Abstract
OBJECTIVE
Despite its growing prevalence in China, the extent to which diabetes leads to excess cardiovascular disease (CVD) mortality and all-cause mortality is unclear.
RESEARCH DESIGN AND METHODS
We compared death rates and causes of death among 630 people with newly diagnosed diabetes (NDD) and 519 with normal glucose tolerance (NGT) who, in 1986, were identified as a result of screening 110,660 adults aged 25–74 years for diabetes in Da Qing, China.
RESULTS
During 23 years of follow-up, 338 (56.5%) participants with NDD and 100 (20.3%) with NGT died. CVD was the predominant cause of death in those with diabetes (47.5% in men and 49.7% in women), almost half of which was due to stroke (52.3% in men and 42.3% in women). The age-standardized incidence of all-cause death was three times as high in those with NDD as in those with NGT with incidences (per 1,000 person-years) of 36.9 (95% CI 31.5–42.3) vs. 13.3 (10.2–16.5) in men (P < 0.0001) and 27.1 (22.9–31.4) vs. 9.2 (7.8–10.6) in women (P < 0.0001). The incidence of CVD deaths in men and women with NDD (17.5 [13.8–21.2] vs. 13.5 [10.5–16.5]) did not differ significantly. Significantly higher death rates attributable to renal disease and infection were also found in the NDD group.
CONCLUSIONS
Diabetes is associated with a substantially increased risk of death in Chinese adults, especially from CVD, almost half of which is due to stroke.
Introduction
In 2010, an estimated 113.9 million adults in China had diabetes (1,2). Whereas excess risk for death among people with diabetes has been well documented in Western populations (3–8), much less information is available in Asian populations, especially in China (5,9–11). Much of the increased risk in Europeans has been attributed to cardiovascular disease (CVD), especially myocardial infarction and heart failure, but also to stroke, renal disease, and infection. Excess mortality related to diabetes has been reported in Chinese origin populations in Singapore, Taiwan, and Mauritius (9,11,12), but the relationship has not been assessed through population-based studies in the Chinese mainland where the only relevant data come from a few short-term studies of hospital-based clinic patients (13,14). We present mortality data from Da Qing, China, comparing the incidence and causes of death in people who in 1986 had newly diagnosed diabetes (NDD) or normal glucose tolerance (NGT) and who were followed up for 23 years.
Research Design and Methods
In 1986, the Da Qing IGT and Diabetes Study was conducted to determine the prevalence of diabetes and associated characteristics among residents of Da Qing, China, a city to which people from all areas of China had migrated since 1960 (15,16). In 1986, Da Qing had 281,589 residents aged 25–74 years who received health care in designated clinics located throughout the city. Residents assigned for care in half of these clinics were selected to be eligible for screening for diabetes. Plasma glucose levels were measured in 110,660 subjects (87.3% of the eligible study population; 55,391 men and 55,269 women) 2 h (±5 min) after each had consumed a standardized breakfast consisting of a 100-g steamed bread bun containing ∼80 g of carbohydrate. Those without previously diagnosed diabetes and with 2-h plasma glucose levels ≥6.7 mmol/L (n = 4,209) after the standard meal were invited to have a 75-g oral glucose tolerance test (OGTT), which included measurement of fasting and 2-h postload plasma glucose levels, and 3,956 participants (94.0%) received this test (15,17). Based on the OGTT results classified by 1985 World Health Organization (WHO) criteria (18), 630 participants (300 men and 330 women) were identified as having NDD (defined as 2-h plasma glucose level ≥11.1 mmol/L).
To determine the effect of diabetes on mortality, we compared death rates in the NDD group to those in a group of 519 people (282 men and 237 women) who had participated in the diabetes screening and had the 75-g OGTT but with NGT, defined as 2-h plasma glucose level <6.7 mmol/L. From these subjects, the group with NGT was randomly selected, by frequency matching, to be of comparable sex, age-group, and size to that of the group with impaired glucose tolerance who subsequently participated in the Da Qing Diabetes Prevention Study (15,19,20). The NGT group had the same baseline age range as the NDD group (25–74 years) but, on average, was somewhat younger.
All participants in the NDD and NGT groups received a baseline examination that included measurements of blood pressure, BMI, a 12-lead electrocardiogram, and plasma lipids. Details of the methods used have been published previously (15,17). Those with diabetes were informed of the diagnosis and referred to their local clinics for continuing medical care, during which most were treated with oral hypoglycemic agents or insulin.
In 2009, we conducted a follow-up study to determine mortality rates and causes of death among both groups of participants. Institutional review boards at WHO and the China-Japan Friendship Hospital approved the study. All surviving study participants and the proxies who served as informants for deceased participants gave written informed consent.
Data Collection
In 2009, we attempted to recontact each participant to determine their vital status. For deceased participants, a verbal autopsy was conducted by interviewing an informant to determine the date, place, and circumstances of death. The informant was also asked to provide information on hospitals, clinics, and physicians where the participant had received medical care around the time of death. The death certificates, medical records, and the informant interviews were reviewed and adjudicated independently by two physicians, blinded to the participant’s 1986 glucose status, to determine the underlying cause of death. Disagreements were resolved by a third senior physician (19–21).
Outcome Classification
We classified underlying causes of death as due to either CVD, which included coronary heart disease (CHD) (myocardial infarction, sudden death, or congestive heart failure) and stroke, or noncardiovascular causes (non-CVD), which included cancer, renal failure, infections, diabetes-related complications (diabetic ketoacidosis or hyperglycemic hyperosmolar coma), chronic obstructive pulmonary disease, cirrhosis, injury (traffic accidents or work-related injuries), and unknown.
Statistical Analyses
The follow-up period was from 1 January 1986 until date of death or 31 December 2009 for those still alive. For those lost to follow-up, the period was truncated to the date of last contact. Death rates and their 95% CIs are expressed as the number of deaths divided by the number of person-years at risk, and relative differences in rates between groups are shown as hazard ratios (HRs) and 95% CI. To control for the difference in age distribution between the NDD and NGT groups, rates and CIs were standardized to the age distribution in the NDD group calculated using the SAS STDRATE procedure. We used Cox proportional hazards regression to obtain the direct age-adjusted survival curve by averaging the estimated survival curves for the observations in the original data (22). Multivariable models adjusted for age, sex, and other CVD risk factors (BMI, blood pressure, smoking, plasma total cholesterol, and previous history of CVD) were used to determine the influence of these factors on all-cause and CVD mortality rates using Cox proportional hazards models. We used the multiple imputation method to impute the missing values for total serum cholesterol. The imputation model included all dependent and independent variables of models in computing the missing values. Fifty sets of multiple imputed data were generated to provide an adequate level of reproducibility of the multiple imputation analysis (22). Differences were considered statistically significant if two-sided P values were <0.05. Data management, multiple imputation, and analyses were performed using SAS version 9.4 (SAS, Cary, NC).
Results
Vital status and cause of death were determined in 598 (94.9%) participants with NDD and 492 (94.8%) with NGT. At baseline, men and women with NDD were, on average, up to 5 years older than those with NGT (Table 1 and Supplementary Table 1). BMI and blood pressure were higher in those with NDD than in those with NGT. The proportion of smokers was much higher in men (59.0 and 67.3%) than in women (12.7 and 18.6%).
Table 1.
Baseline characteristics by glucose tolerance group of participants whose vital status was ascertained in 2009
| NDD (n = 598) |
NGT (n = 492) |
|||
|---|---|---|---|---|
| Men (n = 290) | Women (n = 308) | Men (n = 272) | Women (n = 220) | |
| Age (years) | 50.0 ± 8.6 | 46.6 ± 8.5 | 45.9 ± 9.3 | 41.6 ± 7.9 |
| BMI (kg/m2) | 25.1 ± 3.4 | 26.0 ± 3.8 | 23.5 ± 3.4 | 23.9 ± 3.3 |
| Fasting plasma glucose (mmol/L) | 8.4 ± 2.7 | 8.8 ± 3.3 | 4.8 ± 0.8 | 4.7 ± 0.5 |
| 1-h plasma glucose (mmol/L)* | 15.9 ± 3.3 | 16.1 ± 3.7 | 6.9 ± 1.5 | 6.5 ± 1.4 |
| 2-h plasma glucose (mmol/L)† | 15.1 ± 3.5 | 15.5 ± 3.7 | 4.9 ± 1.3 | 5.2 ± 0.9 |
| SBP (mmHg) | 137.6 ± 24.2 | 133.4 ± 23.3 | 123.9 ± 20.3 | 120.8 ± 22.5 |
| Diastolic blood pressure (mmHg) | 89.1 ± 13.9 | 87.1 ± 14.8 | 82.9 ± 12.8 | 80.7 ± 15.2 |
| Cholesterol (mmol/L) | 5.40 ± 1.67 | 5.24 ± 1.59 | 4.90 ± 1.11 | 4.70 ± 1.12 |
| Triglyceride (mmol/L) | 2.08 ± 1.90 | 2.16 ± 2.44 | 1.36 ± 1.08 | 1.24 ± 0.95 |
| Smokers (%) | 59.0 | 12.7 | 67.3 | 18.6 |
| CVD (%) | 2.76 | 1.95 | 0 | 0.45 |
Data are means ± SD or % of participants in each group.
*Venous plasma glucose concentration 1 h after 75-g oral glucose load;
†venous plasma glucose concentration 2 h after 75-g oral glucose load.
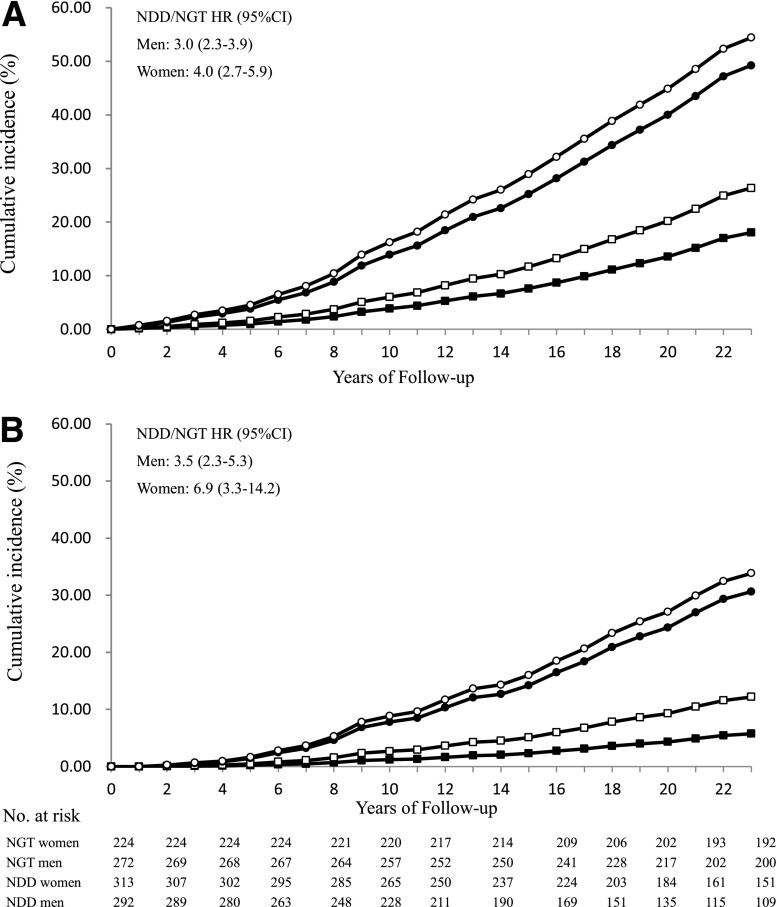
Figure 1A and B shows the age-adjusted cumulative incidence of deaths in the NDD and NGT groups according to sex and length of follow-up, which, in the NDD group, reflects duration of diabetes. The cumulative incidence rises more steeply in both men and women with NDD than in the NGT groups. The cumulative incidence of death in women with NGT is lower than in men, whereas in women with NDD, it approaches the levels seen in men with NDD. By the end of the 23-year follow-up, 338 (56.5%) with NDD and 100 (20.3%) who originally had NGT had died.
Figure 1.
A: Age-adjusted cumulative incidence and age-adjusted HRs of death from all causes in men and women with NGT and NDD over the 23 years of follow-up (white circles, men with NDD; black circles, women with NDD; white squares, men with NGT; black squares, women with NGT). B: Age-adjusted cumulative incidence and age-adjusted HRs of death from CVD in men and women with NGT and NDD over the 23 years of follow-up (white circles, men with NDD; black circles, women with NDD; white squares, men with NGT; black squares, women with NGT).
Table 2 shows underlying causes of death and age-standardized death rates in the NGT and NDD groups by sex. The relative risk of death from all causes was three times as high in the NDD group as in those with NGT. The age-adjusted incidence of all-cause mortality averaged 36.9/1,000 person-years in the NDD vs. 13.3 in the NGT group (P < 0.0001) in men, and in women, the rates were 27.1 vs. 9.2/1,000 person-years, respectively (P < 0.0001).
Table 2.
Death rates (per 1,000 person-years) in men and women according to baseline glucose tolerance groups
| NDD |
NGT* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 290) |
Women (n = 308) |
Men (n = 272) |
Women (n = 220) |
|||||
| pyrs = 4,908 |
pyrs = 5,787 |
pyrs = 5,734 |
pyrs = 4,886 |
|||||
| n | Rate# (95% CI) | n | Rate# (95% CI) | n | Rate# (95% CI) | n | Rate# (95% CI) | |
| CVD | 86 | 17.5 (13.8–21.2) | 78 | 13.5 (10.5–16.5) | 29 | 5.3 (3.3–7.2) | 8 | 3.5 (0.9–6.1) |
| CHD | 41 | 8.4 (5.8–10.9) | 45 | 7.8 (5.7–10.4) | 18 | 3.3 (1.8–4.9) | 1 | 0.3 (0.2–1.1) |
| Stroke | 45 | 9.2 (6.5–11.8) | 33 | 5.7 (3.8–7.6) | 11 | 2.0 (0.8–3.1) | 7 | 3.0 (0.6–5.4) |
| Non-CVD | 95 | 19.4 (15.5–23.2) | 79 | 13.7 (10.6–16.7) | 43 | 8.1 (5.6–10.5) | 20 | 5.7 (2.7–8.7) |
| Cancer | 28 | 5.7 (3.6–7.8) | 20 | 3.5 (2.1–5.3) | 28 | 5.3 (3.3–7.2) | 13 | 2.7 (1.5–4.4) |
| Renal failure | 24 | 4.9 (3.1–7.3) | 28 | 4.8 (3.2–7.0) | 2 | 0.4 (0–1.5) | 1 | 0.2 (0–1.1) |
| Infection | 18 | 3.7 (2.2–5.8) | 10 | 1.7 (0.8–3.2) | 3 | 0.6 (0.1–1.7) | 1 | 1.0 (0.3–2.1) |
| COPD | 4 | 0.8 (0.2–2.1) | 5 | 0.9 (0.3–2.0) | 7 | 1.4 (0.6–2.9) | 0 | |
| Cirrhosis | 8 | 1.6 (0.7–3.2) | 2 | 0.4 (0–1.2) | 1 | 0.2 (0–1.0) | 1 | 0.3 (0–1.1) |
| Injury | 7 | 1.4 (0.6–2.9) | 7 | 1.2 (0.5–2.5) | 2 | 0.3 (0–1.4) | 3 | 0.8 (0.1–1.6) |
| Diabetes§ | 3 | 0.6 (0.1–1.8) | 4 | 0.7 (0.2–1.7) | 0 | 0 | 0 | 0 |
| Unknown† | 3 | 0.6 (0.1–1.7) | 3 | 0.5 (0.1–1.5) | 0 | 0 | 1 | 0.4 (0–0.9) |
| All cause | 181 | 36.9 (31.5–42.3) | 157 | 27.1 (22.9–31.4) | 72 | 13.3 (10.2–16.5) | 28 | 9.2 (7.8–10.6) |
95% CIs calculated using normal approximation from the SAS STDRATE procedure. COPD, chronic obstructive pulmonary disease; n, number of persons or deaths in each group; pyrs, person-years.
*Rates in NGT group are standardized directly to the age distribution of the corresponding NDD group using the SAS STDRATE procedure;
#number of deaths/1,000 person-years;
§deaths from ketoacidosis or hyperosmolar coma;
†unknown causes.
CVD was the most common cause of death in the NDDs, accounting for 47.5% of deaths in men and 49.7% in women. The CVD mortality rates in the men and women with NDD (17.5 vs. 13.5/1,000 person-years) did not differ significantly. CHD accounted for 47.7% of the CVD deaths in men and 57.7% in women with NDD, and the age-standardized CHD mortality rates (8.4 in men vs. 7.8/1,000 person-years in women) were almost identical. In contrast, among those with NGT, CVD mortality rates were much lower (5.3 in men and 3.5/1,000 person-years in women). Stroke also accounted for a large proportion of CVD-related deaths in both men (52.3%) and women (42.3%) with diabetes. Age-adjusted mortality rates attributed to stroke in the NDD group, compared with their same-sex counterparts with NGT, were four times higher among men (9.2 vs. 2.0/1,000 person-years, P < 0.001) and twice as high among women (5.7 vs. 3.0/1,000 person-years, P < 0.05).
Some of the non-CVD causes of death also were more frequent in the NDD than in the NGT group. Most deaths attributed to renal failure (52/55; 94.5%) occurred in the NDD group, with a similar incidence among men and women (4.9 vs. 4.8/1,000 person-years). Deaths attributed primarily to infection occurred in 28 (4.7%) of the NDD group but in only 4 (0.8%) of the NGT group, with an incidence of 2.6 and 0.4/1,000 person-years, respectively (P < 0.001). Death from cancer occurred in 48 (8.0%) participants with NDD and 41 (8.3%) participants with NGT, with death rates per 1,000 person-years of 5.7 vs. 5.3 in men and 3.5 vs. 2.7 in women, respectively. After adjusting for differences in other risk factors (sex, BMI, and smoking), a small but nonsignificant increase in death rates due to cancer was seen among those with NDD (HR 1.2, P = 0.4).
To determine if the effect of diabetes on excess CVD mortality was attributable to diabetes itself, or if the excess could be explained by the presence of other risk factors, multivariable models were used to adjust for other potential cardiovascular risk factors. Table 3 shows adjusted HRs for all-cause and CVD deaths. After adjusting for age, sex, baseline BMI, systolic blood pressure (SBP), smoking, plasma total cholesterol, and previous history of CVD, the risk of death in those with NDD remained significantly higher than within the NGT group (HR 3.5 for CVD and 3.1 for all-cause deaths), and among women the HRs (HR 5.9 for CVD and 4.1 for all-cause deaths) were higher than among the men (HR 2.7 for CVD and 2.6 for all-cause deaths).
Table 3.
HRs for all-cause and CVD mortality in NDD compared with NGT with adjustment for conventional cardiovascular risk factors
| Variable adjusted | HR | 95% CI | P |
|---|---|---|---|
| All-cause mortality in both sexes (438 deaths) | |||
| Unadjusted | 3.7 | 2.9–4.1 | <0.0001 |
| Age and sex | 3.3 | 2.6–4.1 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 3.1 | 2.4–3.9 | <0.0001 |
| Among men (253 deaths) | |||
| Unadjusted | 3.2 | 2.5–4.3 | <0.0001 |
| Age | 2.9 | 2.2–3.9 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 2.6 | 1.9–3.5 | <0.0001 |
| Among women (185 deaths) | |||
| Unadjusted | 5.2 | 3.5–7.7 | <0.0001 |
| Age | 4.1 | 2.7–6.1 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 4.1 | 2.7–6.3 | <0.0001 |
| CVD mortality in both sexes (201 deaths) | |||
| Unadjusted | 4.7 | 3.3–6.8 | <0.0001 |
| Age and sex | 4.2 | 2.9–6.0 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 3.5 | 2.4–5.1 | <0.0001 |
| Among men (115 deaths) | |||
| Unadjusted | 3.8 | 2.5–5.8 | <0.0001 |
| Age | 3.4 | 2.2–5.3 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 2.7 | 1.7–4.2 | <0.0001 |
| Among women (86 deaths) | |||
| Unadjusted | 8.9 | 4.3–16.5 | <0.0001 |
| Age | 6.9 | 3.3–14.4 | <0.0001 |
| Age, sex, BMI, SBP, Chol*, smoking, and previous CVD | 5.9 | 2.8–12.5 | <0.0001 |
HR and 95% CIs calculated using the the SAS PHREG procedure. Chol, total serum cholesterol.
*Multipoint imputation was used to estimate cholesterol levels in 218 individuals with missing values.
At the beginning of follow-up, both the NDD and NGT groups had a wide age range and those with NDD had large variations in age at diagnosis, and over the 23-year follow-up, age and duration of diabetes increased. To examine the influence of each of these factors (aging, age at onset of diabetes, and duration of diabetes) on the likelihood of death, we calculated age-specific and duration-specific death rates (Tables 4 and 5). All-cause and CVD mortality rates increased with age in the NGT and NDD groups, and in the group with NDD, they increased with time since diagnosis within each age-group (except those aged over 70 years with 15 or more years' duration). Age-adjusted all-cause death rates were four times and CVD death rates were seven times higher among those with a diabetes duration of 15 or more years than in those who had diabetes for <5 years. Similar patterns were seen in both men and women (Supplementary Tables 2–5). Thus, longer duration of diabetes, age of onset, and current age each contributed independently to the excess risk of death in those with diabetes.
Table 4.
Age-specific all-cause death rates (per 1,000 person-years) in NDD by duration of diabetes and in NGT*
| Duration (years) | Age-group (years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25–49 |
50–59 |
60–69 |
70+ |
All ages |
||||||
| n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | |
| NDD | ||||||||||
| 0–5 | 5/1,277 | 6.1 | 8/1,031 | 7.8 | 5/335 | 14.9 | 5/55 | 90.9 | 23/2,648 | 8.7 |
| 5–10 | 6/842 | 7.1 | 20/1,131 | 17.7 | 30/653 | 45.9 | 11/112 | 98.7 | 67/2,738 | 22.6 |
| 10–15 | 4/304 | 13.2 | 17/974 | 17.4 | 35/853 | 41.0 | 25/207 | 121.1 | 81/2,338 | 34.6 |
| 15+ | 0/775 | 0.0 | 25/886 | 28.3 | 68/1,344 | 50.6 | 74/667 | 110.9 | 167/2,971 | 56.2 |
| Total | 15/2,450 | 6.1 | 70/4,021 | 17.4 | 138/3,185 | 43.3 | 115/1,040 | 110.6 | 338/10,695 | 31.6 |
| NGT* | 5/3,216 | 1.6 | 21/3,397 | 6.2 | 29/2,392 | 12.1 | 32/757 | 42.3 | 87/9,762 | 8.9 |
Fractional person-years rounded to whole numbers. n, number of deaths; pyrs, person-years.
*Censored at diagnosis of diabetes in those with NGT.
Table 5.
Age-specific CVD death rates (per 1,000 person-years) in NDD by duration of diabetes and in NGT*
| Duration (years) | Age-group (years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25–49 |
50–59 |
60–69 |
70+ |
All ages |
||||||
| n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | n/pyrs | Rate | |
| NDD | ||||||||||
| 0–5 | 0/1,228 | 0.0 | 3/1,031 | 2.9 | 1/335 | 3.0 | 2/55 | 36.7 | 6/2,648 | 2.3 |
| 5–10 | 3/842 | 3.6 | 11/1,132 | 9.7 | 19/653 | 29.1 | 7/112 | 62.8 | 40/2,738 | 14.6 |
| 10–15 | 2/304 | 6.6 | 9/974 | 9.2 | 20/853 | 23.4 | 6/207 | 29.1 | 37/2,338 | 15.8 |
| 15+ | 0/77 | 0.0 | 10/884 | 11.3 | 40/1,344 | 29.8 | 31/667 | 46.5 | 81/2,971 | 27.3 |
| Total | 5/2,449 | 2.0 | 33/4,021 | 8.2 | 80/3,185 | 25.1 | 46/1,040 | 44.2 | 164/10,695 | 15.3 |
| NGT* | 3/3,216 | 0.9 | 7/3,397 | 2.1 | 10/2,392 | 4.2 | 11/757 | 14.5 | 31/9,762 | 3.2 |
Fractional person-years rounded to whole numbers. n, number of deaths; pyrs, person-years.
*Censored at diagnosis of diabetes in those with NGT.
Death below 70 years of age occurred in 223 (66.0%) people with NDD who died compared with 55 people with NGT, with age-adjusted death rates of 20.9 vs. 5.6/1,000 person-years, respectively (P < 0.001) (Table 4). Thus, diabetes was associated with more than a threefold increase in premature mortality, much of which was attributable to death from CVD, in this mainland Chinese population.
Conclusions
Although diabetes is a well-established risk factor for all-cause death and CVD death, few population-based studies have examined the risk for death in people with NDD who were followed over a long time period (12,23–25). This is the first study to do so in China. During the 23-year follow-up, >50% the participants with NDD died, and half of them died of CVD. However, only 20% of those with NGT died, of whom 37% died of CVD. The age-adjusted relative risk of death from all causes in those with diabetes was three times as high as in NGT. The differences in relative risk for all-cause death and CVD death remained significant and largely unchanged after controlling for baseline smoking status and other CVD risk factors. These results indicate that the excess mortality seen in the NDD group was largely due to the effect of diabetes and was not primarily attributable to differences in other cardiovascular risk factors in the NDD and NGT groups. Death rates in those with diabetes were higher in those with longer duration of diabetes within each age-group, so that at any given age, the risk of death was greater among those who had an earlier age of onset. Consequently, delaying the onset of diabetes may reduce the likelihood of death, an observation that provides the basis and strong rationale for diabetes prevention programs. As predicted, the Da Qing Diabetes Prevention Study has recently shown that delaying the onset of diabetes does indeed reduce CVD and all-cause mortality (21).
In China, as in most countries, mortality rates, especially CVD mortality, are higher in men than women. In Western countries, CVD risk among women with diabetes is disproportionately increased with death rates that are similar to those in men without diabetes (6,26). We found a similar pattern of death from CVD in China with similar CVD death rates in men and women with diabetes and with relatively disproportionate increases in the women. Compared with those with NGT, CVD-related mortality in those with diabetes, adjusted for other cardiovascular risk factors, was 2.7-fold higher in men and 5.9-fold higher in women. Notably, in this study, almost half of the diabetes-associated excess CVD mortality was attributable to stroke, whereas in Western countries, a greater proportion is attributable to heart disease.
Our study also demonstrates that diabetes changes the pattern of underlying causes of death in the population. Among people with NGT, cancer accounted for 40% of all deaths, comparable to the 37% due to CVD. However, in those with NDD, although the cancer death rate was similar to that in NGT, only 14% of the deaths were due to cancer and CVD accounted for 48.5%. This excess of cardiovascular and other causes of premature death represent competing causes for death from cancer. Premature death from CVD in subjects with diabetes reduced the likelihood of subsequent deaths due to cancer, which would have occurred if those with diabetes had survived to older ages.
Consistent with our study findings, Chinese people with diabetes in Taiwan also have excess mortality when compared with the general population (9). The main cause of death was CVD, also with half of CVD deaths due to stroke. However, CVD death in the Chinese diabetes population in Taiwan only accounted for 20% of deaths, which is much less than we found in the mainland Chinese, whereas the proportion who died of cancer in Taiwan appears higher than in our study (20 vs. 14%). In the Chinese population residing in Mauritius, comparing those with diabetes to NGT, the relative risk of mortality was lower than seen in our study (12). In the Chinese Mauritians, the HR for all-cause mortality was 2.0 in men and 1.6 in women compared with 2.6 in men and 4.1 in women in our study. For CVD mortality, the ratio in Mauritians was 2.0 in men and 1.8 in women compared with 2.7 in men and 5.9 in women in our study. Reasons for the higher relative mortality rates in our study and for the difference by sex are not clear. The higher rates in our study may be the result of longer follow-up than reported in most other studies as death rates increase and the pattern of causes of death vary with age and increasing diabetes duration. It also seems likely that differences in living environments and variations in medical care may lead to different mortality rates and differences in the pattern of underlying causes of death even within the same ethnic group.
A study published in 2010 reported that >1 million excess deaths per year in China are attributable to diabetes, roughly a quarter of the annual number of such deaths worldwide (27). However, these estimates are based largely on results from small hospital-based studies or on data from Chinese not living in mainland China. Our results, with data from a relatively large, population-based study, should help provide better and more representative estimates of the excess deaths that are attributable to diabetes in mainland China. Our results show that diabetes is associated with a death rate three times as high as in those of similar age without diabetes. Another important finding is the extent to which diabetes resulted in premature mortality. Almost 70% of those with diabetes who died during the study did so below 70 years of age. The high proportion of people with diabetes who die during the active working and productive period of life has major social and economic implications, especially in view of the high and increasing prevalence of diabetes in mainland China. As excess deaths typically occur some 20 years after the diagnosis of diabetes and as the prevalence of diabetes in China has increased fourfold, from 3% in 1990 to 12% in 2010, we project that the number and proportion of deaths attributable to diabetes will increase considerably during the next 20 years.
Strengths and Limitations of Study
Our study had some notable strengths: 1) it was based on a population-based cohort of Chinese adults with NDD identified by screening a well-defined sample of the Da Qing city population; 2) diabetes was diagnosed according to the 1985 WHO criteria from systematic OGTT testing, thus avoiding many of the potential selection biases seen in hospital and clinic-based studies; 3) participants were of uniform ethnic background and most resided in the same area during the follow-up period; 4) the comparison group had well-documented NGT; 5) participants received medical care representative of that provided in China and most received treatment from the same medical care system throughout the study; 6) deaths and cause of death were confirmed by review of their medical records in most subjects; 7) participants were followed for up to 23 years, thereby allowing sufficient time for the development of most of the fatal long-term complications of diabetes; and 8) few of the original participants were lost to follow-up, thereby minimizing nonresponse bias.
The study also had some important limitations: 1) the NGT group, although having the same age range as the group with NDD, was on average younger and contained fewer women (this disparity, and the group’s small size, limits the power of our analysis to detect differences between the groups); 2) participants were not reexamined systematically at defined intervals throughout the follow-up, thereby obviating our ability to adjust for confounding factors that may have changed over time, although both the baseline examination and final follow-up evaluations were systematic; 3) not all deaths were documented by death certificate but the methodology used to determine the vital status of participants has been used successfully in other studies of mortality rates in low- and middle-income countries, and we confirmed deaths and their causes by review of medical records; 4) participants identified as having NDD based on the OGTT results likely developed diabetes some time before the diagnosis, resulting in some underestimation of the true duration of diabetes; 5) 124 people in the NGT group developed diabetes during the follow-up period and 13 of them died during the study (we did not censor follow-up at time of diabetes diagnosis in these subjects for our main analyses; this may have led to some degree of underestimation of the excess risk of death that we attributed to diabetes); and finally, 6) the death rates we report are necessarily retrospective and may have changed due to improvements in medical care or other changing environmental factors.
Conclusion
Our study is the first long-term, population-based cohort study of mortality and causes of death related to diabetes in mainland China. It shows that diabetes was associated with a substantially increased risk of death, especially from CVD, and that in both men and women, almost half of this was due to stroke. Our study can provide more valid estimates of the excess deaths that are attributable to diabetes in mainland China and can be used to predict the future mortality burden due to diabetes for China.
Article Information
Acknowledgments. The authors thank all the participants in the original Da Qing IGT and Diabetes Study and the participants and their relatives who provided information that made the current study possible. The authors thank Yang Wang and Xinran Tang (National Center for Cardiovascular Diseases, Fu Wai Hospital, Chinese Academy of Medical Sciences) for statistical advice. The authors especially thank the late Professor Xiaoren Pan, as this study would not have been possible without his leadership in the design and implementation of the original study.
Funding. This work was supported by the Centers for Disease Control and Prevention/WHO Cooperative Agreement U58/CCU424123-01-02 and the China-Japan Friendship Hospital.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.A. collected data, performed the statistical analysis, and wrote the manuscript. P.Z. coordinated and designed the study, acquired funding, performed the statistical analysis, and wrote the manuscript. J.W. and Q.G. designed the study, collected data, and performed the statistical analysis. E.W.G. and M.M.E. designed the study, acquired funding, performed the statistical analysis, and wrote the manuscript. W.Y. designed the study. H.L., B.Z., Y.S., and Y. Chen collected data. Y. Cheng performed the statistical analysis. Y.H. designed the study and collected data. P.H.B. designed the study, performed the statistical analysis, and wrote the manuscript. G.L. coordinated and designed the study, acquired funding, collected data, performed the statistical analysis, and wrote the manuscript. G.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-2498/-/DC1.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention; the National Heart, Lung, and Blood Institute; and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Xu Y, Wang L, He J, et al.; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed]
- 3.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 1998;21:1167–1172 [DOI] [PubMed] [Google Scholar]
- 4.Sievers ML, Nelson RG, Knowler WC, Bennett PH. Impact of NIDDM on mortality and causes of death in Pima Indians. Diabetes Care 1992;15:1541–1549 [DOI] [PubMed] [Google Scholar]
- 5.Seshasai SR, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen MB, Jensen ML, Carstensen B. Causes of death among diabetic patients in Denmark. Diabetologia 2012;55:294–302 [DOI] [PubMed] [Google Scholar]
- 8.de Vegt F, Dekker JM, Ruhé HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926–931 [DOI] [PubMed] [Google Scholar]
- 9.Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 2004;27:1605–1609 [DOI] [PubMed] [Google Scholar]
- 10.Nakagami T; DECODA Study Group . Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004;47:385–394 [DOI] [PubMed] [Google Scholar]
- 11.Ma S, Cutter J, Tan CE, Chew SK, Tai ES. Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: data from the 1992 Singapore National Health Survey. Am J Epidemiol 2003;158:543–552 [DOI] [PubMed] [Google Scholar]
- 12.Magliano DJ, Söderberg S, Zimmet PZ, et al. Mortality, all-cause and cardiovascular disease, over 15 years in multiethnic Mauritius: impact of diabetes and intermediate forms of glucose tolerance. Diabetes Care 2010;33:1983–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei ZH, Xie XQ, Wei JM. Impact of ageing and related factors on death rate of diabetes mellitus in Beijing. Zhonghua Yu Fang Yi Xue Za Zhi 2005;39:277–279 [in Chinese] [PubMed] [Google Scholar]
- 14.Chi ZS, Lee ET, Lu M, Keen H, Bennett PH. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S82–S86 [DOI] [PubMed] [Google Scholar]
- 15.Pan XR, Hu YH, Li GW, Liu PA, Bennett PH, Howard BV. Impaired glucose tolerance and its relationship to ECG-indicated coronary heart disease and risk factors among Chinese. Da Qing IGT and Diabetes Study. Diabetes Care 1993;16:150–156 [DOI] [PubMed] [Google Scholar]
- 16.Hu YH, Pan XR, Liu PA, Li GW, Howard BV, Bennett PH. Coronary heart disease and diabetic retinopathy in newly diagnosed diabetes in Da Qing, China: the Da Qing IGT and Diabetes Study. Acta Diabetol 1991;28:169–173 [DOI] [PubMed] [Google Scholar]
- 17.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Study Group. Diabetes Mellitus. Report of a WHO Study Group. Geneva, World Health Org., 1985 (Tech. Rep. Ser., no.727) [PubMed] [Google Scholar]
- 19.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 20.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 21.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 22.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis 1982;35:437–443 [DOI] [PubMed] [Google Scholar]
- 23.Sasaki A, Horiuchi N, Hasegawa K, Uehara M. Mortality and causes of death in type 2 diabetic patients. A long-term follow-up study in Osaka District, Japan. Diabetes Res Clin Pract 1989;7:33–40 [DOI] [PubMed] [Google Scholar]
- 24.Kim NH, Pavkov ME, Looker HC, et al. Plasma glucose regulation and mortality in Pima Indians. Diabetes Care 2008;31:488–492 [DOI] [PubMed] [Google Scholar]
- 25.Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998;21:360–367 [DOI] [PubMed] [Google Scholar]
- 26.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 27.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010 (Abstract). Diabetes Res Clin Pract 2010;87:15–19 [DOI] [PubMed] [Google Scholar]