Abstract
Aim
because of the contribution of ethanol and polyunsaturated fatty acids (PUFAs) to alcoholic liver disease, we investigated whether chronic ethanol administration and arachidonic acid (AA) could synergistically mediate Kupffer cell (KC) activation and modulate the stellate cell (HSC) fibrogenic response.
Results
1) Ethanol and AA effects on KC and HSC mono-cultures: cell proliferation, lipid peroxidation, H2O2, O2.−, NADPH oxidase activity, and TNFα were higher in KCethanol than in KCcontrol, and were enhanced by AA; HSCethanol proliferated faster, increased collagen, and showed higher GSH than HSCcontrol, with modest effects by AA. 2) AA effects on the control co-culture: we previously reported (1) the ability of KC to induce a pro-fibrogenic response in HSC via ROS-dependent mechanisms; we now show that AA further increases cell proliferation and collagen in the control co-culture. The latter was prevented by vitamin E (an antioxidant) and by diphenyleneiodonium (a NADPH oxidase inhibitor). 3) Ethanol effects on the co-cultures: co-culture with KCcontrol or KCethanol induced HSCcontrol and HSCethanol proliferation; however, the pro-fibrogenic response in HSCethanol was suppressed due to up-regulation of TNFα and GSH, which was prevented by a TNFα neutralizing Ab and by l-buthionine-sulfoximine, a GSH-depleting agent. 4) Ethanol plus AA effects on the co-cultures: AA lowered TNFα in the HSCcontrol co-cultures allowing for enhanced collagen deposition; furthermore, AA restored the pro-fibrogenic response in the HSCethanol co-cultures by counteracting the up-regulation of TNFα and GSH with a significant increase in GSSG and in pro-fibrogenic TGFβ.
Conclusion
these results unveil synergism between ethanol and AA to the mechanism whereby KC mediate ECM remodeling, and suggest that even if chronic ethanol consumption sensitizes HSC to up-regulate anti-fibrogenic signals, their effects are blunted by a second ‘hit’ such as AA.
Keywords: co-culture, collagen I, oxidative stress, stellate cells, extracellular matrix
Under normal conditions, the space of Disse contains a non electron-dense basement membrane-like matrix which is essential for maintaining the differentiated function of all resident liver cells (2). As the liver becomes fibrotic, the total content of collagens increases and it is accompanied by a shift in the subendothelial space, from normal low density basement membrane, to one enriched in fibrillar collagens type I and III (2). Decades of elegant studies have defined that up-regulation of collagen I synthesis during HSC activation is among the most remarkable molecular responses to injury mediated by multiple signals, many of which remain unknown (1, 3-11).
Paracrine stimuli initiating HSC activation derive from injured hepatocytes (9, 10, 12) and neighboring KC (1, 13) in addition to swift and subtle changes in extracellular matrix (ECM) composition. Evidence for a role of KC early on in ethanol-induced liver injury springs from their remarkable production of reactive oxygen species (ROS), cytokines, and growth factors that provide pivotal effects on all other liver cells (14-24). Recruitment of pro-inflammatory cells also occurs, and upon arriving at the site of injury they release multiple mediators that further activate HSC and enhance collagen I deposition (25). In the final step, maturation and organization of ECM occurs, connective tissue fibers retract, and most of the cellular infiltrates disappear (26). At this stage, when the liver becomes cirrhotic, the end-stage of fibrosis, there is insufficient remodeling, and liver injury becomes nearly irreversible (27). Thus, efforts to understand fibrosis focus primarily on the early events or ‘hits’ that lead to accumulation of scar in hope of identifying therapeutic targets to slow its progression or help its resolution (2).
Chronic injury leading to liver fibrosis takes place in response to alcohol abuse. Indeed, generation of ROS by KC increases under ethanol treatment, and a role for n-3 and n-6 series PUFAs (11, 28-30) needs to be considered as they accelerate the development of alcoholic liver disease. In fact, pathologic changes occur mostly in rats fed ethanol with PUFAs (28). Therefore, both ethanol and PUFAs add an additional layer of complexity to the elaborate crosstalk between KC and HSC.
Understanding the dynamics on how alcohol and PUFAs regulate KC effects on the HSC behavior is of great value for pharmacological design to help prevent liver injury. We have previously shown that KC modulate the fibrogenic response in HSC via ROS-dependent mechanisms (1). To gain additional insight, a co-culture model was established using KC and HSC from control and from ethanol-fed rats and AA, as a representative n-6 series PUFA, was added. The results described herein advance the field by unveiling potential synergism between ethanol and AA to the mechanism whereby KC modulate ECM deposition and remodeling. The data suggest that chronic ethanol consumption sensitizes HSC in the presence of KC to up-regulate two powerful anti-fibrogenic mediators such as TNFα and GSH. These signals may be switched on in HSC in the early stages of alcohol-induced liver injury, even though uncoordinated HSC proliferation may coexist, likely reflecting that KC could drive the HSC phenotype to a more pro-inflammatory and/or proliferative and to a less fibrogenic behavior. However, a second ‘hit’ such as PUFAs (e.g. AA) may tip the balance favoring scarring by lowering TNFα, depleting GSH stores, increasing GSSG, and significantly up-regulating TGFβ, a powerful pro-fibrogenic mediator.
EXPERIMENTAL PROCEDURES
Chronic Alcohol Feeding Model
Rats (300g female Sprague-Dawley, N=10/group) were fed the control or ethanol Lieber-DeCarli diets for 8 months (31). Animals received humane care according to the criteria outlined in the Guide for Care and Use of Laboratory Animals. ALT, AST, ethanol, and non-esterified fatty acids were assayed using kits from Thermo Electron Corporation (Waltham, MA), Sigma (St. Louis, MO), and Wako Chemicals (Richmond, VA), respectively. H&E and TEM sections were evaluated by a liver pathologist.
Co-cultures
Primary rat KC and HSC were isolated by differential centrifugation and elutriation as previously (1). Buoyancy was similar in HSCcontrol and in HSCethanol (Fig. 1C, bottom) allowing for isolation of similar HSC populations in the density gradient. KC were additionally purified by cell sorting using CD163-FITC, a KC specific marker, and by selective attachment. Details on the co-culture model can be found in an earlier publication (1) and in Fig. 4A.
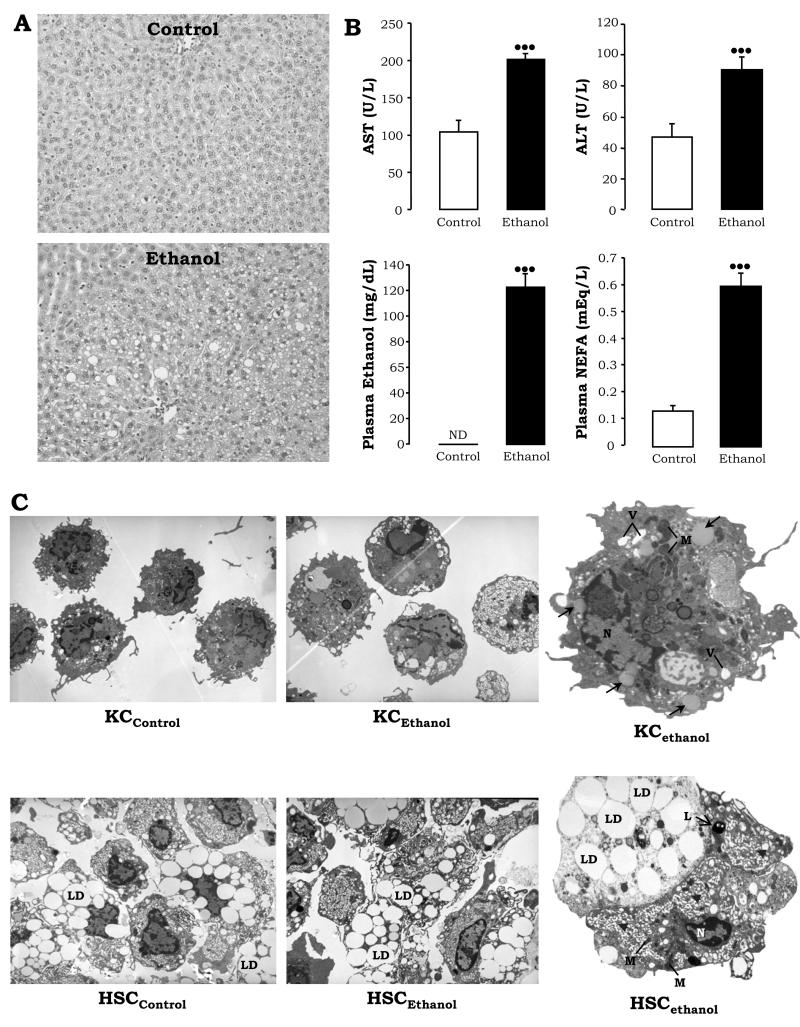
Figure 1. Pathology and Ultrastructural Studies.
H&E staining in livers from control rats showed minimal steatosis (top), while rats fed ethanol showed periportal and pericentral micro- and macro-vesicular steatosis (original magnification= 200×) (A). AST and ALT activities, plasma ethanol levels, and plasma non-esterified fatty acids. Results are average values ± SEM, N=10; •••P<0.001 for ethanol vs. control, ND: not detected (B). Ultrastructural analysis depicting micro- and macrovesicular steatosis (→), vacuolization (V), and electron dense mitochondria (M) in KCethanol vs. KCcontrol (C, top), and similar buoyancy (LD) but more dilated ER (▶), lysosomes (L), and electron sense mitochondria (M) in HSCethanol vs. HSCcontrol (C, bottom).
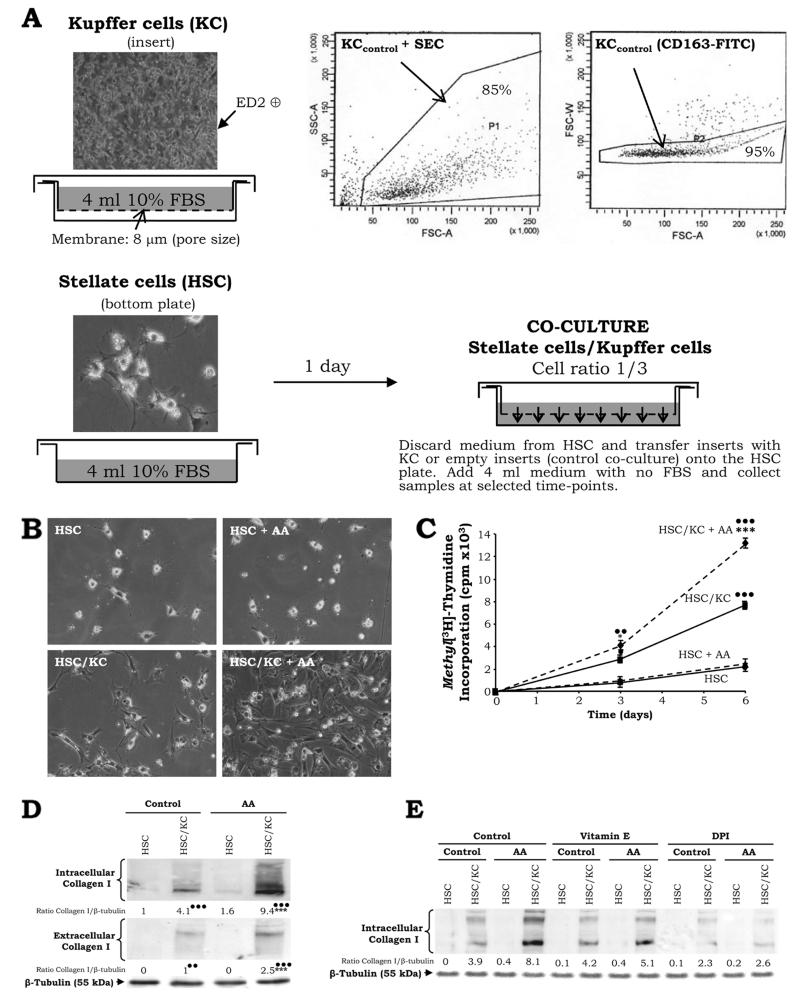
Figure 4. KC Promote HSC Activation, Proliferation, and Collagen I Deposition in the presence of AA.
Co-culture model: After liver perfusion, elutriation, and cell sorting with CD163-FITC (inset), 5 × 105 KC were plated on cell culture inserts and 1.5 × 105 HSC were seeded on the bottom plates in 4ml of DMEM-F12 containing 10% FBS. One hour later, the KC medium was replaced to remove any remaining endothelial cells which do not adhere at short times after plating while KC do. Twenty-four hours after, the medium was discarded and, either empty inserts (co-culture controls) or inserts containing KC were transferred onto the HSC plates. Fresh serum-free medium (4 ml) was added and samples of HSC lysates or culture medium were collected at selected time points (A). Light micrographs of primary HSC cultured alone or with KC in the presence of AA (magnification= ×200) (B). Methyl[3H]-thymidine incorporation into the DNA of HSC cultured alone or with KC in the presence of AA (C). Cells were co-cultured up to 7d in the presence of AA and samples of cell lysate and culture medium were analyzed for intra- and extracellular collagen I and β-tubulin (loading control) by Western blot. Results are in arbitrary densitometry units under the blots, and the quantification of the signal corrected by that of β-tubulin was referred to the non-treated HSC, which was assigned a value of 1 (D). In (C) and (D) results are average values ± SEM of N=4, *P<0.05 and ***P<0.001 for AA-treated vs. non-treated, ••P<0.01 and •••P<0.001 for co-culture vs. control. Primary HSC cultured alone or with KC were incubated with vitamin E (25μM) –an antioxidant- and DPI (1nM) –an inhibitor of NADPH oxidase- 2h prior to addition of AA and intracellular collagen I and β-tubulin (loading control) were evaluated by Western blot. Results are in arbitrary densitometry units under the blots and are average values of N=3 (SEM not shown) (E).
General Methodology
Endotoxin-free AA, to avoid cellular activation, was added in the presence of BSA as a carrier. Cell viability was determined by the MTT assay. Cell proliferation was estimated by methyl[3H]-thymidine incorporation into HSC DNA (9). Apoptosis was measured by flow cytometry with a caspase 3/7 Kit (Invitrogen, Carlsbad, CA). Intra- and extracellular TNFα were evaluated by flow cytometry with anti-rat TNFα PE-conjugated antibody (BD Pharmingen, San Jose, CA) and by ELISA (Biosource, Camarillo, CA), respectively. Intracellular lipid peroxidation was determined by cell sorting using cis-parinaric acid (Molecular Probes). Thiobarbituric acid reactive substances (TBARS) were assayed as described (32). Intracellular hydroperoxides (i.e. H2O2) and O2.− were assessed by flow cytometry using the fluorescent probes 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) and dihydroethidium (DHE), respectively (Invitrogen). Extracellular hydroperoxides (mainly H2O2) were measured by the ferrous oxidation-xylenol orange assay (33). NADPH oxidase activity was determined using lucigenin (34). GSH and oxidized glutathione (GSSG) were determined by the recycling method of Tietze (35) and Anderson (36), respectively.
Western Blot Analysis
Anti-collagen type I Ab was provided by Dr. Schuppan (Harvard Medical School, Boston, MA) (37). Collagen type I was detected as several high MW chains of pro-collagen α1(I) and α2(I) and N-terminally processed pCα1(I) and pCα2(I). For intracellular collagen I, the α1 chain is 139 kDa and the α2 chain is 129 kDa. In the culture media, intact pro-collagen I predominated along with fully processed collagen I (9, 10, 37). The quantification under the blots refers to the sum of bands from all collagen I isoforms. α-Sma and β-tubulin Abs were from Sigma (St. Louis, MO).
Statistical analysis
Data were analyzed by a two-factor ANOVA and results are expressed as means ± SEM (at least N>3).
RESULTS
Chronic Alcohol Feeding Model
Primary HSC and KC were isolated from rats fed with either control or ethanol Lieber-DeCarli diets. H&E staining revealed micro- and macrovesicular steatosis in livers from ethanol-fed rats (Fig. 1A, bottom vs. top). Transaminases and non-esterified fatty acids were elevated ~2-fold and ~6-fold in the ethanol-fed rats, respectively, and plasma ethanol was ~120 mg/dL (Fig. 1B). Ultrastructural analysis depicted micro- and macrovesicular steatosis (→), vacuolization (V), and some electron dense mitochondria (M) in KCethanol vs. KCcontrol (Fig. 1C, top). More dilated ER (▶) and electron dense mitochondria (M) were observed in HSCethanol vs. HSCcontrol while analogous content of lipid droplets (LD) was observed in both (Fig. 1C, bottom). Immediately after perfusion (t=0d), collagen I and α-Sma, markers of HSC activation, as well as HSC counts were higher for HSCethanol compared with HSCcontrol (not shown).
AA and Ethanol Induce Proliferation Rates and Activate KC
Intracellular concentrations of AA in humans are in the μM range and are ~10% of total fatty acids in rat plasma (38), depending on metabolic and nutritional status. Both n-3 and n-6 series PUFAs are effective in increasing collagen I in co-cultures of mouse HSC with pooled hepatocytes, KC, and endothelial cells (11). We chose AA to compare its pro-fibrogenic effects in our model to those described by us in co-cultures of rat HSC with hepatocytes (9). Initially, KCcontrol and KCethanol were incubated with increasing doses of AA up to 10μM and observed dose-dependent cell activation and ROS production (not shown). KC viability was similar in the presence of 0-10μM AA at 24h (Suppl. Fig. 1A). Proliferation rates at 24h were slightly increased in KCethanol compared to KCcontrol and AA further elevated cell proliferation (Suppl. Fig. 1B). Moreover, light micrographs indicated that AA induced phenotypic changes in KC such as stretching and generation of cytoplasmic processes and filopodia, suggestive of cell activation (Suppl. Fig.1C, right vs. left panels). These effects were more apparent in KCethanol than in KCcontrol (Suppl. Fig.1C, bottom vs. top panels).
Ethanol and AA Synergize to Increase Oxidant Stress in KC
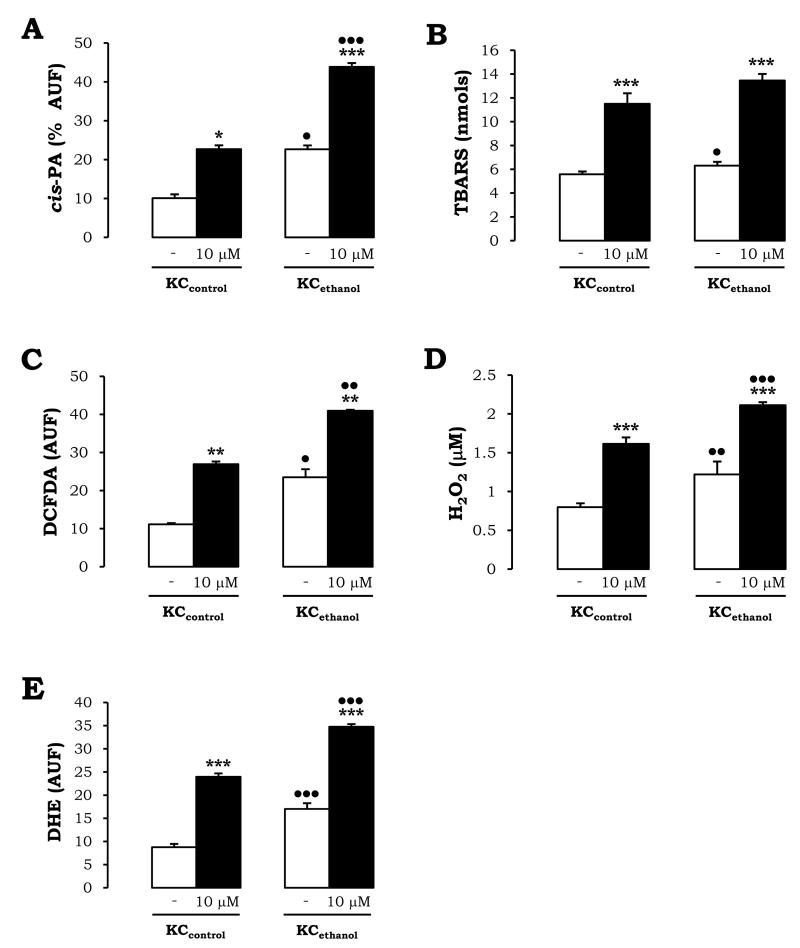
Intracellular lipid peroxidation-end products, hydroperoxides (mostly H2O2), and O2.−, as well as extracellular TBARS and hydroperoxides, which could impact HSC, were assessed to determine if the effects observed in KCcontrol and in KCethanol under AA-treatment were synergistic to those mediated by chronic alcohol feeding. There was a 2.4-fold and a ~12% increase in intra- and extracellular lipid peroxidation-derived products in KCethanol vs. KCcontrol (Fig. 2A-2B, white bars). AA further enhanced ~2-fold lipid peroxidation. Levels were greater in KCethanol (Fig. 2A-2B, black vs. white bars). Moreover, intra- and extracellular hydroperoxides appeared induced ~2.2-fold and ~40% in KCethanol vs. KCcontrol (Fig. 2C-2D, white bars). Likewise, addition of AA elevated hydroperoxides. Values were significantly higher in KCethanol than in KCcontrol (Fig. 2C-2D, black vs. white bars). Lastly, intracellular O2.− was elevated ~2-fold in KCethanol vs. KCcontrol (Fig. 2E, white bars) and were enhanced by ~2.2-fold by AA in both (Fig. 2E, black vs. white bars). Therefore, synergism between ethanol and AA in activating KC and generating ROS appeared likely.
Figure 2. AA Induces Oxidant Stress Mostly in KCethanol.
KCcontrol and KCethanol were incubated with 0-10μM AA for 24h. Intracellular lipid peroxidation assessed by cis-parinaric acid fluorescence (A). Lipid peroxidation end products secreted to the culture medium are shown as nmols of TBARS (B). Intracellular hydroperoxides (mostly H2O2) were measured by flow cytometry using DCFDA (C). Levels of hydroperoxides (mainly H2O2) released to the culture medium were evaluated by the FOX method (D). Intracellular O2.− was determined by flow cytometry using DHE (E). In all panels, results are expressed as means ± SEM of N>3. *P<0.05, **P<0.01, and ***P<0.001 for AA-treated vs. non-treated, and •P<0.05, ••P<0.01, and •••P<0.001 for ethanol vs. control.
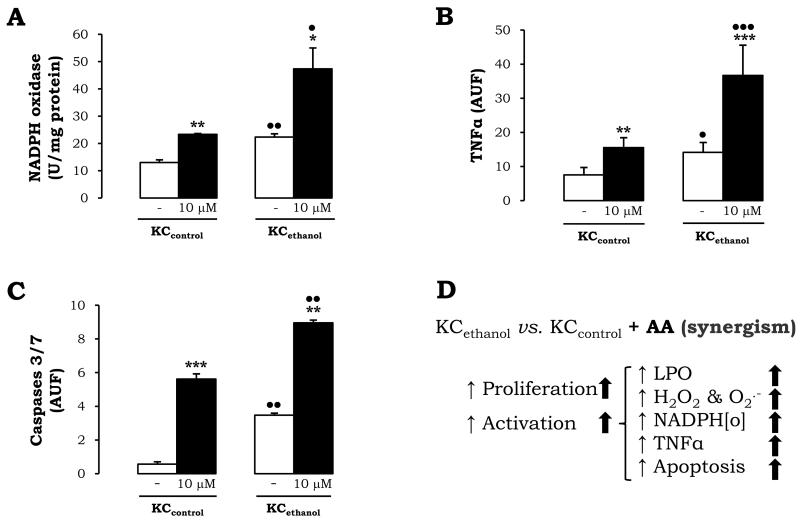
Ethanol and AA Increase NADPH Oxidase Activity, TNFα, and Apoptosis in KC
To determine the source of ROS, we next explored possible events occurring in KC that may play a role in regulating the HSC fibrogenic response. KCcontrol and KCethanol were incubated with 0-10μM AA for 24h and NADPH oxidase activity –a source of ROS-, intracellular TNFα –an anti-fibrogenic agent-, and caspase 3/7 –a marker for apoptosis- were evaluated. KCethanol showed elevated NADPH oxidase activity over that of KCcontrol (Fig. 3A, white bars) and AA further induced it by ~2-fold (Fig. 3A, black vs. white bars). Surprisingly, cytochrome P450 2E1 and xanthine oxidase, remained similar under either ethanol or AA-treatment (not shown). TNFα was 2-fold increased in KCethanol vs. KCcontrol (Fig. 3B, white bars). Moreover, there was a 2-fold up-regulation in intracellular TNFα in KCcontrol and a 2.5-fold increase in KCethanol treated with AA (Fig. 3B, black vs. white bars). Lastly, the 6.5-fold increase in caspase 3/7 activity in KCethanol vs. KCcontrol (Fig. 3C, white bars) was further enhanced by AA in KCcontrol and in KCethanol (Fig. 3C, black vs. white bars). A summary of the main effects on KC can be found in Fig. 3D.
Figure 3. Ethanol and AA increase NADPH Oxidase, TNFα, and Caspase 3/7.
NADPH oxidase activity in KCcontrol and in KCethanol incubated with 0-10μM AA for 24h (A). Intracellular TNFα (B) and caspase 3/7 activities (C) evaluated by flow cytometry. In all panels, results are average values ± SEM of N=4, *P<0.05, **P<0.01, ***P<0.001 for AA-treated vs. non-treated and •P<0.05, ••P<0.01, and •••P<0.001 for ethanol vs. control. Summary of the effects of chronic ethanol consumption and AA on the KC mono-cultures; ↑: up-regulation by ethanol; ⬆: up-regulated by AA (D).
KC Promote HSC Activation, Proliferation, and Collagen I Deposition in the Presence of AA
We previously demonstrated the ability of KC to induce the HSC pro-fibrogenic behavior via ROS-dependent mechanisms (1). To further our knowledge, we questioned whether KC could enhance the pro-fibrogenic behavior of HSC in the presence of AA as well. Using the co-culture model (Fig. 4A) (1), phenotypic changes in HSC co-cultured with KC such as stretching and generation of cytosolic processes establishing contacts among cells, were more apparent in HSC co-cultured with KC than in HSC cultured alone (Fig. 4B, HSC/KC vs. HSC). AA enhanced the activated phenotype in the co-cultured HSC (Fig. 4B, HSC/KC + AA vs. HSC/KC). Consistent with the light micrographs, there was a ~4-fold increase in proliferation of HSC in co-culture compared to HSC cultured alone (Fig. 4C, solid lines). Addition of AA enhanced ~1.7-fold HSC proliferation rates only in the co-culture (Fig. 4C, top dashed line vs. solid line). Likewise, the already elevated ROS levels in the co-culture (1) were synergistically enhanced by AA (not shown).
We previously reported that co-culture with KC increased HSC collagen I expression (1). We now show that AA-treatment further elevated both intra- and extracellular collagen I in the co-culture ~2.3-fold (Fig. 4D, lane 4 vs. lane 2). To establish a link between the availability of AA-derived ROS (Fig. 2, and not shown), activation of NADPH oxidase (Fig. 3A), and the fibrogenic response (Fig. 4D), HSC cultured alone or with KC were incubated with an antioxidant (vitamin E) or a NADPH oxidase inhibitor (diphenyleneiodonium (DPI)) 2h prior to AA addition. Both treatments prevented the increase in collagen I by AA but DPI appeared more effective (Fig. 4E; lanes 8 and 12 vs. lane 4).
Chronic Ethanol Feeding Enhances HSC Activation
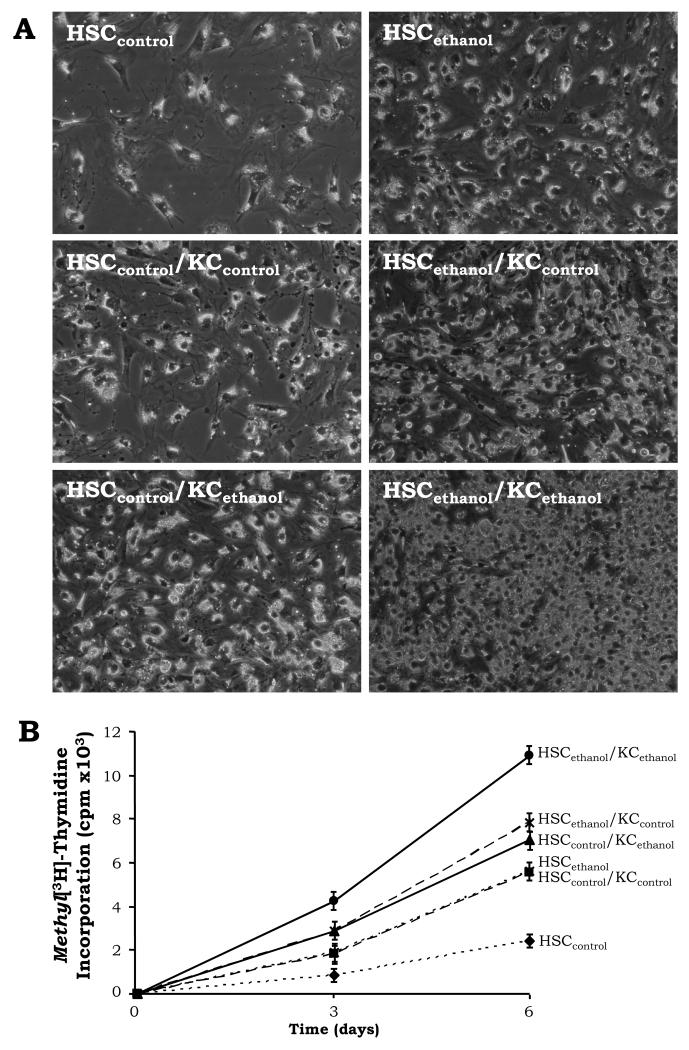
Prior to determine the potential synergistic effects of ethanol and AA on the HSC pro-fibrogenic response, we analyzed the basal effects of chronic ethanol consumption in HSC cultured alone or with KC. Both HSC and KC were isolated from rats fed the control or the ethanol Lieber-DeCarli diets and co-cultured. HSCethanol proliferated faster than HSCcontrol (Fig. 5A-5B, dotted lines). KCcontrol and KCethanol elevated HSCcontrol and HSCethanol proliferation rates but effects were greater in the presence of KCethanol (Fig. 5A-5B, solid vs. dashed lanes for the KCethanol effect, and dashed vs. dotted lines for the KCcontrol effect).
Figure 5. KC Enhance Proliferation Rates mostly in HSCethanol.
A time-course experiment up to 6d was carried out with HSCcontrol and HSCethanol co-cultured with empty inserts (control co-cultures) or with inserts containing KCcontrol or KCethanol (therefore, six culture conditions). Light micrographs of primary HSCcontrol and of HSCethanol co-cultured for 6d with KCcontrol or KCethanol (magnification= ×200) (A). Cell proliferation assessed by the rate of incorporation of methyl[3H]-thymidine into the DNA of HSC. Results are expressed as average values ± SEM of N=4, the significance is not indicated in the graph due limited space (B).
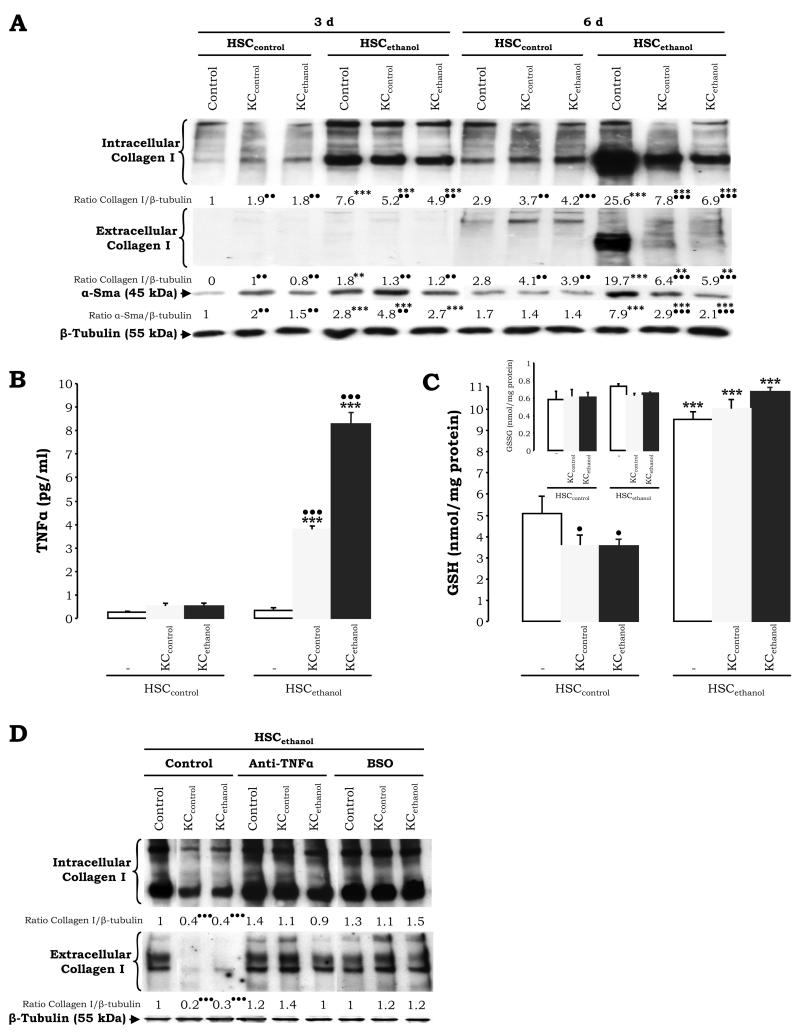
Divergent Response of HSCethanol to the Pro-Fibrogenic Effects Triggered by KC
To address the potential KC-derived effects by ethanol on the fibrogenic response, intra- and extracellular collagen I and α-Sma were analyzed and found to be elevated in HSCethanol compared to HSCcontrol cultured alone (Fig. 6A, compare HSCcontrol and HSCethanol at 3d –lane 4 vs. lane 1- and at 6d –lane 10 vs. lane 7-). This is in agreement with the enhanced proliferation rate of HSCethanol (Fig. 5A-5B) and with the change in phenotype (Fig. 5A, top panels). Co-culture of HSCcontrol with either KCcontrol or with KCethanol increased intracellular and secreted collagen I levels (Fig. 6A, lanes 2 and 3 vs. lane 1 at 3d, and lanes 8 and 9 vs. lane 7 at 6d) (1). However, co-culture of HSCethanol with either type of KC lowered intracellular and secreted collagen I and α-Sma (Fig. 6A, lanes 5 and 6 vs. lane 4 at 3d, and lanes 11 and 12 vs. lane 10 at 6d). Thus, KC had a pro-fibrogenic effect on HSCcontrol but an apparent anti-fibrogenic effect on HSCethanol (1).
Figure 6. TNFα and GSH Up-Regulation Plays a Role in Modulating the Fibrogenic Response in HSCethanol.
HSCcontrol and HSCethanol co-cultures were evaluated for intra- and extracellular collagen I, α-Sma, and β-tubulin (loading control) by Western blot analysis. The quantification of the signal was referred to that of the non-treated HSC at 3d which was assigned a value of 1 (A). Levels of TNFα in the culture medium at 6d of co-culture (B). Intracellular HSC GSH (C) and GSSG levels (C, inset). The HSCethanol co-cultures were incubated with a TNFα neutralizing Ab –to block TNFα- or with l-buthionine sulfoximine (BSO) -to deplete GSH-, and intra- and extracellular collagen I and β-tubulin expression were analyzed by Western blot. Results are expressed as arbitrary densitometry units under the blots; the quantification of the signal was referred to that of the non-treated HSC which was assigned a value of 1 (D). Results are average values ± SEM (SEM not shown in (A) and (D)) N=4, •P<0.05, ••P<0.01, and •••P<0.001 for co-culture vs. control, and **P<0.01, and ***P<0.001 for HSCethanol vs. HSCcontrol.
TNFα and GSH Mediate the Anti-fibrogenic Action of KC on HSCethanol
Due to the ability of ethanol to induce ROS, ROS-sensitive cytokines, and growth factors, we analyzed the expression of candidate anti-fibrogenic signals that could mediate the down-regulation of collagen I in the HSCethanol co-cultures. While a minor increase in TNFα was observed in HSCcontrol co-cultured with KCcontrol or with KCethanol (Fig. 6B, left bars), there was a 11- and a 22-fold increase in secreted TNFα in HSCethanol co-cultured with KCcontrol and with KCethanol, respectively (Fig. 6B, right bars). Although the antioxidant defense in HSC was similar under all culture conditions (not shown), there was a ~2-fold increase in GSH levels in HSCethanol vs. HSCcontrol (Fig. 6C, white bars). A modest decrease in HSC GSH was observed by co-culture with either KC type while GSSG remained similar (Fig. 6C, inset). We then evaluated whether regulating TNFα and GSH levels, collagen I expression could be restored to that of non-co-cultured HCSethanol. Addition of a TNFα neutralizing Ab or treatment with l-buthionine sulfoximine (BSO), which depletes GSH pools, restored intra- and extracellular collagen I expression to that found in non-treated and non-co-cultured HSCethanol (Fig. 6D; lanes 5, 6, 8, and 9 vs. lane 1), suggesting a role for TNFα and GSH on collagen I expression in the HSCethanol co-cultures.
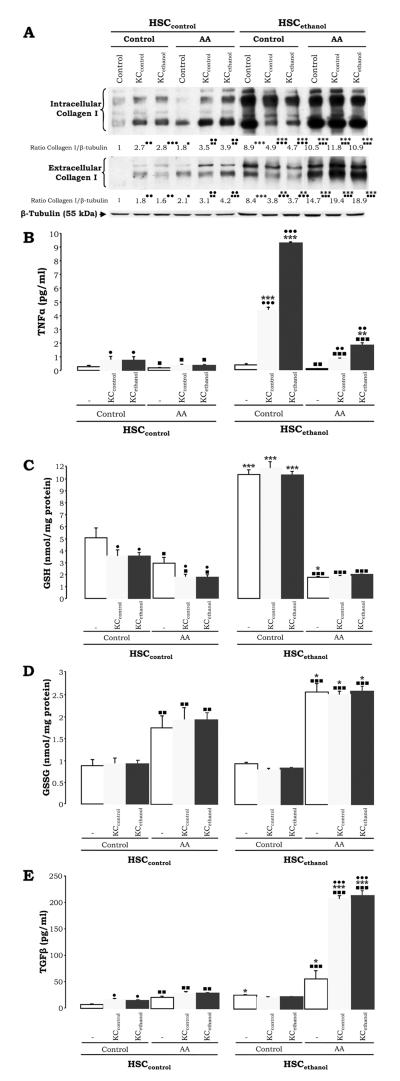
AA Induces Collagen I Expression in the HSCethanol Co-Cultures by Lowering HSCethanol GSH and TNFα and increasing GSSG and TGFβ
To define the potential effects of AA as a second ‘hit’, the control and the ethanol co-cultures were incubated with AA and intra- and extracellular collagen I was analyzed. HSCcontrol were responsive to AA, both when cultured alone or when either KC type were present, increasing collagen I expression (Fig. 7A, lanes 4-6 vs. lanes 1-3). Moreover, HSCethanol, which had lower collagen I in the co-culture with either KC type, appeared responsive to AA, restoring or sustaining collagen I induction above values of non-co-cultured HSCethanol (Fig. 7A, lanes 10-12 vs. lanes 7-9). To determine whether TNFα and GSH played a role in the effects mediated by AA in the HSCethanol co-cultures, secreted TNFα and HSC intracellular GSH were measured. Addition of AA lowered secreted TNFα slightly in the HSCcontrol and considerably in the HSCethanol co-cultures. Similar results were observed for GSH pools, while GSSG increased (Figs. 7B-7D, light and dark grey vs. white bars). More importantly, AA triggered a 2-fold and a 10-fold increase in TGFβ in the HSCcontrol and HSCethanol co-cultures, respectively (Fig. 7E, light and dark grey vs. white bars). Therefore GSSG and TGFβ, a well-known pro-fibrogenic factor, appeared to counteract the anti-fibrogenic signals (i.e. TNFα and GSH) coexisting in the HSCethanol co-culture.
Figure 7. AA Restores the Pro-Fibrogenic Response in the HSCethanol Co-culture.
The co-cultures were treated with AA and intra- and extracellular collagen I and β-tubulin expression were analyzed by Western blot. Results are expressed as arbitrary densitometry units under the blots and are average values of N=3; the quantification of the signal was referred to that of the non-treated HSCcontrol which was assigned a value of 1 (A). Under the same experimental conditions as in (A), levels of TNFα in the culture medium (B), intracellular HSC GSH (C), HSC GSSG (D), and TGFβ (E) are shown. Results are average values ± SEM (SEM not shown in panel A) of N=4, •P<0.05, ••P<0.01, and •••P<0.001 for co-culture vs. control, *P<0.05, **P<0.01, and ***P<0.001 for HSCethanol vs. HSCcontrol, and ■P<0.05, ■■P<0.01, and ■■■P<0.001 for AA-treated vs. non-treated.
DISCUSSION
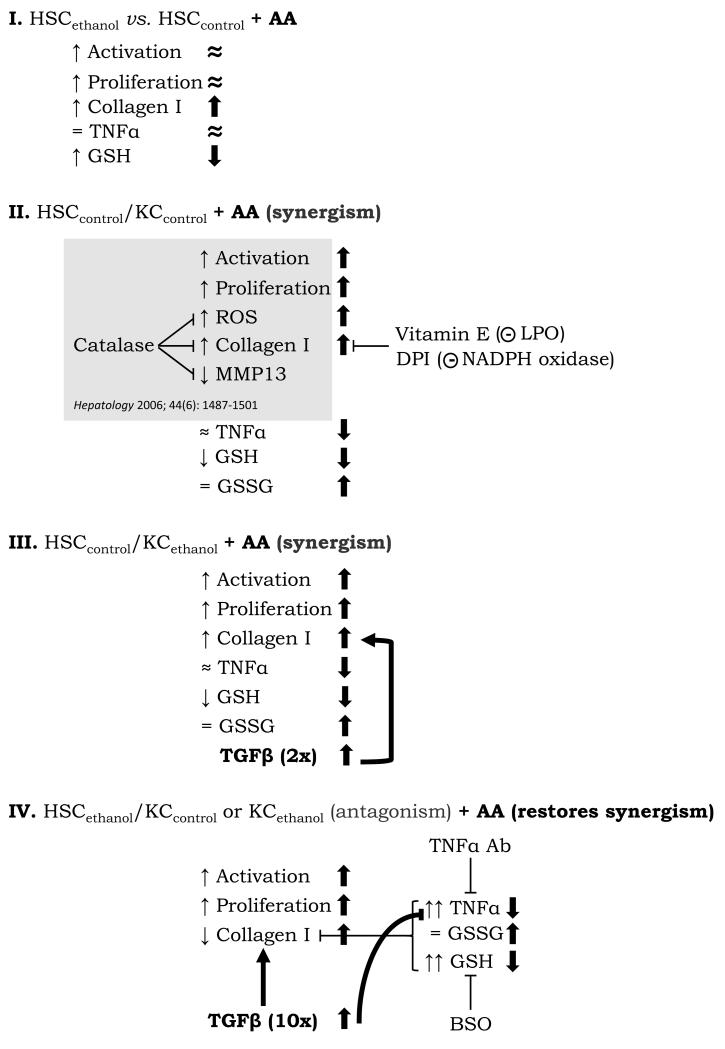
We previously developed a co-culture system to explore the crosstalk between KC and HSC (1). We now focused on delineating potential mechanisms whereby administration of two ‘hits’, chronic ethanol-feeding and a representative PUFA (e.g. AA), could modulate KC activation and the fibrogenic response in HSC and provide additional evidence on the complex intercellular communication that links both cell types with pathological ECM remodeling (for a summary of the main findings see Fig. 8).
Figure 8. Summary of Results and Proposed Model.
I. Effects of chronic alcohol consumption and AA on HSC mono-cultures; II. Effects of AA in the control co-culture, the grey box refers to previous findings published in Hepatology 2006; 44(6);1487-1501; III. Effects of AA in the co-culture of HSCcontrol with KCethanol; IV. Effects of AA in the co-culture of HSCethanol with KCcontrol or KCethanol. The contribution of AA is indicated in bold face. ↑: up-regulation by ethanol, ↓: down-regulation by ethanol, ⬆: up-regulated by AA, ⬇: down-regulated by AA, ≈: slight change.
As a first step, we investigated the effects of chronic ethanol-feeding and addition of AA on KC function and the release of critical mediators that could impact on ECM remodeling. KCethanol had a more activated phenotype, proliferated faster, and were more apoptotic than KCcontrol, and these effects were increased by AA. We then speculated that ROS would be the likely candidates for the above-described phenotype that could drive downstream effects on HSC. As previously reported by others (39, 40), KCethanol generated more ROS than KCcontrol and generation of these species was further enhanced by AA. Likewise, NADPH oxidase activity, a key pro-oxidant enzyme well-known to play a role in alcoholic liver disease (19, 41), increased in KCethanol compared with KCcontrol and AA synergistically up-regulated the activity. Because growth factors are highly ROS-sensitive and, as a corollary, they could regulate ROS production in KC, we evaluated levels of TNFα, known to be increased in alcoholic liver disease (22, 42) and to contribute to collagen I expression (7). Indeed, KCethanol showed elevated TNFα, which was up-regulated by AA as well.
Once the basal effects of ethanol and AA on KC were identified, we determined the contribution of AA to the fibrogenic response in HSC using the control co-culture previously established, and observed that the induced HSC activation, proliferation, ROS generation, and collagen I expression (1) was additionally enhanced by AA. Vitamin E, an antioxidant that inhibits the cascade of lipid peroxidation-derived reactions, prevented the increase in collagen I by AA only in the co-culture. Since vitamin E directly affected the response to AA by lowering ROS production in KC cultured alone, its antifibrogenic potential was likely restricted to KC-derived ROS. However, DPI blunted the up-regulation of collagen I by both the co-culture and the AA-treatment. DPI inhibits NADPH oxidase not only in KC but also in HSC (39), which could as well play a role, and may account for its maximal effects on collagen I down-regulation.
Next, we assessed the involvement of chronic alcohol feeding to the effects mediated by KC on HSC. Chronic ethanol feeding induced activation, cell proliferation, collagen I expression, and GSH levels in HSC cultured alone. The co-cultures established with cells isolated from control and from ethanol-fed rats indicated that HSCcontrol and HSCethanol proliferation rates increase in the co-cultures and are higher in the presence of KCethanol. While HSCcontrol responded to KCcontrol and KCethanol signals up-regulating collagen I; however, HSCethanol were resistant. Based on the above-identified mediators released by KC, we measured GSH and TNFα in the co-cultures as candidate antagonistic signals. Although GSH recycling was considered, GSSG levels remained unchanged; therefore, it is possible that the GSH increase in HSCethanol may derive from up-regulated hepatocyte-generated GSH export as sinusoidal efflux plays a major role in hepatic GSH homeostasis (43, 44). The HSCethanol co-cultures revealed a clear induction of TNFα and similar GSH levels, suggesting a likely defense mechanism triggered by alcohol in HSC. To confirm this possibility, the HSCethanol co-cultures were incubated in the presence of a TNFα neutralizing Ab or l-buthionine sulfoximine, which depletes GSH pools. Both treatments restored collagen I protein to that of HSCethanol cultured alone. Since both cell types produce TNFα and GSH, it is conceivable that the TNFα neutralization had an effect on both KC and HSCethanol; likewise, BSO most likely depleted GSH levels on KC and HSCethanol as well, promoting oxidative stress, and triggering collagen I up-regulation.
To examine the cellular events that could take place by a potential synergism between ethanol and AA, the HSCcontrol and the HSCethanol co-cultures were incubated in the presence of AA. Collagen I protein was up-regulated over levels found in the HSCcontrol co-cultures. Whereas HSCethanol appeared resistant to the effects mediated by the co-culture with KCcontrol or with KCethanol, collagen I expression was restored to above basal levels when AA was present. We then analyzed whether TNFα and/or GSH could play a role in the latter effects. AA significantly decreased TNFα and GSH levels, while in some way, it increased GSSG in HSCethanol, likely leaving HSCethanol vulnerable to lipid peroxidation-end products and other species, which may have triggered the re-establishment of the pro-fibrogenic response. It is feasible that alcohol elevates GSH in HSC through hepatocyte GSH efflux as a protective mechanism, even though hepatocytes could also release pro-fibrogenic and pro-inflammatory signals (9), but when a second ‘hit’ such as AA enters the picture, HSC GSH stores are depleted, GSSG increases, and the pro-fibrogenic phenotype is reinstated. More notably, AA elicited a striking up-regulation of TGFβ, a powerful profibrogenic factor, which antagonizes TNFα and favors collagen I induction (4). Indeed, the TNFα and the TGFβ responsive elements on the COL1A2 promoter overlap (4), and Sp1, a ROS-sensitive transcription factor, is the point of convergence of the TGFβ and TNFα signaling pathways on the COL1A2 gene (4).
In view of the association between ethanol, TNFα, GSH, and collagen I protein expression in HSC, it is tempting to speculate that HSCethanol may develop a mechanism such, that TNFα and GSH may help the cell cope with the pro-oxidant injury, and surpass the increase in TGFβ. These two molecules may act as anti-fibrogenic signals in early liver injury. Likewise, HSCethanol which appear more responsive to self-activation, undergo somehow, a diversion of phenotype in the presence of KC, so that while some HSC may remain proliferative and perhaps pro-inflammatory, others may have greater potential for a pro-fibrogenic response to KC. Similarly, ethanol-feeding may induce HSC-derived mediators such as PDGF-BB which will clearly enhance proliferation while other KC-derived factors such as TNFα or metalloproteinases could act decreasing collagen I.
Activation of ECM-producing HSC when there is active tissue damage, as it occurs in alcoholic liver disease, could reveal chronic activation of the tissue repair process where, as a consequence of the reiterated damage, accumulation of collagen I may be impaired due to effective remodeling. It is also likely that the pro-fibrogenic role of AA in the co-cultures supersede the defense pathway started by ethanol. A potential synergistic role for ethanol and AA to the mechanism of KC-mediated ECM remodeling is suggested whereby chronic ethanol consumption may sensitize HSC to up-regulate antagonistic factors (TNFα and GSH) that may help the cell to cope with injury, while AA-derived reactions may operate as a negative feedback by increasing GSSG and TGFβ.
Supplementary Material
Suppl. Figure 1. AA Induces KCethanol Proliferation and Activation. Primary rat KC were incubated for 24h with 0-10μM AA and cell viability was monitored by the MTT assay (A). Cell proliferation was assessed by the rate of incorporation of methyl[3H]-thymidine into the DNA of KC (B). Results are average values ± SEM of N=9; **P<0.01 and ***P<0.001 for AA-treated vs. non-treated, and ••P<0.01 and •••P<0.001 for KCethanol vs. KCcontrol. Light micrographs from primary KCcontrol and KCethanol cultured for 6d and treated with 0-10μM AA for 24h (magnification= ×200) (C).
ACKNOWLEDGEMENT
The authors are grateful to Dr. Arthur I. Cederbaum for his helpful insight during this project. We thank Drs. Raquel Urtasun and Laura Conde de la Rosa for their critical review of the manuscript.
Financial support: Supported by a Postdoctoral Fellowship from the Spanish Ministry of Education and Science Ref. No. EX2006-0070 (F.J.C) and by the US Public Health Service Grant 5R01DK069286-03 from the National Institute of Diabetes and Digestive and Kidney Diseases (N.N.)
List of Abbreviations
- AA
arachidonic acid
- BSO
l-buthionine sulfoximine
- DPI
diphenyleneiodonium
- ECM
extracellular matrix
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HSCcontrol
control stellate cells
- HSCethanol
stellate cells from ethanol-fed rats
- KCcontrol
control Kupffer cells
- KCethanol
Kupffer cells from ethanol-fed rats
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
- TGFβ
transforming growth factor-β
REFERENCES
- 1.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of rodent Kupffer cells on stellate cells. Hepatology. 2006;44:1487–1501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Greenwel P, Inagaki Y, Hu W, Walsh M, Ramirez F. Sp1 is required for the early response of alpha2(I) collagen to transforming growth factor-beta1. J Biol Chem. 1997;272:19738–19745. doi: 10.1074/jbc.272.32.19738. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- 5.Stefanovic B, Brenner DA. 5′ stem-loop of collagen alpha 1(I) mRNA inhibits translation in vitro but is required for triple helical collagen synthesis in vivo. J Biol Chem. 2003;278:927–933. doi: 10.1074/jbc.M209175200. [DOI] [PubMed] [Google Scholar]
- 6.Lindquist JN, Kauschke SG, Stefanovic B, Burchardt ER, Brenner DA. Characterization of the interaction between alphaCP(2) and the 3′-untranslated region of collagen alpha1(I) mRNA. Nucleic Acids Res. 2000;28:4306–4316. doi: 10.1093/nar/28.21.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iraburu MJ, Dominguez-Rosales JA, Fontana L, Auster A, Garcia-Trevijano ER, Covarrubias-Pinedo A, Rivas-Estilla AM, et al. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology. 2000;31:1086–1093. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Trevijano ER, Iraburu MJ, Fontana L, Dominguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–970. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 9.Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 10.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 11.Nieto N. Ethanol and fish oil induce NFkappaB transactivation of the collagen alpha2(I) promoter through lipid peroxidation-driven activation of the PKC-PI3K-Akt pathway. Hepatology. 2007;45:1433–1445. doi: 10.1002/hep.21659. [DOI] [PubMed] [Google Scholar]
- 12.Nieto N, Cederbaum AI. Increased Sp1-dependent transactivation of the LAMgamma 1 promoter in hepatic stellate cells co-cultured with HepG2 cells overexpressing cytochrome P450 2E1. J Biol Chem. 2003;278:15360–15372. doi: 10.1074/jbc.M206790200. [DOI] [PubMed] [Google Scholar]
- 13.Maher JJ. Leukocytes as modulators of stellate cell activation. Alcohol Clin Exp Res. 1999;23:917–921. [PubMed] [Google Scholar]
- 14.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, Isayama F, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 15.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 16.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, et al. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 17.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 18.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono H, Enomoto N, Connor HD, Wheeler MD, Bradford BU, Rivera CA, Kadiiska MB, et al. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. Am J Physiol Gastrointest Liver Physiol. 2000;278:G467–476. doi: 10.1152/ajpgi.2000.278.3.G467. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, et al. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680–1689. doi: 10.1002/hep.510290633. [DOI] [PubMed] [Google Scholar]
- 21.Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, et al. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13:S39–50. doi: 10.1111/jgh.1998.13.s1.39. [DOI] [PubMed] [Google Scholar]
- 22.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 23.Thurman RG, Gao W, Connor HD, Adachi Y, Stachlewitz RF, Zhong Z, Knecht KT, et al. Role of Kupffer cells in failure of fatty livers following liver transplantation and alcoholic liver injury. J Gastroenterol Hepatol. 1995;10(Suppl 1):S24–30. doi: 10.1111/j.1440-1746.1995.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 24.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 25.Tsukamoto H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol Clin Exp Res. 1999;23:911–916. [PubMed] [Google Scholar]
- 26.Gressner AM. Liver fibrosis: perspectives in pathobiochemical research and clinical outlook. Eur J Clin Chem Clin Biochem. 1991;29:293–311. [PubMed] [Google Scholar]
- 27.Watanabe T, Niioka M, Hozawa S, Kameyama K, Hayashi T, Arai M, Ishikawa A, et al. Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol. 2000;33:224–235. doi: 10.1016/s0168-8278(00)80363-3. [DOI] [PubMed] [Google Scholar]
- 28.Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Tipoe GL, Liong EC, Leung TM, Nanji AA. A voluntary oral-feeding rat model for pathological alcoholic liver injury. Methods Mol Biol. 2008;447:11–31. doi: 10.1007/978-1-59745-242-7_2. [DOI] [PubMed] [Google Scholar]
- 30.Tipoe GL, Liong EC, Casey CA, Donohue TM, Jr., Eagon PK, So H, Leung TM, et al. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin Exp Res. 2008;32:669–682. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 31.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- 32.Fraga CG, Leibovitz BE, Tappel AL. Halogenated compounds as inducers of lipid peroxidation in tissue slices. Free Radic Biol Med. 1987;3:119–123. doi: 10.1016/s0891-5849(87)80006-0. [DOI] [PubMed] [Google Scholar]
- 33.Long L, Evans PJ, Halliwell B. Hydrogen peroxide in human urine: implications for antioxidant defense and redox regulation. Biochem Biophys Res Commun. 1999;262:605–609. doi: 10.1006/bbrc.1999.1263. [DOI] [PubMed] [Google Scholar]
- 34.Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. Faseb J. 2005;19:136–138. doi: 10.1096/fj.04-2291fje. [DOI] [PubMed] [Google Scholar]
- 35.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 36.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 37.Niki T, Rombouts K, De Bleser P, De Smet K, Rogiers V, Schuppan D, Yoshida M, et al. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999;29:858–867. doi: 10.1002/hep.510290328. [DOI] [PubMed] [Google Scholar]
- 38.Nieto N, Giron MD, Suarez MD, Gil A. Changes in plasma and colonic mucosa fatty acid profiles in rats with ulcerative colitis induced by trinitrobenzene sulfonic acid. Dig Dis Sci. 1998;43:2688–2695. doi: 10.1023/a:1026607428716. [DOI] [PubMed] [Google Scholar]
- 39.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varela-Rey M, Fontan-Gabas L, Blanco P, Lopez-Zabalza MJ, Iraburu MJ. Glutathione depletion is involved in the inhibition of procollagen alpha1(I) mRNA levels caused by TNF-alpha on hepatic stellate cells. Cytokine. 2007;37:212–217. doi: 10.1016/j.cyto.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S53–56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 42.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 43.Aw TY, Ookhtens M, Ren C, Kaplowitz N. Kinetics of glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1986;250:G236–243. doi: 10.1152/ajpgi.1986.250.2.G236. [DOI] [PubMed] [Google Scholar]
- 44.Ookhtens M, Hobdy K, Corvasce MC, Aw TY, Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985;75:258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. AA Induces KCethanol Proliferation and Activation. Primary rat KC were incubated for 24h with 0-10μM AA and cell viability was monitored by the MTT assay (A). Cell proliferation was assessed by the rate of incorporation of methyl[3H]-thymidine into the DNA of KC (B). Results are average values ± SEM of N=9; **P<0.01 and ***P<0.001 for AA-treated vs. non-treated, and ••P<0.01 and •••P<0.001 for KCethanol vs. KCcontrol. Light micrographs from primary KCcontrol and KCethanol cultured for 6d and treated with 0-10μM AA for 24h (magnification= ×200) (C).