Abstract
Single nucleotide polymorphism (SNP) rs10911021 at the glutamate-ammonia ligase (GLUL) locus has been associated with an increased risk of coronary heart disease in individuals with type 2 diabetes. The effect of this SNP on mortality was investigated among 1,242 white subjects with type 2 diabetes from the Joslin Kidney Study (JKS) (n = 416) and the Gargano Mortality Study (GMS) (n = 826). During a mean follow-up of 12.8 ± 5.8 and 7.5 ± 2.2 years, respectively, a total of 215 and 164 deaths were observed in the two studies. In both cohorts, the all-cause mortality rate significantly increased with the number of rs10911021 risk alleles, with allelic hazard ratios (HRs) of 1.32 (95% CI 1.07–1.64, P = 0.01), 1.30 (1.10–1.69, P = 0.04), and 1.32 (1.12–1.55, P = 0.0011), respectively, in the JKS, the GMS, and the two studies combined. These associations were not affected by adjustment for possible confounders. In the JKS, for which data on causes of death were available, the HR for cardiovascular mortality was 1.51 (1.12–2.04, P = 0.0077) as opposed to 1.15 (0.84–1.55, P = 0.39) for mortality from noncardiovascular causes. These findings point to SNP rs10911021 as an independent modulator of mortality in patients with type 2 diabetes and, together with the previous observation, suggest that this results from an effect of this variant on cardiovascular risk.
Introduction
Type 2 diabetes (T2D) is one of the leading causes of mortality worldwide (1,2), accounting globally for ∼4.5 million deaths yearly (3). This number is expected to further increase because of the global rise of T2D prevalence (3). Thus, a better understanding of mortality risk factors in diabetic patients is urgently needed in order to target patients who are especially at high risk of death with aggressive prevention strategies.
Among diabetic patients, coronary heart disease (CHD), the risk of which is determined by both genetic and nongenetic factors (4–7), is the principal cause of mortality (8,9). Therefore, one can expect that the genetic determinants of CHD in patients with T2D also act as risk factors of increased mortality among these individuals.
By interrogating the entire genome, we have recently identified a single nucleotide polymorphism (SNP; rs10911021) at the GLUL locus on chromosome 1q25, which is specifically associated with CHD among patients with T2D, with allelic odds ratio of 1.36 (95% CI 1.22–1.51) in diabetic patients compared with 0.99 (95% CI 0.87–1.13) in nondiabetic subjects (10).
Based on this evidence, we investigated the impact of this genetic risk factor on all-cause mortality among patients with T2D.
Research Design and Methods
Study Cohorts
Joslin Kidney Study in Type 2 Diabetes
This cohort consists of a random sample (n = 516) of T2D patients from the Joslin Clinic enriched with individuals with microalbuminuria and macroalbuminuria, who were recruited between 1993 and 1996 at the Joslin Diabetes Center, Boston, MA, as previously described (11). The current study was limited to 416 participants who were self-reported whites and for whom DNA samples were still available in 2013. All subjects had diabetes diagnosed after age 25 years according to World Health Organization criteria and were treated with diet or oral agents for at least 2 years after the diagnosis. The survival status of these subjects was updated as of 31 December 2011 by matching with the National Death Index, and causes of death were extracted for deceased cohort members. A death was ascribed to cardiovascular causes if the primary cause of death was ICD-9 codes 401–448.9 or ICD-10 codes I10–I74.9, or if diabetes or renal failure was listed as the primary cause of death and cardiovascular disease was the secondary cause.
Gargano Mortality Study
This cohort includes 1,028 subjects with T2D (defined according to the American Diabetes Association 2003 criteria) who were consecutively recruited from 1 November 2000 to 30 September 2005 at the Endocrine Unit of the Istituto di Ricovero e Cura a Carattere Scientifico “Casa Sollievo della Sofferenza” in San Giovanni Rotondo, Italy, as previously described (12). The only exclusion criterion was the presence of poor life expectancy due to non–diabetes-related disorders, such as severe infectious illnesses or any type of cancer. The cohort was observed until 2010 by obtaining information on the participants’ vital status by direct contact with patients and/or their relatives or by queries to the registry offices of their cities of residence. Such information was available for 826 individuals, whose data were therefore analyzed in the current study.
Data Collection and Definitions
Clinical data were obtained from standardized interviews and examinations. BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). Data concerning diabetes treatment were confirmed by the review of medical records. The glycated hemoglobin (HbA1c) level at examination was used as an index of glycemic control. Chronic kidney disease (CKD) was defined as a glomerular filtration rate (GFR) (estimated from the serum creatinine level by the MDRD equation [13]) <60 mL/min/1.73 m2. In the Joslin Kidney Study in Type 2 Diabetes (JKS), microalbuminuria was defined as a urinary albumin excretion rate between 30 and 299 μg/min, and macroalbuminuria as a urinary albumin excretion rate ≥300 μg/min. In the Gargano Mortality Study (GMS), microalbuminuria was defined as a urinary albumin creatinine ratio between 2.5 and 29 mg/mmol in females and between 3.5 and 29 mg/mmol in males and macroalbuminuria as an albumin creatinine ratio of ≥30 mg/mmol.
Ethics
Study protocols and informed consent procedures were approved by the Joslin Committee on Human Studies and the Beth Israel Deaconess Medical Center Committee on Clinical Investigations for the JKS, and by the Istituto di Ricovero e Cura a Carattere Scientifico, Casa Sollievo della Sofferenza Institutional Ethic Committee for the GMS. All participants gave written informed consent.
Genotyping
DNA was extracted from whole blood using standard methods. SNP rs10911021 was genotyped by means of a custom TaqMan Assay (Life Technologies, Foster City, CA) at the Joslin Diabetes Center Diabetes and Endocrinology Research Center Genetics Core (for the JKS), and at the Mendel Institute Genetics Core (for the GMS) on 7500 and 7900HT platforms (Applied Biosystems, Foster City, CA), respectively. Genotyping quality was tested by including six blinded duplicate samples in each 384-well assay. The average agreement rate of duplicate samples was >99%. All samples were in Hardy-Weinberg equilibrium.
Statistical Methods
Patients’ baseline characteristics are reported as the mean ± SD and percentages for continuous and categorical variables, respectively.
Survival curves stratified by the rs10911021 genotype were estimated within each cohort by the Kaplan-Meier method. The time variable was defined as the time between the baseline examination and the date of death, or, for subjects who did not die, the date of 31 December 2011 (for the JKS) or of the last clinical follow-up (for the GMS). Mortality rates were estimated as the number of deaths divided by the total number of person-years (py).
The association between rs10911021 genotype and all-cause (in the JKS and GMS) or cardiovascular (in the JKS) mortality during follow-up was evaluated by Cox proportional hazard regression analysis. The following three different models were tested: 1) unadjusted; 2) adjusted for age, sex and race; and 3) adjusted for age, sex, race, duration of diabetes, BMI, HbA1c level, presence of CKD, presence of microalbuminuria/macroalbuminuria, and type of glucose-lowering treatment at baseline. SNP rs10911021 was considered according to an additive model, with the allele previously found to be associated with CHD among diabetic patients (allele “C” [10] coded as the risk allele). Risks were reported as hazard ratios (HRs) along with their 95% CIs. Data were first analyzed by cohort and then in the two cohorts pooled together in an individual patient data meta-analysis (14) (i.e., adjusting for “study cohort”) after no genotype-by-cohort interaction was detected. A P value of 0.05 was considered as significant. All analyses were performed using SAS version 9.2.
Results
Characteristics of Study Cohorts
The baseline characteristics of the two study cohorts are shown in Table 1. Compared with individuals in the GMS, subjects in the JKS included more men, were younger, had received a diagnosis of diabetes at a younger age, had a longer duration of diabetes, had slightly lower BMI and HbA1c levels, had a higher prevalence of macroalbuminuria, and were more frequently treated with insulin. During an average follow-up period of 12.8 and 7.5 years, 215 (51.7%) and 164 (19.5%) subjects, respectively, died in the JKS and the GMS, corresponding to mortality rates of 40.3 and 26.6 per 1,000 py. Male gender, older age at study entry, higher HbA1c level, the presence of CKD, the presence of microalbuminuria or macroalbuminuria, and insulin treatment at baseline were all significantly associated with increased mortality (Supplementary Table 1). The higher mortality observed in the JKS cohort compared with the GMS cohort was accounted for by the higher proportion of macroalbuminuric subjects in this cohort (data not shown).
Table 1.
Clinical characteristics of T2D patients in the two prospective cohorts
| JKS (n = 416) | GMS (n = 826) | |
|---|---|---|
| Males (%) | 57.0 | 49.3 |
| Age at study entry (years) | 57.6 ± 9.5 | 62.3 ± 9.7 |
| Age at diabetes diagnosis (years) | 43.8 ± 9.0 | 51.3 ± 10.4 |
| Diabetes duration (years) | 13.9 ± 8.0 | 11.0 ± 9.1 |
| BMI (kg/m2) | 29.9 ± 6.5 | 31.0 ± 5.8 |
| HbA1c (%) | 8.40 ± 1.6 | 8.65 ± 1.9 |
| CKD | 71 (17.5) | 144 (17.8) |
| Albuminuria | ||
| Normoalbuminuria | 217 (52.1) | 546 (69.5) |
| Microalbuminuria | 114 (27.4) | 196 (24.9) |
| Macroalbuminuria | 85 (20.4) | 44 (5.6) |
| Glucose-lowering therapy | ||
| Diet only | 31 (7.5) | 137 (18.6) |
| Oral agents | 119 (28.6) | 344 (41.6) |
| Insulin with/without oral agents | 266 (63.9) | 345 (41.8) |
| Follow-up | ||
| Years | 12.8 ± 5.8 | 7.5 ± 2.2 |
| py | 5,340.2 | 6,165.6 |
| Deaths, n (%) | 215 (51.7) | 164 (19.9) |
Continuous variables are reported as the mean ± SD; categorical variables are reported as total frequencies, with percentages in parentheses.
rs10911021 and Risk of All-Cause Mortality
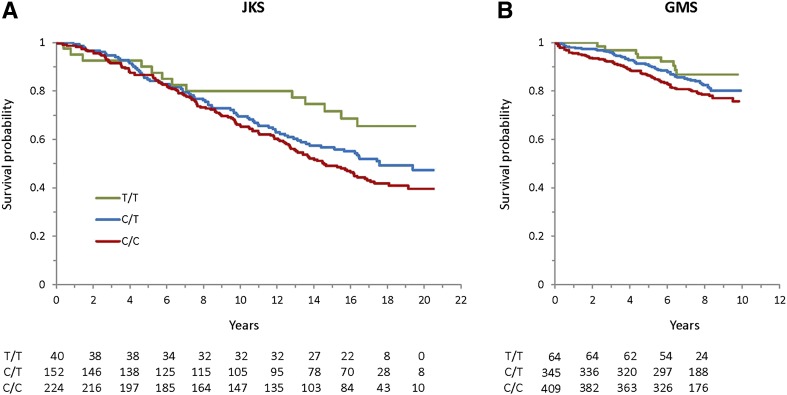
In the JKS, the mortality rate during follow-up progressively increased with the number of rs10911021 risk alleles, with individuals carrying two risk alleles dying at a rate twice as high as those carrying none (Table 2 and Fig. 1A). Although mortality was overall lower in the GMS, a similar relationship was observed in this cohort between mortality rate and the number of rs10911021 risk alleles (Table 2 and Fig. 1B). The allelic HR for mortality was 1.32 (95% CI 1.07–1.64, P = 0.01) in the JKS and 1.30 (95% CI 1.10–1.69, P = 0.044) in the GMS (Table 3). The HR slightly increased in the JKS and decreased in the GMS after adjusting for covariates such as sex, age at study entry, race, diabetes duration, BMI, HbA1c level, CKD, albuminuria, and type of glucose-lowering therapy at baseline (Table 3). Since the HRs were not significantly different between cohorts (P for SNP-by-cohort interaction = 0.95), the two studies were considered together in an individual data meta-analysis including a total of 1,242 individuals and 375 deaths. The summary HR in this combined analysis was 1.32 for the unadjusted model (95% CI 1.12–1.55, P = 0.0011) and 1.35 for the fully adjusted model (95% CI 1.14–1.60, P = 0.0005) (Table 3). The association between rs10911021 and mortality was less apparent in individuals with CKD (GFR <60 mL/min/1.73 m2, n = 213) than in those with preserved kidney function (GFR ≥60 mL/min/1.73 m2, n = 992), although this difference did not reach statistical significance (HR 1.34 [95% CI 1.09–1.64] vs. 1.04 [95% CI 0.79–1.40], P for rs10911021 by CKD interaction = 0.17). No suggestive patterns of interaction with other risk factors (e.g., diabetes duration, HbA1c level, albuminuria) were observed (data not shown).
Table 2.
Mortality rates by rs10911021 genotype in the two cohorts
| rs10911021 | n | Deaths | py | Mortality rate (per 1,000 py) |
|---|---|---|---|---|
| JKS | ||||
| T/T | 40 | 13 | 559.0 | 23.3 |
| T/C | 152 | 75 | 1,978.6 | 37.9 |
| C/C | 224 | 127 | 2,802.5 | 45.3 |
| GMSa | ||||
| T/T | 64 | 8 | 486.8 | 16.4 |
| T/C | 345 | 63 | 2,661.0 | 23.7 |
| C/C | 409 | 89 | 2,962.7 | 30.0 |
ars10911021 genotypes were not available for eight subjects in the GMS cohort.
Figure 1.
Survival curves according to rs10911021 genotype in the JKS (A) and GMS (B) cohorts. The numbers of individuals at risk in each genotype group at each 2-year time point are reported below the curves.
Table 3.
Allelic HR of all-cause and CVD mortality for rs10911021 in the two study cohorts and in the combined analysis
| JKS |
GMS |
JKS + GMS |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| All-cause mortality | ||||||
| Model 1 | 1.32 (1.07–1.64) | 0.010 | 1.30 (1.01–1.69) | 0.044 | 1.32 (1.12–1.55) | 0.0011 |
| Model 2 | 1.33 (1.07–1.65) | 0.0093 | 1.32 (1.02–1.71) | 0.038 | 1.32 (1.12–1.56) | 0.0009 |
| Model 3 | 1.50 (1.19–1.88) | 0.0006 | 1.23 (0.95–1.60) | 0.12 | 1.35 (1.14–1.60) | 0.0005 |
| CVD mortality | ||||||
| Model 1 | 1.51 (1.12–2.04) | 0.0077 | ||||
| Model 2 | 1.50 (1.11–2.03) | 0.0085 | ||||
| Model 3 | 1.72 (1.24–2.38) | 0.0011 | ||||
Model 1, rs10911021; Model 2, rs10911021, sex, age, and race; Model 3, rs10911021, sex, age at study entry, race, BMI, HbA1c, CKD, microalbuminuria/macroalbuminuria, and glucose-lowering therapy.
rs10911021 and Risk of Cardiovascular Mortality in JKS
In the JKS, information on the cause of death was also available. Similar to what was observed for all-cause mortality, cardiovascular mortality progressively increased with the number of risk alleles. The allelic HR was 1.51 (95% CI 1.12–2.04, P = 0.0077) in the unadjusted model and 1.72 (95% CI 1.24–2.38, P = 0.0011) in the fully adjusted model (Table 3). The corresponding HRs for noncardiovascular mortality were 1.15 (95% CI 0.84–1.55, P = 0.39) and 1.28 (95% CI 0.92–1.79, P = 0.14), respectively.
Discussion
In this study, we assessed the association between the rs1011021 variant at the GLUL locus, which we have previously shown to be a marker of increased CHD risk among diabetic patients (10), and all-cause mortality. Our data, obtained by analyzing two independent studies, indicate that, among patients with T2D, mortality rates significantly and progressively increase with the increasing number of rs10911021 risk alleles. The fact that the association between rs10911021 and all-cause mortality was observed in both studies, despite several differences in the characteristics of the two cohorts, makes our finding especially convincing. Combining the two cohorts, we found that each rs10911021 risk allele increases the risk of death by 32%. Such association was robust to the adjustment for several possible confounders, arguing in favor of an independent genetic effect.
Given the association that we previously reported between rs10911021 and CHD risk among diabetic patients (10) and the major role played by CHD on all-cause mortality in T2D (15,16), we sought to also explore the effect of rs109110121 on cardiovascular mortality; this was done in one of the two cohorts (the JKS), in which such information was available. The rs10911021 risk allele was independently associated with a 51–72% increase in the risk of cardiovascular death, depending on the model, thereby suggesting that the effect on all-cause mortality is for the most part mediated by an effect on cardiovascular mortality.
A strength of our study is the availability of two independent prospective cohorts of T2D patients, which allowed us to replicate the genetic association. Since the two cohorts had different baseline clinical features and were from different geographical regions, it is likely that the effect of this locus on mortality is generalizable to all diabetic patients of European ancestry. On the other hand, it is unknown whether a similar effect is also present among diabetic patients of different ancestries. The JKS included a small proportion of nonwhite subjects, but their number (n = 25 with DNA samples available) was too small for an independent analysis. It is also of note that the magnitude of the HR of rs10911021 for both all-cause and cardiovascular mortality is relatively high compared with those of other SNPs thus far reported to be associated with higher mortality in patients with T2D (17) or in the general population (18,19).
Some limitations of our study should be acknowledged. First, although we found that the rs10911021 variant at the GLUL locus is a significant modulator of the risk of death among T2D diabetic patients, the precise mechanisms underlying such modulation are not yet known. Our previous findings (10) have implicated abnormalities of the γ-glutamyl cycle in the effect of this variant on CHD, but further studies are needed to prove that this is indeed the causal pathway. Second, since data on causes of death were available for only one of the cohorts, firm conclusions cannot be drawn on this important aspect. Third, the diabetic subjects included in our study had been exposed to diabetes for a certain amount of time (an average of 14 and 11 years, respectively, in the JHS and GMS) and included a relatively high proportion of individuals with microalbuminuria or macroalbuminuria. While we did not observe significant interactions between rs10911021 and diabetes duration or albuminuria, we cannot draw definitive conclusions on whether the association between rs10911021 and mortality can be generalized to diabetic individuals with different clinical characteristics. Finally, our study was limited to diabetic subjects. Because we previously found that the rs10911021 SNP is associated with CHD only among diabetic subjects (10), one may infer that a similar interaction between this locus and diabetes is present with regard to mortality. However, further studies are required to directly prove this hypothesis.
In conclusion, our data point to the SNP rs10911021 as an independent risk factor of all-cause mortality in patients with T2D of European ancestry and, in combination with our previous findings (10), suggest that this risk is mediated by a specific effect on cardiovascular disease.
Article Information
Acknowledgments. The authors thank the staff and participants of the Joslin Kidney Study and the Gargano Mortality Study for their dedication and contributions.
Funding. This work was supported by the National Institutes of Health (grants R01-HL-073168 and HL-110400 to A.D., K01-DK-090125 to M.P., and P30-DK-036836 to the Joslin Diabetes Research Center [Advanced Genomics and Genetics Core, and a Pilot and Feasibility Grant to M.N.]) and by the Italian Ministry of Health (Ricerca Corrente 2013 and 2014 to S.P. and V.T.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.P. performed and supervised genetic analyses, contributed to the data interpretation, and wrote the manuscript. H.S. performed genetic analyses, contributed to the data interpretation, and reviewed the manuscript. D.B. performed genetic analyses and contributed to the data interpretation. M.P. contributed to the data interpretation and reviewed the manuscript. P.B. performed genetic analyses, contributed to data interpretation, and reviewed the manuscript. L.M. performed genetic analyses and reviewed the manuscript. C.M. performed and supervised genetic analyses and contributed to the data interpretation. S.D.C. was in charge of the clinical work and contributed to the data interpretation. M.N. performed the data analysis and contributed to the data interpretation. V.T. designed the study, contributed to the data interpretation, and wrote the manuscript. A.D. designed the study, contributed to the data interpretation, and wrote the manuscript. A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1653/-/DC1.
References
- 1.Seshasai SR, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005;28:2130–2135 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. Diabetes Atlas [Internet], 2013. 6th ed. International Diabetes Federation, Brussels, Belgium. Available from http://www.idf.org/diabetesatlas/5e/diabetes. Accessed August 23, 2014
- 4.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 5.Wagenknecht LE, Bowden DW, Carr JJ, Langefeld CD, Freedman BI, Rich SS. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes 2001;50:861–866 [DOI] [PubMed] [Google Scholar]
- 6.Lange LA, Bowden DW, Langefeld CD, et al. Heritability of carotid artery intima-medial thickness in type 2 diabetes. Stroke 2002;33:1876–1881 [DOI] [PubMed] [Google Scholar]
- 7.Qi L, Parast L, Cai T, et al. Genetic susceptibility to coronary heart disease in type 2 diabetes: 3 independent studies. J Am Coll Cardiol 2011;58:2675–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011;378:169–181 [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Qi Q, Prudente S, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 2013;310:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria A, Wojcik J, Xu R, et al. Interaction between poor glycemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA 2008;300:2389–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzaghi C, Bacci S, Salvemini L, et al. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PLoS One 2014;8:e64729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 14.Olkin I, Sampson A. Comparison of meta-analysis versus analysis of variance of individual patient data. Biometrics 1998;54:317–322 [PubMed] [Google Scholar]
- 15.Roger VL, Go AS, Lloyd-Jones DM, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Diabetes Data & Trends. Washington, D.C., Centers for Disease Control and Prevention, Department of Health and Human Services, 2009 [Google Scholar]
- 17.Cox AJ, Hsu FC, Freedman BI, et al. Contributors to mortality in high-risk diabetic patients in the Diabetes Heart Study. Diabetes Care 2014;37:2798–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bis JC, Kavousi M, Franceschini N, et al.; CARDIoGRAM Consortium . Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet 2011;43:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell CJ, Kavousi M, Smith AV, et al.; CARDIoGRAM Consortium . Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011;124:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]