Abstract
OBJECTIVE
There is considerable interest in identifying biomarkers that predict high risk for the development of macrovascular complications in patients with diabetes. Therefore, the longitudinal association between subclinical atherosclerosis as measured by internal carotid artery intima-media thickness (IMT) and acute-phase reactants, cytokines/adipokines, thrombosis, and adhesion molecules was examined.
RESEARCH DESIGN AND METHODS
Biomarkers were measured at four time points over 20 years in 886 DCCT/EDIC participants with type 1 diabetes. Four composite scores were created by combining z scores generated from within the data set of individual biomarkers: acute-phase reactants (fibrinogen, C-reactive protein), thrombosis (fibrinogen, active and total plasminogen activator inhibitor [PAI]-1), cytokines/adipokines (tumor necrosis factor receptor-1 and -2, active and total PAI-1, IL-6), and endothelial dysfunction (soluble intracellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, and soluble E-selectin). Internal carotid IMT was measured at EDIC years 1, 6, and 12, with elevated IMT defined at each time point as being in the upper quintile of its distribution.
RESULTS
Logistic regression models indicate that while individual biomarkers were not predictive of or associated with subclinical atherosclerosis, composite scores of acute-phase reactants (odds ratio [OR] 2.78 [95% CI 1.42, 5.42]), thrombolytic factors (OR 2.83 [95% CI 1.45, 5.52]), and cytokines/adipokines (OR 2.83 [95% CI 1.48, 5.41]) measured at our final time point EDIC years 8–11 were associated with higher levels of atherosclerosis at EDIC year 12, but findings were not consistent at early time points. The endothelial dysfunction score was not appreciably predictive of or associated with subclinical atherosclerosis at any of the time points measured.
CONCLUSIONS
The pathophysiologic relationship between higher biomarker levels and progression of subclinical atherosclerosis remains unclear.
Introduction
There is considerable interest in identifying biomarkers that predict high risk for the development of macrovascular and microvascular complications in patients with diabetes (1–12). Patients with diabetes with the same conventional risk factors as nondiabetic patients have increased incidence of macrovascular disease including coronary heart disease and cerebrovascular and peripheral vascular disease (1,3,5,9,12). However, the mechanisms by which diabetes accelerates atherosclerosis are not completely understood, but endothelial cell dysfunction due to diabetic dyslipidemia, increased oxidative stress, and hypertension is considered one of the major players in the development of diabetes complications (1,4,8,10). In the past decade, it has been clearly shown that endothelial cell dysfunction and some of its consequences (i.e., vessel wall inflammation and clotting/fibrinolytic abnormalities) play important roles in atherosclerosis progression and in triggering acute cardiovascular events (1,4,8,10).
Several biomarkers of endothelial cell dysfunction, inflammation, and thrombotic predisposition have been associated with the development of macrovascular complications in diabetes (1–5,8,9,12). However, most studies performed have been on a small number of patients, using a single measurement at a single time point. Moreover, those studies that examined a panel of risk factors developed to predict progression of vascular disease have been based on parameters obtained in patients at different stages of vascular disease.
The larger cohort study investigating the predictive value of risk factors, the EURODIAB Prospective Complications Study, investigated two determinants of inflammatory activity: conventional risk factors for atherothrombosis and plasma concentrations of soluble vascular cell adhesion molecule (VCAM) and E-selectin as an index of endothelial dysfunction (13). Markers of inflammatory activity (C-reactive protein [CRP], interleukin [IL]-6, and tumor necrosis factor [TNF]-α) were associated with conventional risk factors including sex, diabetes duration, level of glycemic control, BMI, HDL cholesterol (inversely), triglycerides, and systolic blood pressure. Plasma levels of the adhesion molecules were also strongly and independently associated with these inflammatory markers, suggesting that inflammatory activity plays an important role in endothelial dysfunction in type 1 diabetes. Subsequent analyses of this cross-sectional data set determined further that soluble VCAM-1 and E-selectin were associated with micro- and macrovascular complications in this cohort of patients with type 1 diabetes (14), but when data were adjusted for confounding variables, only VCAM-1 remained significantly associated with macroalbuminuria. However, because of the cross-sectional design of the study, it is uncertain whether endothelial dysfunction is the result of or precedes vascular complications in type 1 diabetes. Thus, the current literature does not provide a clear picture of the value of biomarkers as predictors of diabetes complications.
We are in a unique position of determining the predictive value of putative risk factors in a large cohort of subjects with type 1 diabetes, the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort, using samples collected longitudinally for over 20 years. Furthermore, this cohort was free of complications at entry into the study except for mild (background) retinopathy and a urinary albumin excretion rate of <139 μg/min (<200 mg/24 h). Our study included classic inflammation markers (CRP, IL-6, and soluble TNF receptors [sTNFR]-1 and -2), endothelial dysfunction markers (soluble intracellular adhesion molecule [sICAM]-1, soluble VCAM [sVCAM]-1, and soluble E-selectin [sE-selectin]), and clotting/fibrinolysis markers (fibrinogen and active and total plasminogen activator inhibitor [PAI]-1), and we used longitudinal carotid intima-media thickness (IMT) measurements as the end point for the severity and progression of atherosclerosis.
Research Design and Methods
The DCCT cohort was randomly assigned to intensive or conventional diabetes therapy and followed for an average of 6.5 years. The DCCT cohort included 1,441 patients who at study entry (1984–1989) were 13–39 years of age and had type 1 diabetes for 1–15 years (15). At DCCT entry, none of the patients had hypertension or dyslipidemia, as defined prior to 1983, and therefore were not on lipid-lowering or antihypertensive therapy. In 1994, after intensive therapy had been demonstrated to have major beneficial effects on microvascular complications, the interventional phase of the study was stopped and the observational phase (EDIC) was initiated (16). During EDIC, patients have been under the care of their personal physicians and encouraged to practice intensive diabetes therapy.
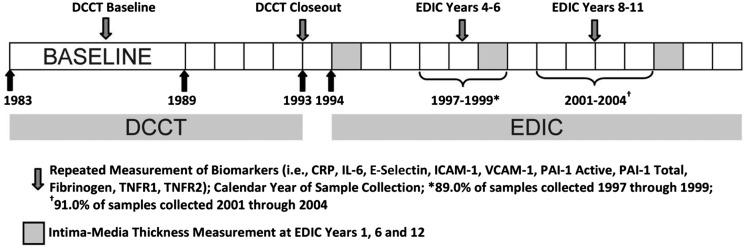
Of the 1,441 DCCT participants, 1,375 of 1,425 surviving members participated in EDIC, and 886 of these individuals participated in a substudy on biomarkers of vascular disease and had blood collected longitudinally. The 886 substudy participants did not differ significantly from the 555 not included with respect to DCCT treatment arm, primary prevention cohort, or age; however, women were less likely to be included in the substudy than men. Biomarkers were measured longitudinally on patients with samples collected at four time points: at enrollment into DCCT (1984–1989), at DCCT closeout (1993), between EDIC years 4 and 6 (1997–1999), and between EDIC years 8 and 11 (2001–2004) (Fig. 1). Analyses were not limited to participants with samples across all time points. The institutional review board of all participating DCCT/EDIC centers approved the DCCT and EDIC studies, and all participants provided written informed consent.
Figure 1.
Schematic depicting timing of biomarker sample collection and IMT outcome measurements.
Assessment of Carotid IMT
Carotid ultrasonography was first performed 1–2 years after initiation of EDIC (EDIC year 1) and repeated at EDIC years 6 and 12. The measurement of IMT in the DCCT/EDIC cohort has previously been described (17,18). In brief, a single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries and three longitudinal views in different imaging planes of each internal carotid artery (ICA) were obtained by certified technicians at the clinical centers, recorded on S-VHS tapes, and read in a central unit (Tufts Medical Center, Boston, MA) by two readers masked to participant characteristics. The maximum IMT (millimeters) of the common carotid arteries was defined as the mean of the maximum IMT for near and far walls on both right and left sides. The maximum IMT of the ICA was defined in the same way, and the results of the three scans (i.e., anterior, lateral, and posterior views of both sides) were averaged.
Biomarker Assays
Serum levels of CRP, total and active PAI-1, sICAM-1, sVCAM-1, sE-selectin, IL-6, sTNFR-1, and sTNFR-2 were assayed using the Signature Plus Protein Array Imaging and Analysis System (Aushon BioSystems) using ArrayVision software for data analysis. Interassay coefficients of variation were, respectively, 2.6% for CRP, 3.4% for total PAI-1, 5.9% for active PAI-1, 3% for sICAM-1, 4% for sVCAM-1, 4% for sE-selectin, 7.5% for IL-6, 5.9% for sTNFR-1, and 2.7% for sTNFR-2. Plasma concentrations of fibrinogen were determined in a Beckman IMMAGE 800 Immunochemistry Analyzer using the Fibrinogen SPQ II Test System (Diagnostica Stago S.A.S, Asnieres sur Seine, France). The coefficient of variation of this assay was 5.6%. The four longitudinal measurements for each patient were analyzed simultaneously using stored serum samples. Supplementary Table 1 details the number of participants with biomarker measurements at each time point. For assessment of sample stability, repeated measurements of sICAM-1, fibrinogen, CRP, and IL-6 were made using the same sample over a 10- to 14-year period (i.e., samples were measured when initially collected [1997–1999] and again, some at 5 and 10 years after storage [sICAM-1 and CRP, n = 18] and some after 12–14 years of storage [fibrinogen and IL-6, n = 20]). There were no statistically significant differences between the results obtained at the different time points among the repeated measurements.
Other Procedures
Demographic and clinical characteristics of the subjects were collected at DCCT enrollment, DCCT closeout, and each EDIC yearly exam; hence, information is available corresponding to each exam during which serum samples were collected for biomarker measurement. At each of the four longitudinal time points, each participant underwent a standardized physical examination and laboratory testing including HbA1c (16,19), fasting lipid profile, and blood pressure (16,20). At DCCT baseline, participants were grouped into one of the two cohorts based on their retinopathy, renal status, and duration of type 1 diabetes (21).
Statistical Analysis
Analyses were carried out in which the levels of biomarkers measured at DCCT enrollment, DCCT closeout, 4–6 years into EDIC, and 8–11 years into EDIC were the exposures of interest. Internal carotid artery IMT at EDIC years 1, 6, and 12 were the primary outcomes of interest. The goal of our analysis was to use the biomarker and outcome information collected at multiple time points to examine the temporal relationship between biomarker levels and development or progression of atherosclerosis. Mean, median, or percentiles were determined for participant demographic, clinical, and biomarker levels for each of the four time points at which biomarkers were measured. All biomarkers were standardized across all time points using z scores. Specifically, z scores were created using the SD obtained from combining data across the four time points, and the analysis results for individual biomarkers represent the association between a difference of 1 SD from the mean in each biomarker and our outcomes of interest.
The influence of higher levels of specific biomarkers on increases in ICA IMT was analyzed at EDIC years 1, 6, and 12. Separate repeated-measures logistic regression models using the methods of generalized estimating equations (22) were applied at each of the four time points when biomarkers were measured to assess the effect of higher biomarker levels on the odds of being in the upper versus lower measurements of ICA IMT at EDIC year 1, EDIC year 6, and EDIC year 12 (i.e., upper quintile versus lower four quintiles defined as ≥0.727 mm at EDIC year 1, ≥0.809 at EDIC year 6, and ≥1.078 at EDIC year 12). The distribution of ICA IMT at EDIC years 1, 6, and 12 is illustrated in Supplementary Fig. 1. Odds ratios (ORs) and asymptotic 95% CIs were computed. Additionally, the odds associated with high progression of ICA IMT from EDIC year 1 to EDIC year 12 (i.e., high progression being defined as being in the upper quintile of ICA IMT change) was assessed using logistic regression models.
Next, composite biomarker scores were created to assess the combined impact of multiple biomarkers believed to be acting on the same pathway. Specifically, four composite scores were created by combining standardized scores (i.e., the average z score) of individual biomarkers: acute-phase reactants (i.e., fibrinogen and CRP), cytokines/adipokines (i.e., sTNFR-1 and -2, active PAI-1, total PAI-1, and IL-6), thrombosis (i.e., fibrinogen, active PAI-1, and total PAI-1), and endothelial dysfunction (sICAM-1, sVCAM-1, and sE-selectin). For the endothelial dysfunction composite score, the inverse z score of sVCAM-1 was used owing to its consistent inverse relationship with outcomes of interest (23) (Supplementary Table 2). Prior to analysis, each composite score was categorized into quartiles that were used as the primary independent variables of interest. The odds predicting each outcome of interest (i.e., high ICA IMT at EDIC years 1, 6, and 12 and high ICA IMT progression from EDIC years 1–12) were determined comparing each quartile of a given composite score with the lowest quartile of that composite score.
All regression models are adjusted for DCCT treatment group (intensive versus conventional), retinopathy cohort (primary prevention versus secondary intervention), sex, and ultrasound IMT reader. Additionally, the following factors measured at the time of biomarker collection were adjusted for in all regression models: age, duration of diabetes, HbA1c levels, LDL cholesterol, HDL cholesterol, systolic blood pressure, and smoking status. Using appropriate interaction terms, we also assessed whether treatment arm modified associations between our composite biomarker scores and outcomes of interest but found no evidence of interaction. All statistical analyses were conducted using SAS, version 9.3 (SAS Institute, Inc., Cary, NC). Significance for all comparisons was set at a two-sided P value of 0.05, and no correction for multiple testing was applied to reported P values.
Results
At DCCT enrollment, the mean age of the study population was 26.9 ± 7.1 years, the mean duration of diabetes was 5.8 ± 4.1 years, 52.1% of the 1,295 participants with biomarker samples available at DCCT enrollment were males, and 50.1% were assigned to the DCCT intensive treatment group. At the time of our final biomarker sample collection, which occurred between EDIC years 8 and 11, the mean age had increased to 44.0 ± 6.9 years and the mean duration of diabetes was 22.2 ± 4.8 years. Demographic, clinical, and biomarker levels across the four time points measured are found in Table 1. Systolic blood pressure levels and BMI increased across the four time points, while HbA1c levels decreased. With respect to the biomarkers of interest, CRP, fibrinogen, IL-6, active PAI-1, and sTNFR-1 levels increased across the four time points, while sE-selectin and sICAM-1 levels decreased over time. Total PAI-1, sTNFR-2, and sVCAM-1 levels did not show a significant trend over time. In comparison with most other biomarkers, which were measured on all available samples at DCCT baseline (i.e., >92% of participants with the exception of sVCAM-1), fibrinogen was measured in only a subset of the DCCT cohort (n = 732). With comparison of DCCT baseline characteristics of these 732 subjects with the remaining DCCT cohort (n = 563), duration of diabetes was shorter among those included (5.5 vs. 6.1 years, P value 0.016) and baseline HbA1c levels were lower (8.76% [72 mmol/mol] vs. 9.01% [75 mmol/mol], P value 0.017). Age, sex, and likelihood of being in the intensive treatment arm or primary prevention cohort were similar in those with and without fibrinogen measured at DCCT baseline.
Table 1.
Demographic, clinical, and biomarker levels across the four time points measured
| Baseline DCCT | Closeout DCCT | EDIC years 4–6 | EDIC years 8–11 | P‡ | |
|---|---|---|---|---|---|
| n | 1,295 | 1,319 | 850 | 869 | |
| Characteristics | |||||
| Age (years) | 26.9 (7.05) | 33.1 (6.96) | 39.8 (6.87) | 44.0 (6.89) | — |
| Male | 52.1 | 52.3 | 55.1 | 55.0 | — |
| Intensive treatment | 50.1 | 49.7 | 50.8 | 50.2 | — |
| Primary prevention cohort | 50.7 | 50.2 | 51.5 | 51.2 | — |
| Duration of T1DM (years) | 5.8 (4.12) | 12.0 (3.93) | 17.9 (4.79) | 22.2 (4.83) | — |
| Smoking status | 20.5 | 18.5 | 16.5 | 14.7 | <0.0001 |
| SBP (mmHg) | 114 (12) | 117 (12) | 121 (14) | 122 (14) | <0.0001 |
| HDL cholesterol (mg/dL) | 51 (12) | 52 (13) | 57 (15) | 55 (15) | <0.0001 |
| LDL cholesterol (mg/dL) | 109 (29) | 114 (29) | 113 (29) | 108 (27) | 0.1566 |
| Triglycerides (mg/dL)† | 69 (54, 93) | 73 (55, 98) | 71 (54, 103) | 72 (53, 101) | 0.0058 |
| AER (mg/24 h)† | 10.1 (7.2, 17.3) | 10.1 (5.8, 15.8) | 11.5 (7.2, 20.2) | 10.1 (7.2, 20.2) | 0.3319 |
| Baseline HbA1c | 8.9 (1.60) | 8.2 (1.58) | 8.1 (1.34) | 7.8 (1.30) | <0.0001 |
| Baseline HbA1c (mmol/mol) | 74 (17.5) | 66 (17.3) | 65 (14.6) | 62 (14.2) | <0.0001 |
| Biomarkers | |||||
| CRP (mg/L)† | 0.15 (0.06, 0.40) | 0.23 (0.09, 0.59) | 0.28 (0.11, 0.71) | 0.26 (0.09, 0.65) | <0.0001 |
| IL-6 (ng/mL)† | 5.51 (3.25, 9.99) | 5.16 (3.31, 8.61) | 5.57 (3.37, 8.71) | 5.77 (3.44, 9.79) | 0.0003 |
| Fibrinogen (ng/mL) | 196 (59) | 218 (60) | 255 (71) | 257 (71) | <0.0001 |
| Total PAI-1 (ng/mL) | 184 (106) | 175 (106) | 175 (101) | 178 (99) | 0.6887 |
| Active PAI-1 (ng/mL) | 8.78 (5.95) | 10.6 (7.47) | 11.1 (8.36) | 11.3 (9.57) | <0.0001 |
| sTNFR-1 (ng/mL)† | 1.38 (1.05, 1.77) | 1.62 (1.28, 2.01) | 1.79 (1.38, 2.22) | 1.69 (1.30, 2.16) | <0.0001 |
| sTNFR-2 (ng/mL)† | 1.37 (1.07, 1.74) | 1.41 (1.10, 1.78) | 1.43 (1.05, 1.86) | 1.33 (1.00, 1.73) | 0.9208 |
| sE-selectin (ng/mL)† | 48 (32, 73) | 46 (28, 77) | 44 (26, 73) | 38 (24, 62) | <0.0001 |
| sICAM-1 (ng/mL) | 359 (131) | 317 (126) | 340 (132) | 320 (132) | <0.0001 |
| sVCAM-1 (ng/mL) | 1,023 (441) | 1,109 (434) | 1,031 (472) | 1,066 (461) | <0.2298 |
Data are means (SD), median (25th, 75th percentile), or percent. AER, albumin excretion rate; SBP, systolic blood pressure; T1DM, type 1 diabetes mellitus.
†Natural log transformations were applied to non–normally distributed variables to assess trend over time;
‡P value for trend over time.
Initially, logistic regression was used to examine the ability of individual biomarkers to predict high ICA IMT at EDIC year 1 (i.e., being in the upper quintile compared with the lower four quintiles of ICA IMT [high IMT ≥0.727 mm]), EDIC year 6 (high IMT ≥0.809 mm), and EDIC year 12 (i.e., high IMT ≥1.07 mm) (Supplementary Table 2). Additionally, high ICA IMT progression from EDIC years 1–12 was examined as an outcome. Presented ORs are those associated with a 1-SD increase in each normally distributed biomarker (i.e., natural log transformations were used when required) for each biomarker measured at each of the four time points in relation to subsequent outcomes of interest. At DCCT baseline and closeout, individual biomarkers were not statistically significantly associated with outcomes of interest with the exception of IL-6, which was marginally associated with ICA IMT progression from EDIC years 1–12 at DCCT baseline (OR 1.17 [95% CI 1.00, 1.37]), and active PAI-1, which was associated with ICA IMT progression from EDIC years 1–12 at DCCT closeout (OR 1.18 [95% CI 1.01, 1.39]). Biomarkers measured on samples obtained in EDIC years 4–6 or EDIC years 8–11 were not associated with outcomes of interest with the exception of active PAI-1 measured during EDIC years 8–11, which was associated with elevated IMT at EDIC year 12 (OR 1.21 [95% CI 1.02, 1.44]).
Switching focus to our composite biomarker scores that were created at each of the four time points of interest, we examined their association with outcomes of interest. At DCCT baseline and at EDIC years 4–6, our composite acute-phase reactant score, which combined information on fibrinogen and CRP levels, was not associated with our outcomes of interest (Table 2). At DCCT closeout, individuals in the highest quartile of our composite acute-phase reactant score had a 2.2-fold increased odds (2.20 [95% CI 1.12, 4.31]) of having high versus normal ICA IMT at EDIC year 12 relative to those in the lowest quartile of the composite score. At our final time point, EDIC years 8–11, individuals in the highest quartile of our composite acute-phase reactant score had increased odds (2.78 [95% CI 1.42, 5.42]) of having high versus normal ICA IMT at EDIC year 12 relative to those in the lowest quartile of the composite score.
Table 2.
Adjusted* ORs (95% CI) from logistic regression† models for quartile of composite acute-phase reactant scores‡ as well as composite thrombosis scores‡‡ measured during DCCT/EDIC in relation to elevated IMT and IMT progression
| Year 1 elevated IMT EDIC (≥0.727 mm) | Year 6 elevated IMT EDIC (≥0.809 mm) | Year 12 elevated IMT EDIC (≥1.078 mm) | Years 1–12 progression (≥0.370 mm) | |
|---|---|---|---|---|
| Composite acute-phase reactant scores | ||||
| Baseline DCCT | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.19 (0.64, 2.21) | 0.81 (0.41, 1.57) | 0.96 (0.52, 1.79) | 0.55 (0.28, 1.07) |
| Quartile 3 | 0.84 (0.44, 1.59) | 0.99 (0.51, 1.92) | 0.90 (0.47, 1.71) | 0.77 (0.40, 1.47) |
| Quartile 4 | 0.80 (0.42, 1.55) | 0.96 (0.50, 1.87) | 1.05 (0.55, 2.00) | 0.72 (0.37, 1.43) |
| DCCT closeout | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 0.90 (0.49, 1.64) | 0.89 (0.46, 1.69) | 1.36 (0.41, 1.36) | 1.12 (0.59, 2.14) |
| Quartile 3 | 0.47 (0.25, 0.87) | 0.46 (0.24, 0.90) | 1.09 (0.70, 2.66) | 0.99 (0.51, 1.92) |
| Quartile 4 | 0.74 (0.39, 1.39) | 0.77 (0.52, 1.77) | 2.20 (1.12, 4.31) | 1.32 (0.66, 2.65) |
| EDIC years 4–6 | ||||
| Lowest quartile | — | 1.00 | 1.00 | — |
| Quartile 2 | — | 1.22 (0.60, 2.49) | 0.99 (0.50, 1.94) | — |
| Quartile 3 | — | 1.38 (0.72, 2.64) | 0.78 (0.40, 1.51) | — |
| Quartile 4 | — | 1.18 (0.61, 2.28) | 1.39 (0.75, 2.60) | — |
| EDIC years 8–11 | ||||
| Lowest quartile | — | — | 1.00 | — |
| Quartile 2 | — | — | 2.14 (1.08, 4.21) | — |
| Quartile 3 | — | — | 1.78 (0.88, 3.59) | — |
| Quartile 4 | — | — | 2.78 (1.42, 5.42) | — |
| Composite thrombosis scores | ||||
| Baseline DCCT | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.20 (0.66, 2.18) | 1.09 (0.58, 2.06) | 0.99 (0.55, 1.78) | 1.00 (0.53, 1.90) |
| Quartile 3 | 0.89 (0.48, 1.65) | 0.76 (0.40, 1.46) | 0.73 (0.39, 1.38) | 0.88 (0.46, 1.70) |
| Quartile 4 | 0.87 (0.46, 1.67) | 0.85 (0.43, 1.65) | 1.03 (0.55, 1.94) | 1.07 (0.56, 1.03) |
| DCCT closeout | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 0.83 (0.43, 1.59) | 0.89 (0.45, 1.75) | 1.54 (0.76, 3.12) | 1.40 (0.71, 2.76) |
| Quartile 3 | 1.13 (0.63, 2.04) | 0.87 (0.45, 1.69) | 1.69 (0.86, 3.33) | 1.19 (0.61, 2.30) |
| Quartile 4 | 1.03 (0.55, 1.92) | 1.07 (0.55, 2.06) | 2.25 (1.14, 4.44) | 1.67 (0.86, 3.27) |
| EDIC years 4–6 | ||||
| Lowest quartile | — | 1.00 | 1.00 | — |
| Quartile 2 | — | 1.06 (0.55, 2.03) | 1.39 (0.70, 2.76) | — |
| Quartile 3 | — | 0.84 (0.43, 1.64) | 1.29 (0.65, 2.54) | — |
| Quartile 4 | — | 0.69 (0.36, 1.33) | 1.46 (0.75, 2.84) | — |
| EDIC years 8–11 | ||||
| Lowest quartile | — | — | 1.00 | — |
| Quartile 2 | — | — | 2.05 (1.04, 4.04) | — |
| Quartile 3 | — | — | 2.01 (1.02, 3.95) | — |
| Quartile 4 | — | — | 2.83 (1.45, 5.52) | — |
*Regression models adjusted for DCCT treatment group (intensive vs. conventional), retinopathy cohort (primary prevention vs. secondary intervention), age, sex, diabetes duration, HbA1c, LDL, HDL, systolic blood pressure, smoking status, and IMT reader.
†At each of the four time points when biomarkers were measured, separate repeated-measures logistic regression models using the methods of generalized estimating equations were applied to assess the effect of increased biomarker levels on the odds of being in the upper versus lower measurements of ICA IMT at EDIC years 1, 6, and 12. Also, the odds associated with high progression of ICA IMT from EDIC years 1–12 (i.e., defined by upper quintile of ICA IMT change) were assessed.
‡Fibrinogen and CRP contributed to the acute-phase reactant score.
‡‡Fibrinogen, active PAI-1, and total PAI-1 contributed to the thrombosis score.
At DCCT baseline and at EDIC years 4–6, our composite thrombosis score that combined information on fibrinogen, active PAI-1, and total PAI-1 was not associated with our outcomes of interest (Table 2). At DCCT closeout, individuals in the highest quartile of our composite thrombosis score had a twofold increased odds (2.25 [95% CI 1.14, 4.44]) of having high versus normal ICA IMT at EDIC year 12 relative to those in the lowest quartile of the composite score. At our final time point, EDIC years 8–11, relative to individuals in the lowest quartile of the composite score those in the second, third, and highest quartile of our composite thrombosis score had more than a twofold increased odds of having high versus normal ICA IMT at EDIC year 12. The OR comparing the highest to lowest quartile was 2.83 (95% CI 1.45, 5.52) at EDIC year 12.
At DCCT baseline, DCCT closeout, and EDIC years 4–6, our composite cytokine/adipokine score, which combined information on TNFR-1, TNFR-2, IL-6, active PAI-1, and total PAI-1, was not associated with our outcomes of interest (Table 3). However, at our final time point, EDIC years 8–11, individuals in both the second and highest quartiles of our composite cytokine/adipokine score had over a twofold increased odds of having high versus normal ICA IMT at EDIC year 12 (OR 3.14 [95% CI 1.69, 5.83] and 2.83 [95% CI 1.48, 5.41], respectively) relative to those in the lowest quartile of the composite score. Our composite endothelial dysfunction score, which combined information on sICAM-1, sVCAM-1, and sE-selectin, was not associated with our outcomes of interest at any of the four time points of interest with one exception (i.e., DCCT baseline, DCCT closeout, EDIC years 4–6, or EDIC years 8–11), the exception being that the composite score measured at DCCT closeout was associated with elevated IMT at EDIC years 6 and 12 when comparing the third (but not fourth) with the first quartile, respective ORs being 1.89 (95% CI 1.05, 3.40) and 2.08 (95% CI 1.14, 3.81).
Table 3.
Adjusted* ORs (95% CI) from logistic regression† models for quartile of composite cytokine/adipokine scores‡ as well as composite endothelial dysfunction scores‡‡ measured during DCCT/EDIC in relation to elevated IMT and IMT progression
| Year 1 elevated IMT EDIC (≥0.727 mm) | Year 6 elevated IMT EDIC (≥0.809 mm) | Year 12 elevated IMT EDIC (≥1.078 mm) | Years 1–12 progression (≥0.370 mm) | |
|---|---|---|---|---|
| Composite cytokine/adipokine scores | ||||
| Baseline DCCT | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.18 (0.76, 1.84) | 1.22 (0.72, 2.07) | 0.89 (0.53, 1.51) | 1.20 (0.76, 1.90) |
| Quartile 3 | 0.92 (0.58, 1.46) | 1.06 (0.61, 1.85) | 1.09 (0.65, 1.84) | 1.01 (0.63, 1.61) |
| Quartile 4 | 0.92 (0.57, 1.47) | 0.73 (0.39, 1.40) | 0.94 (0.51, 1.72) | 1.35 (0.84, 2.17) |
| DCCT closeout | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.18 (0.67, 2.06) | 1.33 (0.73, 2.41) | 1.54 (0.86, 2.78) | 1.46 (0.81, 2.65) |
| Quartile 3 | 1.39 (0.79, 2.44) | 1.37 (0.75, 2.53) | 1.92 (1.07, 3.46) | 1.54 (0.85, 2.78) |
| Quartile 4 | 1.10 (0.70, 1.97) | 1.21 (0.55, 1.97) | 1.10 (0.58, 2.07) | 1.12 (0.60, 2.06) |
| EDIC years 4–6 | ||||
| Lowest quartile | — | 1.00 | 1.00 | — |
| Quartile 2 | — | 1.21 (0.64, 2.26) | 1.08 (0.58, 2.03) | — |
| Quartile 3 | — | 0.94 (0.49, 1.79) | 1.27 (0.69, 2.34) | — |
| Quartile 4 | — | 1.01 (0.54, 1.88) | 1.16 (0.63, 2.11) | — |
| EDIC years 8–11 | ||||
| Lowest quartile | — | — | 1.00 | — |
| Quartile 2 | — | — | 3.14 (1.69, 5.83) | — |
| Quartile 3 | — | — | 1.81 (0.92, 3.55) | — |
| Quartile 4 | — | — | 2.83 (1.48, 5.41) | — |
| Composite endothelial dysfunction scores | ||||
| Baseline DCCT | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.06 (0.61, 1.82) | 1.76 (0.98, 3.17) | 1.66 (0.92, 3.00) | 1.92 (1.07, 3.43) |
| Quartile 3 | 1.06 (0.61, 1.84) | 1.40 (0.76, 2.58) | 1.41 (0.76, 2.61) | 1.35 (0.73, 2.50) |
| Quartile 4 | 0.95 (0.54, 1.69) | 1.55 (0.84, 2.87) | 1.22 (0.65, 2.27) | 1.58 (0.69, 2.39) |
| DCCT closeout | ||||
| Lowest quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 0.80 (0.46, 1.42) | 1.16 (0.62, 2.17) | 1.70 (0.92, 3.15) | 1.48 (0.71, 2.67) |
| Quartile 3 | 1.04 (0.60, 1.80) | 1.89 (1.05, 3.40) | 2.08 (1.14, 3.81) | 1.65 (0.92, 2.98) |
| Quartile 4 | 0.73 (0.40, 1.31) | 0.90 (0.47, 1.70) | 1.10 (0.57, 2.10) | 1.17 (0.63, 2.19) |
| EDIC years 4–6 | ||||
| Lowest quartile | — | 1.00 | 1.00 | — |
| Quartile 2 | — | 0.86 (0.46, 1.64) | 1.03 (0.55, 1.95) | — |
| Quartile 3 | — | 1.22 (0.66, 2.23) | 1.30 (0.71, 2.40) | — |
| Quartile 4 | — | 0.90 (0.48, 1.67) | 0.85 (0.45, 1.60) | — |
| EDIC years 8–11 | ||||
| Lowest quartile | — | — | 1.00 | — |
| Quartile 2 | — | — | 1.33 (0.72, 2.48) | — |
| Quartile 3 | — | — | 1.45 (0.78, 2.70) | — |
| Quartile 4 | — | — | 1.36 (0.73, 2.53) | — |
*Regression models adjusted for DCCT treatment group (intensive vs. conventional), retinopathy cohort (primary prevention vs. secondary intervention), age, sex, diabetes duration, HbA1c, LDL, HDL, systolic blood pressure, smoking status, and IMT reader.
†At each of the four time points when biomarkers were measured, separate repeated-measures logistic regression models using the methods of generalized estimating equations were applied to assess the effect of increased biomarker levels on the odds of being in the upper versus lower measurements of ICA IMT at EDIC years 1, 6, and 12. Also, the odds associated with high progression of ICA IMT from EDIC years 1–12 (i.e., defined by upper quintile of ICA IMT change) were assessed.
‡TNFR-1, TNFR-2, active PAI-1, total PAI-1, and IL-6 contributed to the cytokine/adipokine score.
‡‡sICAM-1, sVCAM-1, and sE-selectin contributed to the endothelial dysfunction score with the inverse z score of sVCAM-1 used to compute the composite score.
Conclusions
The current study examined the longitudinal association between subclinical atherosclerosis as measured by ICA IMT and acute-phase reactants (fibrinogen, CRP), thrombosis (fibrinogen and active and total PAI-1), cytokines/adipokines (TNFR-1 and -2, active and total PAI-1, IL-6), and endothelial dysfunction (sICAM-1, sVCAM-1, and sE-selectin).
Mean carotid artery IMT has been established as an early quantitative marker of generalized atherosclerosis because of its association with cardiovascular outcomes (24,25), cardiovascular risk factors (26,27), and atherosclerosis in other arterial beds (28,29). Mean carotid artery IMT can reflect a combination of arterial characteristics, including an early diffuse preatherosclerotic thickening of the carotid arteries, a single focal thickening of the carotid arteries that contributes disproportionately to the overall mean IMT measured across multiple sites, and at lower levels a nonatherosclerotic thickening that is an adaptive response to altered flow and shear and tensile stress on the arterial wall (30,31). Hence, increased mean ICA IMT measured at the site of maximal wall thickness as was done in the current study likely reflects development of focal carotid artery plaques among older participants, whereas at younger ages it may reflect early diffuse preatherosclerotic thickening. More controversial is the value of carotid IMT progression, which has a high degree of variability, to predict cardiovascular events (32). A recent meta-analysis of individual participant data, which included 22 eligible studies with 36,984 participants, failed to find an association between carotid IMT progression and cardiovascular outcomes (32). In the current study, carotid IMT was measured at two time points 12 years apart (i.e., EDIC years 1 and 12), an extended time period where progression is likely to exceed measurement variability; therefore, we chose to look at both carotid IMT levels and carotid IMT progression.
Notably, our results indicate that individual biomarkers with a couple of marginal exceptions were not predictive of or associated with subclinical atherosclerosis (Supplementary Table 2). Moreover, composite scores of acute-phase reactants, thrombolytic factors, and cytokines/adipokines measured in EDIC years 8–11 were associated with higher levels of atherosclerosis only at EDIC year 12. Interestingly, for each composite score, the risk of subclinical atherosclerosis appeared similarly elevated in the second, third, and fourth quartiles of the composite score (although only statistically significant in fourth quartile) indicating that both mild and severe degrees of inflammation may be associated with the development of atherosclerosis and only individuals in the lowest quartile seem to be protected from elevated ICA IMT. With a couple of exceptions, our longitudinal analyses indicate that only the composite scores obtained from our final biomarker measurement at EDIC years 8–11 were definitively associated with increased subclinical atherosclerosis. Therefore, the temporal relationship between biomarker levels and the progression of subclinical atherosclerosis remains unclear with results suggesting these biomarkers may have little relevance early in the natural history of atherosclerosis.
In a prior study by our group that provided motivation for the current study using linear regression we reported that fibrinogen levels measured at EDIC years 4–6 were associated with progression of ICA IMT from EDIC year 1 to 6 (33). This finding was not replicated in the current analysis, which used logistic regression to examine whether fibrinogen was associated with having elevated ICA IMT at EDIC years 1, 6, or 12. Hence, moving from linear regression, which examined ICA IMT as a continuous variable, to logistic regression, which focused on reaching a threshold and has reduced power, attenuated our prior results. In the current analysis, we focused on logistic rather than linear regression because the clinical utility of a logistic regression model that focused on reaching a threshold (i.e., similar to diagnostic criteria for having a disease) was greater than the clinical utility of a linear regression model. This, however, may have reduced the power to assess the predictive value of the biomarkers studied.
Our study had a number of strengths and limitations. Strengths of the study include the large cohort of patients with type 1 diabetes, longitudinal assessment of patients over >20 years with biomarker measurements at four time points and end point measurements at three time points, and detailed characterization of participants throughout the study period. Limitations of the study include the use of internal z scores to standardize our biomarkers because external reference levels were not available for many of the biomarkers of interest and lack of measurement of the biomarkers in all participants (i.e., incomplete data), which could have introduced selection bias. To overcome potential selection bias, we adjusted for an array of covariates throughout the analysis; however, there may be some residual confounding, which we were unable to account for in our analysis.
The lack of a clear-cut long-term predictive value of any specific biomarker reflecting inflammation, endothelial function, or clotting/fibrinolysis for the presence of subclinical atherosclerosis (as assessed by ICA IMT) in comparison with the observed associations between composite biomarker scores and subclinical atherosclerosis suggests that a single biomarker (likely due to the marked fluctuations in the degree of inflammation in chronic inflammatory processes such as atherosclerosis) is not sufficient to predict the development of disease. Alternatively, our data can be interpreted as meaning that the parameters included in our measurements are likely to reflect the stage of the inflammatory process, endothelial dysfunction, and thrombosis/fibrinolysis activation at the time of the measurements (34–36). Owing to the complexity of the process, it is impossible to definitively establish whether there is merely an association or a predictive relationship between high levels of biomarkers and subclinical atherosclerosis. Regardless, the discriminatory ability of these biomarkers appears limited even in a well-powered study; hence, their relevance in a clinical setting to identify individuals with subclinical atherosclerosis appears quite low.
This contrasts with our previously published reports of the predictive value of the measurements of total modified LDL and its different forms in isolated immune complexes, which exceeded that of classical risk factors (37–39). The proinflammatory properties of immune complexes containing oxidized and other forms of modified LDL have been demonstrated in ex vivo studies (40–44), and all the evidence supports their role as an important initiating factor for the inflammatory process associated with atherosclerosis. They are most likely triggers to initiate the development of atherosclerosis. In contrast, the inflammatory process that is pivotal for the progression of the disease is more complex and variable involving a multitude of players, which renders their use as single and unique biomarkers to assess atherosclerosis rather difficult.
It is possible that the use of carotid artery IMT together with several panels of biomarkers will be able to noninvasively identify patients at high risk of developing cardiovascular disease events. Therefore, future analysis on cardiovascular events will be performed, once this information is released by the DCCT/EDIC group of investigators. Whether the addition of biomarkers that more closely relate to plaque rupture to the present biomarker panels is necessary needs to be determined.
Article Information
Funding. This work was supported by a program project funded by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grant P0NID1-HL55782, by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01-DK081352 and R01-DK088778, and by JDRF grant 2006-49. This work was also supported by the Research Service of the Ralph H. Johnson Department of Veterans Affairs Medical Center. The DCCT/EDIC was sponsored through research contracts from the Division of Diabetes, Endocrinology, and Metabolic Diseases (NIDDK) of the NIH. Additional support was provided by the National Center for Research Resources through the General Clinical Research Center program. The DCCT/EDIC group provided the samples to be analyzed and the clinical data used in data analysis.
Duality of Interest. Additional support was provided by Genentech through a Cooperative Research and Development Agreement with the NIDDK. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.J.H. and N.L.B. wrote the article and were primarily responsible for the statistical analysis of the research data. P.A.C. provided consultation with respect to data analysis and data presentation in the article. R.K. provided the research data and wrote the article. G.V. wrote the article and provided the research data. M.F.L.-V. wrote the article and provided the research data. K.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-2877/-/DC1.
References
- 1.Costacou T, Lopes-Virella MF, Zgibor JC, et al.; The Pittsburgh Epidemiology of Diabetes Complications Study . Markers of endothelial dysfunction in the prediction of coronary artery disease in type 1 diabetes. J Diabetes Complications 2005;19:183–193 [DOI] [PubMed] [Google Scholar]
- 2.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006;55:774–779 [DOI] [PubMed] [Google Scholar]
- 3.Hayaishi-Okano R, Yamasaki Y, Katakami N, et al. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 2002;25:1432–1438 [DOI] [PubMed] [Google Scholar]
- 4.Huvers FC, De Leeuw PW, Houben AJ, et al. Endothelium-dependent vasodilatation, plasma markers of endothelial function, and adrenergic vasoconstrictor responses in type 1 diabetes under near-normoglycemic conditions. Diabetes 1999;48:1300–1307 [DOI] [PubMed] [Google Scholar]
- 5.Klein RL, Hunter SJ, Jenkins AJ, et al.; DCCT/ECIC STUDY GROUP . Fibrinogen is a marker for nephropathy and peripheral vascular disease in type 1 diabetes: studies of plasma fibrinogen and fibrinogen gene polymorphism in the DCCT/EDIC cohort. Diabetes Care 2003;26:1439–1448 [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Hu FB, Rimm EB, Rifai N, Curhan GC. The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int 2006;69:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myrup B, de Maat M, Rossing P, Gram J, Kluft C, Jespersen J. Elevated fibrinogen and the relation to acute phase response in diabetic nephropathy. Thromb Res 1996;81:485–490 [DOI] [PubMed] [Google Scholar]
- 8.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997;6:315–325 [DOI] [PubMed] [Google Scholar]
- 9.Schram MT, Stehouwer CD. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm Metab Res 2005;37(Suppl. 1):49–55 [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Zoppini G, Zenari L, Falezza G. Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in Type 1 diabetic patients without clinically manifest macroangiopathy. Diabet Med 2005;22:999–1004 [DOI] [PubMed] [Google Scholar]
- 11.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Low risk of overt nephropathy after 24 yr of childhood-onset type 1 diabetes mellitus (T1DM) in Norway. Pediatr Diabetes 2006;7:239–246 [DOI] [PubMed] [Google Scholar]
- 12.Shai I, Schulze MB, Manson JE, et al. A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care 2005;28:1376–1382 [DOI] [PubMed] [Google Scholar]
- 13.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD; EURODIAB Prospective Complications Study Group . Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia 2005;48:370–378 [DOI] [PubMed] [Google Scholar]
- 14.Soedamah-Muthu SS, Chaturvedi N, Schalkwijk CG, Stehouwer CD, Ebeling P, Fuller JH; EURODIAB Prospective Complications Study group . Soluble vascular cell adhesion molecule-1 and soluble E-selectin are associated with micro- and macrovascular complications in Type 1 diabetic patients. J Diabetes Complications 2006;20:188–195 [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) . Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan DM, Lachin J, Cleary P, et al.; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group . Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes 1999;48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The DCCT Research Group . Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem 1987;33:2267–2271 [PubMed] [Google Scholar]
- 20.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Early Treatment Diabetic Retinopathy Study Research Group . Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130 [PubMed] [Google Scholar]
- 23.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G; DCCT/EDIC Research Group . Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 2013;36:2317–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997;96:1432–1437 [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol 1997;146:483–494 [DOI] [PubMed] [Google Scholar]
- 26.O’Leary DH, Polak JF, Wolfson SK Jr, et al.; CHS Collaborative Research Group . Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. Stroke 1991;22:1155–1163 [DOI] [PubMed] [Google Scholar]
- 27.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol 1991;134:250–256 [DOI] [PubMed] [Google Scholar]
- 28.Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis 1993;102:99–105 [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DH, Polak JF, Kronmal RA, et al.; The CHS Collaborative Research Group . Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. Stroke 1992;23:1752–1760 [DOI] [PubMed] [Google Scholar]
- 30.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997;28:2442–2447 [DOI] [PubMed] [Google Scholar]
- 31.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 1988;112:1018–1031 [PubMed] [Google Scholar]
- 32.Lorenz MW, Polak JF, Kavousi M, et al.; PROG-IMT Study Group . Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopes-Virella MF, Carter RE, Gilbert GE, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort Study Group . Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 2008;31:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695 [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 37.Lopes-Virella MF, McHenry MB, Lipsitz S, et al.; DCCT/EDIC Research Group . Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis 2007;190:359–369 [DOI] [PubMed] [Google Scholar]
- 38.Lopes-Virella MF, Baker NL, Hunt KJ, Lachin J, Nathan D, Virella G; DCCT/EDIC Research Group . Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis 2011;214:462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes 2011;60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammad SM, Twal WO, Barth JL, et al. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis 2009;202:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwood TA, Virella G. The binding of immune complexes to human red cells: complement requirements and fate of the RBC-bound IC after interaction with human phagocytic cells. Clin Exp Immunol 1986;64:195–204 [PMC free article] [PubMed] [Google Scholar]
- 42.Lappalainen J, Lindstedt KA, Oksjoki R, Kovanen PT. OxLDL-IgG immune complexes induce expression and secretion of proatherogenic cytokines by cultured human mast cells. Atherosclerosis 2011;214:357–363 [DOI] [PubMed] [Google Scholar]
- 43.Virella G, Atchley D, Koskinen S, Zheng D, Lopes-Virella MF; DCCT/EDIC Research Group . Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol 2002;105:81–92 [DOI] [PubMed] [Google Scholar]
- 44.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res 2006;47:1975–1983 [DOI] [PubMed] [Google Scholar]