Abstract
The impact of early donor cell chimerism on outcomes of T-replete reduced-intensity conditioning (RIC) hematopoietic stem cell transplantation (HSCT) is ill-defined. We evaluated day 30 (D30) and 100 (D100) total donor cell chimerism after RIC HSCT undertaken between 2002 and 2010 at our institution, excluding patients who died or relapsed before D30. When available, donor T-cell chimerism was also assessed. The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS), relapse and non-relapse mortality (NRM). 688 patients with hematologic malignancies (48% myeloid; 52% lymphoid) and a median age of 57 years (range, 18-74) undergoing RIC HSCT with T-replete donor grafts (97% peripheral blood; 92% HLA-matched) and median follow-up of 58.2 months (range, 12.6-120.7) were evaluated. In multivariable analysis total donor cell and T-cell chimerism at D30 and D100 each predicted RIC HSCT outcomes, with D100 total donor cell chimerism most predictive. D100 total donor cell chimerism <90% was associated with increased relapse (HR 2.54, 95% CI 1.83-3.51, p<0.0001), impaired PFS (HR 2.01, 95% CI 1.53-2.65, p<0.0001) and worse OS (1.50, 95% CI 1.11-2.04, p=0.009), but not NRM (HR 0.76; 95% CI 0.44-2.27, p=0.33). There was no additional utility of incorporating sustained D30-D100 total donor cell chimerism, or T-cell chimerism. Low donor chimerism early after RIC HSCT is an independent risk factor for relapse and impaired survival. Donor chimerism assessment early after RIC HSCT can prognosticate for long-term outcomes and help identify high-risk patient cohorts that may benefit from additional therapeutic interventions.
Keywords: Allogeneic transplantation, Reduced-intensity, Chimerism
Introduction
Reduced-intensity conditioning (RIC) allogeneic hematopoietic stem cell transplantation (HSCT) is critically dependent on the immunologic graft-versus-tumor (GVT) response for its curative potential. In RIC HSCT, T-cell depletion (TCD) with in-vivo ATG-based conditioning appears associated with increased relapse and impaired survival.1 In T-replete RIC HSCT early donor engraftment and immune reconstitution kinetics remain variable and may predict for the likelihood of GVT response and long-term survival.
The prognostic impact of donor chimerism early after T-replete RIC HSCT is ill-defined. Data on total donor cell chimerism are especially scanty and discordant, as most studies have focused on donor T-cell chimerism. Two small studies found no association of low total donor cell chimerism with outcomes2,3, while other small studies, with varying chimerism thresholds and assessment time-points, described an association with 4-week relapse risk4 or impaired survival5,6. Several small studies have also described an association of donor T-cell chimerism with impaired RIC HSCT outcomes.7-10 However, in the largest study (n=322), lack of full donor T-cell chimerism was a predictor of relapse but not progression-free survival (PFS).11 Additional limitations are that studies often included recipients of TCD (where increased mixed chimerism has been reported), and did not fully adjust for disease relapse likelihood (e.g., Disease Risk Index (DRI)12) or other measures of early engraftment (e.g., absolute lymphocyte count (ALC)13,14, white blood count (WBC) risk score15) which can also affect HSCT outcomes. The comparative utility of total donor cell vs. T-cell chimerism is also ill-defined. Early donor cell chimerism has therefore not been incorporated into RIC HSCT prognostic models.
We undertook a retrospective analysis of adult T-replete RIC HSCT at our institution between 2002 and 2010 in order to comprehensively assess the impact of early donor chimerism on overall survival (OS). We sought to evaluate: 1) the impact of day 30 (D30) and day 100 (D100) total donor cell chimerism; 2) the additional utility of ‘sustained’ total donor cell chimerism between D30-D100; and 3) the additional utility of D30 and D100 donor T-cell chimerism.
Materials and Methods
Patients
At our institution RIC HSCT is offered to adults with advanced and/or aggressive hematologic malignancies unsuitable for myeloablative transplantation due to disease, age or comorbidities. Pre-transplantation eligibility includes ECOG performance status ≤2, adequate organ function (pulmonary, cardiac, renal, hepatic), and no active uncontrolled infections. Recipients are routinely consented to IRB approved data collection.
T-replete RIC HSCT in 688 adult hematologic malignancy patients between 2002 and 2010 are included in the analysis. Patients who relapsed or died prior to the date of D30 or D100 chimerism were excluded from the D30 or D100 analysis respectively.
HSCT regimen
Recipients received RIC fludarabine (30 mg/m2 × 4 days) and busulfan (1.6 mg/kg IV once- (Bu1) or twice-daily (Bu2) × 4 days) followed by T-replete bone marrow or peripheral blood stem cell (BM, PBSC) grafts. GVHD prophylaxis routinely comprised methotrexate on days 1, 3, 6, ±11, with tacrolimus ± sirolimus starting day -3 and tapered through weeks 9-26 in the absence of graft-versus-host disease. All patients received 12 months of infection prophylaxis, surveillance and pre-emptive therapy. Donor lymphocyte infusions (DLI) were at the discretion of the treating physician, but in the absence of relapse were used only in 2 patients to treat falling donor chimerism.
Chimerism Analysis
Post-transplantation D30 (range, 20-50) and 100 (range, 80-120) chimerism was determined using recipient PB or BM samples collected in EDTA. “Total donor cell” chimerism was performed on buffy coat leukocytes. “T-cell” chimerism was on Ficoll Hypaque separated lymphocytes from which purified CD3+ T-cells were isolated using immunomagnetic beads (Stem Cell Technologies Inc.). Pretransplantation PB samples were used to determine the donor and recipient genotypes based on 9 CODIS Short Tandem Repeat loci using the Applied Biosystems Profiler Plus® kit with the ABI 3130 capillary genetic analyzer to determine the alleles at each locus. Informative alleles unique to either donor or recipient were used to calculate % donor chimerism at each locus using the median peak intensity of amplicons attributable to donor divided by the sum of all amplicons at that locus. In cases where there were only unique recipient amplicons, % donor chimerism was calculated as (100 – % recipient chimerism). 95% confidence interval for chimerism was ±5%.
Statistical Methods
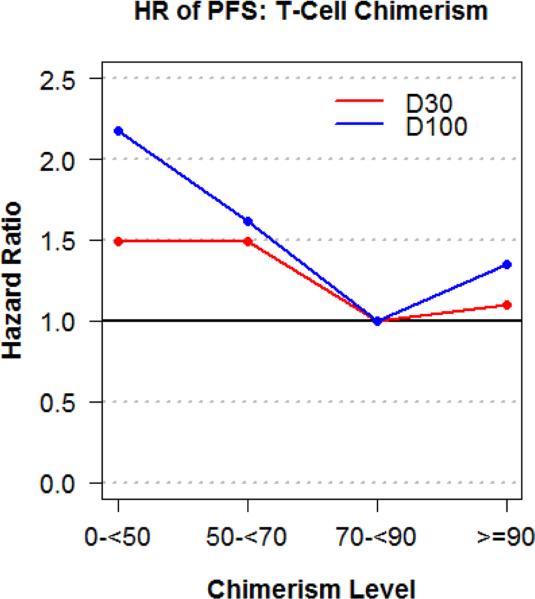
Patient baseline characteristics were reported descriptively. OS and PFS were calculated with the Kaplan-Meier method. OS, PFS, cumulative incidence of relapse and NRM were defined previously.12,15 The log-rank test was used for comparisons of Kaplan-Meier curves. The difference between cumulative incidence curves in the presence of a competing risk was tested with the Gray method.16 Cumulative incidence of graft-versus-host disease (GVHD) was also calculated in the competing risks framework reflecting death or relapse without developing GVHD as a competing event. Potential prognostic factors for OS, PFS, relapse, and NRM were examined in the proportional hazards model as well as in the competing risks regression model.17 The models included pre-transplantation (age, diagnosis, DRI, donor-recipient HLA-match, sex-match, ABO-match, CMV serostatus, prior allotransplant), transplantation (year, conditioning intensity, sirolimus use), and post-transplantation (WBC risk score based on 1- and 3-month WBC values, 1- and 3-month ALC) risk factors (Supplementary Table 1). The proportional hazards assumption for each variable of interest was tested, and interaction terms were examined. The linearity assumption for continuous variables was examined by the use of restricted cubic spline estimates of the relationship between the continuous variable and log relative hazard18, and the cutoff points of these variables determined by the change of the log relative hazards. For total donor cell and T-cell chimerism, this approach is supplemented with calculation of hazard ratios of PFS by intervals of 10 and 20 along with the consideration of the number of events in each interval. From these approaches, the proposed cutoff value for total donor cell chimerism was <90% (low) vs. ≥90%, and <70% (low) vs. ≥70% for donor T-cell chimerism (Figure 1C and 1D). Although the linearity assumption for total donor cell and T-cell chimerism values was not violated, we propose these cutoff values to facilitate the practical use of chimerism data. We compared predictive capacities via the concordance index (C-index).19,20 All P values are 2-sided with a significance level of .05. All calculations were performed with SAS 9.3 (SAS Institute), and R 2.14.1 (the Comprehensive R Archive Network project).
Figure 1C.
Change in log hazard ratio of PFS for D30 and D100 total donor cell chimerism cutoffs
Figure 1D.
Change in log hazard ratio of PFS for D30 and D100 donor T-cell chimerism cutoffs
Results
Patients
Characteristics of the 688 patients are shown in Table 1. Diagnoses were 52% lymphoid and 48% myeloid malignancies. Seventeen percent had undergone prior allogeneic HSCT. Donors were 64% unrelated, 92% 8/8 HLA-matched, and 97% provided PBSC grafts. Median recipient age was 57 years (range, 18-74), with median donor age of 39 years (range, 12-73). Median follow-up in survivors was 58.2 months (range, 12.6-120.7).
Table 1.
Baseline patient, disease and donor characteristics of the T-replete RIC HSCT cohort
| N | % | |
|---|---|---|
| Total | 688 | 100 |
| Patient Age, median (range) | 57 (18,74) | |
| Patient Sex | ||
| Male | 437 | 63.5 |
| Female | 251 | 36.5 |
| Donor Age, median (range) | 39 (12, 73) | |
| Donor Sex | ||
| Male | 400 | 58.1 |
| Female | 288 | 41.9 |
| Patient-Donor Sex Match | ||
| Female-Female | 117 | 17 |
| Female-Male | 134 | 19.5 |
| Male-Female | 171 | 24.9 |
| Male-Male | 266 | 38.7 |
| Diagnosis | ||
| AML | 193 | 28.1 |
| CLL, SLL, PLL | 113 | 16.4 |
| CML | 22 | 3.2 |
| Hodgkin Disease | 52 | 7.6 |
| MM/PCD | 32 | 4.7 |
| ALL | 19 | 2.8 |
| MDS | 94 | 13.7 |
| MPD | 21 | 3.1 |
| Mixed MDS/MPD | 2 | 0.3 |
| NHL | 140 | 20.3 |
| Donor HLA-matching | ||
| 8/8 Matched unrelated | 394 | 57.3 |
| 8/8 Matched related | 242 | 35.2 |
| 7/8 Mismatch unrelated | 49 | 7.1 |
| 7/8 Mismatch related | 3 | 0.4 |
| Stem Cell Source | ||
| Bone marrow | 20 | 2.9 |
| Peripheral blood | 668 | 97.1 |
| Disease Risk Index | ||
| Low | 159 | 23.1 |
| Intermediate | 362 | 52.6 |
| High | 155 | 22.5 |
| Very high | 12 | 1.7 |
| Patient-Donor CMV status | 230 | 33.4 |
| Negative | 230 | 33.4 |
| Any Positive | 458 | 66.6 |
| Acute GVHD prophylaxis | ||
| CNI+Sirolimus based | 483 | 70.2 |
| CNI+Non-Sirolimus based | 193 | 27.9 |
| Other | 12 | 1.7 |
| Prior allogeneic transplant | 114 | 16.6 |
| Conditioning Regimen Intensity | ||
| Bu1 | 559 | 81.3 |
| Bu2 | 129 | 18.8 |
| Year of Transplantation, range | 2002-2010 | |
AML: acute myeloid leukemia, ALL: acute lymphoid leukemia, CLL/SLL/PLL: chronic lymphocytic leukemia/small lymphocytic leukemia/pro-lymphocytic leukemia; CML: chronic myeloid leukemia, MM/PCD: multiple myeloma/plasma cell dyscrasia, MDS: myelodysplastic syndromes, MPD: myeloproliferative diseases, NHL: non-Hodgkin lymphoma, Bu1: once-daily busulfan RIC, Bu2: twice-daily busulfan RIC, CNI: calcineurin inhibitor
Total Donor Cell Chimerism
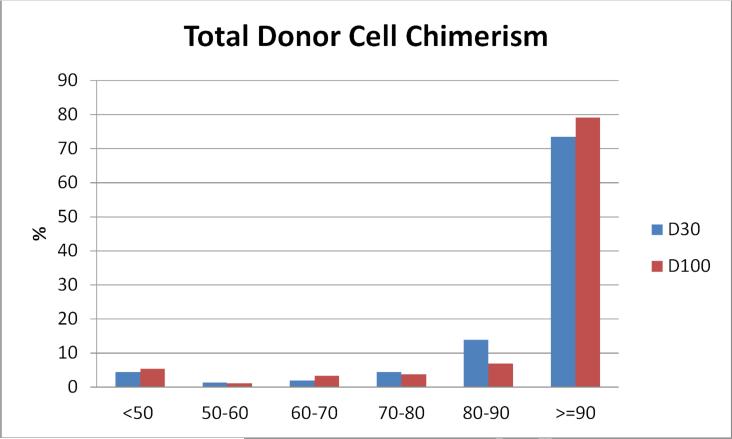
D30 and D100 total donor chimerism data was available for 688 and 530 patients respectively, as 146 patients died or relapsed prior to D100 and 12 had missing D100 chimerism. Low (<90%) total donor cell chimerism occurred in 26% of the cohort at D30 and in 21% at D100 (Figure 1A). Fourteen percent had persistent low total donor cell chimerism at both D30 and D100, identical for lymphoid and myeloid disease.
Figure 1A.
Distribution of D30 and D100 total donor cell chimerism. Columns represent % of patients within each % chimerism category
Total Donor Cell Chimerism and HSCT Outcomes
In univariable analyses total donor cell chimerism associated with better outcomes (Table 2). Low D30 total donor cell chimerism had a higher 2- and 5-year relapse rate of 60% and 67% compared with 44% and 47% respectively (p<0.0001), and a worse 2- and 5-year PFS of 31% and 26% compared with 47% and 36% respectively (p=0.0001). The 5-year NRM was 10% compared with 17% (p=0.047), and 5-year OS was 41% compared with 45% (p=0.046).
Table 2.
Univariable Outcome by D30 and D100 Total Donor Cell Chimerism Level
| D30 Chimerism* |
D100 Chimerism** |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <90 |
≥90 |
p-value | <90 |
≥90 |
p-value | ||||||
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | ||||
| All Patients | 5-yr OS | 182 | 41 (34, 48) | 506 | 45 (40,50) | 0.046 | 110 | 44 (36, 55) | 420 | 56 (51, 61) | 0.0007 |
| 5-yr PFS | 26 (20,33) | 36 (31, 41) | 0.0001 | 27 (18, 36) | 48 (42, 53) | <0.0001 | |||||
| 5-yr CI of NRM | 10 (6, 15) | 17 (13, 21) | 0.047 | 14 (8, 21) | 19 (15, 23) | 0.58 | |||||
| 5-yr CI of Relapse | 67 (56, 70) | 47 (43, 52) | <0.0001 | 60 (49, 68) | 33 (29, 38) | <0.0001 | |||||
| D180 CI of Gr 2-4 aGVHD | 22 (16, 28) | 21 (17. 24) | 0.85 | ||||||||
| 1-yr CI of cGVHD | 46 (36, 55) | 58 (53, 62) | 0.005 | ||||||||
| Myeloid | 5-yr OS | 99 | 33 (24, 43) | 233 | 39 (32, 45) | 0.28 | 58 | 39 (26, 52) | 182 | 53 (45, 60) | 0.012 |
| 5-yr PFS | 25 (17, 34) | 33 (27, 40) | 0.019 | 23 (12, 35) | 49 (41, 56) | <0.0001 | |||||
| 5-yr CI of NRM | 9 (5, 16) | 18 (13, 23) | 0.02 | 11 (4, 21) | 21 (15, 28) | 0.15 | |||||
| 5-yr CI of Relapse | 66 (55, 74) | 49 (42, 56) | 0.0008 | 66 (51, 78) | 30 (24, 37) | <0.0001 | |||||
| D180 CI of Gr 2-4 aGVHD | 17 (10, 25) | 18 (13, 23) | 0.87 | ||||||||
| 1-yr CI of cGVHD | 41 (28, 54) | 54 (46, 61) | 0.025 | ||||||||
| Lymphoid | 5-yr OS | 83 | 50 (38, 61) | 273 | 51 (44, 57) | 0.12 | 52 | 50 (35, 63) | 238 | 59 (52, 66) | 0.029 |
| 5-yr PFS | 28 (19, 38) | 39 (33, 46) | 0.004 | 30 (18, 44) | 47 (40, 54) | 0.0025 | |||||
| 5-yr CI of NRM | 12 (6, 20) | 16 (11, 21) | 0.61 | 17 (8, 29) | 17 (12, 23) | 0.5 | |||||
| 5-yr CI of Relapse | 61 (49, 70) | 45 (39, 52) | 0.003 | 53 (38, 66) | 36 (29, 42) | 0.014 | |||||
| D180 CI of Gr 2-4 aGVHD | 28 (19, 38) | 23 (19, 29) | 0.9 | ||||||||
| 1-yr CI of cGVHD | 50 (36, 63) | 61 (54, 66) | 0.15 | ||||||||
patients who relapsed or died prior to the date of D30 chimerism are excluded
patients who relapsed or died prior to the date of D100 chimerism are excluded
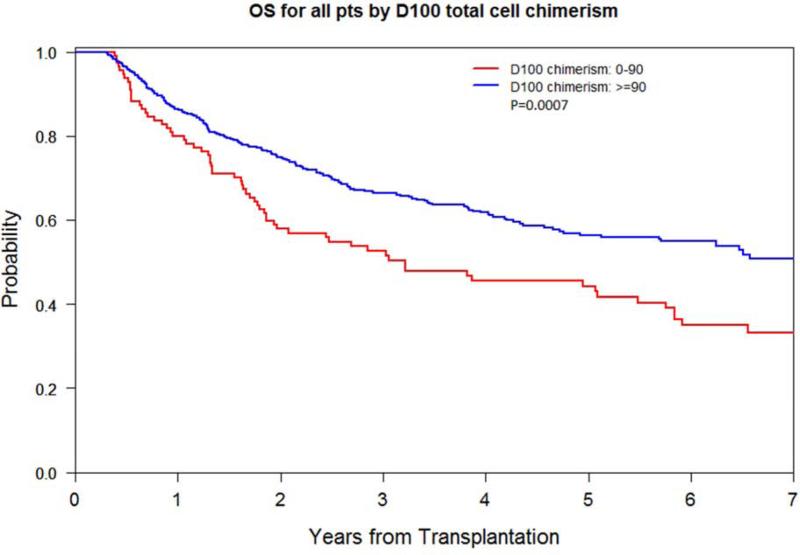
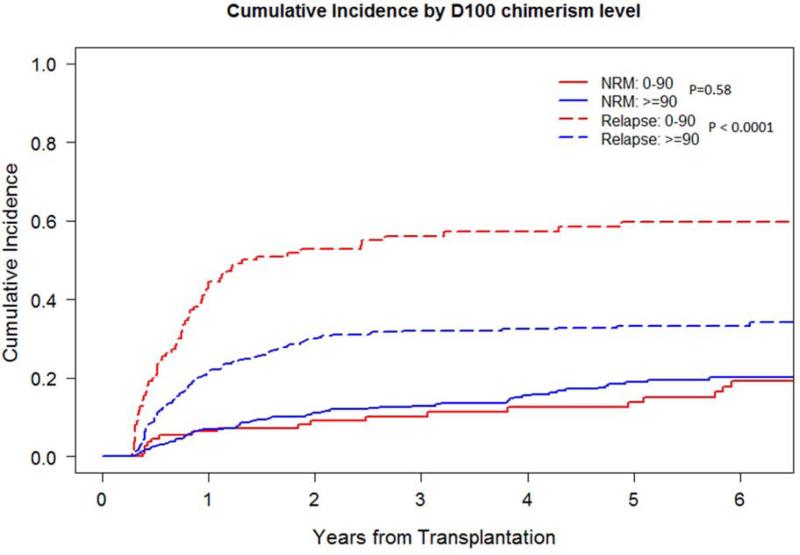
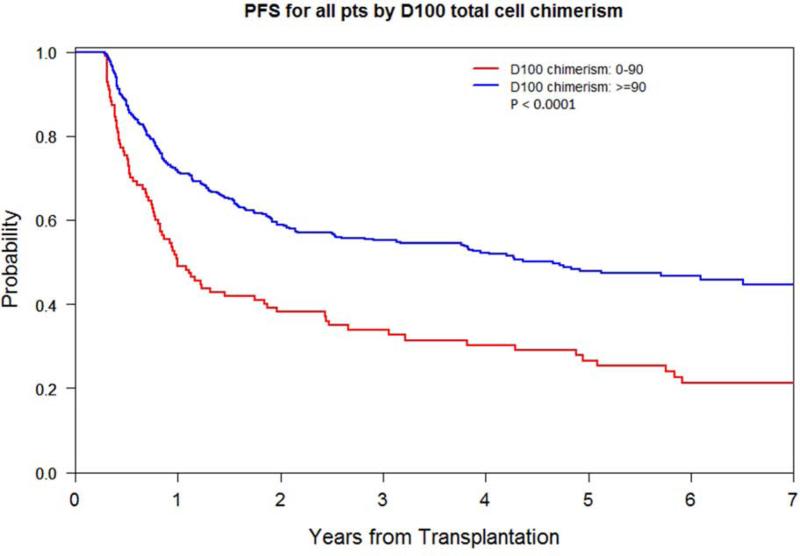
Low D100 total donor cell chimerism had a higher 2- and 5-year relapse rate of 53% and 60% compared with 30% and 33% respectively (p<0.0001), a worse 2- and 5-year PFS of 37% and 27% compared with 59% and 48% respectively (p<0.0001), and a worse 5-year OS of 44% compared with 56% (p=0.0007). The 1-year incidence of chronic GVHD was lower at 46% compared with 58% (p=0.005). The 5-year NRM did not differ (Figure 2A-C). Impact on lymphoid and myeloid disease was similar (Table 2; Supplementary Figure 1A-D).
Figure 2A.
OS by D100 total donor cell chimerism level (<90% vs. ≥90%)
Figure 2C.
Relapse and NRM by D100 total donor cell chimerism level (<90% vs. ≥90%)
In multivariable models, total donor chimerism associated independently with better outcomes. Low D30 total donor cell chimerism associated with higher relapse (HR 1.66, p<0.0001) and impaired PFS (HR 1.41, p=0.0013), but not OS (HR 1.08, p=0.50) (Table 3). Low D100 total donor cell chimerism associated with higher relapse (p<0.0001) and impaired PFS (HR 2.01, p<0.0001) and OS (HR 1.50, p=0.009), but not NRM (HR 0.76, p=0.33) (Table 3; Supplementary Table 1). Myeloid or lymphoid diagnosis did not affect the analysis.
Table 3.
Summary of Multivariable Cox models for OS and PFS and Competing Risks Regression models for NRM and Relapse
| D30 Total Donor Cell Chimerism: <90 vs. ≥90 | D100 Total Donor Cell Chimerism: <90 vs. ≥90 | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| OS | 1.08 | 0.86 | 1.36 | 0.50 | 1.50 | 1.11 | 2.04 | 0.009 |
| PFS | 1.41 | 1.14 | 1.74 | 0.0013 | 2.01 | 1.53 | 2.65 | <.0001 |
| NRM | 0.58 | 0.35 | 0.99 | 0.032 | 0.76 | 0.44 | 2.27 | 0.33 |
| Relapse | 1.66 | 1.30 | 2.11 | <0.0001 | 2.54 | 1.83 | 3.51 | <0.0001 |
We compared the predictive capacity of D30 and D100 total donor cell chimerism, for 530 patients who were alive and relapse-free at D100, using the C-index, the probability of concordance between predicted and observed survival among all usable pairs. The multivariable C-index of low vs. ≥90% D30 total donor cell chimerism for PFS and OS was 0.626 and 0.631 respectively, while at D100 it was higher at 0.646 and 0.644 respectively. C-index utilizing chimerism as a continuous variable also indicated that D100 total donor cell chimerism had greater predictive capacity (data not shown).
Sustained D30-D100 Total Donor Chimerism
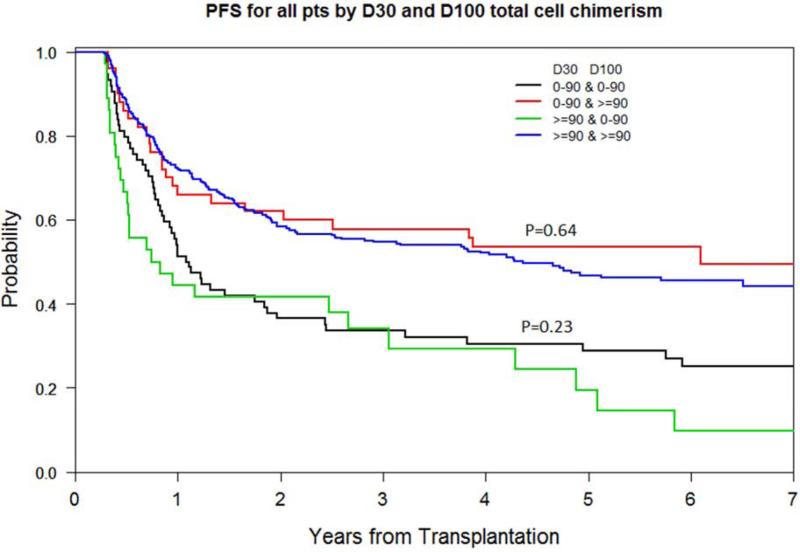
Of the 530 patients alive and relapse-free at D100, few experienced a change of total donor cell chimerism category between D30-D100: 50 (9.4%) had a rise from a low D30 to a ≥90% D100 chimerism, and 36 (6.7%) had a fall from ≥90% D30 to a low D100 chimerism. PFS and OS at 5 years were determined by D100 chimerism and did not differ significantly between those remaining within their chimerism categories between D30-D100 vs. those moving between categories (Figure 2D, and data not shown). We also compared the predictive capacity of D100 vs. 4 categories of D30-D100 total donor chimerism (i.e., <90% & <90% (stable low), <90% & ≥90% (rising), ≥90% & <90% (falling), ≥90% & ≥90% (stable high)). The C-index of low vs. ≥90% D100 total donor cell chimerism was 0.646 and 0.644 for PFS and OS respectively while for D30-D100 it was 0.646 and 0.646 respectively, indicating negligible utility of incorporating sustained D30-D100 total cell chimerism.
Figure 2D.
PFS by D30-D100 sustained vs. non-sustained total donor cell chimerism levels (<90% vs. ≥90%)
Donor T-cell Chimerism
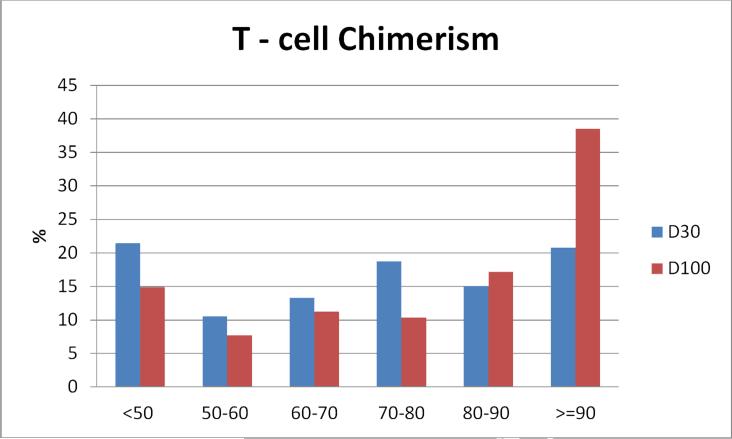
D30 and D100 donor T-cell chimerism was available for 293 and 337 patients respectively. Importantly, there was no difference in 5-year PFS and OS for patients with available vs. missing D30 or D100 T-cell chimerism (p=NS, data not shown). Low (<70%) donor T-cell chimerism occurred in 55% at D30 and 37% at D100 (Figure 1B). There was limited correlation between donor T-cell and total donor cell chimerism, with D30 and D100 Spearman Correlation Coefficients of 0.57 and 0.63 respectively.
Figure 1B.
Distribution of D30 and D100 donor T-cell chimerism. Columns represent % of patients within each % chimerism category
Donor T-cell Chimerism and Outcomes
In univariable analyses early donor T-cell chimerism was associated with better outcomes. Low D30 donor T-cell chimerism had a higher 5-year relapse rate of 59% compared with 40% (p=0.0016) and worse 5-year PFS of 30% compared with 39% (p=0.014). NRM at 5 years was 11% compared with 21% (p=0.12) and 5-year OS was 41% compared with 45% (p=0.33).
Low D100 donor T-cell chimerism had a higher 5-year relapse rate of 52% compared with 30% (p<0.0001) and impaired 5-year PFS of 38% compared with 48% (p=0.004). NRM at 5 years was 10% compared with 22% (p=0.078) and 5-year OS was 55% compared with 54% (p=0.34).
In multivariable models, donor T-cell chimerism also associated independently with better outcomes. Low D100 T-cell chimerism associated with higher relapse (p=0.0003) and impaired PFS (p=0.01), but not OS (p=0.58). Myeloid or lymphoid diagnosis did not affect the analysis (data not shown).
We evaluated the predictive capacity of D100 total donor vs. T-cell chimerism for patients with both data available using the C-index. The C-index of low vs. ≥90% D100 total donor cell chimerism for PFS and OS was 0.646 and 0.662 respectively, but for D100 T-cell chimerism was lower at 0.633 and 0.652 respectively. We also evaluated the predictive capacity of combining D100 total donor plus T-cell chimerism for the same set of patients, and the C-index was 0.649 and 0.66 for PFS and OS respectively, indicating negligible additional utility of incorporating donor T cell chimerism.
Discussion
Hematologic malignancy relapse after T-replete RIC HSCT remains the major hazard and determinant of long-term survival. One predictor of relapse is pre-transplantation disease risk, but the identification of additional potentially modifiable predictors early in the post-transplantation period, before the onset of morphologic relapse, will be important for potential intervention strategies.
Total and T-subset donor cell chimerism early after RIC HSCT is a routine measure of engraftment kinetics, and most studies have focused on T-subset chimerism as a measure of immunologic graft-vs.-tumor capacity. However, results thus far have been mixed. For instance, the largest prior study (n=322) in this context documented the impact of T-cell chimerism on relapse but not on survival.11 Further, chimerism threshold cutoffs, time points, and utility of total vs. T-cell chimerism have not been systematically assessed. The survival impact of donor chimerism early after RIC HSCT remains ill-defined. Early donor chimerism is therefore not usually included in prognostic models of RIC HSCT outcome.
We undertook a systematic analysis of adult T-replete RIC HSCT at our institution from 2002-2010 to better address this important issue. All 688 hematologic malignancy patients with available chimerism data were included. The large cohort is homogeneous with regards therapy: Busulfan/Fludarabine RIC without in-vivo TCD, predominantly PBSC grafts with methotrexate, tacrolimus ± sirolimus GVHD prophylaxis. We evaluated D30 and D100 total donor cell chimerism, and in subset analysis, T-cell chimerism, with a primary endpoint of OS. The goal was to determine chimerism threshold cutoffs; the survival impact of D30 and D100 total donor cell chimerism; the additional utility of ‘sustained’ D30-D100 total donor cell chimerism; and the additional utility of donor T-cell chimerism.
We identified total donor cell and T-cell chimerism cutoffs of 90% and 70% respectively, based on change of log relative hazard ratio of PFS. We found that >70% of patients achieved total donor cell chimerism of ≥90% at D30 and D100. The distribution of donor T-cell chimerism was more broadly spread, from <50% to 100%, particularly at D30. Furthermore, patients with ≥90% donor T-cell chimerism were not better off compared to those with 70-90% donor T-cell chimerism.
Early donor chimerism was an independent prognostic predictor of long-term outcomes in T-replete RIC HSCT. Low D100 total donor cell chimerism was associated with increased relapse and impaired PFS and OS. The magnitude of the C-index of D100 total donor cell chimerism may appear modest (0.644), likely a reflection of heterogeneous disease, patient and transplant characteristics in allogeneic HSCT studies. We note that the predictive capacity for OS reported here is in the range of that for the widely used HCT-CI (0.624) and the recently proposed DRI (0.643).21,22
D100 provided greater predictive capacity than D30 chimerism, which however remains important, as many patients died or relapsed prior to D100. Indeed, D30 may represent a timely point for therapeutic interventions to forestall early relapse (e.g. immunosuppression taper, DLI, or novel therapies) as the apparent lower cumulative incidence of NRM in the low D30 chimerism cohort is largely due to a higher number of relapses in this group. We refer those interested to a detailed description of competing risks data analysis per Gray (1988) and Kalbfleisch and Prentice (2002).16,23 Incorporating ‘sustained’ D30-D100 total donor cell chimerism did not add predictive capacity. In subset analysis donor T-cell chimerism was independently associated with relapse and PFS, but not OS. Donor T-cell chimerism alone or in combination did not add predictive capacity above that of D100 total donor cell chimerism alone.
Our study has limitations inherent to retrospective analyses, including potential selection bias, and questions regarding the generalizability of single-center analyses. The strengths of this study, namely a large homogeneous cohort of adult hematologic malignancy patients treated at a single center by the same physicians over an extended period with uniform T-replete RIC, predominantly PBSC grafts and standard-of-care methotrexate/tacrolimus-based GVHD prophylaxis, with ‘hard’ endpoints of relapse, NRM and survival, help minimize concerns to the extent possible. Importantly, prior risk factors (e.g., age, DRI, WBC risk score, ALC) retained significance in multivariable models, with early donor chimerism proving a novel independent variable. Transplantation variables (e.g., transplantation year, conditioning intensity, sirolimus use) did not impact the findings, allaying some of the concerns regarding applicability of the findings to other T-replete RIC regimens. Additionally, in multivariable landmark analyses excluding patients with early morphologic relapse within 30 days after chimerism assessment, total donor cell chimerism retained independent prognostic significance: D30 associated with PFS (p=0.019), and D100 associated with PFS and OS (p<0.001; p=0.024 respectively). Validation of our findings in other datasets however remains important.
This study represents the largest systematic assessment of the impact of early donor chimerism on long-term outcomes. We document that early total donor chimerism independently predicts long-term relapse and survival. We also document that total donor cell chimerism is sufficient and assessing T-subset chimerism is of no additional value to predicting these endpoints. Based on these findings, prospective interventions to enhance donor chimerism, reduce relapse, and improve long-term survival should be considered early after T-replete RIC HSCT.
Supplementary Material
Figure 2B.
PFS by D100 total donor cell chimerism level (<90% vs. ≥90%)
Acknowledgements
We thank the Dana-Farber Cancer Institute's HSCT program NPs, RNs, and our patients. J.K. is a Scholar in Clinical Research of the Leukemia & Lymphoma Society. This study is funded in part by the Jock and Bunny Adams Research and Education Endowment, and by P01 CA142106-06A1 (JHA) and R01 CA183559-01 (RJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–70. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galimberti S, Benedetti E, Morabito F, et al. Chimerism does not influence graft-versus-myeloma and graft-versus-host disease in reduced intensity setting. Transpl Immunol. 2005;15:173–7. doi: 10.1016/j.trim.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Blau IW, Schmidt-Hieber M, Leschinger N, et al. Engraftment kinetics and hematopoietic chimerism after reduced-intensity conditioning with fludarabine and treosulfan before allogeneic stem cell transplantation. Ann Hematol. 2007;86:583–9. doi: 10.1007/s00277-007-0294-6. [DOI] [PubMed] [Google Scholar]
- 4.Lange T, Hubmann M, Burkhardt R, et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia. 2011;25:498–505. doi: 10.1038/leu.2010.283. [DOI] [PubMed] [Google Scholar]
- 5.Holtan SG, Hogan WJ, Elliott MA, et al. CD34+ cell dose and establishment of full donor chimerism at day +100 are important factors for survival with reduced-intensity conditioning with fludarabine and melphalan before allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplant. 2010;45:1699–703. doi: 10.1038/bmt.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huisman C, de Weger RA, de Vries L, Tilanus MG, Verdonck LF. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant. 2007;39:285–91. doi: 10.1038/sj.bmt.1705582. [DOI] [PubMed] [Google Scholar]
- 7.Saito AM, Kami M, Mori S, et al. Prospective phase II trial to evaluate the complications and kinetics of chimerism induction following allogeneic hematopoietic stem cell transplantation with fludarabine and busulfan. Am J Hematol. 2007;82:873–80. doi: 10.1002/ajh.20977. [DOI] [PubMed] [Google Scholar]
- 8.Mohty M, Avinens O, Faucher C, Viens P, Blaise D, Eliaou JF. Predictive factors and impact of full donor T-cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Haematologica. 2007;92:1004–6. doi: 10.3324/haematol.10971. [DOI] [PubMed] [Google Scholar]
- 9.Saito B, Fukuda T, Yokoyama H, et al. Impact of T cell chimerism on clinical outcome in 117 patients who underwent allogeneic stem cell transplantation with a busulfan-containing reduced-intensity conditioning regimen. Biology of Blood and Marrow Transplantation. 2008;14:1148–55. doi: 10.1016/j.bbmt.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer BC, Modric M, Stetler-Stevenson M, et al. Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia. Exp Hematol. 2013;41:772–8. doi: 10.1016/j.exphem.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savani BN, Mielke S, Rezvani K, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2007;13:1216–23. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Blanc K, Barrett AJ, Schaffer M, et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biology of Blood and Marrow Transplantation. 2009;15:1108–15. doi: 10.1016/j.bbmt.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HT, Frederick D, Armand P, et al. White blood cell recovery after allogeneic hematopoietic cell transplantation predicts clinical outcome. Am J Hematol. 2014 doi: 10.1002/ajh.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–54. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 19.Harrell FE, Jr., Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247:2543–6. [PubMed] [Google Scholar]
- 20.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation: a study from the CIBMTR. Blood. 2014 doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley & Sons; New York; Chichester: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.