Abstract
OBJECTIVE
Controversy surrounds appropriate risk factor targets in older adults with diabetes. We evaluated the proportion of older adults with diabetes meeting different targets, focusing on possible differences by race, and assessed whether demographic and clinical characteristics explained disparities.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional study of 5,018 participants aged 67–90 years (1,574 with and 3,444 without diagnosed diabetes) who attended visit 5 of the Atherosclerosis Risk in Communities (ARIC) study (2011–2013). Risk factor targets were defined using both stringent (and less stringent) goals: hemoglobin A1c (HbA1c) <7%, <53 mmol/mol (<8%, <64 mmol/mol); LDL cholesterol (LDL-c) <100 mg/dL (<130 mg/dL); and blood pressure (BP) <140/90 mmHg (<150/90 mmHg). We used Poisson regression to obtain prevalence ratios (PRs).
RESULTS
Most older adults with diabetes met stringent (and less stringent) targets: 72% (90%) for HbA1c, 63% (86%) for LDL-c, and 73% (87%) for BP; but only 35% (68%) met all three. A higher proportion of whites than blacks met targets, however defined. Among people treated for risk factors, racial disparities in prevalence of meeting stringent targets persisted even after adjustment: PRs (whites vs. blacks) were 1.03 (95% CI 0.91, 1.17) for HbA1c, 1.21 (1.09, 1.35) for LDL-c, 1.10 (1.00, 1.21) for BP, and 1.28 (0.99, 1.66) for all three. Results were similar but slightly attenuated using less stringent goals. Black women were less likely than white women to meet targets for BP and all three risk factors; this disparity was not observed in men.
CONCLUSIONS
Black-white disparities in risk factor control in older adults with diabetes were not fully explained by demographic or clinical characteristics and were greater in women than men. Further study of determinants of these disparities is important.
Introduction
The prevalence of type 2 diabetes in adults aged 65 years or older in the U.S. is ∼20–25% (1–3). There is currently much controversy regarding the best approaches to treatment and management of diabetes in older adults, particularly appropriate targets for glycemic and cardiovascular risk factor control (4–6).
Randomized clinical trials have demonstrated that in adults with diabetes, lowering hemoglobin A1c (HbA1c) and blood pressure (BP) reduces the risk of microvascular disease, and controlling BP and lipids reduces cardiovascular disease (CVD) risk (7,8). Citing evidence that simultaneously controlling multiple risk factors reduces CVD risk (9,10), the American Diabetes Association (ADA) has established targets for three key modifiable risk factors for people with diabetes: HbA1c <7% (<53 mmol/mol); LDL cholesterol (LDL-c) <100 mg/dL; and systolic BP (SBP) <140 mmHg and diastolic BP (DBP) <90 mmHg (11).
Previous studies have shown that ∼20–30% of adults of all ages with diagnosed diabetes in the general population meet all three risk factor targets and that this proportion has increased over the past decade (12–14). Nonetheless, there is evidence for disparities in risk factor control in racial/ethnic minorities compared with whites (12,15–18). Characterizing risk factor control in older adults is particularly important since treatment targets are especially controversial (4,19). The evidence base for current treatment targets comes largely from randomized clinical trials in middle-aged adults. These findings may not apply to older adults with diabetes who may not live long enough to experience the full microvascular benefits of tight glycemic control. Further, adverse effects of pharmacologic treatment and tight control are of particular concern in older adults (20–22). Indeed, hypoglycemia is one of the most common side effects of glucose-lowering treatment and is associated with substantial morbidity and mortality (23–26). In older adults, the risks of stringent glycemic control, in particular, may outweigh the benefits. Individualized, less stringent risk factor targets have been proposed for older adults, for whom the presence of comorbidities and functional status vary greatly (4,11).
We conducted a cross-sectional study in community-dwelling older adults with diabetes to 1) evaluate the prevalence of glycemic, lipid, and BP control, overall and by race (black or white), and 2) to investigate correlates of meeting treatment targets and evaluate if racial differences in risk factor control could be explained by demographic and clinical characteristics.
Research Design and Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a community-based cohort of 15,792 participants recruited from Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD (27). Visits 1–5 took place from 1987 to 1989, 1990 to 1992, 1993 to 1995, 1996 to 1998, and 2011 to 2013, respectively. Institutional review boards at each site approved all procedures, and all study participants provided written informed consent.
There were 6,538 ARIC participants who attended visit 5 (2011–2013) (all participants were >65 years of age at the time of this visit). We excluded participants who were nonwhite or nonblack (n = 18), missing key covariates (additional n = 1,295), or not fasting for ≥8 h (additional n = 207), resulting in 5,018 participants. Our primary analysis was restricted to participants with diagnosed diabetes (n = 1,574). We identified people as having diagnosed diabetes if they self-reported a physician diagnosis of diabetes or use of glucose-lowering medication at any of the first four visits or during any of the annual telephone calls conducted after visit 4 or if they were taking any glucose-lowering medications at the visit 5 examination (Supplementary Fig. 1). Self-reported diabetes in the ARIC study has been previously reported to have moderate sensitivity (>55%), high specificity (>80%), and high reliability (>92%) (28).
Laboratory Measurements
HbA1c was measured in whole blood samples using a Tosoh G7 automated analyzer (Tosoh Medics, Inc., San Francisco, CA) using a high-performance liquid chromatography method standardized to the Diabetes Control and Complications Trial assay. LDL-c was calculated using the Friedewald equation, which includes total cholesterol, HDL cholesterol, and triglycerides, all of which were measured in stored plasma samples by an enzymatic method using a Beckman Coulter Olympus AU 480 (Beckman Coulter, Inc., Brea, CA). Serum creatinine was measured using a Jaffe reaction, which is a colorimetric method, also on the Beckman Coulter Olympus AU 480 (Beckman Coulter, Inc.).
Additional Variables of Interest
The following characteristics were self-reported during visit 5, unless stated otherwise: age; race/ethnicity; sex; alcohol consumption; current smoking status; education level (asked at visit 1); physical activity level, assessed using the sport index of the Baecke physical activity questionnaire (29); household income; health insurance coverage besides Medicare; self-rated health; and use of glucose-lowering, cholesterol-lowering, and antihypertensive medications. Diabetes duration was calculated as the amount of time between the date of the first report of either physician diagnosis of diabetes or glucose-lowering medication use and the date of the visit 5 exam. BMI was calculated as measured weight (in kilograms) divided by measured height (in meters squared). SBP and DBP were recorded as the mean of the second and third readings.
Physical function was assessed using the Short Physical Performance Battery (SPPB), with higher scores indicating better function (30–32). Functional disability status was defined as self-reported difficulty completing 1 of 12 tasks, based on questionnaires administered during the annual and semiannual phone calls prior to visit 5 (33–36). Reduced renal function was defined as creatinine-based estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (37). Prevalent CVD was defined as history of coronary heart disease, stroke, or heart failure as determined by either self-reported history at visit 1 or an adjudicated event at or prior to the visit 5 exam.
Statistical Analysis
Stringent risk factor targets were defined using 2015 ADA-recommended cut points: glycemic control, HbA1c <7.0% (<53 mmol/mol); lipid control, LDL-c <100 mg/dL; and BP control, SBP <140 and DBP <90 mmHg (11). Less stringent targets were defined as follows: HbA1c <8% (<64 mmol/mol); LDL-c <130 mg/dL; and SBP <150 and DBP <90 mmHg (11,38–40). We compared characteristics of people with and without diabetes, separately, further stratifying those with diabetes by glucose-lowering medication use. We used Student t tests for comparisons of continuous variables and χ2 tests for comparisons of categorical variables. All subsequent analyses were restricted to people with diabetes. We calculated the prevalence of risk factor control (i.e., proportion of people meeting treatment targets) in people with diabetes overall and stratified by race and sex. We used Poisson regression models with robust variance to assess unadjusted associations of demographic and clinical characteristics with meeting risk factor targets and to obtain prevalence ratios (PRs) and 95% CIs for meeting treatment targets in whites versus blacks. We compared four models. Model 1 was adjusted for age (years) and sex (male or female). Model 2 was adjusted for all variables in model 1 plus annual household income (<$25,000, $25,000 to <$50,000, ≥$50,000, or not reported), education level (<high school, high school or college, or >college), and health insurance status in addition to Medicare (yes or no). Model 3 was adjusted for all variables in model 2 plus cigarette smoking status (current, former, never, or indeterminate), alcohol consumption (current, former, or never), physical activity, BMI (kg/m2), prevalent CVD (yes or no), self-rated health (fair to poor or good to excellent), eGFR <60 mL/min/1.73 m2 (yes or no), any functional disability (yes or no), physical function, and duration of diabetes (years). Model 4 was adjusted for all variables in model 3 plus medication use (glucose-lowering, cholesterol-lowering, and BP-lowering medication use) for risk factors other than the outcome of interest (e.g., adjusted for cholesterol-lowering and BP-lowering medication use in analyses of glycemic control). In model 4, we tested the statistical significance of the inclusion of the interaction between race and sex using a Wald test.
We conducted analyses in all people with diabetes, regardless of medication use, as well as restricted to people with diabetes who were treated for each risk factor. As a sensitivity analysis, we calculated the prevalence of meeting risk factor targets by duration of diabetes (<5, 5–15, and ≥15 years).
All analyses were conducted using Stata version 13.0 (College Station, TX).
Results
Characteristics of the Study Population
Among the 5,018 participants with complete data at visit 5, 1,574 (31%) had diagnosed diabetes. Among people with diagnosed diabetes, the mean age was 75 years (SD 5.0; range 67–89 years), 44% were male, and 29% were black. People with diabetes were more likely to be taking cholesterol- or BP-lowering medications compared with people without diabetes (P < 0.01). Among people with diabetes, those who were taking glucose-lowering medication were more likely to be black, male, and obese and have lower education or income compared with those not taking glucose-lowering medication (P < 0.05 for all) (Table 1). Additionally, 24% of people with treated diabetes were taking insulin.
Table 1.
Study population characteristics, ARIC visit 5 (2011–2013)
| P value* | Diagnosed diabetes |
P value† | |||
|---|---|---|---|---|---|
| No history of diagnosed diabetes (n = 3,444) |
No glucose-lowering medication (n = 569) | Current glucose-lowering medication (n = 1,005) |
|||
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |||
| Age, years | 75.4 (5.1) | 0.16 | 75.7 (5.3) | 75.0 (4.9) | 0.01 |
| White | 82.4 | <0.01 | 80.5 | 65.8 | <0.01 |
| Male | 40.3 | 0.03 | 40.1 | 45.5 | 0.04 |
| <High school education | 10.7 | <0.01 | 14.6 | 18.9 | 0.03 |
| Annual household income <$25,000 | 22.2 | <0.01 | 26.5 | 33.9 | <0.01 |
| Health insurance in addition to Medicare | 89.0 | <0.01 | 86.3 | 83.1 | 0.09 |
| Self-rated health ≥good‡ | 92.5 | <0.01 | 85.8 | 79.5 | <0.01 |
| Current alcohol consumption | 55.0 | <0.01 | 49.0 | 35.7 | <0.01 |
| Current smoking | 5.7 | 0.96 | 5.8 | 5.6 | 0.85 |
| Obese (BMI ≥30 kg/m2) | 28.8 | <0.01 | 38.7 | 52.9 | <0.01 |
| HbA1c, % | 5.6 (0.4) | <0.01 | 5.9 (0.6) | 7.0 (1.2) | <0.01 |
| HbA1c, mmol/mol | 38.2 (4.3) | <0.01 | 40.8 (6.4) | 52.5 (12.6) | <0.01 |
| LDL-c, mg/dL | 109.2 (34.1) | <0.01 | 101.3 (32.7) | 88.1 (32.1) | <0.01 |
| SBP, mmHg | 130.1 (17.3) | 0.65 | 128.6 (18.1) | 130.5 (18.6) | 0.05 |
| DBP, mmHg | 67.2 (10.4) | <0.01 | 65.6 (10.8) | 65.0 (10.4) | 0.23 |
| % meeting stringent targets | |||||
| HbA1c <7% (<53 mmol/mol) | 99.4 | <0.01 | 95.8 | 59.2 | <0.01 |
| LDL-c <100 mg/dL | 41.7 | <0.01 | 52.6 | 69.7 | <0.01 |
| SBP <140 and DBP <90 mmHg | 73.5 | 0.57 | 75.2 | 71.3 | 0.10 |
| All three | 31.2 | <0.01 | 38.7 | 33.5 | 0.04 |
| % meeting less stringent targets | |||||
| HbA1c <8% (<64 mmol/mol) | 100.0 | <0.01 | 99.3 | 85.4 | <0.01 |
| LDL-c <130 mg/dL | 74.9 | <0.01 | 79.3 | 89.8 | <0.01 |
| SBP <150 and DBP <90 mmHg | 86.5 | 0.90 | 87.7 | 86.1 | 0.36 |
| All three | 65.1 | 0.02 | 70.0 | 67.5 | 0.31 |
| Medication use | |||||
| Glucose lowering | N/A | N/A | 0 | 100 | <0.01 |
| Cholesterol lowering | 48.1 | <0.01 | 59.9 | 75.0 | <0.01 |
| BP lowering | 60.5 | <0.01 | 76.3 | 85.6 | <0.01 |
| All of the above | N/A | N/A | 0 | 66.6 | <0.01 |
| Baecke sport index | 2.7 (0.8) | <0.01 | 2.5 (0.8) | 2.4 (0.7) | 0.10 |
| Any functional disability | 47.8 | <0.01 | 61.2 | 66.5 | 0.03 |
| Physical function score (SPPB) | 9.6 (2.3) | <0.01 | 9.1 (2.6) | 8.5 (2.8) | <0.01 |
| Prevalent CVD | 15.7 | <0.01 | 22.7 | 27.0 | 0.06 |
| eGFR <60 mL/min/1.73 m2 | 25.5 | <0.01 | 32.0 | 33.7 | 0.48 |
| Duration of diabetes | |||||
| <5 years | N/A | N/A | 28.3 | 18.8 | <0.01 |
| 5–15 years | N/A | 65.0 | 54.1 | ||
| ≥15 years | N/A | 6.7 | 27.1 | ||
P values were calculated using Student t tests for continuous variables and χ2 tests for categorical variables. N/A, not applicable.
*P value for people with vs. without diabetes;
†P value for people treated with glucose-lowering medication vs. untreated;
‡good, very good, or excellent (vs. fair or poor).
Unadjusted Prevalence of Meeting Risk Factor Targets
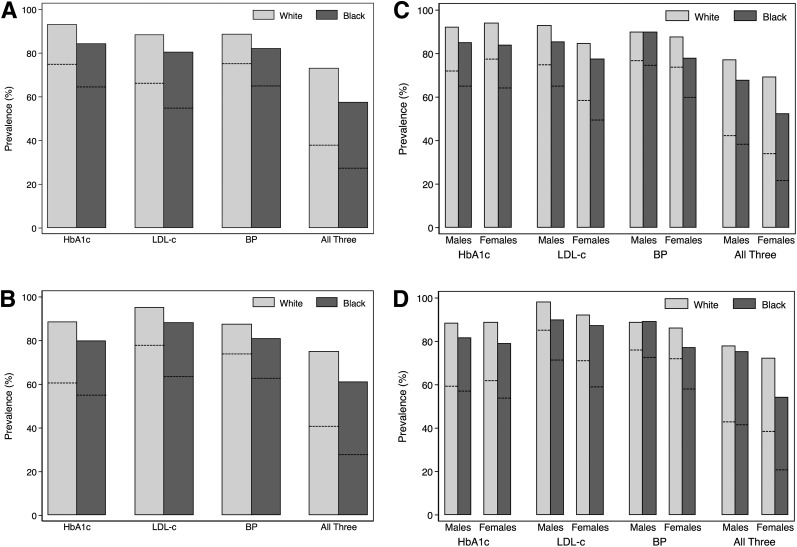
Among all people with diabetes, the percentages of those who met stringent (and less stringent) targets were 72% (90%) for HbA1c, 63% (86%) for LDL-c, 73% (87%) for BP, and 35% (68%) for simultaneous control of all three risk factors. Among people who were pharmacologically treated for the individual risk factors, the percentages were 59% (85%) for HbA1c, 75% (93%) for LDL-c, 71% (85%) for BP, and 37% (70%) for all three. Overall, whites were more likely than blacks to meet risk factor targets for any of the three risk factors (Fig. 1A and Supplementary Table 5). Racial disparities were similar among people who were taking glucose-, cholesterol-, or BP-lowering medication, although the association of race with control of HbA1c was not statistically significant in treated people (Fig. 1B and Table 2).
Figure 1.
Prevalence of meeting risk factor targets in older adults with diabetes. Stringent targets were HbA1c <7% (<53 mmol/mol), LDL-c <100 mg/dL, SBP <140 mmHg, and DBP <90 mmHg. Less stringent targets were HbA1c <8% (<64 mmol/mol), LDL-c <130 mg/dL, SBP <150 mmHg, and DBP <90 mmHg. Bars indicate the proportion of participants meeting less stringent targets. Dashed lines indicate the proportion of participants meeting stringent targets. A: By race, according to either stringent or less stringent risk factor targets. n = 1,119 white and n = 455 black participants. B: By race, according to either stringent or less stringent risk factor targets, and restricted to people pharmacologically treated for risk factors. n = 661 white and n = 344 black participants treated for glucose; n = 800 white and n = 295 black participants treated for lipids; n = 880 white and n = 414 black participants treated for BP; n = 443 black and n = 226 white participants treated for all three risk factors. C: By race and sex, according to either stringent or less stringent risk factor targets. n = 528 white male, n = 157 black male, n = 591 white female, and n = 298 black female participants. D: By race and sex, according to either stringent or less stringent risk factor targets, and restricted to people pharmacologically treated for risk factors. n = 339 white male, n = 118 black male, n = 322 white female, and n = 226 black female participants treated for glucose; n = 384 white male, n = 107 black male, n = 416 white female, and n = 188 black female participants treated for lipids; n = 414 white male, n = 134 black male, n = 466 white female, and n = 280 black female participants treated for BP; n = 228 white male, n = 76 black male, n = 215 white female, and n = 150 black female participants treated for all three risk factors.
Table 2.
Adjusted associations of race (white vs. black) with meeting stringent risk factor targets* among participants treated for risk factors
| HbA1c (n = 1,005) | LDL-c (n = 1,095) | BP (n = 1,294) | HbA1c, LDL-c, and BP (n = 669) | |
|---|---|---|---|---|
| PR (95% CI) for being at target for white vs. black | PR (95% CI) for being at target for white vs. black | PR (95% CI) for being at target for white vs. black | PR (95% CI) for being at target for white vs. black | |
| Unadjusted | 1.10 (0.98, 1.23) | 1.22 (1.11, 1.34) | 1.18 (1.08, 1.28) | 1.46 (1.15, 1.85) |
| Model 1 | 1.09 (0.97, 1.22) | 1.19 (1.09, 1.31) | 1.18 (1.08, 1.28) | 1.39 (1.09, 1.76) |
| Model 2 | 1.03 (0.91, 1.17) | 1.16 (1.05, 1.29) | 1.13 (1.03, 1.24) | 1.30 (1.00, 1.68) |
| Model 3 | 1.05 (0.92, 1.19) | 1.18 (1.06, 1.31) | 1.11 (1.00, 1.22) | 1.28 (0.99, 1.66) |
| Model 4 | 1.03 (0.91, 1.17) | 1.21 (1.09, 1.35) | 1.10 (1.00, 1.21) | N/A |
Poisson regression with robust variance (sandwich estimator) was used to obtain PRs. Bolded results are statistically significant (P < 0.05). Model 1 was adjusted for age and sex. Model 2 was adjusted for all variables in model 1 plus income, education, and health insurance. Model 3 was adjusted for all variables in model 2 plus smoking status, alcohol consumption, physical activity, BMI, prevalent CVD, self-rated health, eGFR <60 mL/min/1.73 m2, any functional disability, physical function score, and diabetes duration. Model 4 was adjusted for all variables in model 3 plus glucose-lowering medication use, cholesterol-lowering medication use, and BP-lowering medication use (if not the risk factor of interest). N/A, not applicable.
*At target defined as follows: HbA1c <7% (<53 mmol/mol); LDL-c <100 mg/dL; SBP <140 and DBP <90 mmHg.
Of the race-sex groups, black women were least likely to have risk factors that were at or below treatment targets (Fig. 1C). Indeed, greater black-white disparities in risk factor control were observed in women as compared with men (Fig. 1C). Patterns in race-sex differences were similar when restricting analyses to people with diabetes who were treated for risk factors (Fig. 1D).
People with diabetes of ≥15 years duration were less likely to meet treatment targets for BP and HbA1c compared with people with a shorter duration of diabetes (Supplementary Fig. 2A).
Correlates of Meeting Targets in Older Adults With Diabetes Who Were Pharmacologically Treated for Risk Factors
Older people with diabetes who met risk factor targets were generally more likely to be male, to be white, to have a higher income, to have higher physical function scores, and to have diabetes for a shorter duration (Table 3). These patterns were similar when using less stringent cut points (Supplementary Table 1) (see Supplementary Tables 2 and 3 for study population characteristics stratified by whether meeting stringent or less stringent targets).
Table 3.
Unadjusted associations of participant characteristics with meeting stringent risk factor targets* in participants with diagnosed diabetes treated for risk factors
| HbA1c (n = 1,005) | LDL-c (n = 1,095) | BP (n = 1,294) | HbA1c, LDL-c, and BP (n = 669) | |
|---|---|---|---|---|
| PR (95% CI) for at target vs. not at target |
PR (95% CI) for at target vs. not at target |
PR (95% CI) for at target vs. not at target |
PR (95% CI) for at target vs. not at target |
|
| White (vs. black) | 1.10 (0.98, 1.23) | 1.22 (1.11, 1.34) | 1.18 (1.08, 1.28) | 1.46 (1.15, 1.85) |
| Age (per 5 years) | 1.05 (0.99, 1.10) | 1.03 (1.00, 1.07) | 0.95 (0.92, 0.99) | 1.04 (0.94, 1.15) |
| Male (vs. female) | 1.00 (0.90, 1.11) | 1.22 (1.14, 1.30) | 1.13 (1.05, 1.21) | 1.36 (1.11, 1.65) |
| Education (vs. <high school) | ||||
| Some college | 0.98 (0.85, 1.12) | 1.08 (0.97, 1.20) | 1.17 (1.05, 1.30) | 1.11 (0.83, 1.48) |
| More than college | 0.99 (0.86, 1.14) | 1.12 (1.00, 1.25) | 1.11 (0.99, 1.24) | 1.16 (0.87, 1.56) |
| Household income (vs. <$25,000) | ||||
| $25,000–49,999 | 1.23 (1.07, 1.41) | 1.10 (1.00, 1.21) | 1.12 (1.02, 1.23) | 1.42 (1.07, 1.88) |
| ≥$50,000 | 1.26 (1.10, 1.44) | 1.18 (1.08, 1.30) | 1.16 (1.06, 1.27) | 1.75 (1.34, 2.29) |
| Not reported | 1.12 (0.89, 1.41) | 1.19 (1.05, 1.35) | 1.08 (0.93, 1.24) | 1.53 (1.02, 2.29) |
| Additional health insurance (vs. Medicare only) | 1.02 (0.89, 1.17) | 1.14 (1.01, 1.28) | 1.11 (1.00, 1.24) | 1.12 (0.84, 1.48) |
| Good health or better (vs. fair or poor health) | 1.08 (0.94, 1.23) | 1.08 (0.98, 1.19) | 1.07 (0.98, 1.18) | 1.14 (0.89, 1.46) |
| Alcohol consumption (vs. current) | ||||
| Former | 0.95 (0.85, 1.08) | 0.97 (0.90, 1.05) | 0.93 (0.87, 1.01) | 0.87 (0.70, 1.09) |
| Never | 1.01 (0.88, 1.15) | 0.87 (0.79, 0.96) | 0.86 (0.78, 0.94) | 0.77 (0.60, 1.01) |
| Smoking status (vs. current) | ||||
| Former | 0.95 (0.77, 1.18) | 1.02 (0.88, 1.19) | 0.95 (0.83, 1.10) | 0.92 (0.60, 1.40) |
| Never | 0.97 (0.78, 1.20) | 0.94 (0.80, 1.09) | 0.89 (0.77, 1.03) | 0.86 (0.56, 1.32) |
| Indeterminate | 0.76 (0.55, 1.04) | 0.99 (0.81, 1.21) | 0.84 (0.68, 1.03) | 0.46 (0.23, 0.94) |
| BMI (per kg/m2) | 0.99 (0.98, 1.00) | 1.00 (0.99, 1.01) | 1.00 (1.00, 1.01) | 0.99 (0.97, 1.00) |
| Glucose-lowering medication | N/A | 1.20 (1.10, 1.31) | 0.96 (0.89, 1.03) | N/A |
| Cholesterol-lowering medication | 1.11 (0.98, 1.26) | N/A | 1.14 (1.04, 1.24) | N/A |
| BP-lowering medication | 0.91 (0.80, 1.04) | 1.09 (0.97, 1.22) | N/A | N/A |
| Baecke sport index | 1.05 (0.98, 1.12) | 1.04 (0.99, 1.09) | 1.02 (0.98, 1.07) | 1.07 (0.94, 1.23) |
| Any functional disability | 0.87 (0.79, 0.97) | 0.90 (0.84, 0.97) | 1.00 (0.93, 1.08) | 0.79 (0.65, 0.96) |
| Physical function score (SPPB) | 1.02 (1.00, 1.04) | 1.00 (0.99, 1.02) | 1.03 (1.01, 1.04) | 1.07 (1.03, 1.11) |
| Prevalent CVD | 0.89 (0.79, 1.01) | 1.18 (1.11, 1.26) | 0.95 (0.88, 1.03) | 0.98 (0.79, 1.22) |
| eGFR <60 mL/min/1.73 m2 | 0.98 (0.88, 1.09) | 1.04 (0.97, 1.12) | 0.96 (0.89, 1.03) | 0.88 (0.71, 1.08) |
| Duration of diabetes (per 5 years) | 0.83 (0.80, 0.87) | 1.02 (0.99, 1.05) | 0.96 (0.93, 0.99) | 0.81 (0.75, 0.87) |
Bolded results indicate P < 0.05. N/A, not applicable.
*Risk factor targets defined as follows: HbA1c <7% (<53 mmol/mol); LDL-c <100 mg/dL; SBP <140 and DBP <90 mmHg.
In unadjusted models, black-white disparities were statistically significant for meeting all targets except for HbA1c (white vs. black: PR 1.10 [95% CI 0.98, 1.23] for HbA1c, 1.22 [1.11, 1.34] for LDL-c, 1.18 [1.08, 1.28] for BP, and 1.46 [1.15, 1.85] for all three). In fully adjusted models, whites remained more likely to meet LDL-c targets as compared with blacks (PR 1.21 [1.09, 1.35]) (Table 2). There was evidence of a statistical interaction of race and sex with meeting targets for BP and all three risk factors simultaneously (P values for interaction were 0.08 and 0.03, respectively). Among men, there was no significant difference in meeting BP targets in whites versus blacks (PR 0.98 [0.86, 1.13]) (Supplementary Table 4). However, white women were more likely than black women to meet BP targets (PR 1.18 [1.04, 1.35]) (Supplementary Table 4). Likewise, the prevalence of meeting targets for all three risk factors simultaneously was similar in white and black men (PR 0.95 [0.69, 1.32]), whereas white women were more likely than black women to meet all three targets (1.58 [1.08, 2.32]) (Supplementary Table 4). Results in all people with diabetes regardless of medication use were similar (Supplementary Table 5).
When using less stringent cut points, adjusted associations of race (white vs. black) with meeting risk factor targets were marginally significant for HbA1c (PR 1.07 [95% CI 1.00, 1.15]) and statistically significant for LDL-c (1.06 [1.01, 1.11]) (Supplementary Table 6). Results were similar in all participants with diabetes, regardless of whether or not they were treated for risk factors (PRs ranged from 1.04 to 1.10 and P values ranged from 0.01 to 0.21) (Supplementary Table 7).
Conclusions
Each of the stringent (ADA 2015) targets for HbA1c, LDL-c, or BP was met by approximately two-thirds of older adults with diabetes in the ARIC study. However, only about one-third of older adults met targets for all three risk factors. A much larger proportion of older adults with diabetes met less stringent risk factor targets. Whites were more likely to meet LDL-c targets than blacks. Adjustment for demographic and clinical characteristics, including functional status and comorbidities, did not appreciably change the association of race with meeting targets. There were also sex differences in racial disparities for meeting risk factor targets. Among women, whites were more likely than blacks to meet targets for BP and for all three risk factors simultaneously; however, these racial disparities were not observed in men.
Comparing treated versus untreated people, the prevalence of meeting targets was lower for HbA1c and higher for LDL-c. Glucose-lowering medication may be a marker of disease severity, and we therefore observed worse glycemic control in people who were treated compared with those who were being managed with diet and/or lifestyle only. In contrast, the large majority of ARIC participants with diagnosed diabetes at visit 5 (70%) reported using cholesterol-lowering medications, and we observed lower LDL-c levels in treated people. Furthermore, a higher proportion of people reporting use of cholesterol-lowering medications had a history of CVD. Guidelines suggest more aggressive treatment targets for LDL-c in people with a history of CVD, which could have also contributed to our observation of lower lipid levels in treated participants.
Our findings extend those from previous studies that have reported racial and socioeconomic disparities in risk factor control in people with diabetes. Studies conducted in middle-aged (17,18,41,42) and older (43) adults with diabetes have shown that ethnic minority populations are less likely to meet glycemic, lipid, or BP targets. In a recent analysis of adults of all ages with diabetes from the National Health and Nutrition Examination Survey, white race (compared with black race) was associated with meeting HbA1c targets, and a higher level of education was associated with meeting targets for HbA1c and BP (16). However, in contrast to our study, the authors reported no associations of race or education with lipid control (16). Furthermore, the authors suggested that racial disparities in glycemic control have increased over the past couple of decades and may be driven by improved glycemic control in people with higher education levels, whereas rates of glycemic control have remained stable in people with less than a high school education (16). However, in our study of older adults with diabetes, we did not find that educational differences entirely explained racial differences in risk factor control. There could be racial differences in access to health care or treatment approaches, as well as medication adherence, which could contribute to the observed racial disparities in risk factor control.
Previous studies have also reported sex differences in risk factor control. A recent study of veterans with diabetes found that women had higher lipid levels than men and were less likely to be on cholesterol-lowering medication (44), and another found that among adults with diabetes, women had worse control of cardiovascular risk factors than men (45). Our study extends these findings and suggests there may be important sex disparities in approaches and/or adherence to care.
Some guidelines, such as those from the American Geriatrics Society, recommend different treatment targets based on age or comorbidity status (39,46). Heterogeneity in risk of complications is clearly important in older adults. Many older adults may not reap the full benefits of tight risk factor control, particularly glycemic control, and may be overtreated. Older adults may be at particularly high risk of hypoglycemia and/or hypotension, and risks of tight treatment targets may outweigh the benefits. The competing risk of death and other conditions may make an emphasis on microvascular disease prevention less relevant. Whereas there was evidence from the Steno-2 Study that simultaneous tight control of all three risk factors reduced the risk of vascular complications and death in middle-aged adults (10), the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial reported that intensive treatment to low glucose targets did not reduce the risk of cardiovascular events and actually increased the risk of mortality (22). There is growing emphasis on the need for individualized treatment targets but it is unclear how to optimize treatment in older adults to maximize health benefits and minimize adverse outcomes (47–49). It remains unclear whether and how to most appropriately consider less stringent treatment targets in older adults.
There are several limitations that should be considered in the interpretation of our results. We used risk factor treatment targets recommended by the ADA in 2015. However, BP targets have changed over the past several years and were different at the time of participation in visit 5 of the ARIC study, from 2011 to 2013. Therefore, participants may have actually been treated to lower BP targets during that time period. The large majority of black participants in the ARIC study were recruited from two of four study sites (Jackson, MS and Forsyth County, NC). Thus, we cannot definitively separate race and geographic differences. We were also unable to fully account for potential racial differences in access to health care, treatment approaches, or medication adherence. This was a cross-sectional study, and attrition (loss to follow-up) resulting in selection bias is a salient concern. People with poorly controlled diabetes and severe comorbidities may have been less likely to attend ARIC visit 5 than their healthier counterparts and would not have been included in this study. Indeed, cross-sectional studies have found older adults to have better risk factor control than younger individuals (12,14,50,51). Strengths of this study include the large, biethnic community–based population of older adults in the contemporary era and the rigorous measurement of diabetes and cardiovascular risk factors. Our results are highly relevant and may be generalizable to other contemporary community-dwelling populations of older adults living with diabetes.
Among older adults with diabetes, the association of race with meeting targets for lipids was not fully explained by demographic and clinical characteristics. Our results suggest a need to improve care in ethnic minorities, particularly black women, to narrow this racial disparity. However, older adults are a heterogeneous group, and the benefit of treatment to very low risk factor targets is unclear. To define appropriate treatment approaches and risk factor targets in older adults with diabetes, randomized clinical trials in this population may be needed. Additional studies should examine the effects of treating to tight versus less stringent risk factor targets on macrovascular and microvascular outcomes and mortality and should include people with comorbidities to assess the potential benefits of individualized treatment targets.
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding. C.M.P. is supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Cardiovascular Epidemiology training grant T32-HL-007024. J.G.G. is supported by NIH/National Institute on Aging Epidemiology and Biostatistics of Aging training grant T32-AG-000247. E.S. is supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-089174. The ARIC study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.P. designed the study, analyzed and interpreted the data, and wrote the manuscript. I.R. designed the study, analyzed and interpreted the data, and reviewed and edited the manuscript. J.G.G., M.D.M., and K.M. interpreted the data and reviewed and edited the manuscript. E.S. designed the study, interpreted the data, and reviewed and edited the manuscript. E.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the American Heart Association's Epidemiology and Prevention and Lifestyle and Cardiometabolic Health 2015 Scientific Sessions, Baltimore, MD, 3–6 March 2015.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0016/-/DC1.
References
- 1.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 4.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 5.Turnbull FM, Abraira C, Anderson RJ, et al.; Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes [published correction appears in Diabetologia 2009;52:2470]. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 9.Buse JB, Ginsberg HN, Bakris GL, et al.; American Heart Association; American Diabetes Association . Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007;30:162–172 [DOI] [PubMed] [Google Scholar]
- 10.Gaede P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 12.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335–342 [DOI] [PubMed] [Google Scholar]
- 14.Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014;161:681–689 [DOI] [PubMed] [Google Scholar]
- 15.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 16.Chatterji P, Joo H, Lahiri K. Racial/ethnic- and education-related disparities in the control of risk factors for cardiovascular disease among individuals with diabetes. Diabetes Care 2012;35:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egede LE, Gebregziabher M, Lynch CP, Gilbert GE, Echols C. Longitudinal ethnic differences in multiple cardiovascular risk factor control in a cohort of US adults with diabetes. Diabetes Res Clin Pract 2011;94:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland AT, Zhao B, Wong EC, Choi SE, Wong ND, Palaniappan LP. Racial/ethnic differences in control of cardiovascular risk factors among type 2 diabetes patients in an insured, ambulatory care population. J Diabetes Complications 2013;27:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002-2012 literature review. J Am Geriatr Soc 2013;61:2027–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghissi E. Management of type 2 diabetes mellitus in older patients: current and emerging treatment options. Diabetes Ther 2013;4:239–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Diabetes Public Health Resource. Emergency department visit rates for hypoglycemia as first-listed diagnosis per 1,000 diabetic adults aged 18 years or older, by age, United States, 2006–2009, 2012. Available from http://www.cdc.gov/diabetes/statistics/hypoglycemia/fig5byage.htm. Accessed 16 October 2014
- 22.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014;174:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 25.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014;174:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 28.Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 2012;176:738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 33.Kuo HK, Bean JF, Yen CJ, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. J Gerontol A Biol Sci Med Sci 2006;61:380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo H-K, Leveille SG, Yen C-J, et al. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am J Phys Med Rehabil 2006;85:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Guo X. Obesity and functional disability in elderly Americans. J Am Geriatr Soc 2008;56:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care 2010;33:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520 [DOI] [PubMed] [Google Scholar]
- 39.Moreno G, Mangione CM, Kimbro L, Vaisberg E; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 41.Wang Y, Katzmarzyk PT, Horswell R, et al. Racial disparities in cardiovascular risk factor control in an underinsured population with type 2 diabetes. Diabet Med 2014;31:1230–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertoni AG, Clark JM, Feeney P, et al.; Look AHEAD Research Group . Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complications 2008;22:1–9 [DOI] [PubMed] [Google Scholar]
- 43.Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM. Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med 2014;371:2288–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vimalananda VG, Miller DR, Palnati M, Christiansen CL, Fincke BG. Gender disparities in lipid-lowering therapy among veterans with diabetes. Womens Health Issues 2011;21(Suppl.):S176–S181 [DOI] [PubMed] [Google Scholar]
- 45.Strom Williams JL, Lynch CP, Winchester R, Thomas L, Keith B, Egede LE. Gender differences in composite control of cardiovascular risk factors among patients with type 2 diabetes. Diabetes Technol Ther 2014;16:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imran SA, Rabasa-Lhoret R, Ross S. Targets for glycemic control. Can J Diabetes 2013;37(Suppl. 1):S31–S34 [DOI] [PubMed]
- 47.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the US population of patients with diabetes mellitus. Arch Intern Med 2010;170:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laiteerapong N, Fairchild PC, Chou CH, Chin MH, Huang ES. Revisiting disparities in quality of care among US adults with diabetes in the era of individualized care, NHANES 2007-2010. Med Care 2015;53:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selvin E, Parrinello CM. Age-related differences in glycaemic control in diabetes. Diabetologia 2013;56:2549–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care 2006;29:2415–2419 [DOI] [PubMed] [Google Scholar]