Abstract
OBJECTIVE
Ranolazine is an antianginal drug that mediates its effects by inhibition of cardiac late sodium current. Although ranolazine is not approved for the treatment of type 2 diabetes, in post hoc analyses of pivotal angina trials, ranolazine was associated with reductions in percent glycosylated hemoglobin (HbA1c) in subjects with type 2 diabetes. The study prospectively assessed the safety and efficacy of ranolazine in subjects with type 2 diabetes with inadequate glycemic control managed by lifestyle alone.
RESEARCH DESIGN AND METHODS
The study was conducted worldwide in 465 subjects, with baseline HbA1c of 7–10% (53–86 mmol/mol) and fasting serum glucose of 130–240 mg/dL, randomized to placebo versus ranolazine.
RESULTS
Compared with placebo, there was a greater decline in HbA1c at week 24 from baseline (primary end point) in subjects taking ranolazine (mean difference −0.56% [−6.1 mmol/mol]; P < 0.0001). Moreover, the proportion of subjects achieving an HbA1c <7.0% was greater with ranolazine (25.6% vs. 41.2%; P = 0.0004). Ranolazine was associated with reductions in fasting (mean difference −8 mg/dL; P = 0.0266) and 2-h postprandial glucose (mean difference −19 mg/dL; P = 0.0008 vs. placebo). Subjects taking ranolazine trended toward a greater decrease from baseline in fasting insulin (P = 0.0507), a greater decrease in fasting glucagon (P = 0.0003), and a lower postprandial 3-h glucagon area under the curve (P = 0.0031 vs. placebo). Ranolazine was safe and well tolerated.
CONCLUSIONS
Compared with placebo, use of ranolazine monotherapy over 24 weeks, in subjects with type 2 diabetes and inadequate glycemic control on diet and exercise alone, significantly reduced HbA1c and other measures of glycemic control.
Introduction
Type 2 diabetes is a global health problem with increasing prevalence, associated with significant cardiovascular morbidity and mortality. The incidence of type 2 diabetes is increased in patients with acute coronary syndrome (ACS) (1), and such patients also have increased risk for coronary artery disease (CAD), CAD-related death, and stroke (2). The presence of type 2 diabetes is associated with a worse prognosis in patients with stable and unstable CAD (3,4). Thus, it may be desirable to have drugs that target both type 2 diabetes and CAD.
Ranolazine is a first-in-class antianginal drug with cardioprotective properties that does not affect heart rate or blood pressure (5). The proposed mechanism of its anti-ischemic effects is inhibition of late sodium current due to blockade of the cardiac isoform of the sodium channel, Nav1.5 (6). Ranolazine has been shown to be safe and effective in treating chronic angina both as monotherapy (Monotherapy Assessment of Ranolazine In Stable Angina [MARISA] trial) and in combination with commonly prescribed cardiovascular drugs (Combination Assessment of Ranolazine In Stable Angina [CARISA] and Efficacy of Ranolazine In Chronic Angina [ERICA] trials) in subjects with established CAD, including subgroups of those with diabetes (5,7–9).
In clinical trials in chronic angina, ranolazine treatment was associated with significant reductions in glycosylated hemoglobin (HbA1c) (5,9–11). Data from the CARISA study showed that ranolazine, in a dose-dependent manner, lowered HbA1c in subjects with chronic angina and type 2 diabetes (9). Data from the MERLIN-TIMI-36 study revealed that ranolazine lowered HbA1c in subjects with diabetes and reduced the incidence of newly elevated HbA1c in initially normoglycemic subjects (10,11).
In nonclinical studies, ranolazine was found to lower fasting and nonfasting glucose levels and preserve pancreatic β-cells in streptozotocin-treated mice and Zucker diabetic fatty rats (12,13). More recent data show that ranolazine inhibits glucagon secretion by blocking the Nav1.3 isoform of sodium channels in pancreatic α-cells, leading to glucagon- and glucose-lowering effects in animal models of diabetes (13). Given that increases in glucagon secretion by α-cells and the failure of glucagon suppression following oral glucose are well documented in type 2 diabetes (14,15), these data suggest a novel and plausible mechanism for ranolazine’s putative antidiabetic properties.
Although the HbA1c-lowering effect of ranolazine has been observed in four previous clinical studies, those studies were not prospectively designed to determine the effect of ranolazine on glycemic parameters. In addition, the effect of ranolazine on HbA1c in these trials was studied in the presence of other antidiabetic medications (which were not controlled). We present the first double-blind, randomized, placebo-controlled trial evaluating the safety and efficacy of ranolazine monotherapy in subjects with type 2 diabetes and inadequate glycemic control with diet and exercise alone.
Research Design and Methods
The ranolazine monotherapy study (ClinicalTrials.gov identifier NCT01472185) was one of three randomized, double-blind, placebo-controlled, multicenter phase 3 clinical trials conducted to investigate the effect of ranolazine on glycemic control. This study enrolled subjects with inadequately controlled type 2 diabetes who were either treatment naïve or had been washed off of their background antihyperglycemic medications.
Subjects
Eligible subjects were men and women aged 18–75 years, with an established diagnosis of type 2 diabetes, an HbA1c of 7–10% (53–86 mmol/mol), and a fasting serum glucose (FSG) of 130–240 mg/dL at screening and at the end of a prerandomization qualifying period (see Study Design below). Other inclusion criteria included a BMI of 25–45 kg/m2 and a fasting serum C-peptide ≥0.8 ng/mL. Key exclusion criteria included type 1 diabetes, severe hypoglycemia, myocardial infarction (MI), ACS, coronary revascularization, stroke, or transient ischemic attack (TIA) within 3 months, inadequately controlled hypertension (i.e., >160/100 mmHg), a QT interval >500 ms, bariatric surgery or significant weight change within 2 months, an estimated glomerular filtration rate <30 mL/min/1.73 m2, and prior ranolazine treatment.
Study Design
The study consisted of a screening period, a 14-day qualifying period, a 24-week treatment period, and a 14-day safety follow-up period. Potentially eligible subjects had to be either treatment naïve or washed off of all antihyperglycemic therapy for 90 days (24 weeks for thiazolidinediones). During the qualifying period, subjects were treated with single-blind placebo, receiving one tablet twice daily. At the end of the qualifying period, subjects who were ≥80% compliant with dosing and continued to meet all eligibility criteria (including HbA1c 7–10% and FSG 130–240 mg/dL) proceeded to randomization.
Subjects entering the treatment period were randomized in a 1:1 ratio to receive either ranolazine or matching placebo for 24 weeks. Randomization was stratified by baseline HbA1c (≤8 vs. >8% [64 mmol/mol]); enrollment of subjects with HbA1c ≤8% was capped at half the total enrollment. Ranolazine was started at 500 mg twice daily and then uptitrated to 1,000 mg twice daily after 7 days. Subjects taking placebo were given matching tablets during treatment. Subjects took 1,000 mg twice daily throughout the treatment period but were permitted to downtitrate to 500 mg twice daily (or matched placebo) for tolerability at the discretion of the site investigator. Subjects who continued to experience intolerance to study drug underwent an early termination (ET) visit and were discontinued from the study.
During the treatment period, subjects returned to the clinic for efficacy and safety assessments at weeks 6, 12, 18, and 24 and ET visits for subjects who prematurely discontinued the study. HbA1c, FSG, fasting serum insulin, fasting serum C-peptide, fasting plasma glucagon, and safety laboratory values were measured. In addition, 12-lead electrocardiograms (ECGs), fasting lipid profiles, and mixed-meal tolerance testing (MMTT) were performed at weeks 12 and 24 and at ET visits.
For the MMTTs, subjects were required to fast for 8 h and take their dose of study drug (single-blind placebo at randomization, double-blind ranolazine, or placebo at weeks 12 and 24 and ET visits) at the study site 60 min prior to the MMTT. Subjects consumed a standard glucose-containing drink (i.e., Boost Plus) containing 15% protein, 50% carbohydrate, and 35% fat, with a caloric content of 360 kcal per 240 mL. Serial postprandial blood samples were collected 15, 30, 45, 60, 120, 150, and 180 min after the meal for determination of serum glucose, serum insulin, serum C-peptide, and plasma glucagon.
During the treatment period, subjects found to be persistently hyperglycemic were given open-label rescue medication, in addition to their blinded treatment regimen, to treat ongoing hyperglycemia. Eligibility for hyperglycemic rescue therapy consisted of two consecutive FSG measurements >270 mg/dL spaced ≤7 days apart from day 1 to week 6, or two consecutive FSGs >240 mg/dL spaced ≤7 days apart from weeks 7 to 24. Subjects who continued to be hyperglycemic and required the addition of a second antihyperglycemic agent were discontinued from the study.
Following the last completed visit (week 24 or ET), subjects were contacted 14 days after the last dose of study drug. In addition, randomized subjects who discontinued the study early were contacted 24 weeks after the first dose of study drug for ascertainment of vital status.
The study was conducted according to the guidelines of Good Clinical Practice and the Declaration of Helsinki. The protocol, amendments, and associated materials were approved by relevant regulatory agencies, institutional review boards, and ethics committees. All subjects provided written informed consent prior to study entry.
Study End Points
The primary end point was the 24-week change from baseline in HbA1c. Prespecified ordered secondary end points (all at week 24) were 1) change from baseline in FSG, 2) proportion of subjects with HbA1c <7.0% (53 mmol/mol), and 3) change from baseline in 2-h postprandial glucose (PPG). Additional non–alpha-controlled end points included by-visit changes from baseline in the primary and secondary end points; change from baseline in incremental change of 2-h PPG; changes from baseline in fasting insulin, C-peptide, and glucagon; change from baseline in 3-h area under the curve (AUC) for PPG, insulin, C-peptide, and glucagon; and change from baseline in body weight.
Safety assessments included adverse events (AEs) (including adjudicated MACE/MACE+; see below), the incidence and severity of hypoglycemia (per American Diabetes Association [ADA] criteria [16]), discontinuation for hyperglycemia, use of rescue medication for hyperglycemia, vital signs, body weight, physical exam, ECG parameters, and laboratory evaluations (including complete blood chemistry, hematology, and urinalysis).
Safety data were monitored by an independent data safety monitoring board. Additionally, an independent clinical events committee adjudicated whether AEs were major adverse cardiac/cerebrovascular events (MACE and MACE+). Components of MACE were cardiovascular-related mortality, MI, and stroke/TIA. Components of MACE+ included all MACE as well as unstable angina requiring hospitalization or urgent revascularization.
Statistical Methods
Four hundred subjects provided ≥90% power to detect a statistically significant treatment difference in change from baseline in HbA1c of −0.5% at week 24, assuming a common SD of 1.2% and a two-sided type I error rate of 0.05.
Primary and secondary efficacy analyses were performed on the following analysis sets.
Full Analysis Set
All randomized subjects received at least one dose of study treatment, with a baseline and at least one postbaseline HbA1c measurement, and no major eligibility violations.
MMTT Full Analysis Set
All randomized subjects received at least one dose of study treatment, with a baseline and at least one postbaseline 2-h PPG measurement, and no major eligibility violations.
MMTT AUC Full Analysis Set
All randomized subjects received at least one dose of study treatment, with glucose measurements at −5, 60, and 120 min (or later) following the start of the MMTT during the baseline visit and at least one postbaseline visit, and no major eligibility violations.
Primary and secondary efficacy end points were tested using a fixed-sequence closed testing procedure to adjust for multiplicity and preserve the family-wise type I error rate of 0.05. Continuous efficacy end points were analyzed using a mixed-models repeated-measures approach, which accounts for correlations among observations within a subject, allows for baseline adjustment, and uses all available data collected at various time points, including data from participants not completing week 24. Effects included baseline HbA1c, baseline value of the end point (if not HbA1c), treatment, visit, and treatment by visit interaction term using unstructured correlation matrices. Subgroup analyses included additional effects for subgroup, subgroup by treatment, subgroup by visit, and subgroup by treatment by visit; subgroup analysis by baseline HbA1c did not include baseline HbA1c. For the proportion of subjects with HbA1c <7%, treatments were compared using a Cochran-Mantel-Haenszel test stratified by baseline HbA1c (≤8 vs. >8%). For efficacy analyses, measurements after the antihyperglycemic rescue medication were excluded. Values from ET, rescue, or unscheduled visits occurring within 3 weeks of week 24 were substituted for missing week 24 values.
Safety analyses were performed on a safety analysis set consisting of all randomized subjects receiving at least one dose of study treatment.
Results
Subject Disposition
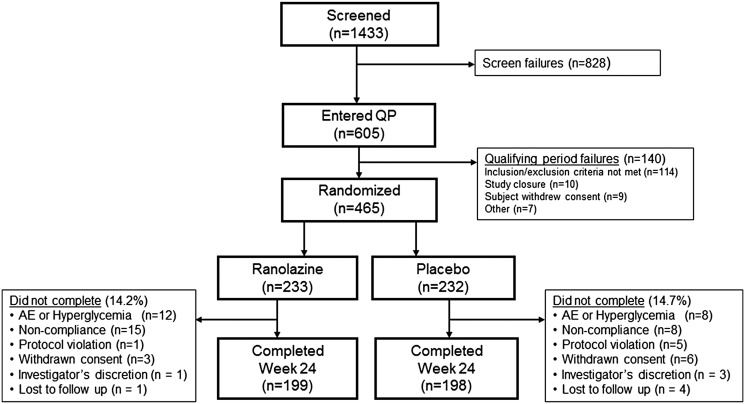
The study was conducted in 133 sites from nine countries worldwide between November 2011 and October 2013. Detailed country-specific enrollment metrics can be found in Supplementary Table 1. Subjects (n = 1,433) entered screening for the study (Fig. 1). Of these, 605 subjects entered the qualifying period and 465 were randomized (232 to placebo and 233 to ranolazine). A total of 198 placebo subjects and 199 ranolazine subjects completed the study, and 34 (14.7%) placebo and 33 (14.2%) ranolazine subjects were prematurely discontinued from the study. The most frequent reason for ET was subject noncompliance (8 [3.4%] placebo and 15 [6.5%] ranolazine), followed by nonhyperglycemic AEs (3 [1.3%] placebo and 10 [4.3%] ranolazine). The most common nonhyperglycemic AE leading to permanent study drug discontinuation was nausea (0 placebo and 3 [1.3%] ranolazine), followed by dizziness (0 placebo and 2 [0.9%] ranolazine). One participant from the ranolazine group discontinued the study drug due to hypoglycemia. Otherwise, no other nonhyperglycemic AE resulting in early study drug discontinuation occurred in more than one subject.
Figure 1.
Disposition of subjects. QP, qualifying period.
Baseline Characteristics
Overall, baseline characteristics were similar between treatment groups (Table 1). Subjects had a mean age of 56 years, with a range of 20–75 years. Approximately half (50.9%) were female. Subjects had a mean BMI of 32.8 kg/m2, and a mean waist circumference of 105.8 cm. The mean baseline HbA1c was 8.04% (64 mmol/mol) (8.01% [64 mmol/mol] placebo and 8.06% [65 mmol/mol] ranolazine), and the mean FSG was 171.8 mg/dL (171.5 mg/dL placebo and 172.1 mg/dL ranolazine). The mean fasting serum insulin, fasting serum C-peptide, and fasting plasma glucagon were also similar between treatment groups. Most subjects (78.0% placebo and 80.2% ranolazine) were treatment naïve to previous diabetes medications; the most frequently used classes of antihyperglycemic agents prior to study entry were metformin (17.2% placebo and 15.9% ranolazine) and sulfonylureas (4.7% for both groups). Similar numbers of subjects were on statins (17.7% and 18.1% in the placebo and ranolazine groups, respectively). The overall duration of diabetes prior to enrollment was similar (3.0 years). Few subjects reported a history of MI (4.7% placebo and 5.6% ranolazine) or stroke (3.0% placebo and 2.2% ranolazine).
Table 1.
Demographics and baseline characteristics
| Placebo (n = 232) | Ranolazine (n = 232) | |
|---|---|---|
| Age, years (mean ± SD) | 56 ± 9.3 | 55 ± 9.5 |
| Female | 48.7% | 53.0% |
| White race | 90.1% | 91.8% |
| Ethnicity | ||
| Hispanic or Latino | 13.4% | 12.5% |
| Not Hispanic or Latino | 86.6% | 87.1% |
| Not reported | 0 | 0.4% |
| Weight, kg (mean ± SD) | 92.1 ± 17.5 | 93.2 ± 16.4 |
| BMI, kg/m2 (mean ± SD) | 32.8 ± 4.85 | 32.8 ± 4.75 |
| eGFR†, mL/min/1.73 m2 (mean ± SD) | 83.3 ± 18.4 | 84.5 ± 18.8 |
| HbA1c, % (mean ± SD) | 8.01 ± 0.727 | 8.06 ± 0.732 |
| HbA1c, mmol/mol (mean) | 64 | 65 |
| FSG, mg/dL (mean ± SD) | 171.5 ± 34.45 | 172.1 ± 34.32 |
| Diabetes duration, years (mean ± SD) | 3.0 ± 4.00 | 3.0 ± 4.29 |
| Took prior antihyperglycemic agent (any) | 22.0% | 19.8% |
| Sulfonylureas | 4.7% | 4.7% |
| Biguanides | 17.2% | 15.9% |
| Concomitant lipid-lowering therapy (any) | 21.1% | 21.1% |
| Statins | 17.7% | 18.1% |
| Fibrates | 3.0% | 2.2% |
| Niacin | 0 | 0 |
| Other | 3.0% | 2.2% |
eGFR, estimated glomerular filtration rate.
Efficacy Assessments
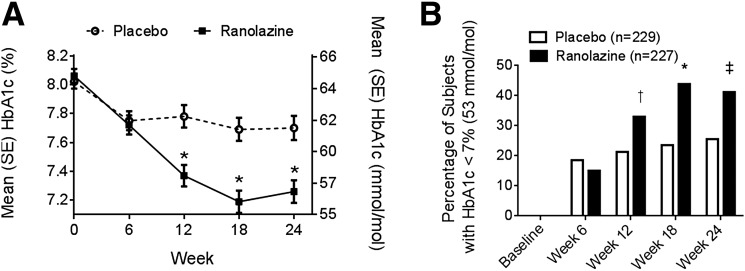
Compared with placebo, there was a greater decline in HbA1c at week 24 from baseline (primary efficacy end point) in subjects taking ranolazine monotherapy (placebo-corrected least squares mean [LSM] difference −0.56% [−6.1 mmol/mol]; P < 0.0001) (Table 2). This difference was first evident at week 12 and persisted through week 24 (Fig. 2A). The number of subjects achieving an HbA1c <7.0% at week 24 was greater in the ranolazine group (25.6% placebo and 41.2% ranolazine; P = 0.0004) (Fig. 2B). Subgroup analyses (i.e., by age, sex, region, renal function, baseline HbA1c, diabetes duration, and prior diabetes medications) generally showed that subjects in the ranolazine group had greater mean reductions from baseline in HbA1c at week 24 than placebo subjects (Supplementary Fig. 1 and Supplementary Table 2). Treatment-naïve subjects tended to have a greater response to ranolazine than those previously treated for type 2 diabetes (P = 0.0865); otherwise there were no significant interactions noted for any single subgroup.
Table 2.
Efficacy and safety results
| Efficacy data | ||
|---|---|---|
| Treatment assignment | Placebo | Ranolazine |
| HbA1c | ||
| n | 195 | 199 |
| Baseline mean ± SD (%) | 8.02 ± 0.728 | 8.06 ± 0.735 |
| Baseline mean (mmol/mol) | 64 | 65 |
| Week 24 mean ± SD (%) | 7.70 ± 1.183 | 7.26 ± 1.101 |
| Week 24 mean (mmol/mol) | 61 | 56 |
| LS change from baseline mean ± SE (%) | −0.20 ± 0.073 | −0.76 ± 0.073 |
| LS change from baseline mean (mmol/mol) | −2.2 | −8.3 |
| Placebo-corrected LS change from baseline mean (95% CI) (%) | −0.56 (−0.76, −0.36) | |
| Placebo-corrected LS change from baseline mean (95% CI) (mmol/mol) | −6.1 (−8.3, −3.9) | |
| P value vs. placebo | 0.0001 | |
| FSG (mg/dL) | ||
| n | 191 | 197 |
| Baseline mean ± SD | 171 ± 34.6 | 172 ± 34.5 |
| Week 24 mean ± SD | 169 ± 40.3 | 165 ± 40.4 |
| LS change from baseline mean ± SE | 2 ± 2.7 | −6 ± 2.6 |
| Placebo-corrected LS change from baseline mean (95% CI) | −8 (−16, −1) | |
| P value vs. placebo | 0.0266 | |
| Fasting plasma glucagon (pg/mL) | ||
| n | 188 | 192 |
| Baseline mean ± SD | 86 ± 26.1 | 85 ± 28.9 |
| Week 24 mean ± SD | 93 ± 32.3 | 84 ± 28.5 |
| LS change from baseline mean ± SE | 9 ± 1.8 | −1 ± 1.8 |
| Placebo-corrected LS change from baseline mean (95% CI) | −09 (−14, −4) | |
| P value vs. placebo | 0.0003 | |
| Fasting serum insulin (μIU/mL) | ||
| n | 166 | 168 |
| Baseline mean ± SD | 14.84 ± 10.79 | 13.85 ± 9.081 |
| Week 24 mean ± SD | 13.18 ± 10.46 | 11.66 ± 7.484 |
| LS change from baseline mean ± SE | −1.04 ± 0.510 | −2.45 ± 0.509 |
| Placebo-corrected LS change from baseline mean (95% CI) | −1.41 (−2.83, 0.00) | |
| P value vs. placebo | 0.0507 | |
| Fasting serum C-peptide (ng/mL) | ||
| n | 186 | 189 |
| Baseline mean ± SD | 2.65 ± 1.106 | 2.55 ± 1.002 |
| Week 24 mean ± SD | 2.68 ± 1.175 | 2.46 ± 0.943 |
| LS change from baseline mean ± SE | 0.09 ± 0.059 | −0.11 ± 0.059 |
| Placebo-corrected LS change from baseline mean (95% CI) | −0.20 (−0.36, −0.04) | |
| P value vs. placebo | 0.0158 | |
| Safety data | ||
| Treatment assignment (n) | Placebo (n = 232) | Ranolazine (n = 232) |
| AEs | ||
| Subjects with any AE, n (%) | 89 (38.4%) | 97 (41.8%) |
| Hyperglycemia, n (%) | 23 (9.9%) | 19 (8.2%) |
| Constipation, n (%) | 10 (4.3%) | 12 (5.2%) |
| Nausea, n (%) | 2 (0.9%) | 9 (3.9%) |
| Dizziness, n (%) | 2 (0.9%) | 6 (2.6%) |
| AEs related to study drug | ||
| Subjects with any related AE, n (%) | 9 (3.9%) | 21 (9.1%) |
| Constipation, n (%) | 3 (1.3%) | 5 (2.2%) |
| Headache, n (%) | 2 (0.9%) | 6 (2.6%) |
| Dizziness, n (%) | 1 (0.4%) | 5 (2.2%) |
| SAEs, n (%) | 7 (3.0%) | 6 (2.6%) |
| AE leading to premature study drug discontinuation, n (%) | 8 (3.4%) | 12 (5.2%) |
| Deaths during study, n (%) | 0 | 1 (0.4%) |
P value indicates difference in placebo-corrected LSM change from baseline; n of change from baseline at week 24. LS, least squares; SAEs, serious adverse events.
Figure 2.
A: Effect of ranolazine on HbA1c by visit. B: Effect of ranolazine on proportion of subjects achieving HbA1c <7.0%. Placebo, n = 229; ranolazine, n = 227. *P = 0.0046 vs. placebo. †P < 0.0001 vs. placebo. ‡P = 0.0004 vs. placebo.
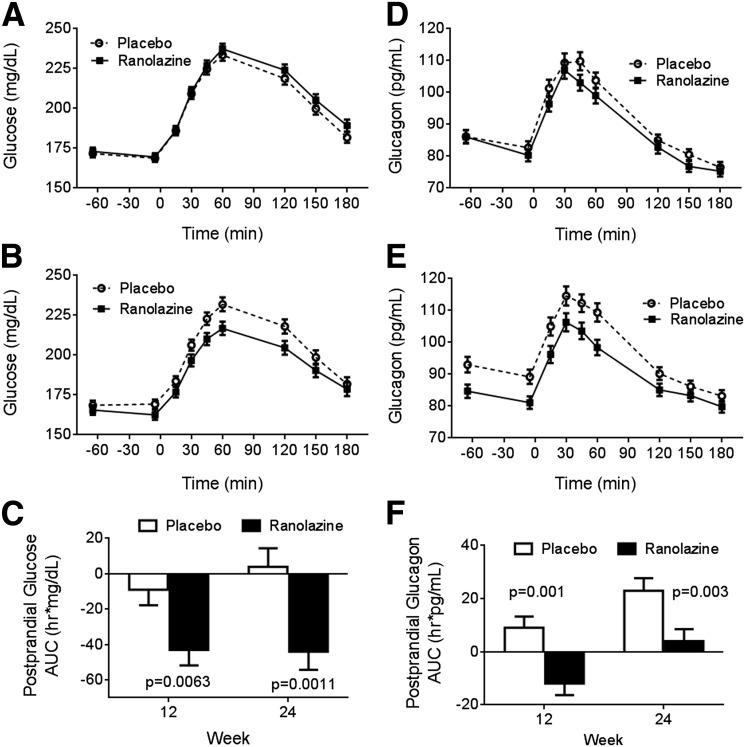
At week 24, FSG was decreased in subjects taking ranolazine compared with subjects taking placebo (change from baseline LSM difference −8 mg/dL; P = 0.0266) (Table 2). Similar to fasting glucose, the 2-h PPG (LSM difference −19 mg/dL; P = 0.0008) and incremental change in 2-h PPG (LSM difference −9 mg/dL; P = 0.0130) were also improved with ranolazine (Fig. 3A–C). Additionally, the placebo-corrected change from baseline in total and incremental 3-h glucose AUCs derived from MMTTs were significantly decreased in the ranolazine group at week 12 and remained decreased at week 24.
Figure 3.
FSG and glucose response to MMTT at baseline (A) and week 24 (B), as well as PPG change from baseline at weeks 12 and 24 (C). Fasting plasma glucagon and glucagon response to MMTT at baseline (D) and week 24 (E), as well as postprandial glucagon change from baseline at weeks 12 and 24 (F). All values listed are least squares means ± SE.
Ranolazine lowered both fasting and postprandial glucagon levels compared with placebo (Fig. 3D–F). The LSM differences between treatment groups for the changes in fasting plasma glucagon from baseline to weeks 6–24 ranged from −9 to −4 pg/mL; the treatment difference was statistically significant at weeks 6, 12, and 24 (P ≤ 0.0035) but not week 18 (P = 0.1165). During MMTT, change from baseline in the total 3-h AUC for glucagon was lower with ranolazine versus placebo, with an LSM difference of −20 h · pg/mL (P = 0.0010) at week 12 and −19 h · pg/mL (P = 0.0031) at week 24 (Supplementary Table 2), but the changes from baseline in incremental 3-h AUC for glucagon were not statistically different between treatments.
Relative to placebo, subjects taking ranolazine had a trend toward a greater decrease from baseline in fasting serum insulin at week 24 (LSM difference −1.41 μIU/mL; P = 0.0507) (Supplementary Table 3). Changes from baseline in postprandial serum insulin 3-h AUC were similar between ranolazine and placebo. However, fasting serum C-peptide levels were significantly lower in the ranolazine group versus placebo at week 24 (change from baseline LSM difference −0.20 ng/mL; P = 0.0158). Yet, no treatment differences were observed in postprandial C-peptide 3-h AUC.
Safety Assessments
In general, ranolazine was well tolerated and safe (Table 2). The overall number of subjects experiencing an AE was similar between groups (38.4% placebo and 41.8% ranolazine). Although the proportion of subjects with study drug–related AEs was greater in the ranolazine group (3.9% placebo and 9.1% ranolazine), the number of subjects with serious AEs (3.0% placebo and 2.6% ranolazine) and drug discontinuations due to AEs (3.4% placebo and 5.2% ranolazine) was broadly comparable between treatment arms. No serious AEs were related to study drug, and no single serious AE occurred in more than one subject.
The most frequently reported AEs were hyperglycemia (9.9% placebo and 8.2% ranolazine), headache (4.3% vs. 5.2%), constipation (1.3% vs. 4.7%), and nausea (0.9% vs. 3.9%). The most commonly reported AEs related to study drug (either ranolazine or placebo) were constipation, headache, nausea, and dizziness. Four subjects, all randomized to ranolazine, experienced hypoglycemia during the treatment period. No subject had severe or documented symptomatic hypoglycemia.
Two subjects (one on placebo with unstable angina requiring hospitalization and one on ranolazine with urgent revascularization and unstable angina requiring hospitalization) had AEs that were adjudicated by the clinical events committee as major cardiovascular events. Otherwise there were no adjudicated MIs, strokes/TIAs, or cardiovascular deaths. One death occurred during the study, 92 days after the last dose of study drug. The subject died of cardiorespiratory failure as a consequence of pneumonia and was not considered related to study drug. The subject, who was in the ranolazine group, had been inappropriately enrolled into the study with advanced chronic kidney disease. The subject’s enrollment was considered a protocol violation, and study drug was discontinued after 5 days.
There were no notable changes from baseline or differences between treatment groups in blood chemistries and hematology (including hemoglobin, white blood cell count, and platelets). No increases in urinary glucose, creatinine, or microalbumin were observed. Similarly, there were no changes in heart rate or blood pressure. Although subjects taking ranolazine had decreased body weight at week 12 (LSM difference −0.49 kg vs. placebo; P = 0.0388), this difference was not sustained through week 24. During ECG monitoring, ranolazine had no effect on PR, RR, or QRS; a 7-ms increase in QTc was seen in the ranolazine group, consistent with prior observations (17).
Conclusions
CAD and diabetes are nominally two distinct disease entities; however, the two are mechanistically and epidemiologically linked. It is well appreciated that the metabolic derangements inherent in diabetes (including hyperglycemia, insulin resistance, and excess free fatty acids) lead to endothelial dysfunction, impaired vascular smooth muscle function, and hypercoagulability, conditions that ultimately lead to atherogenesis and coronary events (18). It has been estimated that 5.7 million U.S. adults ≥35 years with diabetes have coincident cardiovascular disease (19). A more recent analysis in 1,957 adults with CAD from the National Health and Nutrition Examination Survey (NHANES) cohort revealed that 619 (28%) had a diagnosis of diabetes (20). Of these, 44% also had angina, suggesting a significant overlap in the patient populations affected by these two diseases. Such overlap substantially increases the complexity of the management of patients with both conditions. For instance, although β-blockers are an established cornerstone of antianginal therapy, to varying extents they also worsen glycemic control and mask the symptoms of hypoglycemia (21,22). On the other hand, the development and use of effective pharmacological agents for the treatment of patients with type 2 diabetes has been limited by concerns over the cardiovascular safety of such agents (23). Hence, given the high prevalence of CAD and diabetes in contemporary society, there is thus a need for antianginal and antihyperglycemic medications that do not have the safety and tolerability liabilities associated with currently available agents. In concert with the results of the Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina (TERISA) clinical trial (24), which demonstrated the antianginal efficacy of ranolazine in subjects with comorbid type 2 diabetes, the results of the current study suggest that ranolazine could potentially address both needs. It should be noted, however, that ranolazine is not approved for the treatment of diabetes.
This study is the first to demonstrate that ranolazine monotherapy reduced HbA1c as a measure of ambient glycemia assessed prospectively in a randomized, placebo-controlled fashion in subjects with type 2 diabetes. This effect was seen by 12 weeks in the absence of other glucose-lowering medications and was sustainable up to 24 weeks. In addition, the percentage of subjects achieving a guideline-directed HbA1c goal of <7.0% was nearly twice as high with ranolazine as placebo. This level of glycemic control is accepted by the ADA as a reasonable goal for most patients with type 2 diabetes (25). Overall, ranolazine proved safe, with a low dropout rate that was similar between groups.
The mechanism underlying the antidiabetic effects of ranolazine has been described in detail in a recent preclinical study (13). Ranolazine was shown to inhibit glucagon secretion from pancreatic α-cells by inhibiting their electrical activity via blockade of sodium channels (Nav1.3 isoform) (13). In animal models of diabetes, ranolazine lowered postprandial and basal glucagon levels, which were associated with a reduction in hyperglycemia and lowering of HbA1c (13). The current study provides analogous findings in a population of subjects with type 2 diabetes, suggesting confirmation of this proposed mechanism of action in the clinical setting.
The relationship of glucagon to type 2 diabetes merits discussion, as this would suggest that ranolazine exhibits antihyperglycemic effects via a novel mechanism. Hyperglucagonemia increases the rates of glycogenolysis and gluconeogenesis (26), thereby increasing hepatic glucose production in both fasting and postprandial states, ultimately leading to increases in both fasting glucose and PPG levels (14). The importance of hyperglucagonemia as a contributor to the pathophysiology of type 2 diabetes (27) is further highlighted by the finding that glucagon receptor deficiency is sufficient to abolish hyperinsulinemia and hyperglycemia seen in fat-fed and leptin receptor–deficient mice (27). The concomitant reduction of both glucagon and glucose by ranolazine in this trial suggests that ranolazine might reduce blood glucose by decreasing glucagon secretion from α-cells, as described above, and lowering circulating glucagon levels. Additional effects of ranolazine on other elements known to influence either glucose or glucagon levels (such as glucagon-like peptide 1 and dipeptidyl peptidase 4) have not been ruled out.
Notably, ranolazine had a relatively greater effect in lowering HbA1c compared with the reductions in FSG or PPG. This raises the question as to whether ranolazine lowers HbA1c via one or more mechanisms other than reducing glucose levels. However, several lines of evidence argue against such alternate mechanisms. First, direct interference by ranolazine on the HbA1c assay was investigated and eliminated in experiments measuring HbA1c in the presence or absence of ranolazine (Gilead Sciences, data on file). Second, ranolazine does not affect the glycation of hemoglobin independent of changes in glucose concentration (Gilead Sciences, data on file). Third, ranolazine has not been associated with anemia in this study or other clinical trials (5,7,28), making increased red cell turnover an unlikely cause for the association. Fourth, ranolazine has little to no effect on patients with lower blood/plasma glucose (11). Finally, there are greater ranolazine-associated reductions in HbA1c seen in patients with higher glucose levels and more severe type 2 diabetes (insulin use vs. nonuse) (9,11). Importantly, FSG and PPG as measured in this study may not be ideal surrogates for mean glucose. Considering that the elimination half-life of ranolazine is 7 h, a blood sample drawn 12 h after dosing of ranolazine (i.e., at trough level) might yield a minimal reduction in glucose relative to samples drawn at earlier time points (at higher drug concentrations). Future studies evaluating glucose more intensively, either through multiple-point testing or continuous glucose monitoring, should be considered to fully understand ranolazine’s effect on glucose. The totality of current evidence suggests that the lowering of HbA1c by ranolazine is likely due to reductions in glucose. Regarding the management of patients with type 2 diabetes, the ADA and the European Association for the Study of Diabetes (EASD) recommend using HbA1c as the primary target for glycemic control.
To have a complete picture of ranolazine’s antihyperglycemic properties, it is important to evaluate its efficacy not only as monotherapy but also as an add-on to established therapies such as biguanides and sulfonylureas. Trials evaluating ranolazine on a background of either glimepiride (NCT01494987) or metformin (NCT01555164) have been conducted. The results of these two studies are available on www.clinicaltrials.gov, with publication of their results forthcoming.
In summary, in subjects with type 2 diabetes on no glucose-lowering pharmacological agents, ranolazine lowered HbA1c by a mean difference of −0.56% with a near doubling of subjects achieving an HbA1c <7.0%. Ranolazine also decreased fasting glucose and PPG and fasting and postprandial glucagon levels. The results of this study suggest ranolazine could be an option for the management of patients with concurrent chronic angina and type 2 diabetes. Given the neutral-to-negative glycemic effects of currently available antianginal drugs, such patients may be uniquely suited to be managed with ranolazine.
Article Information
Duality of Interest. R.H.E. has received consulting fees from Amgen, Amylin Pharmaceuticals, Esperion, Johnson & Johnson, Novo Nordisk, FoodMinds, Regeneron, Sanofi, Vivus, and Eli Lilly and Company and has received research funding from GlaxoSmithKline, Sanofi, Esperion, and Janssen. R.R.H. has received consulting/advisory board honoraria from Amgen, Boehringer Ingelheim, Eli Lilly and Company, F. Hoffmann-La Roche/Genentech, Gilead Sciences, Intarcia, Isis, Johnson & Johnson, Merck, Novo Nordisk, and Sanofi; has received research funding from Amylin Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Medtronic, and Sanofi; and has received speaker honoraria from Boehringer Ingelheim. A.D., L.B., P.Y., P.W., and P.J. are employees and stockholders of Gilead Sciences. J.S.S. is chairman of the Type 1 Diabetes TrialNet clinical trials network, supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; has served as a board member for Amylin Pharmaceuticals, Dexcom, Moerae Matrix, and Paean Therapeutics; has received consulting fees from BD Technologies, Boehringer Ingelheim, Bristol-Myers Squibb, Cebix, DiaVacs, Elcelyx, Exsulin, Gilead Sciences, Halozyme Therapeutics, Ideal Life, Intarcia Therapeutics, MannKind, Mellitech, Merck, Orgenesis, Sanofi, Sekris Biomedical, Takeda, Valeritas, and ViaCyte; has received research funding from Halozyme Therapeutics, Intuity Medical, Mesoblast, and Osiris Therapeutics; has received speaker honoraria from Novo Nordisk and Sanofi; has received royalties for editing a book from Springer; and is a stockholder of Amylin Pharmaceuticals, Dexcom, Ideal Life, Moerae Matrix, OPKO Health, Patton Medical Devices, and Tandem Diabetes Care. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.H.E. and A.D. drafted the manuscript. R.R.H., L.B., and J.S.S. designed the study. P.Y. participated in the conduct of the study and drafted the manuscript. P.W. and P.J. designed the study and participated in the conduct of the study. All authors analyzed the data and provided editorial assistance with the manuscript. All authors are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
Clinical trial reg. no. NCT01472185, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-2629/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol 2005;96:363–365 [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 3.Herlitz J, Wognsen GB, Karlson BW, et al. . Mortality, mode of death and risk indicators for death during 5 years after coronary artery bypass grafting among patients with and without a history of diabetes mellitus. Coron Artery Dis 2000;11:339–346 [DOI] [PubMed] [Google Scholar]
- 4.Duarte R, Castela S, Reis RP, et al. . Acute coronary syndrome in a diabetic population–risk factors and clinical and angiographic characteristics. Rev Port Cardiol 2003;22:1077–1088 [PubMed] [Google Scholar]
- 5.Chaitman BR, Pepine CJ, Parker JO, et al.; Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators . Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291:309–316 [DOI] [PubMed] [Google Scholar]
- 6.Belardinelli L, Shryock JC, Fraser H. The mechanism of ranolazine action to reduce ischemia-induced diastolic dysfunction. Eur Heart J 2006;8:A10–A13 [Google Scholar]
- 7.Chaitman BR, Skettino SL, Parker JO, et al.; MARISA Investigators . Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–1382 [DOI] [PubMed] [Google Scholar]
- 8.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L; ERICA Investigators . Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48:566–575 [DOI] [PubMed] [Google Scholar]
- 9.Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27:42–48 [DOI] [PubMed] [Google Scholar]
- 10.Morrow DA, Scirica BM, Chaitman BR, et al.; MERLIN-TIMI 36 Investigators . Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009;119:2032–2039 [DOI] [PubMed] [Google Scholar]
- 11.Chisholm JW, Goldfine AB, Dhalla AK, et al. . Effect of ranolazine on A1C and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care 2010;33:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning Y, Zhen W, Fu Z, et al. . Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther 2011;337:50–58 [DOI] [PubMed] [Google Scholar]
- 13.Dhalla AK, Yang M, Ning Y, et al. . Blockade of Na+ channels in pancreatic α-cells has antidiabetic effects. Diabetes 2014;63:3545–3556 [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, Defronzo RA, Glass L, et al. . Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 2002;51:1111–1119 [DOI] [PubMed] [Google Scholar]
- 15.Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007;50:797–805 [DOI] [PubMed] [Google Scholar]
- 16.Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranexa (ranolazine) extended-release tablets. U.S. prescribing information. Foster city, CA, Gilead Sciences, Inc., December 2013
- 18.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003;108:1527–1532 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Prevalence of self-reported cardiovascular disease among persons aged > or =35 years with diabetes—United States, 1997-2005. MMWR Morb Mortal Wkly Rep 2007;56:1129–1132 [PubMed] [Google Scholar]
- 20.Wong ND, Hui G. Angina prevalence and characteristics in coronary artery disease patients with and without diabetes. J Am Coll Cardiol 2014;63:A1538 [Google Scholar]
- 21.Rizos CV, Elisaf MS. Antihypertensive drugs and glucose metabolism. World J Cardiol 2014;6:517–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakris G, Stockert J, Molitch M, et al.; STAR Investigators . Risk factor assessment for new onset diabetes: literature review. Diabetes Obes Metab 2009;11:177–187 [DOI] [PubMed] [Google Scholar]
- 23.Green JB. Understanding the type 2 diabetes mellitus and cardiovascular disease risk paradox. Postgrad Med 2014;126:190–204 [DOI] [PubMed] [Google Scholar]
- 24.Kosiborod M, Arnold SV, Spertus JA, et al. . Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol 2013;61:2038–2045 [DOI] [PubMed] [Google Scholar]
- 25.Williamson C, Glauser TA, Burton BS, Schneider D, Dubois AM, Patel D. Health care provider management of patients with type 2 diabetes mellitus: analysis of trends in attitudes and practices. Postgrad Med 2014;126:145–160 [DOI] [PubMed] [Google Scholar]
- 26.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E671–E678 [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Berglund ED, Yu X, et al. . Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA 2014;111:13217–13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al.; MERLIN-TIMI 36 Trial Investigators . Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007;297:1775–1783 [DOI] [PubMed] [Google Scholar]