Abstract
Objective
This retrospective study evaluates the influence of serum platelet count on chemotherapy response rates among women with endometrial cancer.
Methods
From three separate cancer centers, a total of 318 patients with endometrial cancer who received post-operative chemotherapy between June 1999 and October 2009 were retrospectively identified. Endometrioid, serous, clear cell, and carcinosarcoma histologies were included. Subjects were classified as having an elevated platelet count if their serum platelet count was greater than 400 × 109/L at the time of initial diagnosis. Primary outcome was chemotherapy response, classified as either complete or partial/refractory. Secondary outcomes were disease free and disease specific survival (DFS, DSS). Chi-square and Student t-tests were performed as appropriate. Kaplan-Meier curves and Cox proportional hazards models were used to assess serum platelet effect on survival.
Results
There were 125 deaths, 76 recurrences, and 48 disease progressions. Of the total group, 53 (16.7%) were categorized as having an elevated platelet count. An elevated platelet count was associated with a lower chemotherapy response rate in univariate analysis (HR 2.8; 95% CI 1.46, 5.38; p <0.01). Multivariate analysis showed elevated platelets to be independently associated with decreased DFS (HR 2.24; 95% CI 1.26, 3.98; p<0.01) but not DSS (HR 1.03, 95%CI 0.56, 1.88, p=0.93).
Conclusions
Endometrial cancer patients with an elevated serum platelet count > 400 × 109/L may have lower chemotherapy response rates and are at increased risk for recurrence when compared to patients with a count within normal range.
Introduction
The association of platelets with cancer biology is well-established [1, 2, 3] with approximately one-third of all cancer patients presenting with thrombocytosis [4]. Serum platelet levels > 400 × 109/L have correlated with more aggressive tumor biology and decreased survival in multiple different cancer types [5–10]. Tumor cells activate platelets through IL-6 secretion inducing a thrombogenic environment [11], and platelets have been shown to directly contribute to a tumor’s growth, metastatic potential [12–18] and vasculature homeostasis [19, 20].
Platelets are a source of both pro- and anti-angiogenic factors suggesting that they may play a critical role in the regulation of vessel formation during tumorigenesis. Platelets are a rich source of the pro-angiogenic factors, platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and thrombospondin-1 (TSP-1). Additionally, multiple tumor cell types, including breast and osteosarcoma cells, have been shown to adhere to TSP-1 suggesting an additional role for platelets in promoting cell adhesion and invasion [21–23]. In terms of therapy, antiplatelet treatments have been shown to reduce metastases and to provide a survival benefit both in murine models and in human clinical trials of cancer subjects [20, 24].
In regards to gynecologic malignancies, negative correlations between elevated platelets and outcomes in ovarian, cervical, vulvar and endometrial cancers have been described [25–31]. Thrombocytosis is an independent poor prognostic factor for locally advanced cervical cancer [29] and for advanced stage/recurrent epithelial ovarian cancers [26, 32]. Several small retrospective studies also show elevated platelets to correlate with decreased survival in endometrial cancers of both endometrioid and papillary serous histologies [26, 27, 30, 33]. Additionally, in endometrial cancers, thrombocytosis was associated with poor prognostic features such as advanced stage, cervical involvement, unfavorable grade, and non-endometrioid histology [25, 34, 35]. One study of 61 patients found no correlation between thrombocytosis and survival [36].
Studies evaluating the influence of platelet levels on chemotherapy response are sparse. Literature review found only one study of approximately 1000 non-small cell lung cancer subjects that investigated prognostic factors influencing response to platinum derivatives. The authors found that a normal platelet count is independently associated with a higher objective response rate [37]. As far as we know, no data exists for gynecologic malignancies. The aims of our study are as follows: 1) to investigate the association of serum platelet levels on chemotherapy response rates among women with endometrial cancer and 2) to evaluate the influence of serum platelets on survival in this same cohort.
Methods
Study Population and Clinical Data
Study subjects consisted of patients treated at Washington University School of Medicine (Saint Louis, MO), Stephenson Oklahoma Cancer Center (Oklahoma City, OK), or Atlantic Health System Hospitals (Morristown, NJ). Washington University prospectively gathers clinical and demographic information for endometrial cancer patients treated at our facility. Blood and tumor specimens are collected on participating subjects at the time of study enrollment. Tissue specimen and data collection began in 1991 and continues with active curation of clinical data. We retrospectively identified all endometrial cancer patients enrolled between June 1999 and September 2009. All participants consented to molecular analyses and follow-up as part of a Washington University Medical Center Human Studies Committee-approved protocol (93-0828). Patients treated at the Stephenson Oklahoma Cancer Center were retrospectively identified as women diagnosed with endometrial cancer between January 2000 and January 2008 who underwent surgery and chemotherapy and had complete follow-up information. Approval was obtained from the Human Research Protection Office at University of Oklahoma, IRB # 14872. Similarly, patients included from the Atlantic Health System Hospitals were identified retrospectively as women diagnosed with endometrial cancer between May 1999 and October 2009 who underwent surgery and received chemotherapy. The study was approved by their institution’s Internal Review Board. Eligibility criteria included women 18 years or older with any stage endometrial cancer who underwent primary surgical resection followed by chemotherapy and who had medical record data available. Only histologies consistent with either epithelial endometrial carcinoma (endometrioid, papillary serous, clear cell, or mixed histology) or carcinosarcoma were included.
Pathologic evaluation of all tissue specimens was performed by gynecologic pathologists at each institution. Demographic, chemotherapy, and follow-up data were extracted from the research database and hospital records. Accurate surgical stage for each subject was confirmed during data collection for our study according to the FIGO (International Federation of Gynecology and Obstetrics) 2009 criteria (38). Completeness of staging was not captured in data collection.
Adjuvant therapy was administered on an individual basis at the discretion of the treating physician, and consisted of chemotherapy, radiation therapy, hormonal therapy, or some combination of these treatment modalities. Radiation therapy was determined at the discretion of the treating physician and may have included brachytherapy, external beam radiation (EBRT), or a combination of both. At completion of treatment subjects were typically followed at 3-month intervals for the first two years, at six-month intervals for 3 additional years, and yearly thereafter. Standard surveillance at all institutions included physical examination and Pap test for at least 3 years after initial treatment with performance of additional imaging studies and directed biopsies as indicated. All recurrent and progressive disease was histologically and/or radiographically confirmed.
Primary outcome was chemotherapy response. Response was dichotomized for analysis: complete response or partial/refractory response. Complete response was defined as normalization of the CA-125 level with a normal computed tomography (CT) scan post completion of the last cycle of chemotherapy. Partial response was defined as decreased, but elevated post-treatment CA-125 level and/or decreased, but persistent disease on follow-up CT imaging, or if noted as a partial response per the treating physician within the clinical record. Refractory disease was defined as progression of disease during chemotherapy or within 3 months post treatment.
Secondary outcomes were disease specific survival (DSS) and disease-free survival (DFS). DSS was defined as the time from completion of chemotherapy to date of cancer-specific death. Deaths were confirmed using the online social security death index (http://ssdi.rootsweb.ancestry.com/). Survivors were censored at the date of last contact. DFS was defined as the time from chemotherapy completion to date of first disease recurrence or progression.
Statistical Analyses
Power was estimated assuming a chemotherapy response rate of 60% for control group based off data for combination treatment consisting of carboplatin and paclitaxel [39, 40]. This was the most commonly used chemotherapy regimen for endometrial cancer at each institution. A total of 196 subjects (98 per group) would provide our study with 80% power to detect a difference of 20% or greater between treatment group and control group using a two tailed chi-square test with significance defined as p<0.05. Subjects were divided into 2 groups for analysis based on their serum platelet level at time of diagnosis. Subjects with a serum platelet level > 400 × 109/L were categorized as having an elevated platelet level. All others were categorized as normal. Although the National Heart Lung and Blood Institute (NHLBI) defines thrombocytosis as platelet count > 450 × 109/L (http://www.nhlbi.nih.gov/health/dci/Diseases/thrm/thrm_what.html), 400 × 109/L has been shown to be a clinically meaningful cut off in multiple studies involving gynecologic malignancies [5–8].
Association between serum platelet level and variables of interest were assessed by univariate logistic regression. Multivariate logistic regression was conducted to detect whether elevated serum platelet level was independently associated with chemotherapy response while adjusting for possible confounders.
DSS and DFS between group with elevated platelet and group with normal platelet were compared by Kaplan-Meier curve. The hazard ratio for each variable of interest is estimated from univariate Cox proportional hazards model. Multivariate Cox models were fitted to find the hazard ratio between patient with elevated platelet and normal platelet while adjusting for other confounders for DSS and DFS. Statistical software SAS 9.2 was used for data analysis.
Results
The demographic and clinicopathologic characteristics of the 318 included in this study are presented in Table 1. Of the total group, 53 (16.7%) were categorized as having an elevated platelet count (mean 487 ± 98.2). Of those with data available, the majority (n=280, 88%) received carboplatin alone or in combination with paclitaxel. The mean age at diagnosis was 64 and 62 years for the normal and elevated platelet groups respectively. Thirty-five subjects (11%) had histology consistent with carcinosarcoma, 68 (35%) had pure serous or a serous component, 20 (10%) had pure clear cell or a clear cell component, and 11 (5.6%) had both clear cell and serous components. Subjects categorized as having elevated platelets were of higher stage than those categorized as normal. Median follow-up time was 25.6 and 23.1 months for the normal and elevated platelet groups respectively.
Table 1.
| Normal Plt (n=265) |
Elevated Plt (n=53) |
P | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | 0.192 | ||||
| Mean ± Standard deviation | 64.2 ± 11.0 | 61.9 ± 10.0 | |||
| Serum Platelet Level (K/ml) | <0.0001 | ||||
| Mean ± Standard deviation | 272.4 ± 63.4 | 487.2 ± 98.2 | |||
| BMI (Kg/m2) | 0.340 | ||||
| Mean ± Standard deviation | 32.7 ± 9.5 | 31.3 ± 8.5 | |||
| CA-125 (U/ml) | 0.320 | ||||
| Mean ± Standard deviation | 113.0 ± 387 | 159.5 ± 237 | |||
| Race | 0.192 | ||||
| White | 222 | 84.4 | 48 | 92.3 | |
| Black/Other | 41 | 15.6 | 4 | 7.7 | |
| FIGO Stage | 0.044 | ||||
| I–II | 132 | 73.7 | 26 | 57.8 | |
| III–IV | 47 | 26.3 | 19 | 42.2 | |
| Grade | 0.680 | ||||
| 1 | 43 | 18.0 | 7 | 14.6 | |
| 2–3 | 196 | 82.0 | 41 | 85.4 | |
| Radiation Treatment | 0.879 | ||||
| Yes | 119 | 46.1 | 23 | 44.2 | |
| No | 139 | 53.9 | 29 | 55.8 | |
| LVSI | 0.056 | ||||
| Present | 181 | 68.8 | 40 | 83.3 | |
| Absent | 82 | 31.2 | 8 | 16.7 | |
| Histology | 0.273 | ||||
| Endometrioid | 92 | 35.0 | 23 | 43.4 | |
| Non-Endometrioid* | 173 | 65.0 | 30 | 56.6 | |
Totals of each category of discrete variables may not equal 318 due to missing data. For continuous variables, there is no missing data for age or platelet level. For BMI, N=8 missing data, and for CA-125 N=88 for missing data.
Discrete variables were analyzed with Fisher’s exact contingency tables, continuous variables were analyzed with sample t-test
includes carcinosarcoma
During the observation period, there were 125 deaths, 76 recurrences, and 48 disease progressions. In regards to chemotherapy, 55 subjects were categorized as having a partial/refractory response. An elevated platelet count was found to be associated with a lower chemotherapy response in univariate analysis (p< 0.01) (Table 2). Stage, grade, radiation therapy and CA-125 level were also found to be significantly associated with chemotherapy response. After controlling for all variables with p < 0.05 on univariate analysis, multivariate analysis did not confirm an independent association between a platelet count > 400 × 109/L and lower chemotherapy response rates (OR 2.59; 95% CI 0.96, 6.99; p =0.06) (Table 3).
Table 2.
Univariate Analysis of Clinicopathologic Factors and Chemotherapy Response
| HR | 95% CI | P | |
|---|---|---|---|
| Patient Characteristics | |||
| Age | 1.00 | 0.97, 1.02 | 0.75 |
| BMI | 0.98 | 0.95, 1.01 | 0.2 |
| Race* | 0.83 | 0.37, 1.87 | 0.65 |
| Radiation therapy± | 0.49 | 0.27, 0.87 | 0.02 |
| Serum Platelet Level | 2.80 | 1.46, 5.38 | <0.01 |
| Serum CA-125 Level† | 1.01 | 1.00,1.03 | 0.03 |
| Tumor Characteristics | |||
| FIGO Stage | 2.48 | 1.32, 4.65 | <0.01 |
| Grade | 4.88 | 1.47, 16.13 | 0.01 |
| LVSI | 1.81 | 0.93, 3.51 | 0.08 |
| Histology | 1.11 | 0.62, 1.97 | 0.73 |
white versus black/other
Received radiation versus none (includes EBRT, vaginal brachytherapy, or combination of both)
Analyzed as continuous variable
Table 3.
Multivariate Analysis of Serum Platelet Level and Chemotherapy Response
| OR | 95% CI | P | |
|---|---|---|---|
| Patient Characteristics | |||
| Radiation Therapy† | 0.23 | 0.09, 0.57 | < 0.01 |
| Serum Platelet Level | 2.59 | 0.96, 6.99 | 0.06 |
| Serum CA-125 Level* | 1.00 | 1.00, 1.00 | 0.29 |
| Tumor Characteristics | |||
| FIGO Stage | 1.89 | 0.72, 4.98 | 0.19 |
| Grade | 4.67 | 1.01, 21.74 | 0.05 |
Only variables significant in univariate analysis included
Analyzed as continuous variable
Includes EBRT, vaginal brachytherapy, or a combination of both
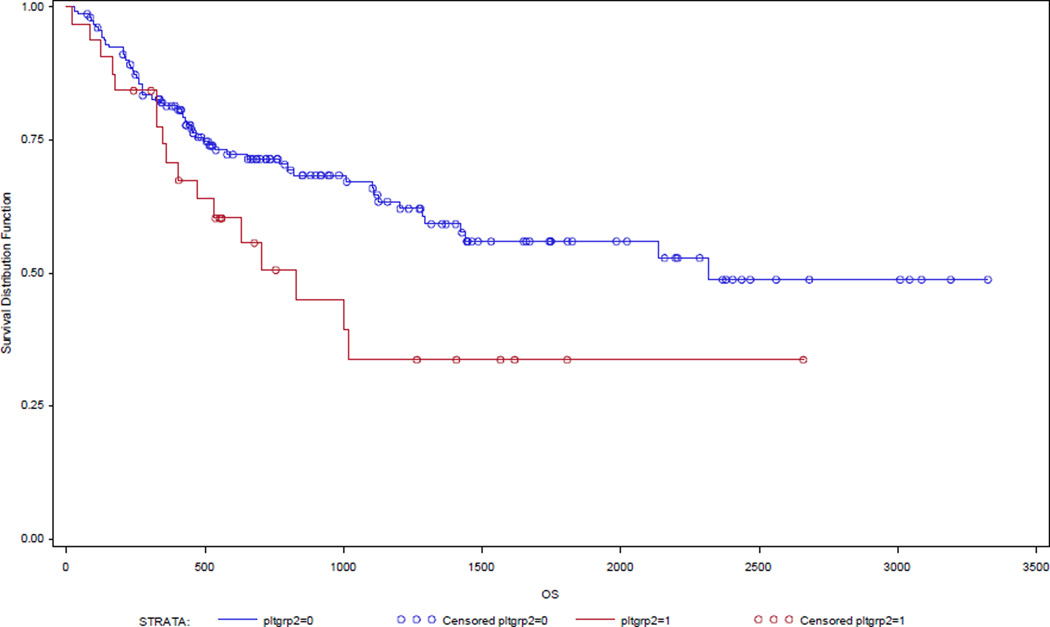
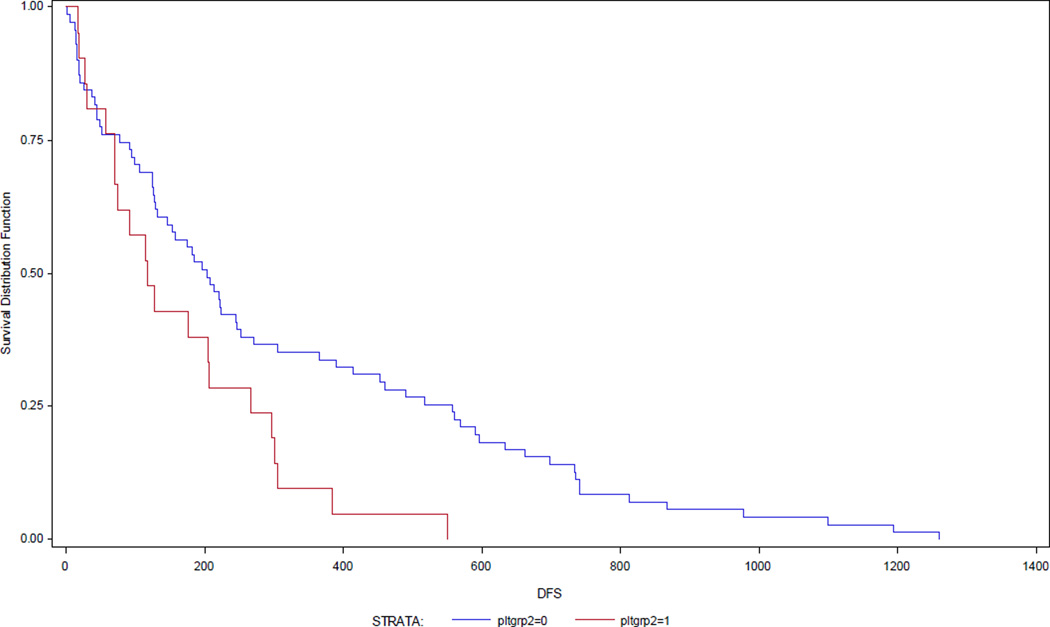
Survival analyses revealed elevated serum platelet levels to be correlated with decreased disease specific (HR 1.55; 95% CI 1.01, 2.38; p= 0.04) and disease free survival (HR 2.02; 95% CI 1.31, 3.13; p< 0.01) (Table 4). Median survival time for the normal platelet group was 74.5 months compared to 33.5 months for the elevated platelet group. Stage, grade, lymphovascular space invasion (LVSI), CA-125 level, race, and radiation therapy were also associated with a statistical improvement of DSS and DFS.
Table 4.
Univariate Analysis of Clinicopathologic Factors and Survival
| Disease Free Survival | Disease Specific Survival | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Patient Characteristics | ||||||
| Age* | 1.02 | 1.00, 1.04 | 0.04 | 1.02 | 1.00, 1.04 | 0.04 |
| BMI * | 1.00 | 0.99, 1.03 | 0.66 | 0.99 | 0.97, 1.01 | 0.27 |
| Race† | 1.09 | 0.66, 1.80 | 0.73 | 1.82 | 1.16, 2.84 | 0.01 |
| Radiation therapyµ | 0.45 | 0.31, 0.66 | <0.001 | 0.46 | 0.32, 0.67 | <0.001 |
| Serum platelet level | 2.02 | 1.31, 3.13 | <0.01 | 1.55 | 1.01, 2.38 | 0.04 |
| Serum CA-125 level* | 1.00 | 1.00, 1.00 | <0.001 | 1.00 | 1.00, 1.00 | <0.01 |
| Tumor Characteristics | ||||||
| FIGO Stage | 2.67 | 1.80, 3.98 | <0.001 | 2.13 | 1.43, 3.19 | <0.001 |
| Grade | 5.62 | 2.28, 13.89 | <0.001 | 5.75 | 2.34, 4.09 | <0.001 |
| LVSI | 2.49 | 1.54, 4.05 | <0.001 | 2.48 | 1.55, 4.00 | <0.001 |
| Histology | 1.34 | 0.90, 2.00 | 0.15 | 1.34 | 0.91, 1.99 | 0.15 |
White versus Black/Other
Analyzed as continuous variable
Includes EBRT, vaginal brachytherapy, or a combination of both
Covariates with a p <0.05 were included in multivariate analysis for DFS and DSS After adjusting for confounders, an elevated platelet count was independently associated with decreased DFS (HR 2.24; 95% CI 1.26, 3.98; p<0.01), but not DSS (HR 1.03, 95%CI 0.56, 1.88, p=0.93).
Discussion
To the best of our knowledge, this is the first clinical study to primarily evaluate the influence of platelet count on chemotherapy response rates in a gynecologic malignancy. Our results demonstrate that endometrial cancer patients with an elevated platelet count > 400 × 109/L have a higher risk for recurrence than patients with a platelet count within normal range. There was a trend towards lower chemotherapy response rates as well. This result may have reached significance had we been able to achieve our target goal of 96 patients per group.
Our findings could have important clinical implications for the treatment of endometrial cancer. Although the majority of patients will have a good prognosis, those patients who receive chemotherapy are considered a separate “higher risk” group with a much lower overall 5-year survival. Tailoring chemotherapy to improve response rates and survival within this cohort is important. These higher risk endometrial cancer patients who also have elevated platelet levels may require more aggressive chemotherapy treatment, combination treatment with anti-platelet therapy, and/or closer follow-up. However, recommendations for modifications to current treatment strategies are beyond the scope of this study.
How elevated serum platelets may influence cancer prognosis is not fully understood. Thrombocytosis could represent a more biologically aggressive tumor type or the platelets could be directly involved in regulation of molecular pathways contributing to chemotherapy resistance. Since platelets have been shown to promote angiogenesis and shield tumors from immune responses, it is reasonable to think they may contribute to chemotherapy response rates. Additionally platelets contribute to the process of basement membranes by releasing proteinases and via proteolytic activity of platelet derived growth factors (PDGF) and thus may contribute to the metastatic potential of tumors.
Few clinical studies exist in the literature addressing the effect of serum platelet levels on chemotherapy response rates. Borges et al retrospectively evaluated approximately 1000 lung cancer patients and demonstrated that an elevated platelet count > 440 × 103/ mmm3 was associated with decreased response rates (RR 0.6; 95% CI 0.43–0.92, p=0.02) [37]. More recently, a phase I study of an integrin α-2 inhibitor showed stable disease responses in a wide variety of heavily pretreated cancers [41]. As integrins are necessary for platelet aggregation and clot formation the authors are investigating effects of this drug on platelet function. This study did not include a significant portion of patients with gynecologic malignancies. Twenty-seven percent were colorectal cancers, 21.6% were “other” cancers, and 8.1% were renal cell cancers.
There is also new evidence within murine models suggesting that anti-platelet therapy may enhance chemotherapy response rates. Demers et al used a mammary carcinoma murine model to show that platelet depletion enhanced bleeding specifically at tumor sites; thus allowing improved rates of intratumoral drug accumulation and slower growth. The authors suggest that selective induction of tumor hemorrhage by targeting platelet function may facilitate the delivery of chemotherapeutic agents to tumors [42]. Another study showed that platelet depletion was associated with decreased tumor growth and that higher platelet counts were associated with worse survival and resistance to docetaxel-induced apoptosis in a murine model of ovarian cancer [43]. In vitro work within the same study demonstrated that ovarian cancer cell proliferation and migration was increased when exposed to platelet-enriched media. These findings were corroborated by recent work from another group who created an ovarian cancer model using murine xenografts and demonstrated that treatment with anti-platelet therapy decreased overall tumor volume [44].
It is also well known that there exists an intricate interplay between platelets and the actual molecular pathways that drive angiogenesis, one of the cardinal processes in the development of cancer. Platelets can induce angiogenesis in vivo and are also a rich source of proangiogenic factors, such as vascular endothelial growth factor (VEGF). They also store and release angiogenesis inhibitors and thus are key regulators of the total process. Additionally, researchers demonstrated a correlation between inflammation and interleukin- 6 (IL6) dependent release of VEGF from platelets among breast and prostate cancer patients [45]. Previously mentioned work within an ovarian cancer mouse model also demonstrated that treatment with an anti-IL6 antibody reduced platelet serum levels while enhancing response to paclitaxel treatment [43].
Clinical studies focusing on anti-angiogenic therapies are already being actively pursued among many gynecologic malignancies. Specifically, bevacizumab, a humanized anti-VEGF antibody, has been shown to have effectiveness in ovarian, cervical, and endometrial cancers and is frequently prescribed clinically [46–53]. More recently, brivanib, a dual tyrosine kinase inhibitor of VEGF and FGFR (Fibroblast growth factor receptor) signaling, has shown promise for the treatment of recurrent or persistent endometrial cancer [54]. Perhaps, routine upfront treatment with anti-angiogenic drugs and/or combination therapy with anti-platelet inhibitors could further increase response rates among endometrial cancer patients, especially those with elevated serum platelets.
In general, our survival results are consistent with previously published literature [5–10]. However, we did not find a significant correlation between platelet count and overall survival, likely secondary to lack of power due to small sample size despite a multi-institutional approach.
Due to the retrospective nature of the study, assessment of chemotherapy response was not uniform between all patients and varied over the 10 year study period. Since we dichotomized chemotherapy response for analysis, it is less likely that mis-interpretation and thus misclassification of response occurred. Similarly, we did not account for variation of treatment strategies and modalities over the study. Our target sample may have been biased as subjects without medical record data were excluded. Additionally, we only had information regarding the exact type of chemotherapy received on 283 subjects (82.6%). Influence of platelets on response may vary according to type of chemotherapy given. Our study analyzed all patients as one group and were not separated out according to tumor histology. Differences may exist between carcinosarcomas, serous, clear cell, and endometrioid histologies.
Overall, we found increased risk for recurrence among endometrial cancer patients with an elevated serum platelet count > 400 × 109/L. In addition, patients with higher platelet counts may have reduced chemotherapy response rates. Our results suggest that these patients should potentially be targeted in future clinical trials as they may benefit most from new therapeutic strategies. Additional investigation into the biologic mechanisms behind platelet influence on chemotherapy response is also warranted. Prospective studies are needed to confirm our research findings.
Figure 1.
Cancer specific survival according to platelet group.
Log-rank test p=0.03
Figure 2.
Decreased Disease-free survival in patients with thrombocytosis at initial diagnosis.
Log-rank p= 0.02
Table 5.
Multivariate Analysis of Serum Platelet Level and Survival †
| Disease Free Survival | Disease Specific Survival | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Patient Characteristics | ||||||
| Age | 1.01 | 0.99, 1.03 | 0.2 | 1.01 | 0.98, 1.03 | 0.61 |
| Race | * | * | * | 0.8 | 0.38, 1.68 | 0.56 |
| Radiation therapyµ | 0.19 | 0.11, 0.34 | <0.001 | 0.33 | 0.19, 0.6 | <0.001 |
| Serum platelet level | 2.24 | 1.26, 3.98 | <0.01 | 1.03 | 0.56, 1.88 | 0.93 |
| Serum CA-125 level | 1.00 | 1.00, 1.00 | 0.001 | 1.001 | 1.000, 1.001 | 0.014 |
| Tumor Characteristics | ||||||
| Stage | 1.54 | 0.89, 2.65 | 0.12 | 1.35 | 0.76, 2.42 | 0.31 |
| Grade | 5.13 | 1.59, 16.67 | <0.01 | 8.29 | 2.01, 4.25 | <0.01 |
| LVSI | 1.72 | 0.78, 3.80 | 0.18 | 1.27 | 0.63, 2.56 | 0.5 |
Only those variables significant in univariate analysis are included
no data presented because not included in analysis.
Includes EBRT, vaginal brachytherapy, or a combination of both
Acknowledgements
The authors wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Conflict of Interest Statement:
None of the authors has a conflict of interest to disclose in regards to this study and its findings.
References
- 1.Trousseau A. Clinique Medicale de l’Hotel-Dieu de Paris. 2nd ed. Paris France: JB Bailliere & Fils; 1865. Phlegmasia alba dolens; pp. 654–712. [Google Scholar]
- 2.Sorensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis of pulmonary embolism. NEJM. 1998;338(17):1169–1173. doi: 10.1056/NEJM199804233381701. [DOI] [PubMed] [Google Scholar]
- 3.Tranum BL, Haut A. Thrombocytosis: platelet kinetics in neoplasia. J Lab Clin Med. 1974;84(5):615–619. [PubMed] [Google Scholar]
- 4.Naschitz JE, Heshurum D, Eldar S, Lev LM. Diagnosis of cancer-associated vascular disorders. Cancer. 1996;77:1759–1767. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1759::AID-CNCR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Scholz HS, Petru E, Gucer F, Haas J, Tamussino K, Winter R. Preoperative thrombocytosis is an independent prognostic factor in stage III and IV endometrial cancer. Anticancer Res. 2000;20(5C):3983–3985. [PubMed] [Google Scholar]
- 6.Ayhan A, Bozdag G, Taskiran C, Gultekin M, Yuce K, Kucukali T. The value of preoperative platelet count in the prediction of cervical involvement and poor prognostic variables in patients with endometrial carcinoma. Gynecol oncol. 2006;103(3):902–905. doi: 10.1016/j.ygyno.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez E, Lavine M, Dunton CJ, Gracely E, Parke J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992;69(12):2975–2977. doi: 10.1002/1097-0142(19920615)69:12<2975::aid-cncr2820691218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Zeimet AG, Marth C, Muller-Holzner E, Daxenbichler G, Dapunt O. Significance of thrombocytosis in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1994;170(2):549–554. doi: 10.1016/s0002-9378(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda M, Furukawa H, Imamura H, Shimizu J, Masutani S, Tatsuta M, Satomi T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 10.Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, Takeda A, Gunji Y, Ochiai T. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004;198(5):737–741. doi: 10.1016/j.jamcollsurg.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Gastl G, Plante M, Finstad CL, Wong Gy, Federici MG, Bander NH, Rubin SC. High IL-6 levels in ascetic fluid correlate with reactive thrombocytosis in patients with epithelial ovarian cancer. Brit J Haematol. 1993;83:433–441. doi: 10.1111/j.1365-2141.1993.tb04668.x. [DOI] [PubMed] [Google Scholar]
- 12.Karpatkin S, Pearlstein E, Ambrigio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1998;81(4):1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpatkin S, Ambrogio C, Pearlstein E. The role of tumor-induced platelet aggregation, platelet adhesion and adhesive proteins in tumor metastasis. Prog Clin Biol Res. 1988;283:585–606. [PubMed] [Google Scholar]
- 14.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA. 1968;61(1):46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3(2):99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- 16.Erpenbeck L, Nieswandt B, Schon M, Pozgajova M, Schon MP. Inhibition of platelet GPIbalpha and promotion of melanoma metastasis. J Invest Dermatl. 2010;130(2):576–586. doi: 10.1038/jid.2009.278. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 18.Amirkhosravi A, Mousa SA, Amaya M, Blaydes S, Desai H, Meyer T, Francis JL. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost. 2003;90(3):549–554. doi: 10.1160/TH03-02-0102. [DOI] [PubMed] [Google Scholar]
- 19.Ho-Tin-Noé B, George T, Cifuni SM, Duershmied D, Wagner DD. Platelet granule secretion continuously prevents intratumo hemorrhage. Cancer Res. 2008;68(16):6851–6858. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho-Tin-Noé B, George T, Wagner DD. Platelets: guardians of tumor vasculature. Cancer Res. 2009;69(14):5623–5626. doi: 10.1158/0008-5472.CAN-09-1370. [DOI] [PubMed] [Google Scholar]
- 21.Decker S, van Halen F, Vischer P. Adhesion of osteosarcoma cells to the 70-kDa core region of thrombospondin-1 is mediated by the α4β1 integrin. Biochem Biophys Res Comm. 2002;293(1):86–92. doi: 10.1016/S0006-291X(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg V, Roberts DD. Glycoconjugates and cell adhesion: the adhesive proteins laminin, thrombospondin and von Willebrand’s factor bind specifically to sulfated glycolipids. Biochimie. 1988;70(11):1651–1659. doi: 10.1016/0300-9084(88)90300-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang TN, Qian XH, Granick MS, Solomon MP, Rothman VL, Berger DH, Tuszynski GP. Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res. 1996;63(1):39–43. doi: 10.1006/jsre.1996.0219. [DOI] [PubMed] [Google Scholar]
- 24.Erpenbeck L, Schon M. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamussino KF, Gucer F, Reich O, Moser F, Petru E, Scholz HS. Pretreatment hemoglobin, platelet count, and prognosis in endometrial carcinoma. Int J Gynecol Cancer. 2001;11:236–240. doi: 10.1046/j.1525-1438.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 26.Scholz HS, Petru E, Gucer F, Haas J, Tamussino K, Winter R. Preoperative thrombocytosis is an independent prognostic factor in stage III and IV endometrial cancer. Anticancer Res. 2000;20:3983–3985. [PubMed] [Google Scholar]
- 27.Gucer F, Moser F, Tamussino K, Reich O, Haas J, Arikan G, Petru E, Winter R. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol Oncol. 1998;70:210–214. doi: 10.1006/gyno.1998.5078. [DOI] [PubMed] [Google Scholar]
- 28.Hefler L, Mayerhofer K, Leibman B, Obemair A, Reinthaller A, Kainz C, et al. Tumor anemia and thrombocytosis in patients with vulvar cancer. Tumor Biol. 2000;21:309–314. doi: 10.1159/000030136. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez E, Donohue KA, Anderson LL, Heller PB, Stehman FB. The significance of thrombocytosis in women with vulvar carcinoma. Gynecol Oncol. 2000;78:137–142. doi: 10.1006/gyno.2000.5838. [DOI] [PubMed] [Google Scholar]
- 30.Li AJ, Madden AC, Cass I, Leuchter RS, Lagasse LD, Karlan BY. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004;92(1):211–214. doi: 10.1016/j.ygyno.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Lavie O, Comerci G, Daras V, Bolger BS, Lopes A, Monaghan JM. Thrombocytosis in women with vulvar carcinoma. Gynecol Oncol. 1999;72(1):82–86. doi: 10.1006/gyno.1998.5225. [DOI] [PubMed] [Google Scholar]
- 32.Nather A, Mayerhofer K, Grimm C, Hefler L, Leodolter S, Obermair A, Joura EA. Thrombocytosis and anaemia in women with recurrent ovarian cancer prior to a second-line chemotherapy. Anticancer Res. 2003;23(3C):2991–2994. [PubMed] [Google Scholar]
- 33.Lerner DL, Walsh CS, Cass I, Karlan BY, Li AJ. The prognostic significance of thrombocytosis in uterine papillary serous carcinomas. Gynecol Oncol. 2007;104:91–94. doi: 10.1016/j.ygyno.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Menczer J, Schejter E, Geva D, Ginath S, Zakuth H. Ovarian carcinoma associated thrombocytosis. Correlation with prognostic factors and with survival. Eur J Gynaecol Oncol. 1998;19(1):82–84. [PubMed] [Google Scholar]
- 35.Bozkurt N, Yuce K, Basaran M, Kose F, Ayhan A. Correlation of platelet count with second-look laparotomy results and disease progression in patients with advanced epithelial ovarian cancer. Obstet Gynecol. 2004;103(1):82–85. doi: 10.1097/01.AOG.0000102703.21556.0B. [DOI] [PubMed] [Google Scholar]
- 36.Metindir J, Dilek GB. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol. 2009;135:125–129. doi: 10.1007/s00432-008-0430-2. [DOI] [PubMed] [Google Scholar]
- 37.Borges M, Sculier JP, Paesmans M, Richez M, Bureau G, Dabouis G, Lecomte J, Michel J, Van Cutsem O, Schmerber J, Giner V, Berchier MC, Sergysels R, Mommen P, Klastersky J. Prognostic factors for response to chemotherapy containing platinum derivatives in patients with unresectable non-small cell lung cancer (NSCLC) Lung Cancer. 1996;16:21–33. doi: 10.1016/s0169-5002(96)00609-5. [DOI] [PubMed] [Google Scholar]
- 38.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105(2):109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Hoskins PJ, Swenerton KD, Pike JA, Wong F, Lim P, Acuino-Parsons C, Lee N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase II study. JCO. 2001;19(20):4048–4053. doi: 10.1200/JCO.2001.19.20.4048. [DOI] [PubMed] [Google Scholar]
- 40.Price FV, Edwards RP, Kelley JL, Kunschner AJ, Hart LA. A trial of outpatient paclitaxel and carboplatin for advanced, recurrent, and histologic high-risk endometrial carcinoma: preliminary report. Semin Oncol. 1997;24:S15-78–S15-82. [PubMed] [Google Scholar]
- 41.Mita M, Kelly KR, Mita A, et al. Phase I study of E7820, an oral inhibitor of integrin α-2 expression with antiangiogenic properties, in patients with advanced malignancies. Clin Cancer Res. 2011;17:193–200. doi: 10.1158/1078-0432.CCR-10-0010. [DOI] [PubMed] [Google Scholar]
- 42.Demers M, Ho-Tin-Noe, Schatzberg, et al. Increased efficicacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-2038. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone RL, Nick AM, McNeisch IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. NEJM. 2012 Feb 16;366(7):610. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaun L, Lui X. Platelets are associated with xenograft tumor growth and the clinical malignancy of ovarian cancer through an angiogenesis-dependent mechanism. Mol Med Rep. 2014 Dec 11; doi: 10.3892/mmr.2014.3082. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caine GJ, Lip GY, Blann AD. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: further evidence for a role of platelets in tumour angiogenesis. Ann Med. 2004;36(4):273–277. doi: 10.1080/07853890410026098. PMID:15224653[PubMed - indexed for MEDLINE] [DOI] [PubMed] [Google Scholar]
- 46.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D. Ray-Coquard I Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014 May 1;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. Epub 2014 Mar 17.PMID:24637997[PubMed - indexed for MEDLINE] [DOI] [PubMed] [Google Scholar]
- 47.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, McGuire W. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007 Nov 20;25(33):5180-6. Erratum. J Clin Oncol. 2008 Apr 1;26(10):1773. doi: 10.1200/JCO.2007.12.0782. PMID:18024865[PubMed - indexed for MEDLINE] /pubmed/18024865. [DOI] [PubMed] [Google Scholar]
- 48.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007 Nov 20;25(33):5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 49.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D, Scudder S, Morgan R, Chen H, Lenz HJ, Oza AM. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008 Jan 1;26(1):76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 50.Tewari KS1, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014 Feb 20;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schefter T, Winter K, Kwon JS, Stuhr K, Balaraj K, Yaremko BP, Small W, Jr, Sause W, Gaffney D Radiation Therapy Oncology Group (RTOG) RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys. 2014 Jan 1;88(1):101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Simpkins F, Drake R, Escobar PF, Nutter B, Rasool N, Rose PG. A phase II trial of paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma (EMCA) Gynecol Oncol. 2014 Dec 6;(14):01559-5. doi: 10.1016/j.ygyno.2014.12.004. pii: S0090-8258 [Epub ahead of print]PMID:25485782[PubMed - as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- 53.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, Rotmensch J, Barnes MN, Hanjani P, Leslie KK. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. 2011 Jun 1;29(16):2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell MA, Sill MW, Goodfellow PJ, Benbrook DM, Lankes HA, Leslie KK, Jeske Y, Mannel RS, Spillman MA, Lee PS, Hoffman JS, McMeekin DS, Pollock PM. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014 Oct;135(1):38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]