable expectations of a favorable balance of risks and benefits, provided the ATN with a compelling rationale for conducting ATN113 without parental consent. Nevertheless, the study did confront researchers and IRBs with a host of ethical challenges.
Ethical considerations generally revolved around the principle of respect for persons. Although adolescents with legal authority to consent under state law were assumed to be fully autonomous, careful consideration was given to adolescents’ capacity to fully understand the risks of participation, including the risks of failing to adhere to the study protocol. Informants also expressed concern about whether adolescents would have a realistic view of the medication's benefits, be influenced by monetary reimbursements, and/or underestimate risks of study participation to health or family relationships. Thus, the provision of a legal basis for adolescent self-consent did not erase vulnerability from the considerations, such as risk compensation and preventive misconception,19,20 that inform restrictions on adolescent research participation in the first place.
Ethical considerations also focused on the principle of beneficence and the balance of potential harms and benefits. The benefit of participation most often identified was the prevention of HIV. Identified risks included coercion and concerns about drug safety. Informants also noted that privacy issues related to the use of social media for recruitment could emerge if adolescents inadvertently, or without sufficient consideration of the consequences, were to share information about the ATN113 participation on the social media tools used to recruit them. Informants also expressed concerns about the potential for the development of drug resistance if partially-adherent participants were to acquire HIV, remain undiagnosed and continue taking the study drug intermittently, resulting in ineffective treatment for the virus.
Implementation of ATN113 hinged primarily on IRB interpretations of state minor consent laws. While most states allow minors to consent to medical diagnosis or treatment of sexually transmitted infections, few expressly allow self-consent for preventive services,15 and legal interpretations extending the laws to prevention have not yet been tested in most state court systems. While some IRBs interpreted the statutory language of their respective states to include prevention, many did not.
Informants made great efforts to better understand and educate their IRBs about their states’ minor consent laws, and advised future researchers to do the same. They stressed the importance of researching the law in advance, and engaging knowledgeable experts (ethicists, attorneys, compliance officers and State Attorneys General) to provide guidance that may proactively be shared. Our finding that two IRBs reached opposite conclusions, referencing statutory language that was almost exactly the same to support their divergent positions, illustrates just how widely interpretations of law may differ. Although it is expected and appropriate for IRB dispositions to vary across sites based on the cultures and values of their respective institutions and communities, the inconsistent application of law in this case highlights a genuine need for better-articulated standards regarding adolescent participation in preventive biomedical research. Such standards may help address procedural justice issues that directly affect adolescent access to both the risks and benefits of research participation.21
As with all research, this study has several limitations. Informants were from major medical centers in large U.S. cities with well-established adolescent research programs and strong IRB relationships, so findings may therefore typify the initiation process at centers such as these rather than that experienced at smaller centers with developing programs. Also, interviews were conducted after IRB decisions were rendered, where applicable, and responses relied on informant recall. It is possible that IRB dispositions may have influenced informant perceptions; those whose IRBs failed to approve the protocol may have tried to rationalize the rejection by emphasizing challenges, whereas those from approved sites may have minimized them. A different, although arguably less efficient, approach may have been to collect data at several different points in time to minimize such interactions. This limitation was mitigated, however, by the collection of verbal communication logs and other communications throughout the protocol initiation process, and the triangulation of interviews and official IRB memoranda with these data. The goal of this study was to examine factors related to the process by which IRBs and researchers make decisions regarding whether to approve and implement biomedical HIV prevention studies, we elected to assess these factors primarily from the perspective of researchers. A more comprehensive understanding may be obtained if future studies were to include IRB members as engaged subjects.
These findings demonstrate the complex factors researchers and IRBs must consider in determining whether to conduct biomedical HIV prevention trials in which high-risk minor youth consent on their own behalf. They also clearly demonstrate that such protocols may be approved and implemented in situations where compelling justifications exist for not engaging parents, and provide guidance about how best to proceed. Researchers working with adolescents in this context should carefully consider relevant law, particularly in states with minor consent laws that do not contain language specific to prevention. They may also need to work with local authorities and policymakers to help educate them about adolescent research needs, and how various laws, regulations and policies influence the implementation of clinical trials with this vulnerable population. Additional standards, such as formal agency guidance or a comprehensive toolkit developed by the ATN in collaboration with investigators and other stakeholders at each of its sites, would go far in ensuring the responsible conduct of future biomedical HIV prevention research with minor participants.
IMPLICATIONS AND CONTRIBUTION.
This study illustrates the complexity of practical, legal and ethical factors that researchers and IRB members must consider in determining whether to implement biomedical HIV prevention trials with high-risk adolescent populations; demonstrates that such protocols have the potential to be approved; and highlights a need for clearer standards.
Acknowledgements
The authors would like to thank the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN), with special thanks to C. Wilson and C. Partlow at the ATN Coordinating Center, J. Korelitz and B. Driver at the ATN Data and Operations Center at Westat and all ATN site-specific research personnel who participated as study informants. This sub-study was supported by the ATN with funding from the National Institutes of Health (U01 HD 040533 and U01 HD 040474) through the National Institute of Child Health and Human Development ([NICHD] B. Kapogiannis and S. Lee), and with supplemental funding from the National Institutes on Drug Abuse (K. Davenny, R. Jenkins) and Mental Health (P. Brouwers and S. Allison). Additional support was provided by the National Institute of Nursing Research (2T32 NR007066, A. Knopf). The manuscript was scientifically reviewed by the ATN's Community Prevention Leadership Group and the NICHD. The comments and views of the authors do not necessarily represent the views of the NICHD or those of the Department of Health and Human Services. Findings were presented at the April 2014 Network Meeting of the Adolescent Trials Network in Bethesda, Maryland.
Abbreviations
- HIV
Human Immunodeficiency Virus
- PrEP
Pre-Exposure Chemoprophylaxis
- FTC/TDF
Emtricitabine/Tenofovir
- FDA
U.S. Food and Drug Administration
- YMSM
young men who have sex with men
- IRB
Institutional Review Board
- ATN
The Adolescent Medicine Trials Network for HIV/AIDS Interventions
- ATN113
ATN Protocol 113
- QD
Qualitative Descriptive methods
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Food US, Drug Administration [July 23, 2014];News & events: FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2012 Jul 16; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm312210.htm.
- 2.Public Health Service US. [September 24, 2014];Preexpsoure prophylaxis for the prevention of HIV infection in the United States - 2014: A clinical practice guideline. 2014 May 20; http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
- 3.Centers for Disease Control Monitoring selected national HIV prevention and care objectives by using HIV surveillance data – United States and 6 U.S. dependent areas – 2010. HIV Surveillance Supplemental Report. 2012;17((3)(A)) [Google Scholar]
- 4.Wilson CW, Wright PF, Safrit JT, Rudy B. Epidemiology of HIV infection and risk in adolescents and youth. J Acquir Immune Defic Syndr. 2012;54:S5–S6. doi: 10.1097/QAI.0b013e3181e243a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace JE, Siberry GK, Hazra R, Kapogiannis BG. Preexposure prophylaxis for adolescents and young adults at risk for HIV infection: Is an ounce of prevention worth a pound of cure? CID. 2013;56:1149–1155. doi: 10.1093/cid/cis1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS (UNAIDS) Ethical Considerations in Biomedical HIV Prevention Trials. UNAIDS; Geneva, Switzerland: 2012. [Google Scholar]
- 7.DiClemente RJ, Ruiz MS, Sales JM. Barriers to adolescents’ participation in HIV biomedical prevention research. J Acquir Immune Defic Syndr. 2012;54:S12–S17. doi: 10.1097/QAI.0b013e3181e1e2c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson RM, Lewis LL, Struble K, Wood SF. Ethical and regulatory considerations for the inclusion of adolescents in HIV biomedical prevention research. J Acquir Immune Defic Syndr. 2012;54:S18–S23. doi: 10.1097/QAI.0b013e3181e2012e. [DOI] [PubMed] [Google Scholar]
- 9.Hester CJ. Adolescent consent: Choosing the right path. Issues Comp Pediatr Nurs. 2003;27:27–37. doi: 10.1080/01460860490279536. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico Emilie, Julien Danielle. Disclosure of sexual orientation and gay, lesbian, and bisexual youths’ adjustment: Associations with past and current parental acceptance and rejection. Journal of GLBT Family Studies. 2012;8(3):215–242. [Google Scholar]
- 11.D'Augelli AR, Grossman AH, Starks MT. Families of lesbian, gay, and bisexual youth: What do parents and siblings know and how do they react? Journal of GLBT Family Studies. 2008;4:95–115. [Google Scholar]
- 12.Flicker S, Guta A. Ethical approaches to adolescent participation in sexual health research. J Adolesc Hlth. 2008;42:3–10. doi: 10.1016/j.jadohealth.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.45 CFR 46 . Human subjects protection. Department of Health and Human Services; Washington, D.C.: 2009. [Google Scholar]
- 14.21 CFR 50 . Protection of human subjects. Food and Drug Administration; Washington, D.C.: 2012. [Google Scholar]
- 15.English A, Bass L, Boyle AD, Eshragh F. State minor consent laws: A summary. 3rd ed. Center for Adolescent Health and the Law; Chapel Hill, NC: 2010. [Google Scholar]
- 16.21 CFR 56 . Institutional review boards. Food and Drug Administration; Washington, D.C.: 2014. [Google Scholar]
- 17.Sandelowksi M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. Third edition Sage Publications, Inc.; Thousand Oaks, CA: 2014. [Google Scholar]
- 19.Hosek SG, Zimet GD. Behavioral considerations for engaging youth in HIV clinical research. J Acquir Immune Defic Syndr. 2010;54(Suppl 1):S25–30. doi: 10.1097/QAI.0b013e3181e15c22. [DOI] [PubMed] [Google Scholar]
- 20.Simon AE, Wu AW, Lavori PW, et al. Preventive misconception: its nature, presence, and ethical implications for research. Am J Prev Med. 2007;32:370–4. doi: 10.1016/j.amepre.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine Committee on the Ethical and Legal Issues Relating to the Inclusion of Women in Clinical Studies . Chapter 3: Justice in Clinical Studies: Guiding Principles. In: Mastroianni AC, Faden R, Federman D, editors. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Vol. 1. National Academies Press; 1994. [PubMed] [Google Scholar]