Abstract
OBJECTIVE
To assess the prevalence of diabetes and prediabetes and the associated risk factors in two Asian Indian populations living in different environments.
RESEARCH DESIGN AND METHODS
We performed cross-sectional analyses, using representative samples of 2,305 Asian Indians aged 40–84 years living in Chennai, India, from the Centre for cArdiometabolic Risk Reduction in South-Asia study (CARRS) (2010–2011), and 757 Asian Indians aged 40–84 years living in the greater San Francisco and Chicago areas from the U.S. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study (2010–2013). Diabetes was defined as self-reported use of glucose-lowering medication, fasting glucose ≥126 mg/dL, or 2-h glucose ≥200 mg/dL. Prediabetes was defined as fasting glucose 100–125 mg/dL and/or 2-h glucose 140–199 mg/dL.
RESULTS
Age-adjusted diabetes prevalence was higher in India (38% [95% CI 36–40]) than in the U.S. (24% [95% CI 21–27]). Age-adjusted prediabetes prevalence was lower in India (24% [95% CI 22–26]) than in the U.S. (33% [95% CI 30–36]). After adjustment for age, sex, waist circumference, and systolic blood pressure, living in the U.S. was associated with an increased odds for prediabetes (odds ratio 1.2 [95% CI 0.9–1.5]) and a decreased odds for diabetes (odds ratio 0.5 [95% CI 0.4–0.6]).
CONCLUSIONS
These findings indicate possible changes in the relationship between migration and diabetes risk and highlight the growing burden of disease in urban India. Additionally, these results call for longitudinal studies to better identify the gene-environment-lifestyle exposures that underlie the elevated risk for type 2 diabetes development in Asian Indians.
Introduction
Asian Indians appear to have a higher propensity toward developing type 2 diabetes than other race/ethnic groups. India is home to the second-largest population of individuals with type 2 diabetes worldwide (1). Furthermore, immigration to developed countries is traditionally associated with higher type 2 diabetes risk (2–4), and Asian Indian immigrants have a higher prevalence of type 2 diabetes than the general U.S. population (4–6). However, given that India has recently undergone rapid economic and nutrition transitions (7,8), it is unclear whether diabetes risk among Asian Indians immigrants in the U.S. differs from that of Asian Indians in urban India. Such a comparison of two genetically similar populations living in different environmental settings could shed light on the behavioral and environmental factors associated with increased diabetes risk in this ethnic group. We therefore compared the age-specific prevalence of type 2 diabetes and prediabetes in two current population-based studies of urban Asian Indians aged ≥40 years: n = 2,305 residents of Chennai, India, using data from the Centre for cArdiometabolic Risk Reduction in South-Asia study (CARRS) (2010–2011) (9), and n = 757 from the U.S.-based Mediators of Atherosclerosis in South Asians Living in America (MASALA) study (2010–2013) (10). We also analyzed the relative associations of demographic and anthropometric characteristics on prevalent glycemic status in urban Asian Indians in both India and the U.S.
Research Design and Methods
The design, sampling strategy, recruitment, enrollment, and questionnaire and examination components of the MASALA and CARRS studies have previously been described in detail (9,10). In brief, CARRS is a multisite cohort study that recruited participant populations from three urban megacities in India and Pakistan (Delhi, Chennai, and Karachi). The baseline examination for this cohort included a representative cross-sectional survey conducted in each city between 2010 and 2011. For the purposes of this study, data were analyzed from the Chennai study site only, as this site was the only one to perform an oral glucose tolerance test (OGTT) in order to identify diabetes accurately. Households were selected for participation using multistage random sampling technique in order to be representative of the city of Chennai (9). A total of 6,920 individuals were screened for participation, of whom 6,906 (99%) provided questionnaire data. Fasting plasma glucose was obtained from 5,952 participants (86%) and 2-h post–glucose challenge on 4,051 participants. For this study, we limited our population to the 4,865 (70%) participants who were previously diagnosed with diabetes as determined by questionnaire data or who provided fasting and 2-h postchallenge glucose measurements.
Participants with existing cardiovascular disease as ascertained through self-report (n = 283) and those of age <40 years (n = 2,277) were excluded from the CARRS study for valid comparisons with MASALA.
MASALA is based on a community-based sample of South Asians living in the greater Chicago and San Francisco Bay areas. Data collection and assessment occurred between 2010 and 2013. The MASALA study was modeled to be similar to the Multi-Ethnic Study of Atherosclerosis (MESA) cohort study (11), and only individuals without a known history of cardiovascular disease were eligible. Recruitment was conducted using telephone-based recruitment methods, similar to the MESA study (11). Sampling frames were created by clinical site (either the University of California, San Francisco, or Northwestern University) and included all nine counties of the San Francisco Bay Area and the seven census tracts closest to the Northwestern University medical center, as well as suburban locations around Chicago where census data revealed high proportions of Asian Indian residents. Name, address, and telephone number were obtained for ∼10,000 households in the targeted census tracts from commercial mailing list companies (InfoUSA, Omaha, NE, and Marketing Systems Group, Horsham, PA). Random samples of South Asian surnames from the desired geographic locations were created using a specific cultural coding algorithm to identify 162 ethnicities, 16 ethnic groups, 80 language preferences, 21 countries of origin, and 12 religions using a five-step matching process to classify a person's first and last name, thereby reducing selection bias among participants with uncommon South Asian surnames (10). All participants were screened by telephone and were invited to either the University of California, San Francisco, or the Northwestern University clinical field center for a 6-h baseline clinical examination. In total, 9,097 households were attempted to be reached. Within these households, 3,053 individuals were reached and 1,801 (59%) were eligible for participation (10). Of all those eligible, a total of 906 individuals participated in the study. However, for the purposes of our analysis, data were analyzed only for individuals who identified as being born in India (n = 757). Details regarding the eligibility criteria, questionnaire, and examination components in CARRS and MASALA are shown in Table 1.
Table 1.
Eligibility, questionnaire, and exam components in CARRS and MASALA
| Eligibility criteria | CARRS-Chennai | MASALA |
|---|---|---|
| Inclusion criteria | • Aged 20 years or older | • Self-identify as South Asian |
| • Permanently residing in the selected household | • Age range 40–84 years | |
| • Ability to speak and read English, Hindi, or Urdu. | ||
| Exclusion criteria | • Pregnant women were excluded from the study, as were bedridden individuals. | • Those with history of physician-diagnosed myocardial infarction (MI), stroke, or transient ischemic attack; with a history of heart failure, angina, or use of nitroglycerin; or with a history of cardiovascular procedures |
| • Current atrial fibrillation, active cancer treatment, or life expectancy <5 years; impaired cognitive ability as judged by the reviewer; plans to move out of the study region in the next 5 years; currently living in or on the wait list for a nursing home | ||
| • Individuals weighing >136 kg (300 lb) were also excluded owing to limitations with the computed tomography scanner | ||
| Questionnaires | • Questionnaires were used to gather demographic information including language use, family history of type 2 diabetes, medical history, and current medication use | • Questionnaires were used to gather demographic information including language use, medical history, family history of type 2 diabetes, and current medication use |
| Blood pressure | • Three seated blood pressure measurements were taken using an electronic sphygmomanometer | • Three seated blood pressure measurements were taken using an automated blood pressure monitor |
| • An average of the last two readings was used to assess systolic and diastolic blood pressure | • An average of the last two readings was used to assess systolic and diastolic blood pressure | |
| Weight | • Participant weight was measured using a standing balance beam scale | • Participant weight was measured using a standing balance beam scale or digital weighing scale |
| Height | • Height was measured using a portable stadiometer | • Height was measured using a stadiometer |
| Waist circumference | • Waist circumference was measured using a nonstretch measuring tape at the site of maximum circumference halfway between the lower ribs and the anterior superior iliac spine | • Waist circumference was measured using a flexible tape measure at the site of maximum circumference halfway between the lower ribs and the anterior superior iliac spine |
In both studies, after at least a 9-h overnight fast, a 75-g OGTT was administered to participants without previously diagnosed diabetes who were willing and able to participate in the glucose challenge. Blood samples were obtained from a peripheral vein just before glucose ingestion (time 0) and at 30 and 120 min post–glucose challenge for plasma glucose measurements. Serum glucose was measured using the hexokinase method in both studies. Type 2 diabetes was defined similarly as self-reported use of glucose-lowering medication (either an oral agent or insulin), fasting glucose ≥126 mg/dL, or 2-h postchallenge glucose ≥200 mg/dL; prediabetes was defined as fasting glucose 100–125 mg/dL and/or a 2-h postchallenge glucose 140–199 mg/dL (12). BMI was classified by World Health Organization criteria (13). Normal weight was classified as BMI 18.5–24.99 kg/m2, overweight was classified as BMI 25–29.99 kg/m2, and obese was classified as BMI ≥30 kg/m2. Asian-specific cut points for BMI classification were also used for sensitivity analyses (14).
Statistical Analysis
Prevalence values and 95% CIs were estimated by study site, sex, age-group, and BMI category. Participant characteristics were stratified by sex and were compared by study using χ2 test or ANOVA as appropriate. The non–normally distributed variables of fasting and 2-h plasma glucose were log transformed. The effect of location of residence (India or the U.S.) on the odds of prediabetes and type 2 diabetes compared with normal glucose tolerance was assessed using standardized polytomous regression. Initially, an unadjusted regression model was created to compare the individual association between study location and prevalent glycemic status. Subsequent multivariable models were then created to adjust for covariates including age, sex, blood pressure, waist circumference, educational status, and years since migration to the U.S. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Table 2 displays participant characteristics by sex and study. Of the 2,305 participants from CARRS-Chennai, 54% were women. Of the 757 participants from MASALA, 46% were women. The mean duration of residence in the U.S. for MASALA study participants was 27.8 ± 10.8 years for men and 26.5 ± 10.8 years for women. Participants in the MASALA Study were on average older than those in CARRS-Chennai and had higher educational attainment. On average, for both sexes, participants in MASALA were taller and had greater weight and waist circumference measurements than those in CARRS-Chennai. Additionally, men in the MASALA study had a higher mean BMI than men in CARRS-Chennai; however, this was reversed among women. In both studies, fasting glucose was obtained from all participants who were willing to provide a sample; however, a 75-g OGTT was only administered to participants without a prior diagnosis of type 2 diabetes (MASALA N = 617, CARRS N = 1,674). Participants in the MASALA study had lower log fasting glucose values than participants in CARRS-Chennai but higher log fasting 2-h glucose values. Those in MASALA also had lower systolic and diastolic blood pressure levels and took more blood pressure–lowering medications than participants in CARRS-Chennai. Of those with a prior diagnosis of type 2 diabetes, participants in MASALA had on average a longer duration since diagnosis.
Table 2.
Baseline participant characteristics by study center*
| Men |
Women |
|||
|---|---|---|---|---|
| CARRS-Chennai (N = 1,055) | MASALA (N = 408) | CARRS-Chennai (N = 1,250) | MASALA (N = 349) | |
| Age (years) | 51.2 (9.2)† | 56.3 (10.0)† | 49.7 (8.4)† | 54.6 (8.7)† |
| Education: Bachelor’s degree or higher | 11.0† | 93.1† | 3.8† | 87.4† |
| Weight (kg) | 64.6 (12.6)† | 74.2 (11.6)† | 61.8 (11.9)† | 64.0 (10.8)† |
| Height (cm) | 163.1 (3.3)† | 169.8 (4.1)† | 150.1 (5.5)† | 157.0 (5.9)† |
| BMI (kg/m2) | 24.2 (4.3)† | 25.9 (4.4)† | 27.4 (4.9)† | 26.0 (4.0)† |
| Waist circumference (cm) | 88.8 (11.4)† | 95.7 (9.2)† | 84.2 (11.0)† | 88.9 (9.7)† |
| Log fasting glucose (mg/dL)§ | 4.7 (0.3)† | 4.6 (0.2)† | 4.7 (0.3)‡ | 4.5 (0.1)‡ |
| Log 2-h glucose (mg/dL)‖ | 4.7 (0.4)† | 4.8 (0.3)† | 4.7 (0.3)† | 4.8 (0.3)† |
| Systolic blood pressure (mmHg) | 131.0 (21.0)‡ | 126.8 (14.7)‡ | 127.5 (20.7)‡ | 123.0 (17.0)‡ |
| Diastolic blood pressure (mmHg) | 85.4 (12.4)† | 76.6 (8.7)† | 83.3 (11.7)† | 70.0 (9.8)† |
| Use of blood pressure–lowering medication | 10.9† | 36.8† | 15.9† | 26.3† |
| Self-reported diabetes diagnosis (%) | 66.9 | 70.1 | 74.5† | 56.8† |
| Years since diagnosis | 6.4 (6.5)‡ | 11.2 (10.1)‡ | 6.0 (5.6)† | 8.7 (6.3)† |
Data are mean (SD) or % unless otherwise indicated.
*Adjusted for age.
†P < 0.01.
‡P < 0.0001.
§Log fasting glucose: men, CARRS-Chennai (N = 1,027), MASALA (N = 402); women, CARRS-Chennai (N = 1,215), MASALA (N = 345).
‖Log 2-h glucose: men, CARRS-Chennai (N = 780), MASALA (N = 323); women, CARRS-Chennai (N = 894), MASALA (N = 294).
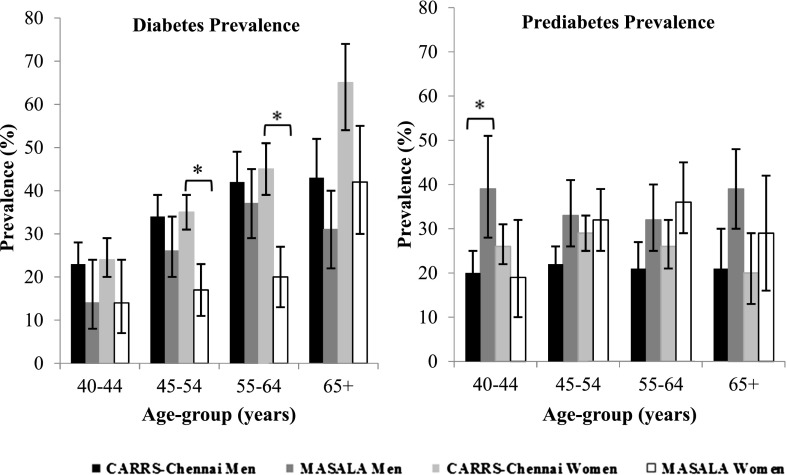
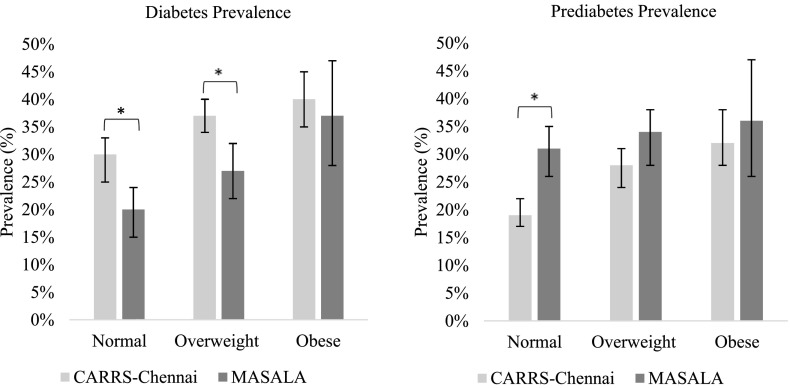
Age-adjusted type 2 diabetes prevalence was higher among Indians in CARRS-Chennai than those in the MASALA Study both overall (38% [95% CI 36–40] vs. 24% [95% CI 21–27]) and by sex (men 36% [95% CI 33–39] vs. 27% [95% CI 23–31]; women 42% [95% CI 39–45] vs. 23% [95% CI 19–28]). Of participants with type 2 diabetes, 65% of Asian Indians living in the U.S. and 71% of Asian Indians living in India had a previous diagnosis of diabetes. Age-adjusted prediabetes prevalence was lower in Asian Indians in Chennai than in the U.S. (overall 24% [95% CI 22–26] vs. 33% [95% CI 30–36], men 21% [95% CI 19–24) vs. 35% [95% CI 31–40], and women 25% [95% CI 23–28] vs. 29% [95% CI 24–34]). These patterns were consistent across age- and sex groups, but differences in type 2 diabetes prevalence by age were more significant in women (Fig. 1). In all categories of BMI, the prevalence of diabetes was higher in Asian Indians living in India than in Asian Indians living in the U.S. (Fig. 2). Differences in diabetes prevalence between the groups were significant in normal and overweight participants but were not significant in participants who were obese. In all categories of BMI, the prevalence of prediabetes was lower in native Asian Indians than those in the U.S. and was significantly different in participants with normal BMI. The pattern of higher diabetes prevalence and lower prediabetes prevalence in Asian Indians living in India than Asian Indians in the U.S. in all BMI categories was consistent using the Asian BMI cut points. However, when using the Asian specific cut points, the prevalence of diabetes and prediabetes was most significantly different in participants who were overweight.
Figure 1.
Age-specific prevalence of diabetes and prediabetes by study and sex. *P < 0.05.
Figure 2.
Prevalence of diabetes and prediabetes by study and BMI category. *P < 0.05.
Of the 757 participants from MASALA, 189 (25%) had origins from one of four of the South Indian states of Tamil Nadu, Karnataka, Andhra Pradesh, or Kerala. After restriction of participants from MASALA to only those with origins from South India, age-adjusted type 2 diabetes prevalence was again higher among Indians in CARRS-Chennai than those in the MASALA Study both overall (38% [95% CI 36–40] vs. 25% [95% CI 20–32]) and by sex (men 36% [95% CI 33–39] vs. 27% [95% CI: 19–35]; women 42% [95% CI 39–45] vs. 25% [95% CI 15–34]). Age-adjusted prediabetes prevalence was again lower in Asian Indians in Chennai than in those in the U.S. with origins from South India specifically (overall 24% [95% CI 22–26] vs. 33% [95% CI 26–39], men 21% [95% CI 19–24) vs. 36% [95% CI 27–45], and women 25% [95% CI 23–28] vs. 27% [95% CI 19–38]). These patterns were again consistent in all age- and sex groups, but differences in diabetes prevalence between Asian Indians in Chennai compared with Asian Indians in the U.S. with origins in South India were more significant than differences in prediabetes prevalence between these groups.
Table 3 shows the association of place of residence (either India [Chennai] or the U.S. [greater San Francisco and Chicago areas]) with glycemic status. After adjustment for age, sex, waist circumference, and systolic blood pressure, Asian Indians in the MASALA Study had a 50% (95% CI 0.4–0.6) decreased odds of type 2 diabetes but a 20% (95% CI 0.9–1.5) increased odds of prediabetes than those in CARRS-Chennai. The inclusion of education and years since migration in multivariable models somewhat attenuated the effect of place of residence on the odds of having diabetes compared with normal glucose tolerance. Income could not be assessed in the models, as it was found to be collinear with place of residence. The inclusion of height in multivariable models as a proxy for socioeconomic status prior to migration did not alter the effect of place of residence on the odds of having diabetes or prediabetes compared with normal glucose tolerance between the groups. However, the inclusion of height and education together in multivariable models significantly attenuated the effect of place of residence on the odds of having diabetes.
Table 3.
Risk factors associated with prediabetes and type 2 diabetes
| Model | Covariates | Prediabetes |
Type 2 diabetes |
P | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| 1 | Migrant AI* | 1.39 | (1.14, 1.69) | 0.73 | (0.59, 0.90) | <0.01 |
| 2 | Migrant AI* | 1.18 | (0.93, 1.50) | 0.46 | (0.36, 0.59) | <0.01 |
| Age-group (years) | 1.21 | (1.08, 1.38) | 1.55 | (1.38, 1.74) | <0.01 | |
| Sex** | 1.48 | (1.18, 1.85) | 1.47 | (1.19, 1.84) | <0.01 | |
| Waist circumference (cm) | 1.03 | (1.02, 1.04) | 1.05 | (1.04, 1.06) | <0.01 | |
| SBP (mmHg) | 1.01 | (1.00, 1.02) | 1.02 | (1.01, 1.03) | <0.01 | |
| 3 | Migrant AI* | 1.52 | (0.85, 2.73) | 0.73 | (0.39, 1.35) | 0.07 |
| Age-group (years) | 1.23 | (1.08, 1.40) | 1.55 | (1.37, 1.75) | <0.01 | |
| Sex** | 1.46 | (1.16, 1.84) | 1.43 | (1.14, 1.78) | <0.01 | |
| Waist circumference (cm) | 1.03 | (1.02, 1.04) | 1.05 | (1.04, 1.06) | <0.01 | |
| SBP (mmHg) | 1.01 | (1.01, 1.02) | 1.02 | (1.01, 1.03) | <0.01 | |
| Education | 0.88 | (0.60, 1.31) | 0.64 | (0.43, 0.94) | 0.06 | |
| Years since migration | 0.99 | (0.98, 1.01) | 1.00 | (0.99, 1.02) | 0.81 | |
| 4 | Migrant AI* | 1.50 | (0.97, 2.32) | 0.88 | (0.57, 1.35) | 0.05 |
| Age-group (years) | 1.20 | (1.06, 1.37) | 1.51 | (1.33, 1.71) | <0.01 | |
| Sex** | 1.24 | (0.90, 1.72) | 1.04 | (0.75, 1.43) | 0.41 | |
| Height | 0.99 | (0.98, 1.01) | 0.98 | (0.96, 1.0) | 0.09 | |
| Waist circumference (cm) | 1.03 | (1.02, 1.04) | 1.05 | (1.03, 1.06) | <0.01 | |
| SBP (mmHg) | 1.01 | (1.01, 1.02) | 1.02 | (1.01, 1.03) | <0.01 | |
| Education | 0.83 | (0.55, 1.26) | 0.56 | (0.37, 0.85) | 0.02 | |
AI, Asian Indian; SBP, systolic blood pressure.
*Asian Indians living in India (CARRS-Chennai study) were used as the referent group.
**Males were used as the referent group.
Conclusions
Few studies have compared Asian Indians in India to those who have immigrated to the U.S. In this study comparing middle- to older-aged urban Asian Indians, we found that a community-based sample of Asian Indians in the U.S. had a lower prevalence of type 2 diabetes but a higher prevalence of prediabetes than Asian Indians living in urban south India. This was observed despite Asian Indians in the U.S. being older and heavier than those in India. Asian Indians in the U.S. also had better blood pressure levels than those in India, possibly explained by their higher usage of blood pressure–lowering medications. However, the adjustment for age, sex, waist circumference, and systolic blood pressure did not fully explain the increased odds of type 2 diabetes in Asian Indians in the CARRS-Chennai Study.
It is possible that India is in an early stage of the type 2 diabetes epidemic wherein those who are most susceptible to the disease develop it the earliest (15). It is also possible that Asian Indians who have immigrated to the U.S. have adopted more positive dietary and exercise habits, thereby lowering their risk for progression from prediabetes to overt type 2 diabetes (16). Contrary to previous findings that Asian Indians who migrate to the U.S. have poorer metabolic profiles than their counterparts in India (17,18), our results indicate that while Asian Indians in India had lower BMI and waist circumference measurements than those living in the U.S., they still had a higher prevalence of type 2 diabetes even at normal levels of BMI and in both sexes, thereby suggesting a shift in the association between migration and type 2 diabetes risk in this population. Paradoxically, both the overall and the age-specific prevalence of prediabetes were lower in Asian Indians living in India than in the U.S., which may be due to a more rapid conversion through the natural history of disease in Asian Indians living in India. Our results also add strength to the notion that factors besides age and central adiposity play a large role in type 2 diabetes development in Asian Indians (7) in both developed and developing country settings, since the adjustment for age, sex, waist circumference, and systolic blood pressure did not explain differences in the odds of prediabetes or type 2 diabetes between the two groups. Furthermore, while the prevalence of type 2 diabetes was lower in Asian Indians living in the U.S. than in India, it was still considerably higher than the general U.S. population (19–21), despite Asian Indians having an overall lower BMI.
Risk factors for type 2 diabetes development such as high-carbohydrate and/or -fat diets and sedentary lifestyles were once considered to influence those who had migrated to developed countries leading to an increased prevalence of diabetes in migrants than in those who remained in developing country settings (17,18). The results of our study are among the first to highlight a higher prevalence of diabetes in individuals living in India than their counterparts who have immigrated to the U.S. It is therefore possible that, given the rapid economic and nutritional transitions currently taking place in India (7,8), these factors now exacerbate risks in Asian Indians both in India and abroad. It is also possible that with more increased knowledge of beneficial diet and lifestyle choices, migrant Asian Indians may be shifting toward more health-promoting dietary patterns. A more thorough understanding of the dietary transitions taking place in India and in diaspora Indians could provide important insights into the development of type 2 diabetes in nonobese phenotypes. It is possible that Asian Indians in the U.S. may also have increased knowledge regarding diabetes prevention and greater access to health care (16,22) than Asian Indians in India. Such factors may serve to protect immigrant populations against type 2 diabetes risk; however, further research is needed in this area.
Our study directly compared the age-specific prevalence of prediabetes, type 2 diabetes, and the associated risk factors between Asian Indians living in the U.S. and India. While there were differences in the sampling frames and sociodemographic characteristics between the two studies, both are large population-based samples with similar anthropometric and laboratory measures that are representative of Asian Indians in large urban centers either in India or in the U.S. Additionally, while participants from CARRS are primarily of South Indian origin and participants from MASALA migrated from all parts of India, it is possible that the differences in type 2 diabetes prevalence between the groups could be attributed to differences in regional origins. However, when we restricted our analyses to participants from MASALA with origins in South India only, the finding of a high prevalence of diabetes and a relatively lower prevalence of prediabetes in Asian Indians from CARRS compared with Asian Indians from MASALA remained virtually unchanged. These results suggest that the differences in type 2 diabetes prevalence between the groups are likely not attributable to region of origin.
Furthermore, while there were large differences in education status as well as height between Asian Indians living in India and the U.S., adjustment for education and height in multivariable models as proxy measures for socioeconomic status prior to migration attenuated the effect of migration on the odds of diabetes between the two groups. These results suggest a possible healthy migrant effect, whereby individuals with greater access to education as well as early maternal and childhood nutrition were more likely to have the means for migration. However, while participants from the MASALA study had high levels of educational attainment, diabetes prevalence in this group was still considerably higher than that in the general U.S. population (20,21), thereby suggesting that factors besides education attainment play a large role in diabetes risk in Asian Indians.
Being that our study directly compares two distinct Asian Indian populations from differing geographic regions (Chennai, India, and the greater San Francisco and Chicago areas of the U.S.) the results cannot be generalized to Asian Indians living in other parts of India or the U.S. However, several studies have noted an increasingly high prevalence of diabetes in urban India (23–25) with recent evidence indicating a rise of diabetes in rural areas of India as well (26). Therefore, the high prevalence of diabetes in one urban Indian city as reported in this study may be indicative of an even larger burden of disease in India yet to come. Furthermore, the diabetes prevalence in MASALA study participants was similar to what was found in a recently published study of Asian Indians in Michigan (27). However, additional national level data are needed to assess the prevalence of diabetes among Asian Indians living in the U.S.
Our findings point to a high prevalence of type 2 diabetes in urban India with a paradoxically low prevalence of prediabetes compared with urban Asian Indians in the U.S. Furthermore, the increased type 2 diabetes prevalence in Asian Indians in India is evident in both sexes, in all age-groups, and at all levels of BMI and therefore cannot be explained by differences in anthropometry or age alone. These findings suggest the need for collaborative longitudinal research efforts between India and the U.S. Such collaborations could help identify the gene-environment-lifestyle exposures that underlie the elevated risk for type 2 diabetes development in Asian Indians.
Article Information
Funding. The CARRS study is funded in whole or in part by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under contract no. HHSN268200900026C. The MASALA study was supported by NIH grant 1 R01 HL093009. Data collection at University of California, San Francisco (UCSF), was also supported by NIH/National Center for Research Resources UCSF Clinical and Translational Science Institute grant UL1 RR024131.
Duality of Interest. The CARRS study is also funded in whole or in part by the UnitedHealth Group, Minneapolis, MN. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. U.P.G. analyzed data, wrote the manuscript, drafted tables and figures, and revised the manuscript and approved the final manuscript for submission. K.M.V.N. contributed to concept, design, analysis, and interpretation of the data; reviewed and revised the manuscript; and approved the final manuscript for submission. R.G.P. and M.D. oversaw the CARRS research operations and contributed to the design and data collection of the CARRS study. M.K.A. obtained the funding for the CARRS study, contributed to the design of the CARRS study, contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. R.M.A. contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. N.R.K. contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. V.M. contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. A.M.K. obtained the funding for the MASALA study; collected the data; contributed to concept, design, analysis, discussion, and interpretation of the data; reviewed and revised the manuscript; and approved the final manuscript for submission. U.P.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014, and at the U.S. Investigators’ Global Non-Communicable Diseases Research Network Symposium, Atlanta, GA, 8–9 September 2014.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 2.Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of diabetes in New York City, 2002-2008: comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care 2011;34:1791–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oza-Frank R, Ali MK, Vaccarino V, Narayan KM. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care 2009;32:1644–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra R, Patel T, Kotha P, et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications 2010;24:145–153 [DOI] [PubMed] [Google Scholar]
- 5.Creatore MI, Moineddin R, Booth G, et al. Age-and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ 2010;182:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choukem SP, Fabreguettes C, Akwo E, et al. Influence of migration on characteristics of type 2 diabetes in sub-Saharan Africans. Diabetes Metab 2014;40:56–60 [DOI] [PubMed] [Google Scholar]
- 7.Shetty PS. Nutrition transition in India. Public Health Nutr 2002;5:175–182 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths PL, Bentley ME. The nutrition transition is underway in India. J Nutr 2001;131:2692–2700 [DOI] [PubMed] [Google Scholar]
- 9.Nair M, Ali MK, Ajay VS, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 2012;12:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanaya AM, Kandula N, Herrington D, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol 2013;36:713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report on a WHO Consultation on Obesity, Geneva, 3–5 June, 1997. Geneva, World Health Org., 2000 (Tech. Rep. Ser., no. 894) [PubMed]
- 14.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157. [DOI] [PubMed] [Google Scholar]
- 15.Qiao Q, Hu G, Tuomilehto J, et al.; DECODA Study Group . Age-and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 2003;26:1770–1780 [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh S, Weatherspoon LJ, Kaplowitz SA, Song WO. Acculturation and glycemic control of Asian Indian adults with type 2 diabetes. J Community Health 2013;38:78–85 [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar D, Anand IS, Durrington PN, et al. Coronary risk factors in people from the Indian subcontinent living in west London and their siblings in India. Lancet 1995;345:405–409 [DOI] [PubMed] [Google Scholar]
- 18.Patel JV, Vyas A, Cruickshank JK, et al. Impact of migration on coronary heart disease risk factors: comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 2006;185:297–306 [DOI] [PubMed] [Google Scholar]
- 19.Gujral UP, Narayan KM, Kahn SE, Kanaya AM. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: The MASALA study. J Diabetes Complications 2014;28:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JWR, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 2011;34:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 2014;37:1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Gagne JC, Oh J, So A, Haidermota M, Lee SY. A mixed methods study of health care experience among Asian Indians in the southeastern United States. J Transcult Nurs. 6 May 2014 [Epub ahead of print] DOI: 10.1177/1043659614526247 [DOI] [PubMed] [Google Scholar]
- 23.Mohan V, Deepa M, Deepa R, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban south India – the Chennai Urban Rural Epidemiology Study (CURES-17). Diabetologia 2006;49:1175–1178 [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran A, Snehalatha C, Kapur A, et al.; Diabetes Epidemiology Study Group in India (DESI) . High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001;44:1094–1101 [DOI] [PubMed] [Google Scholar]
- 25.Anjana RM, Pradeepa R, Deepa M, et al.; ICMR-INDIAB Collaborative Study Group . Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia 2011;54:3022–3027 [DOI] [PubMed] [Google Scholar]
- 26.Hwang CK, Han PV, Zabetian A, Ali MK, Narayan KM. Rural diabetes prevalence quintuples over twenty-five years in low-and middle-income countries: a systematic review and meta-analysis. Diabetes Res Clin Pract 2012;96:271–285 [DOI] [PubMed] [Google Scholar]
- 27.Wu TY, Wang J, Chung S. Cardiovascular disease risk factors and diabetes in Asian Indians residing in Michigan. J Community Health 2012;37:395–402 [DOI] [PubMed] [Google Scholar]