Abstract
GLP-1 receptor (GLP-1R) agonists may improve endothelial function (EF) via metabolic improvement and direct vascular action. The current study determined the effect of GLP-1R agonist exenatide on postprandial EF in type 2 diabetes and the mechanisms underlying GLP-1R agonist–mediated vasodilation. Two crossover studies were conducted: 36 participants with type 2 diabetes received subcutaneous exenatide or placebo for 11 days and EF, and glucose and lipid responses to breakfast and lunch were determined; and 32 participants with impaired glucose tolerance (IGT) or diet-controlled type 2 diabetes had EF measured before and after intravenous exenatide, with or without the GLP-1R antagonist exendin-9. Mechanisms of GLP-1R agonist action were studied ex vivo on human subcutaneous adipose tissue arterioles and endothelial cells. Subcutaneous exenatide increased postprandial EF independent of reductions in plasma glucose and triglycerides. Intravenous exenatide increased fasting EF, and exendin-9 abolished this effect. Exenatide elicited eNOS activation and NO production in endothelial cells, and induced dose-dependent vasorelaxation and reduced high-glucose or lipid-induced endothelial dysfunction in arterioles ex vivo. These effects were reduced with AMPK inhibition. In conclusion, exenatide augmented postprandial EF in subjects with diabetes and prevented high-glucose and lipid-induced endothelial dysfunction in human arterioles. These effects were largely direct, via GLP-1R and AMPK activation.
Introduction
Endothelial dysfunction plays a crucial role in the development of atherosclerosis and cardiovascular events (1–3). It is present in type 2 diabetes (4,5) and is associated with postprandial blood glucose and triglyceride concentrations (6–8). Both postprandial hyperglycemia and hypertriglyceridemia are ameliorated by GLP-1 receptor (GLP-1R) agonists (9–13), indicating the potential of these diabetes medications to improve endothelial function (EF).
In our previous study, a single injection of the GLP-1R agonist exenatide inhibited postmeal increases in glucose and triglyceride concentrations and improved EF after single high-fat meal in subjects with impaired glucose tolerance (IGT) and newly diagnosed, diet-controlled type 2 diabetes (14). Although the improvement of EF was similar in patients with IGT and diabetes, the pattern of EF change appeared different, and whereas IGT patients demonstrated an absolute increase in postprandial vasodilation with exenatide, patients with diabetes showed an amelioration of the meal-induced decline in EF. Whether exenatide can improve EF in patients with type 2 diabetes throughout subsequent day meals, and whether these vascular benefits would be present in patients with type 2 diabetes of longer duration, and perhaps greater vascular dysfunction, is largely unknown.
Although we found that a portion of exenatide’s effect on EF was accounted for by a reduction in plasma triglyceride concentrations (14), some of the exenatide-induced improvement of EF was independent of reductions in plasma glucose and triglycerides, supporting the concept of direct, endothelium-dependent vasorelaxation by GLP-1R agonists (15–24). Direct vascular action of GLP-1R agonist may involve several mechanisms. Whereas earlier studies in rodents implicated a role of a non-GLP-1R–mediated pathway activated mainly by GLP-1 degradation products (19,20), more recent in vitro and in vivo data indicate a direct action via endothelial GLP-1Rs (22,24). In support of the latter, several kinase pathways known to activate endothelial nitric oxide (NO) synthase (eNOS) and increase NO production are in vitro upregulated by various GLP-1R agonists (25–28). However, which of these specific postreceptor pathways could account for the effect of exenatide on EF in humans remains largely unknown.
To clarify these questions, we studied 1) whether exenatide has favorable effects on EF throughout sequential meals in type 2 diabetes, 2) whether these effects also occur in patients with more established type 2 diabetes, and 3) to what extent exenatide’s effects could be explained by direct action of exenatide through GLP-1Rs. In addition, we researched molecular pathways responsible for GLP-1R agonist action using human arterioles and endothelial cells.
Research Design and Methods
Two randomized, double-blind, crossover studies were approved by the institutional review board. All subjects provided informed consent prior to participation. Patients in both studies were on stable doses of blood pressure and lipid-lowering medications for 3 months prior to enrollment.
Study 1
Participants with a history of type 2 diabetes of <3 years, as in our previous study (14), or >5 years (to ensure a difference between groups in duration of diabetes) and relatively good glycemic control (hemoglobin A1c [HbA1c] ≤8.0%, 64 mmol/mol) on diet, metformin, sulfonylureas, or long-acting insulin (alone or in combination) were randomized to twice daily subcutaneous injection of exenatide (Byetta) or placebo for 11 days, with a 14-day washout period between the two treatments. The injected dose of exenatide was 5 µg on days 1–5 and was increased to 10 µg on days 6–11, if tolerated. The dosing regimen and duration of exenatide therapy were chosen to reduce the occurrence and severity of nausea that was common and relatively severe in our previous study after a single 10-µg dose (14) and to avoid significant weight loss. On the morning of day 11, overnight-fasted (≥10 h) participants were admitted to the clinical research unit. Thirty minutes after arrival, baseline EF was measured followed by injection of study medication and ingestion of 650 mg acetaminophen to assess gastric emptying (29), and a standardized solid breakfast meal (400 kcal/m2 of body surface area; 45% fat, 40% carbohydrates, and 15% protein) was provided to be consumed within 15 min. EF was measured 2 and 4 h after breakfast. Immediately after the 4-h test, subjects ate a lunch of similar caloric and macronutrient composition as breakfast that also contained 15 g of d-xylose to estimate postlunch gastric emptying. EF was measured again 2 and 4 h after lunch. Blood samples were collected through an indwelling catheter immediately after completion of each EF measurement.
Study 2
Participants with IGT or recently diagnosed (<1 year) type 2 diabetes with good glycemic control (HbA1c ≤7.0%, 53 mmol/mol) on diet alone were enrolled. Each subject was studied three times over a period of 6 weeks, receiving after an overnight fast, in random order, intravenous infusions of exenatide with saline or exenatide with the GLP-1R inhibitor exendin-9 or placebo with saline. All sessions started at the same time for a given patient between 7:00 and 10:00 a.m. After baseline EF measurement, catheters were placed in the antecubital vein (for medication infusion) and dorsal hand vein (for blood sampling) of the dominant and nondominant arm, respectively. Sixty minutes after the baseline EF measurement, a primed (6,000 pmol/L/kg) continuous (600 pmol/L/kg ⋅ min) infusion of exendin-9 (Bachem, Bubendorf, Switzerland) or equivalent volume of saline was initiated. Thirty minutes thereafter, an intravenous infusion of exenatide (50 ng/min) or equivalent volume of placebo was introduced. This rate of exenatide infusion was shown to provide a rapid and stable concentration similar to peak concentrations after typical subcutaneous exenatide injections (30). The solutions were prepared by a research-dedicated pharmacist to ensure study personnel remained blinded. EF measurement was repeated 30 min into exenatide/placebo infusion. The intravenous use of exendin-9 and exenatide was approved under the U.S. Food and Drug Administration IND 108.117 (J.K.). Blood samples for glucose and insulin measurements were taken before each infusion step and 10 min prior to the second EF measurement, after which the blood sampling line was withdrawn to avoid possible interference with the EF measurement.
Measurement of EF
EF was assessed by peripheral arterial tonometry (PAT) (ENDO-PAT2000; Itamar Medical, Caesarea, Israel) as detailed previously (14). Continuous pulsatile blood volume responses from both index fingers were recorded during a 5-min equilibration period, a 5-min period including suprasystolic inflation of blood pressure cuff on nondominant arm, and a 5-min postinflation period. The reactive hyperemia index (RHI) values were normalized to the readings from the contralateral arm. The average intrasubject coefficient of variation of RHI measurement in our laboratory is 3% on the same day sequentially and 10% on two separate days.
Studies in Endothelial Cells
Human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) (Lonza; CC-2535 and CC-2517, respectively, Walkersville, MD) were maintained in endothelial basal medium (CC-3162; Lonza) supplemented with supplied growth factors at 37°C in a humidified incubator supplemented with 5% CO2.
Passage 6–8 HAECs at 90–95% confluence were treated with exendin-4 (i.e., exenatide) with or without pretreatment with AMPK inhibitor compound-C (CC) or exendin-9 (Sigma-Aldrich, St. Louis, MO), washed with PBS, and lysed with suitable phosphatase and protease inhibitors. Total and phosphorylated AMPKα (phosphorylation site Thr172), cAMP-dependent protein kinase (PKA, Thr197), Akt-kinase (Ser473), and eNOS (Ser1177) were measured by Western blots, using antibodies from Cell Signaling Technology (Beverly, MA) and normalized to α-tubulin.
AMPKα1-specific small interfering RNA (siRNAs) (TriFECTa Kit; IDT Inc., Coralville, IA) or control siRNA (IDT Inc.) were used to examine whether AMPK mediated the effects of NO production. HUVECs were used for ease of transfection using HiPerFect Transfection Reagent (Qiagen, Valencia, CA). Passage 3–5 cells at 75–80% confluence were transfected for 24 h with anti-AMPKα1 siRNAs or control siRNA before treatment with 10 nmol/L exendin-4.
NO was measured using 4,5-diaminofluorescein-diacetate (DAF-2DA; Calbiochem, EMD Millipore, Billerica, MA). HAECs and HUVECs that had reached a confluence of 80% on 24-well plates were incubated with 5 µmol/L DAF-2DA for 15 min prior to the completion of the respective treatment, washed with PBS (pH 7), and fixed in 4% paraformaldehyde. The plates were imaged on EVOS (Life Technologies, Grand Island, NY), and the fluorescence intensity of NO was analyzed using ImageJ.
Vasoreactivity Studies
Arterioles were isolated from subcutaneous abdominal adipose tissue biopsies from research study volunteers as detailed previously (31) or from subjects undergoing elective abdominal hernia surgery without known diabetes or cardiovascular diseases. All provided consent to donate adipose tissue samples for research purposes. Up to four arterioles per sample were isolated, cannulated, and pressurized to 60 mmHg pressure without flow, as previously described (32,33). With the use of a video microscope (VIA-100; Boeckeler, Tucson, AZ), the vessels were preconstricted to ∼60% of maximum diameter using endothelin-1 and then treated with gradually increasing doses of acetylcholine followed by papaverine (4 min each step). Arterioles that dilated >70% of maximum diameter were washed out and exposed to intra- and extraluminal high glucose (33 mmol/L) or VLDL lipolysis products (VLDL briefly mixed with lipoprotein lipase; final added mixture contained 150 μmol/L fatty acids) for 2 h prior to the second dilator response to acetylcholine and papaverine as described above. Exendin-4 was added into media 1 h into high-glucose or VLDL treatments. Some replicates were treated with CC. In additional experiments, preconstricted vessels were, instead of acetylcholine, exposed to increasing doses of exendin-4 or GLP-1 (Sigma-Aldrich) before and after pretreatment for 30 min with eNOS inhibitor l-NG-nitro-l-arginine methyl ester (l-NAME), CC, or vehicle alone. Vasodilation responses were calculated as the percent diameter change between postendothelin-1 diameter and maximum observed diameter.
Laboratory Assays
Plasma glucose concentrations were measured bedside using a YSI 2700D glucose analyzer (Yellow Springs, OH). Samples for other measurements in plasma or serum were stored at −80°C until assayed for plasma lipids (Abbott, Lake Forest, IL), plasma insulin (EMD Millipore), apolipoprotein B48 (apoB48; Biovendor, Asheville, NC) and acetaminophen (Immunalysis, Pomona, CA), and d-xylose concentrations (34).
Statistical Analyses
SAS v9.2 (SAS Institute, Cary, NC) statistical package was used. For descriptive statistics, the groups were compared by independent Student t test or Wilcoxon rank sum test for continuous data and by χ2 test for categorical variables. The effect of treatment on study outcomes was evaluated by mixed-model ANCOVA, adjusting for subject-specific random effect and fixed effects of treatment sequence and additional variables pertinent for the study design. Data were log10 transformed if not normally distributed. Two-tailed P values <0.05 were considered statistically significant. Thirty-six subjects in study 1 and 32 subjects in study 2 provided 90 and 80% statistical power, respectively, to detect a difference of 0.04 in log10 RHI between exenatide and placebo, assuming within-subject SD of 0.05 as indicated in our previous study (14).
Results
Forty-two and 34 subjects were enrolled in studies 1 and 2, respectively (Fig. 1). Participants in both studies were predominantly obese, white males with a high prevalence of hypertension and use of lipid-lowering agents (Table 1).
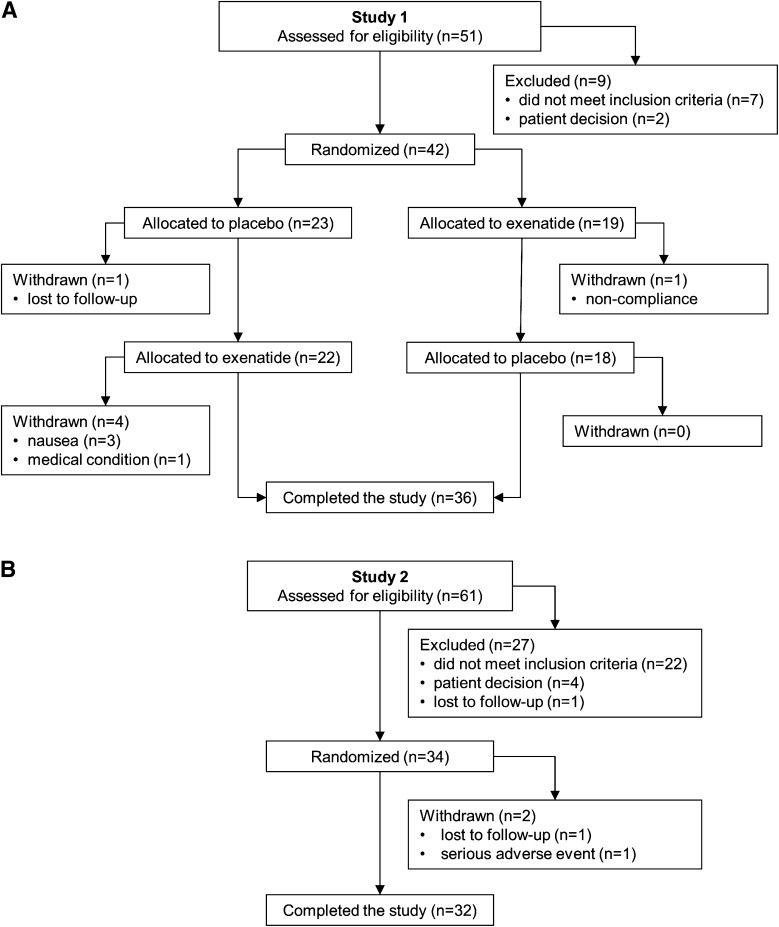
Figure 1.
Participant flow for studies 1 (A) and 2 (B).
Table 1.
Baseline clinical characteristics of the participants completing studies 1 and 2
| Study 1 all | Study 1 <3 years diabetes | Study 1 >5 years diabetes | Study 2 | |
|---|---|---|---|---|
| n | 36 | 16 | 20 | 32 |
| Age (years) | 63 ± 6 | 63 ± 6 | 62 ± 6 | 60 ± 6 |
| BMI (kg/m2) | 33 ± 6 | 34 ± 6 | 32 ± 7 | 33 ± 12 |
| Race (% whites) | 89% | 100% | 80% | 85% |
| Sex (% males) | 100% | 100% | 100% | 97% |
| HbA1c (%) | 6.4 ± 0.8 | 6.3 ± 0.5 | 6.5 ± 1.0 | 6.1 ± 0.5 |
| HbA1c (mmol/mol) | 47 ± 9 | 46 ± 6 | 48 ± 11 | 43 ± 5 |
| Duration of diabetes (years) | 5.5 (1–8) | 1 (0.5–2) | 7.5 (6–9.5) | <1 |
| Systolic blood pressure (mmHg) | 128 ± 15 | 127 ± 17 | 128 ± 14 | 125 ± 12 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 78 ± 9 | 78 ± 9 | 80 ± 8 |
| History of hypertension (%) | 81% | 69% | 90% | 50% |
| Lipid-lowering therapy (%) | 86% | 81% | 90% | 59% |
Data are means ± SD or medians (25th–75th percentile).
Study 1
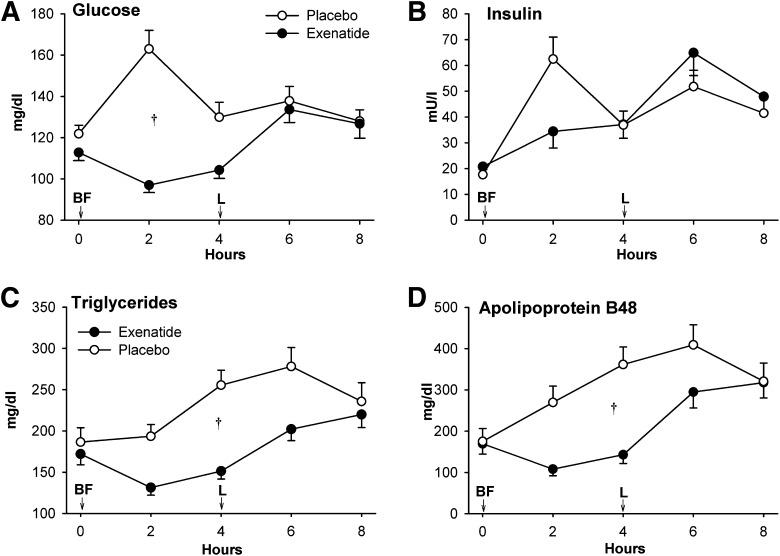
Thirty-six subjects completed both treatment arms, one withdrew while on placebo, and five withdrew while on exenatide (Fig. 1A). Two patients had nausea with 10 µg exenatide and completed the study on 5 µg exenatide. Clinical characteristics did not differ between diabetes duration subgroups (Table 1). Exenatide treatment was followed by modest reductions in body weight, systolic blood pressure, fasting glucose, and total and HDL cholesterol concentrations (Table 2). All participants consumed the entire test meals. Postbreakfast glucose and insulin responses were lower, and postlunch insulin responses were higher after exenatide (Fig. 2A and B). Postprandial excursions of triglycerides and apoB48 were markedly reduced after exenatide (Fig. 2C and D). Acetaminophen concentrations showed a significant interaction between treatment and time, trending lower 2 h postbreakfast after exenatide (P = 0.01) whereas postlunch d-xylose concentrations were not different between the treatments (Supplementary Fig. 1).
Table 2.
Clinical characteristics and fasting blood concentrations of several metabolic variables after 10 days of exenatide and placebo treatment in 36 patients completing crossover study 1
| Placebo | Exenatide | Change* | P value | |
|---|---|---|---|---|
| Body weight (kg) | 101.4 ± 22 | 100.7 ± 21.5 | −0.95 (−2 to 0.6) | 0.01 |
| Systolic blood pressure (mmHg) | 131 ± 11 | 126 ± 13 | −5.5 (−14 to 9) | 0.03 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 76 ± 10 | 1 (−5 to 6.5) | 0.9 |
| Heart rate (bpm) | 69 ± 12 | 71 ± 11 | 1.5 (−5.5 to 5.5) | 0.2 |
| Glucose (mg/dL) | 122 ± 25 | 113 ± 23 | −7.5 (−17.5 to 3.5) | 0.01 |
| Insulin (mU/L) | 12.3 (8.6–24) | 13.2 (8.8–25.6) | 0.8 (−1.5 to 4.4) | 0.2 |
| Triglycerides (mg/dL) | 186 ± 104 | 172 ± 77 | −16 (−51 to 34) | 0.4 |
| Total cholesterol (mg/dL) | 163 ± 36 | 150 ± 33 | −10 (−27 to 3) | 0.002 |
| LDL cholesterol (mg/dL) | 80 ± 10 | 81 ± 28 | 7 (−11 to 21) | 0.8 |
| HDL cholesterol (mg/dL) | 38 ± 8 | 36 ± 7 | −1 (−6 to 2) | 0.048 |
Data are means ± SD or medians (25th–75th percentile). P values indicate comparison between the two treatment arms by repeated-measures ANCOVA adjusted for treatment sequence and diabetes duration group.
*Exenatide minus placebo.
Figure 2.
The effect of exenatide on plasma glucose (A), insulin (B), triglycerides (C), and apoB48 (D) concentrations in study 1. On day 11 of therapy, immediately after initial blood sampling (time 0), study drug was injected and participants ingested a breakfast meal (BF) and 4 h later a lunch meal (L). Data are means ± SE. †P < 0.05, exenatide vs. placebo.
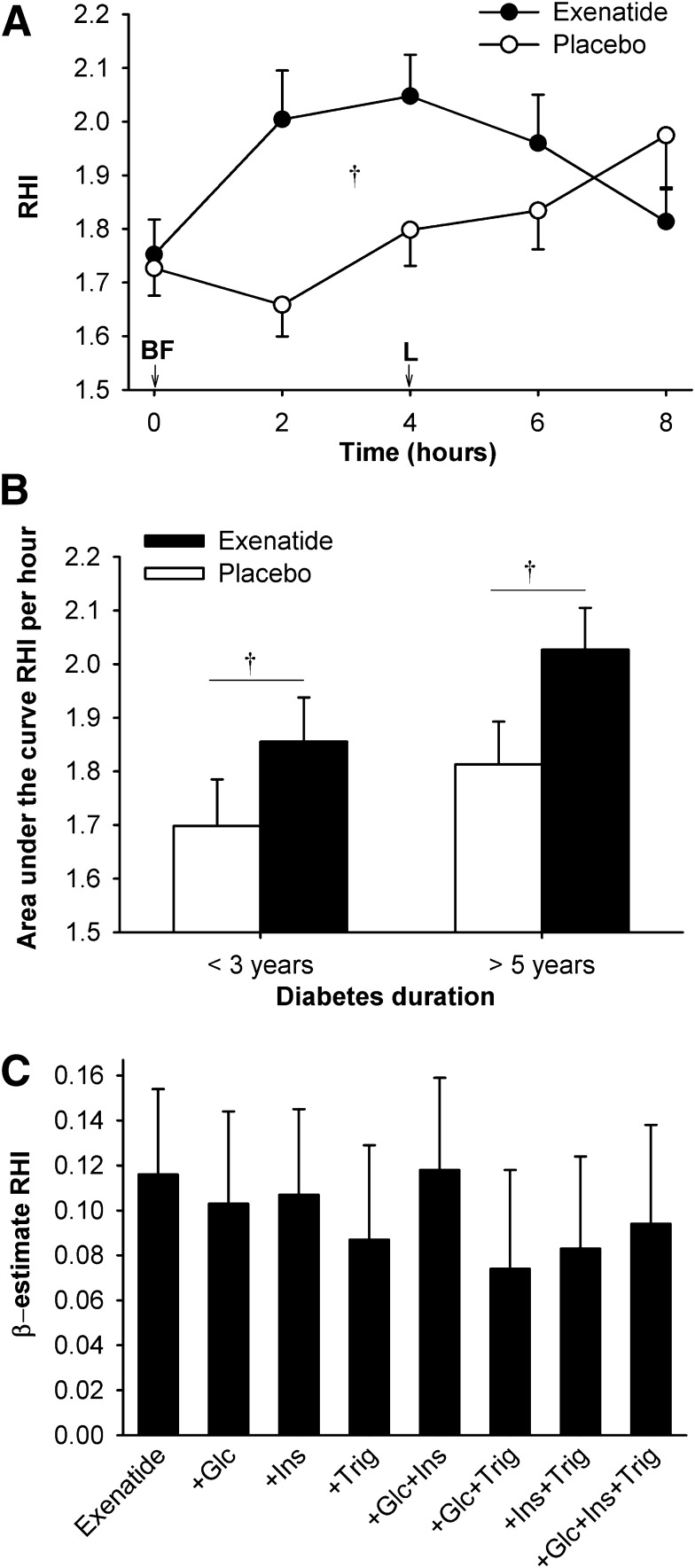
Exenatide increased overall RHI compared with placebo (Fig. 3A), and this was similar in the two diabetes duration subgroups (Fig. 3B). The increase in RHI after exenatide remained significant after adjustment for baseline HbA1c, change in body weight over the study, or changes in glucose, triglyceride, and insulin concentrations. It was modestly attenuated (P < 0.1) in a model including both glucose and triglyceride concentrations, explaining ∼36% of exenatide’s effect (Fig. 3C). The effect of exenatide did not show significant interactions with history of hypertension or use of lipid-lowering therapy (P > 0.5 both).
Figure 3.
The effect of exenatide on EF (RHI) in study 1. RHI was calculated as the ratio of the average amplitude of the PAT signal over a 30 s time interval starting 90 s after blood pressure cuff deflation divided by the average amplitude of the PAT signal of a 3.5-min time period before cuff inflation. On day 11 of therapy, immediately after initial blood sampling (time 0), study drug was injected and participants ingested a breakfast meal (BF) and 4 h later a lunch meal (L). A: RHI over the 8-h test period. B: RHI area under the curve (normalized to 1 h, by trapezoid method) according to the duration of diabetes. Data are means ± SE. †P < 0.05, exenatide vs. placebo. C: Multivariate models of exenatide’s effect on RHI. The modeled effect is shown as β estimates and SE of exenatide’s effect on RHI before and after adjustment for plasma glucose (Glc), insulin (Ins), and triglyceride (Trig) concentrations. Adjustments for individual or combinations of these variables did not significantly reduce the effect of exenatide.
Study 2
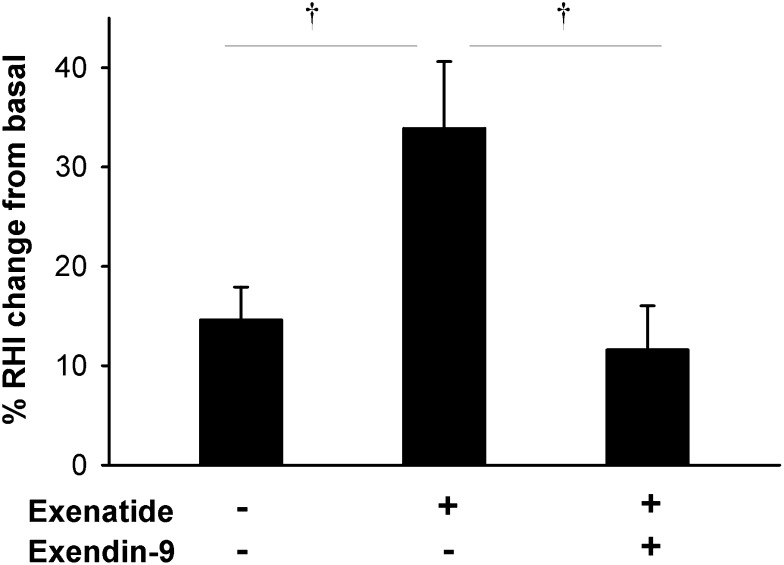
Thirty-two participants completed the study, one participant had a serious, study-unrelated adverse event between the test sessions, and one withdrew from the study (Fig. 1B and characteristics in Table 1). In all three test sessions, RHI was increased after the treatment (P < 0.0001 vs. baseline) (Fig. 4), consistent with rising EF during the morning (35). The increment was greater with exenatide than with placebo, and this was completely abolished with exendin-9 (Fig. 4). The increase in RHI with exenatide alone remained significant after adjustment for glucose (P = 0.02) and insulin (P = 0.007), or both glucose and insulin (P = 0.02) plasma concentrations. There were no differences in responses to treatments between those with IGT and type 2 diabetes (P = 1.0).
Figure 4.
Percent change in RHI after intravenous infusion of exenatide, placebo, or exenatide + GLP-1R inhibitor exendin-9 in study 2. RHI was calculated as the ratio of the average amplitude of the PAT signal over a 30-s time interval starting 90 s after blood pressure cuff deflation divided by the average amplitude of the PAT signal of a 3.5-min time period before cuff inflation. Data are means ± SE. †P < 0.05 between treatments.
Experiments in Endothelial Cells
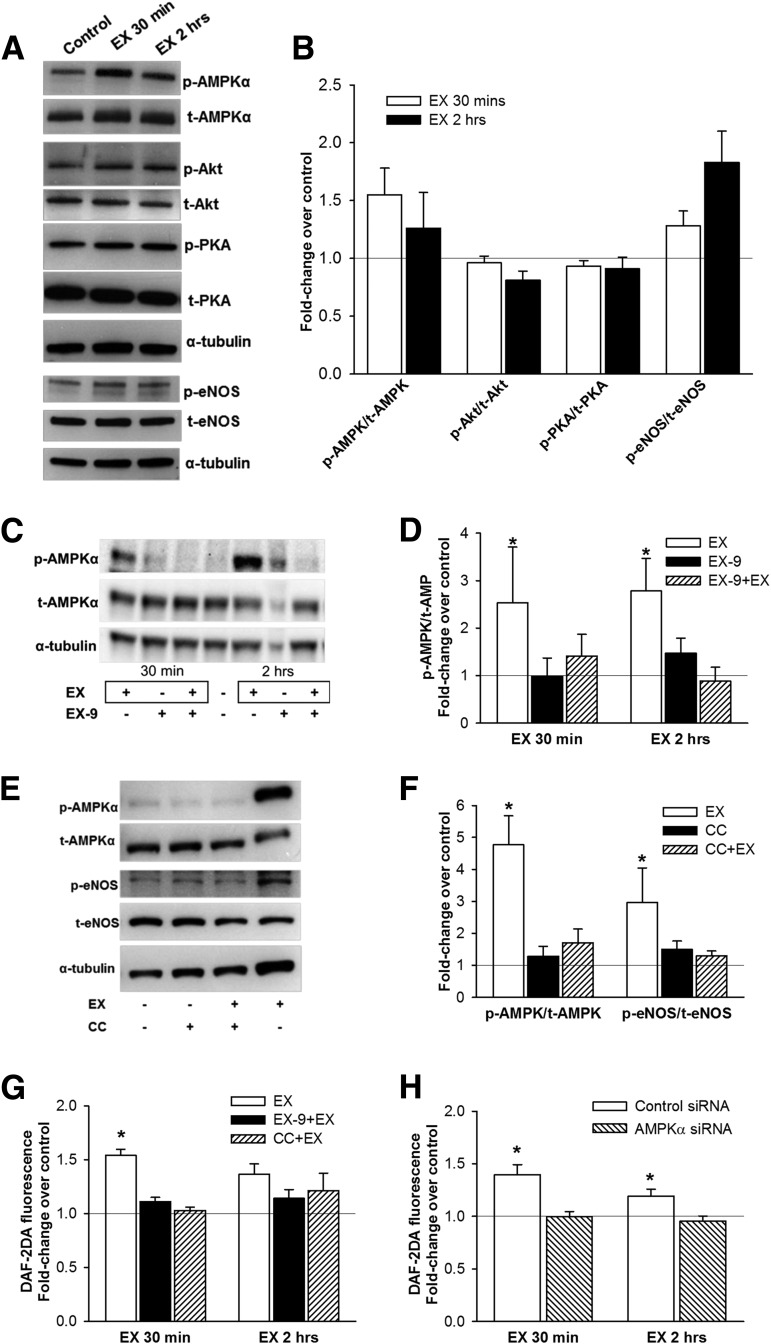
AMPKα phosphorylation was increased after 30 min of incubation with exendin-4, PKA phosphorylation was unchanged after incubation with exendin-4, and Akt phosphorylation was modestly reduced after 2-h exendin-4 treatment (Fig. 5A and B). eNOS phosphorylation trended higher after 30 min (P = 0.09) and was significantly increased after 2-h exendin-4 treatment (Fig. 5A and B). The increase in AMPK phosphorylation with exendin-4 was inhibited by pretreatment with exendin-9 (Fig. 5C and D). Stimulation by exendin-4 of both AMPK and eNOS phosphorylation was abolished with CC (Fig. 5E and F). DAF-2DA fluorescence was significantly increased after both 30-min and 2-h incubation with exendin-4, whereas pretreatment with both exendin-9 and CC blunted this effect of exendin-4 (Fig. 5G and Supplementary Fig. 2). The effect of exendin-4 on DAF-2DA fluorescence was also blocked in HUVECs with knockdown of AMPKα protein (Fig. 6H and Supplementary Fig. 3).
Figure 5.
The effects of exenatide in vitro in human endothelial cells. A and B: Phosphorylation of AMPKα (Thr172), PKA (Thr197), Akt kinase (Ser473), and eNOS (Ser1177) in HAECs after treatment with 10 nmol/L exendin-4 (EX) for 30 min and 2 h (A: representative Western blot; B: densitometry analysis [means ± SE]; n = 6). C and D: AMPKα phosphorylation in HAECs after EX (30 min and 2 h) with or without pretreatment for 30 min with 1 μmol/L GLP-1R inhibitor EX-9 (C: representative Western blot; D: densitometry analysis; n = 7–8). E and F: AMPKα and eNOS phosphorylation in HAECs after EX (2 h) with or without 1-h pretreatment with 5 mol/L of AMPKα inhibitor CC (E: representative Western blot; F: densitometry analysis; n = 8–9). The effect of EX on NO production (by DAF-2DA fluorescence) in HAECs with or without pretreatment with EX-9 or CC (n = 4–7) (G) and in HUVECs with knocked-down AMPKα gene expression (siRNA, n = 6) (H). Phosphorylated bands were normalized to total bands and α-tubulin. Control, untreated cells. Data are means ± SE. *P < 0.05 vs. control.
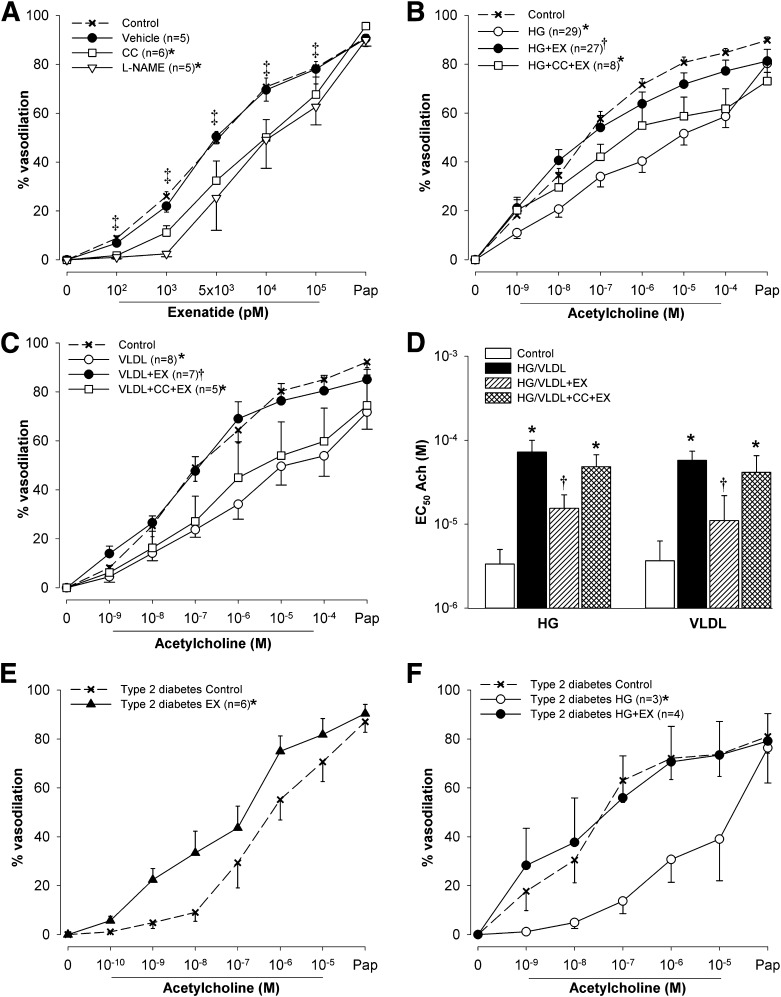
Figure 6.
The effects of exenatide (EX) ex vivo in isolated human adipose tissue arterioles. A: Vasodilation responses to increasing doses of EX followed by papaverine before (control) and after treatment with vehicle, eNOS inhibitor l-NAME (5 µmol/L), or AMPK inhibitor CC (1 µmol/L). B: Vasodilation responses to acetylcholine before (control) and after exposure to high glucose for 2 h (HG, 33 mmol/L), HG with addition of 10 nmol/L EX after 1 h (HG+EX), and HG+EX pretreated with 1 µmol/L CC (HG+EX+CC). C: Vasodilation responses to acetylcholine before (control) and after exposure to VLDL lipolysis products mixture for 2 h (VLDL, 150 μmol/L fatty acids), VLDL with addition of 10 nmol/L EX after 1 h (VLDL+EX), and VLDL+EX pretreated with 1 µmol/L CC. D: EC50 of acetylcholine from experiments shown in panels B and C. EC50 was calculated by nonlinear regression and variable slope (four parameters) and least squares fit (GraphPad Prism 5.0, San Diego CA). If the vessels dilated to <50% with maximum dose of acetylcholine, EC50 was set at 10−4 mol/L. Acetylcholine induced vasodilation in adipose tissue arterioles from subjects with type 2 diabetes before (control) and after 1-h exposure to 10 nmol/L EX (E) and HG and HG+EX (F). Data are means ± SE. *P < 0.05 vs. control; †P < 0.05 vs. HG or VLDL; ‡P < 0.05 vs. EX 0 pmol/L (tested in control only).
Vasoreactivity Studies
Adipose tissue arterioles were obtained from 43 research subjects and 2 subjects undergoing hernia surgery (mean age 53 years [SD ±8]; 40 males, 4 with type 2 diabetes). Dose-dependent arteriole dilation was observed with both exendin-4 (Fig. 6A) and GLP-1 (Supplementary Fig. 4A). Exendin-4–induced vasodilation was attenuated by l-NAME and CC (Fig. 6A). GLP-1–induced vasodilation was also attenuated with CC (Supplementary Fig. 4A). High glucose reduced vasodilation responses to acetylcholine, and this was completely prevented by exendin-4 (Fig. 6B). The AMPK activator AICAR also prevented high glucose–induced impairment of acetylcholine vasodilation (Supplementary Fig. 4B). Consistent with the role of AMPK signaling in these events, the ability of exendin-4 to reverse the effect of high glucose was largely abolished by CC (Fig. 6B). VLDL lipolysis products also inhibited acetylcholine-induced vasodilation; this was reversed by exendin-4 and the effect of exendin-4 was blocked by CC (Fig. 6C). Improved vasodilation with exendin-4 was also evident when the half-maximal effective concentration (EC50) for acetylcholine-induced arteriole dilation was calculated (Fig. 6D). To determine if exendin-4 could improve vasodilation even in patients with known vascular dysfunction, it was tested on adipose tissue arterioles from individuals with type 2 diabetes. Direct addition of exendin-4 improved vasodilation in arterioles from these patients (Fig. 6E) and prevented high glucose–induced attenuation of acetylcholine vasodilation (Fig. 6F).
Discussion
In our previous study, a single dose of exenatide had favorable effects on both postprandial lipid levels and EF in participants with IGT or new-onset diabetes. However, several key questions remained unanswered. For example, it was not clear whether the nonglycemic benefits of exenatide would extend beyond a single acute dose. This was clearly answered in the affirmative in the current study, as similar robust changes in triglyceride concentrations and EF occurred following the more chronic use of exenatide in study 1. Although modest weight loss did occur with longer use of exenatide, this small change did not account for the substantial improvements in metabolic and vascular outcomes.
GLP-1R agonists can reduce intestinal nutrient absorption by slowing gastric emptying (12,36–38). Thus, it was possible that by simply delaying nutrient absorption, exenatide might reduce the postprandial rise of plasma lipids and glucose concentrations, and postpone their peak until after subsequent meals. We therefore assessed gastric emptying by measuring absorbed acetaminophen and d-xylose with the breakfast and lunch meals, respectively. Consistent with previous studies showing slower gastric emptying with GLP-1R agonists (37,39), postbreakfast acetaminophen concentrations were reduced after exenatide injection. However, this reduction was modest and was observed only at the 2-h time point. The corresponding early delay in the postprandial glucose peak is in agreement with the involvement of gastric emptying in glucose-lowering effects of exenatide (37,39). In contrast, discordant postmeal rises between plasma apoB48 or triglycerides and acetaminophen or d-xylose indicate additional mechanisms of triglyceride lowering by exenatide, such as inhibition of intestinal lipoprotein production and triglyceride release as shown previously (40).
We considered the possibility that fat and carbohydrates from the lunch meal, in addition to delayed absorption of nutrients from the preceding breakfast meal, might produce a late surge in blood glucose and triglyceride concentrations that would overcome the benefits of exenatide treatment on EF in the postlunch period. This was supported by a previous report of equivalent rises in triglycerides after exenatide and placebo after a midday meal in subjects with type 2 diabetes (41). In contrast, triglyceride concentrations in the current study were still reduced 2 h postlunch with exenatide. EF was also still improved with exenatide at the 2-h postlunch period, consistent with the concept that GLP-1R agonists protect against high-fat meal–induced endothelial dysfunction through reduction in postprandial triglycerides. The similar triglyceride concentrations and EF in both treatment groups at 4 h postlunch may reflect waning exenatide concentrations. However, it is likely that the next exenatide dose in a typical 2×/day regimen would reinstate the mealtime benefit of exenatide, as indicated by significant reductions in postdinner glucose and triglyceride concentrations reported by Schwartz et al. (41). Moreover, prolonged duration of action of newer GLP-1R agonists may further diminish the likelihood of any rebounds in triglyceride concentrations and EF impairment.
In our previous study, EF was only tested in individuals with IGT or diabetes of very short duration (<1 year) (14). Thus, it was not known whether exenatide would be effective in patients with more established type 2 diabetes and potentially greater vascular dysfunction. As the improvement of EF in study 1 was demonstrated in patients with diabetes of both short and more prolonged duration, the beneficial effect of exenatide on EF appears to occur across the full range of IGT to diabetes of at least moderate duration. The pattern of postmeal changes in EF was similar in both groups. Of note, the pattern of exenatide improvement in patients with type 2 diabetes in the current study was slightly different than in our previous study. These minor differences may be explained by a lower fat content of the breakfast meal (400 vs. 600 kcal/m2) and/or higher systemic exenatide levels resulting from more chronic therapy (11 days vs. single dose) in the current study.
More than one-third of exenatide’s effect on EF in our previous study remained unexplained by changes in plasma nutrients (14). Although it was possible that the unexplained portion simply reflected imprecise matching of time points of EF measurement and blood draws for plasma glucose and lipid concentrations, we suspected that there may be a direct effect of exenatide on EF. Even with temporally linked EF and plasma measures in the current study, improvement of EF after exenatide was only partly related to changes in plasma triglycerides and glucose concentrations, suggesting an additional vascular action of exenatide. To test this point, exenatide was infused under fasting conditions in study 2 at a rate known to achieve exenatide concentrations comparable to those obtained with standard dosing of subcutaneous exenatide injection (30). EF was enhanced by almost 20% during exenatide infusion compared with placebo. This is in agreement with a previous report showing improved EF after subcutaneous exenatide injection (24) and earlier studies demonstrating EF elevations after intravenous GLP-1 infusion (17,18).
We also hypothesized that GLP-1R agonists improve EF directly via endothelial GLP-1Rs (19). Previous in vitro studies showed the GLP-1R inhibitor exendin-9 antagonized exenatide-induced activation of eNOS in cultured endothelial cells (26). Consistent with this, exendin-9 blunted exenatide-induced eNOS activation and NO production in HAECs. Moreover, exendin-9 completely abolished the effect of exenatide infusion on EF, providing important in vivo evidence for GLP-1R involvement in the vasodilation effect of GLP-1R agonists.
GLP-1R agonists have been previously shown to improve vasodilation responses of rodent vessels ex vivo (15,16,22). In our study, human arterioles dilated in a concentration-dependent manner with increasing doses of exenatide or GLP-1. Importantly, exenatide was already effective at concentrations similar to those achieved with therapeutic dosing (42). The vasodilation was inhibited by eNOS inhibitor l-NAME, indicating an NO-dependent mechanism of exenatide action. Exenatide also restored endothelium-mediated vasodilation that was attenuated by high glucose or VLDL lipolysis products. Exenatide’s protection against high glucose–induced impairment of EF concurs with a previously reported effect of GLP-1 during in vivo hyperglycemia (21). We now demonstrate the novel finding that exenatide directly prevents lipid-induced endothelial dysfunction.
Of the several GLP-1R downstream kinase pathways previously postulated as relevant for eNOS activation (25–27), only AMPK was activated by exenatide in HAECs. AMPK activation appeared to precede phosphorylation of eNOS, consistent with reports of this pathway sequence (43,44). Supporting a key role of the AMPK in endothelial vasodilation by GLP-1R agonists, AICAR reproduced the effect and CC inhibited exenatide- and GLP-1–induced vasodilation in human arterioles. Moreover, CC was nearly as effective as l-NAME in blocking exenatide vasodilation and also abolished exenatide’s protection against high glucose– or lipid-induced impairment of endothelium-mediated vasodilation ex vivo. In HAECs, CC also blocked exenatide-induced eNOS activation and NO production. To exclude the possibility that CC might have modulated exenatide’s action through inhibition of other kinase pathways (45), we knocked down transcription of AMPKα in HUVECs and found reductions in exenatide-induced NO production in cells lacking AMPKα.
There are several potential limitations of the study. The majority of participants were male and we cannot confidently extend our in vivo results to females. Although prior evidence (46) suggests it is unlikely that exendin-9 has vascular consequences beyond its interference with exenatide action, this cannot be entirely excluded. PAT was chosen for this study because of its excellent reproducibility during repeated measurements, good correlation with established gold standard methods (brachial flow-mediated dilation, direct measurement of coronary artery EF, and forearm plethysmography), and proven association with cardiovascular risk in large populations (47–49).
In conclusion, our in vivo and ex vivo studies showed that 1) the GLP-1R agonist exenatide improved EF over two sequential meals in individuals with type 2 diabetes of short to moderate duration, 2) exenatide improved EF via a direct vascular GLP-1R–mediated mechanism, 3) exenatide via direct vascular action ameliorated both high glucose– and lipid-induced endothelial dysfunction, and 4) exenatide stimulated endothelial AMPK pathway activity, resulting in greater eNOS activation and NO production. Since endothelial dysfunction is associated with progression of atherosclerosis (50) and predicts clinical cardiovascular events (1–3), our findings provide further conceptual and mechanistic support for the potential use of GLP-1R agonists in cardiovascular protection in type 2 diabetes.
Article Information
Acknowledgments. The authors acknowledge the excellent project assistance provided by Linda McDonald RN, Irina Moroz, Keith Rasmussen, Ashley Haile, and Dewayne C. Thurmond (all at the Phoenix VA Health Care System).
Funding. This work was supported in part by the American Diabetes Association (1-10-CT-31 to J.K.), the Amyloidosis Foundation (to R.Q.M.), the National Institutes of Health (R21-HL-092344-01 to R.Q.M.), VA Merit (BLRD I01BX007080 to R.Q.M.), and the Office of Research and Development, Department of Veterans Affairs (1I01CX000598 to P.D.R.). Amylin Lilly provided study medications at no costs.
The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Duality of Interest. P.D.R. has received research grants from Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.K. designed the study, obtained funding, generated research data, and prepared the manuscript. M.S. and C.B. researched data and reviewed and edited the manuscript. K.M.D. designed the experiments, generated research data, and edited the manuscript. K.R. collected the data and edited the manuscript. J.L. generated research data and edited the manuscript. S.T. and D.A.F. designed the experiments and edited the manuscript. E.A.S. helped design the experiments, collected the data, and edited the manuscript. D.C.S. helped design the study and edited the manuscript. D.D. consulted on the study design and reviewed the manuscript. R.Q.M. helped design the experiments, obtained funding, and reviewed and edited the manuscript. P.D.R. helped design the study and experiments, obtained funding, and reviewed and edited the manuscript. J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013, and the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
Clinical trial reg. no. NCT01181986, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0976/-/DC1.
See accompanying article, p. 2319.
References
- 1.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658 [DOI] [PubMed] [Google Scholar]
- 2.Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 2002;105:1567–1572 [DOI] [PubMed] [Google Scholar]
- 3.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007;115:2390–2397 [DOI] [PubMed] [Google Scholar]
- 4.McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992;35:771–776 [DOI] [PubMed] [Google Scholar]
- 5.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1996;27:567–574 [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A, Taboga C, Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation 2002;106:1211–1218 [DOI] [PubMed] [Google Scholar]
- 7.Bae JH, Bassenge E, Lee HJ, et al. Impact of postprandial hypertriglyceridemia on vascular responses in patients with coronary artery disease: effects of ACE inhibitors and fibrates. Atherosclerosis 2001;158:165–171 [DOI] [PubMed] [Google Scholar]
- 8.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999;34:146–154 [DOI] [PubMed] [Google Scholar]
- 9.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008;294:E846–E852 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 [DOI] [PubMed] [Google Scholar]
- 11.Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001;281:E155–E161 [DOI] [PubMed] [Google Scholar]
- 12.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003;88:3082–3089 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Gethmann A, Götze O, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006;49:452–458 [DOI] [PubMed] [Google Scholar]
- 14.Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care 2010;33:1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter G, Feddersen O, Wagner U, Barth P, Göke R, Göke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol 1993;265:L374–L381 [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 2003;21:1125–1135 [DOI] [PubMed] [Google Scholar]
- 17.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007;293:E1289–E1295 [DOI] [PubMed] [Google Scholar]
- 19.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 20.Nathanson D, Erdogdu O, Pernow J, Zhang Q, Nyström T. Endothelial dysfunction induced by triglycerides is not restored by exenatide in rat conduit arteries ex vivo. Regul Pept 2009;157:8–13 [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011;34:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaspari T, Liu H, Welungoda I, et al. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diab Vasc Dis Res 2011;8:117–124 [DOI] [PubMed] [Google Scholar]
- 23.Chai W, Dong Z, Wang N, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012;61:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha SJ, Kim W, Woo JS, et al. Preventive effects of exenatide on endothelial dysfunction induced by ischemia-reperfusion injury via KATP channels. Arterioscler Thromb Vasc Biol 2012;32:474–480 [DOI] [PubMed] [Google Scholar]
- 25.Dong Z, Chai W, Wang W, et al. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 2013;304:E222–E228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 2010;325:26–35 [DOI] [PubMed] [Google Scholar]
- 27.Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 2011;54:1214–1223 [DOI] [PubMed] [Google Scholar]
- 28.Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014;9:e97554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clements JA, Heading RC, Nimmo WS, Prescott LF. Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther 1978;24:420–431 [DOI] [PubMed] [Google Scholar]
- 30.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005;90:5991–5997 [DOI] [PubMed] [Google Scholar]
- 31.Goldfine AB, Conlin PR, Halperin F, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia 2013;56:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migrino RQ, Truran S, Gutterman DD, et al. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol 2011;301:H2305–H2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 2007;292:H93–H100 [DOI] [PubMed]
- 34.Eberts TJ, Sample RH, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem 1979;25:1440–1443 [PubMed] [Google Scholar]
- 35.Gaenzer H, Sturm W, Neumayr G, et al. Pronounced postprandial lipemia impairs endothelium-dependent dilation of the brachial artery in men. Cardiovasc Res 2001;52:509–516 [DOI] [PubMed] [Google Scholar]
- 36.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008;151:123–129 [DOI] [PubMed] [Google Scholar]
- 37.Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 2008;93:4909–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–221 [DOI] [PubMed] [Google Scholar]
- 39.Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005;62:173–181 [DOI] [PubMed] [Google Scholar]
- 40.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012;32:1513–1519 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz SL, Ratner RE, Kim DD, et al. Effect of exenatide on 24-hour blood glucose profile compared with placebo in patients with type 2 diabetes: a randomized, double-blind, two-arm, parallel-group, placebo-controlled, 2-week study. Clin Ther 2008;30:858–867 [DOI] [PubMed] [Google Scholar]
- 42.Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 2003;26:2370–2377 [DOI] [PubMed] [Google Scholar]
- 43.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem 2007;282:20351–20364 [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Peng I-C, Sun W, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 2009;104:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J 2007;408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 2008;478:136–142 [DOI] [PubMed] [Google Scholar]
- 47.Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004;44:2137–2141 [DOI] [PubMed] [Google Scholar]
- 48.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003;146:168–174 [DOI] [PubMed] [Google Scholar]
- 49.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117:2467–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halcox JP, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation 2009;119:1005–1012 [DOI] [PubMed] [Google Scholar]