Abstract
Regular physical activity and exercise training have long been known to cause adaptations to white adipose tissue (WAT), including decreases in cell size and lipid content and increases in mitochondrial proteins. In this article, we discuss recent studies that have investigated the effects of exercise training on mitochondrial function, the “beiging” of WAT, regulation of adipokines, metabolic effects of trained adipose tissue on systemic metabolism, and depot-specific responses to exercise training. The major WAT depots in the body are found in the visceral cavity (vWAT) and subcutaneously (scWAT). In rodent models, exercise training increases mitochondrial biogenesis and activity in both these adipose tissue depots. Exercise training also increases expression of the brown adipocyte marker uncoupling protein 1 (UCP1) in both adipose tissue depots, although these effects are much more pronounced in scWAT. Consistent with the increase in UCP1, exercise training increases the presence of brown-like adipocytes in scWAT, also known as browning or beiging. Training results in changes in the gene expression of thousands of scWAT genes and an altered adipokine profile in both scWAT and vWAT. Transplantation of trained scWAT in sedentary recipient mice results in striking improvements in skeletal muscle glucose uptake and whole-body metabolic homeostasis. Human and rodent exercise studies have indicated that exercise training can alter circulating adipokine concentration as well as adipokine expression in adipose tissue. Thus, the profound changes to WAT in response to exercise training may be part of the mechanism by which exercise improves whole-body metabolic health.
Introduction
Of the numerous benefits of physical activity on health, one of the most important is the ability of exercise to improve whole-body glucose homeostasis. In fact, physical exercise is widely accepted as a clinical modality to prevent type 2 diabetes and decrease blood glucose concentrations in people with type 2 diabetes. Exercise training improves whole-body glucose homeostasis and insulin sensitivity, and adaptations in skeletal muscle are considered central to this effect because this tissue is responsible for the majority of glucose disposal (1). Although skeletal muscle is important to the beneficial effects of physical activity on metabolic homeostasis, exercise training also results in adaptations to numerous other tissues, including white adipose tissue (WAT).
WAT: Depot-Specific Effects on Metabolic Health
WAT plays a role in lipid storage, hormone production, immune function, and local tissue architecture (2) and is classified into two major depots: visceral (vWAT) and subcutaneous (scWAT). vWAT refers to the adipose tissue that surrounds the internal organs, whereas scWAT is primarily found around the thighs and buttocks. The specific type of adipose tissue that accumulates in the body is critically important with regard to health risks. An accumulation of vWAT is associated with insulin resistance, an increased risk of type 2 diabetes, dyslipidemia, progression of atherosclerosis, and mortality (3–5), whereas an accumulation of scWAT is associated with improved insulin sensitivity and a reduced risk for developing type 2 diabetes (6,7).
An important area of investigation has been to determine the factors responsible for the differences in vWAT and scWAT that result in their distinctly different effects on metabolic health. Transplantation of scWAT or vWAT from donor mice into the subcutaneous or visceral cavity of recipient mice has been used to delineate whether differences in metabolic function of scWAT and vWAT were due to anatomic location or intrinsic differences of these adipose tissue depots (8). This study showed that mice transplanted with vWAT in either the visceral or subcutaneous cavity had no improvements in metabolic health. In contrast, mice transplanted with scWAT into the visceral cavity had increased insulin sensitivity, decreased body weight, decreased fat mass, and decreased circulating glucose and insulin concentrations 12 weeks after transplantation. Mice transplanted with scWAT into the subcutaneous cavity also had lower body weights and increased insulin sensitivity but not to the extent of the mice transplanted with scWAT in the visceral cavity. These findings suggest that scWAT exerts beneficial effects on metabolic health (8).
The underlying mechanisms for the various metabolic effects of transplanting scWAT and vWAT are likely to be related to the distinct molecular properties of these adipose tissue depots. Subcutaneous and visceral adipocytes have been shown to develop from different progenitor cell lines, have the capacity to differentiate at varying rates, and develop distinct cell-autonomous properties, establishing unique gene expression profiles (9,10). Compared with vWAT, scWAT has higher expression of many genes involved in glucose homeostasis and insulin action (e.g., Glut1, Igf-1, Igfbp3, Pparγ), as well as genes involved in lipid metabolism (e.g., Hsl, β-adrenergic receptors, hydroxymethylglutaryl CoA synthase) (11). scWAT also has increased expression of PRDM16, a transcriptional coregulatory protein responsible for the development of brown adipocytes in both brown adipose tissue (BAT) and scWAT. Expression of PRDM16 in vWAT, however, is minimal. The deletion of PRDM16 in scWAT results in the scWAT adopting the metabolic and morphological characteristics of vWAT (12). Mice deficient in adipose tissue PRDM16 develop altered fat distribution with an increased accumulation of scWAT. PRDM16-deficient scWAT mice also exhibit an increased expression of inflammatory genes and increased macrophage accumulation when fed a high-fat diet, similar to that of vWAT. Taken together, these studies demonstrate intrinsic differences between vWAT and scWAT depots. These distinct properties could account for the different effects of these adipose depots on metabolic health (12,13). Although PRDM16 is clearly an important gene with regard to the differences in the metabolic phenotypes of scWAT and vWAT, additional genes are likely to confer these differential characteristics.
Exercise Training–Induced Adaptations to WAT
Exercise training, defined as repeated bouts of exercise over a period of days, weeks, or even years, can have profound effects on WAT morphology and biochemical properties. Exercise training can decrease adipocyte size (14,15) and reduce lipid content (14,15), resulting in decreased adiposity. Studies have shown that exercise training can increase expression of several key metabolic proteins, including GLUT4 and PGC-1α, among others (14–18). Of note, many of these metabolic adaptations to adipose tissue can occur independently of significant changes in weight loss (14,16).
The exercise-induced reduction in adipocyte size and lipid content as well as increased GLUT4 and PGC-1α occurs in both scWAT and vWAT (15,17,18,19). Additionally, there are exercise training–induced adaptations specific to each adipose tissue depot. Here, we discuss various adaptations to adipose tissue as a result of exercise training as well as distinct exercise-specific adaptations to scWAT and vWAT.
Exercise Training Causes a Beiging of scWAT
Recent studies have demonstrated that a number of conditions result in the presence of brown fat–like adipocytes in scWAT. These adipocytes have been termed “adaptive brown fat cells,” “recruitable brown fat cells,” “beige cells,” or “brite cells” (20–22), and the increased presence of the these cells within the scWAT is referred to as “browning” or “beiging.” Beige cells are distinct from white adipocytes in that they express uncoupling protein 1 (UCP1) and have a multilocular morphology. They also have a distinct molecular signature, including the expression of Tbx1, Tmem26, and Cd137, gene markers not expressed in mature brown or white adipocytes (23). Beige cells are found interspersed in the WAT of humans and rodents (19–22), and beiging occurs predominantly in scWAT. In mice, beiging occurs in response to a number of stimuli, including cold exposure (21), β3-selective adrenergic agonists (22), exercise (16,18,24–26), and exposure to an enriched environment (26). The factors involved in the determination of beige adipocyte fate are not fully understood, and a number of different theories have been hypothesized, including that beige adipocytes 1) are derived from the maturation of a brown adipocyte precursor within WAT (21), 2) differentiate from an existing white adipocyte precursor (27,28), 3) transdifferentiate from an existing white adipocyte (29–31), and 4) are derived from a distinct smooth muscle cell precursor (32). Beiging results in more metabolically active cells, making these cells an attractive obesity therapeutic (22), although the increased heat production that can occur in these cells is not favorable as a treatment strategy.
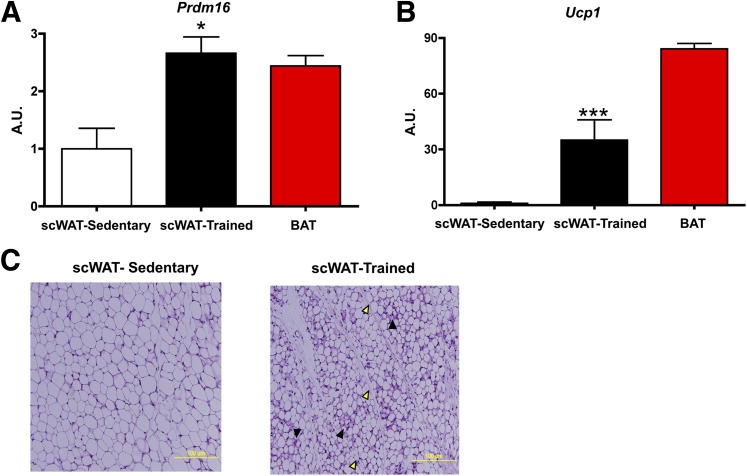
As mentioned above, exercise training results in an increased expression of beige adipocytes in scWAT (16,18,24–26). In rodent studies of 3–4 weeks of exposure to an enriched environment, which included the presence of a running wheel, beige cells emerged in scWAT marked by an increase in Ucp1, Prdm16, and other markers of BAT or beiging (25,26). Our recent study demonstrated that only 11 days of exercise training by voluntary wheel running results in a marked upregulation of brown and beige adipocyte marker genes, including Ucp1 and Prdm16 (Fig. 1A and B) (16). In fact, the analysis by quantitative PCR showed that although expression of Prdm16 mRNA in sedentary scWAT was approximately one-half that of intrascapular BAT, 11 days of exercise training increased Prdm16 mRNA expression in scWAT to a level similar to that observed in BAT (Fig. 1A). Several other genes known to be indicative of brown or beige adipocytes such as Cidea, Elovl3, Pgc1α, Pparγ, Cox8b, Dio2, and otopetrin were also significantly increased with exercise training as well as the beige-specific marker Tbx1. We found that 11 days of training dramatically increased UCP1 immunofluorescence and resulted in the presence of multilocular cells in the scWAT from the trained mice (Fig. 1C), all consistent with the beiging of scWAT. The number of blood vessels also increased in the scWAT from trained mice, consistent with microarray data indicating an increase in markers of vascularization (e.g., Vegfa, Pdgf, Angptl2).
Figure 1.
Exercise training increases the beiging of scWAT. A–C: Mice were housed in wheel cages for 11 days of exercise training, and scWAT was analyzed. Prdm16 (A) and Ucp1 (B) mRNA of trained scWAT was increased compared with sedentary scWAT, and Prdm16 expression was increased to the expression level of BAT (n = 7/group). *P < 0.05, ***P < 0.001. (C) Hematoxylin-eosin staining revealed the presence of multilocular droplets in the trained subcutaneous adipose tissue (solid arrowheads indicate the presence of multilocular droplets; open arrowheads indicate blood vessels). Adapted with permission from Chechi et al. (31). A.U., arbitrary unit.
The cause of the exercise training–induced beiging has been the focus of several investigations. Of note, most nonexercise stimuli, including cold exposure and numerous pharmaceutical agents, are believed to cause beiging of scWAT through increased heat loss and possible compensatory adrenergic stimulation (33). This heat loss results in increased thermogenic demand resulting in increased sympathetic tone and expression of UCP1 to increase heat production (33). It is clear that exercise does not work through this mechanism. Although the function of beiging as a result of exercise training is not fully understood, one hypothesis is that the decrease in cell size and lipid content in scWAT that occurs with exercise training decreases insulation of the body, necessitating increased heat production through the beiging of scWAT (33,34). Several hypotheses have been proposed for the underlying molecular mechanisms that cause the beiging. For example, because exercise is known to increase sympathetic innervation in scWAT, the increased sympathetic innervation could contribute to the beiging of scWAT (33,35), or exercise-induced adaptations to other tissues may be responsible for the beiging of scWAT. One study concluded that exercise training–induced beiging occurs in response to increased secretion of hypothalamic brain-derived neurotrophic factor (26), whereas other studies have suggested that various myokines released from skeletal muscle during exercise can be responsible for beiging (36). These myokines include irisin (25), meteorin-like 1 (37), myostatin (38), and β-aminoisobutyric acid (39). Although all these hypotheses are intriguing, further investigation is needed to fully elucidate the mechanism responsible for the exercise-induced beiging of scWAT.
Exercise Training Increases Mitochondrial Activity in WAT
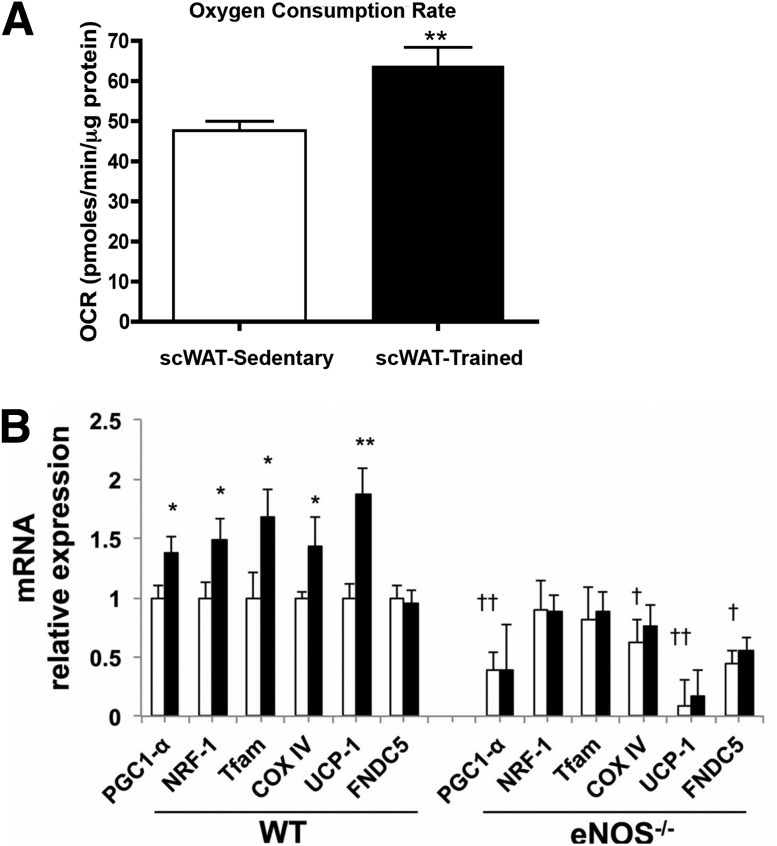
It has long been established that exercise training induces mitochondrial adaptations in adipose tissue. In one study, mitochondrial activity, as measured by activity of the respiratory chain enzyme cytochrome c oxidase and tricarboxylic acid cycle enzyme malate dehydrogenase, was significantly increased in vWAT in response to 8 weeks of swim training in rats (17). Of note, this increase was exercise specific because cold exposure over the same 8-week period did not result in increased activity of cytochrome c oxidase and malate dehydrogenase in the vWAT (17). Although most prior studies have measured mitochondrial activity by determining expression of mitochondrial genes and mitochondrial enzyme activity (17,18,24), we recently assessed mitochondrial function in trained scWAT by measurement of oxygen consumption rates (OCRs) using respirometry. Eleven days of training by voluntary wheel-running exercise significantly increased basal rates of OCR in scWAT, directly demonstrating an increase in mitochondrial activity (Fig. 2A) (16,40).
Figure 2.
Exercise training increases mitochondrial biogenesis in WAT. A: OCR was significantly increased in trained scWAT (11 days of wheel cage running) compared with scWAT from sedentary mice (n = 7/group). **P < 0.01. Adapted with permission from Chechi et al. (31). B: Genes involved in mitochondrial biogenesis after 30 days of exercise training in scWAT in wild-type (WT) and eNOS−/− mice (n = 8) *P < 0.05, **P < 0.01 relative to sedentary controls; †P < 0.05, ††P < 0.01 relative to WT mice. Adapted with permission from Craig et al. (15).
Expression of Pgc1α often is used as a marker for mitochondrial biogenesis. A single bout of exercise has been shown to increase expression of Pgc1α mRNA in both scWAT and vWAT (18), suggesting that an increase in Pgc1α after exercise training could be the accumulation of the individual exercise sessions that result in the increased mitochondrial biogenesis in WAT. Two-weeks of swim training in mice resulted in a significant increase in Pgc1α expression in scWAT and vWAT (18). To determine the mechanism for this increase, mice were exercised in the presence or absence of a β-blocker to inhibit adrenergic stimulation. Of note, the presence of a β-blocker inhibited Pgc1α expression in vWAT but not scWAT, indicating that the mechanism for increased Pgc1α expression with exercise training, and presumably mitochondrial biogenesis, is different in each adipose tissue depot (18).
Endothelial nitric oxide synthase (eNOS) has been proposed to function in training-induced increases in mitochondrial biogenesis in scWAT (24). Thirty days of swim training in wild-type mice significantly increased mitochondrial biogenesis in scWAT as suggested by an increased expression of Pgc1α, Nrf1, Tfam, and CoxIV and mitochondrial DNA content. In contrast, exercise training in eNOS−/− mice failed to increase expression of these genes or mitochondrial DNA content (Fig. 2B) (24), and there was no beiging of scWAT in the eNOS−/− mice. Adrenergic stimulation by norepinephrine treatment of wild-type mice significantly increased expression of Pgc1α and cytochrome c, and this effect was significantly attenuated in eNOS−/− mice. Because it has been hypothesized that exercise-induced beiging occurs through increased sympathetic innervation, it is interesting that eNOS−/− mice do not have an increase in beiging in response to either exercise or adrenergic stimulation. This indicates that although some effects of exercise on scWAT are mediated by sympathetic innervation, a second mechanism independent of sympathetic drive may be essential in the regulation of mitochondrial number and activity (18,24,41). Although these data point to an important role of exercise and regulation of mitochondrial activity in scWAT, it is important to note that these effects were observed in mice that were whole-body knockouts, not adipose tissue–specific deletions in eNOS; thus, this effect may not be adipose tissue specific (24,41).
All these studies consistently demonstrated that exercise training has marked effects on mitochondrial gene expression and activity in scWAT. Moreover, the changes in mitochondrial gene expression in scWAT occurred in response to various modalities of exercise, including swimming, treadmill running, and voluntary wheel running, as well as various training durations ranging from as few as 11 days to up to 8 weeks. Further investigation is needed to fully determine the molecular mechanisms underlying exercise training–induced mitochondrial regulation and how this regulation is specific to each adipose tissue depot.
Exercise Training Has Profound Effects on scWAT Gene Expression
We recently determined the effects of exercise training on the complete gene expression profile of scWAT (16). The goal of this experiment was to determine the degree of plasticity of scWAT in response to training and to determine whether other cellular functions are altered with exercise training. Compared with the scWAT from sedentary mice, exercise training by voluntary wheel running significantly increased the expression of 1,844 genes and significantly decreased the expression of 1,156 genes. Gene set enrichment analysis (P < 0.05 and Q < 0.25) showed that exercise training resulted in significant increases in scWAT genes involved in metabolism, mitochondrial biogenesis, oxidative stress and signaling, membrane transport, cell stress, proteolysis, apoptosis, replication, and glycoproteins. Furthermore, genes involved in Wnt signaling and Pgc1α-related pathways were significantly increased in scWAT with exercise training. Remarkably, the number of genes upregulated by exercise training in scWAT was substantially greater than what has been reported to be increased in skeletal muscle with exercise training (42–44). This degree of plasticity in scWAT suggests that scWAT plays a prominent role in whole-body adaptations to exercise training.
Exercise and Adipokines
Secreted proteins make up ∼10–15% of encoded proteins of the human genome (45) and include serum proteins, extracellular matrix proteins, digestive enzymes, and milk proteins. In contrast, growth factors, cytokines, and hormones are in low abundance, but these types of secreted molecules are considered highly bioactive (46). Adipose tissue secretes cytokines and other molecules termed “adipokines,” factors that can modulate inflammation, lipid and glucose metabolism, blood pressure, and atherosclerosis (47).
Several studies in humans and rodents investigated the effects of exercise training on adipokine expression and secretion. Two of the most well-studied adipokines are leptin and adiponectin (48–50). Leptin is secreted by adipocytes and helps to regulate energy balance by acting as an appetite suppressant. Circulating leptin is correlated to changes in adiposity, and exercise training–induced decreases in adiposity result in decreased circulating leptin in both rodents and humans (51–56). Although this correlation between adipose tissue mass and leptin has been very well established, the role of exercise training on adipose tissue depot–specific expression of leptin is unclear. Adiponectin is another well-studied adipokine but in contrast to leptin, adiponectin concentrations in the circulation are inversely correlated to fat mass (57,58). Adiponectin modulates glucose and fatty acid regulation and increases insulin sensitivity (57,58). Because adiponectin is inversely correlated to fat mass and related to improved insulin sensitivity, it has been hypothesized that exercise increases circulating adiponectin concentrations. However, studies in both rodent models and humans have not come to a consensus on the effects of exercise training on adiponectin concentrations.
There have also been conflicting data on the effects of exercise training on the regulation of adipokine expression within scWAT. Human subjects who underwent varying lengths of exercise training (4–12 weeks) showed increased leptin, adiponectin, IL-6, and TNFα mRNA expression in scWAT (59,60), whereas other studies showed little change in adipokine mRNA expression (60–62). The function of the exercise training–induced adipokines and how exercise regulates the concentration of circulating adipokines are topics of intense investigation. It is possible that increased expression of these adipokines may function to enhance free fatty acid supply to working skeletal muscle (50–52) or play a yet-to-be-identified role in the regulation of whole-body glucose homeostasis.
Transplantation of scWAT From Trained Mice Improves Metabolic Homeostasis
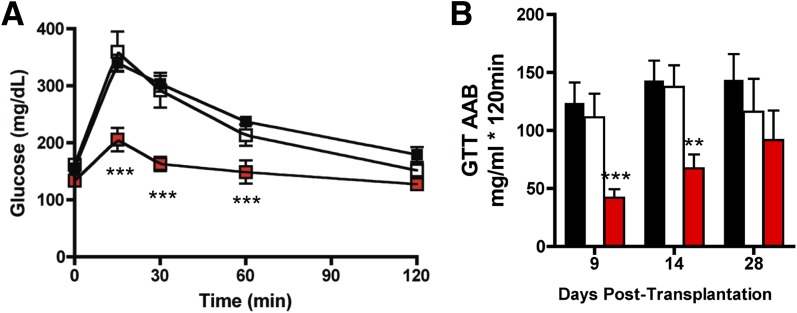
As stated above, exercise training increases metabolic activity and mitochondrial function of adipose tissue, alters adipokine expression within adipose tissue, and affects circulating concentrations of adipokines. In general, the training-induced changes in adipose tissue are more pronounced in scWAT. These findings have led us to hypothesize that trained scWAT could have beneficial metabolic effects on whole-body metabolism (16). To test this hypothesis, sedentary recipient mice were transplanted with scWAT from donor mice trained by voluntary wheel running for 11 days. Control mice were transplanted with scWAT from sedentary mice or sham operated. Transplantation of scWAT from exercise-trained or sedentary mice was not associated with changes in body weight, food intake, energy expenditure, or spontaneous activity in the recipient mice. However, 9 days posttransplantation, significant improvement was seen in glucose tolerance in mice transplanted with scWAT from trained mice compared with both sham-operated mice and mice transplanted with scWAT from sedentary mice (Fig. 3A). This dramatic effect on glucose tolerance was transient because there was no significant improvement in glucose tolerance 4 weeks after transplantation (Fig. 3B). In addition to the marked improvement in glucose tolerance, mice transplanted with trained scWAT exhibited a decrease in fasting blood glucose, insulin, and cholesterol concentrations 9 days posttransplant. In contrast to these marked effects of transplanting scWAT from trained mice, there was no effect of transplanting vWAT from trained mice, demonstrating that the beneficial metabolic effects of transplanting exercise-trained adipose tissue is depot specific.
Figure 3.
Transplantation of trained scWAT improves glucose tolerance and increases whole-body insulin sensitivity. A and B: Mice were transplanted with 0.85 g scWAT from trained or sedentary mice or were sham operated. For glucose tolerance tests (GTTs), mice were injected with glucose 2 g/kg body weight i.p. A: GTT at 9 days posttransplantation. B: Glucose area above baseline (AAB) at 9, 14, and 28 days posttransplantation. Data are mean ± SEM (n = 5–12/group). **P < 0.01, ***P < 0.001. Adapted with permission from Chechi et al. (31).
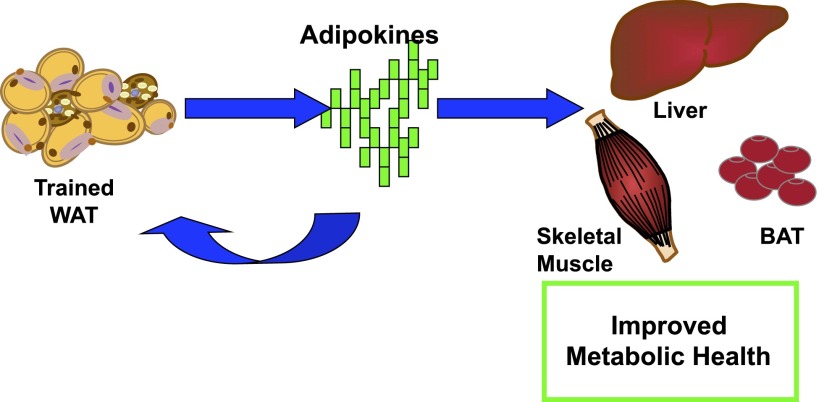
To understand the mechanisms underlying the effects of transplanting exercise-trained scWAT, a number of control experiments were performed (16). An additional cohort of mice transplanted with a cell-size control was studied to determine whether the improvements in glucose tolerance were due to the decreased adipocyte size resulting from exercise training or exercise-specific adaptations to the scWAT. These mice were transplanted with scWAT from 6-week-old sedentary mice with the same-sized adipocytes as scWAT from trained 12-week-old mice. Interestingly, the mice transplanted with the cell-size control had a worsening in glucose tolerance compared with all other groups. These data indicate that the improved glucose tolerance is due to exercise-specific adaptations to scWAT and not merely transplantation of smaller adipocytes. We also showed that there was no effect of transplanting trained scWAT on glucose uptake into the transplanted adipose tissue. However, measurements of insulin-stimulated glucose disposal in vivo revealed increased rates of glucose uptake into oxidative skeletal muscles and BAT in mice transplanted with trained scWAT. The finding that transplantation of trained scWAT results in short-term improvements in glucose tolerance, along with data showing that this transplantation increases glucose uptake in other tissues in the body, suggests that trained scWAT has endocrine effects. These endocrine effects would likely be mediated by the release of adipokines from the trained scWAT. The specific adipokine(s) released from trained adipose tissue and responsible for increasing glucose uptake in muscle and BAT have not yet been identified, but interestingly, microarray analysis revealed numerous putative secreted proteins that are increased in scWAT from exercise-trained mice (16). Although in recent years a major focus of research has been on the concept that skeletal muscle is a source of circulating factors (myokines) that function in tissue-to-tissue communication, these experiments suggest that adipose tissue from exercise-trained mice is also a source of circulating factors (adipokines), and these exercise-induced adipokines may have beneficial effects on systemic metabolism (Fig. 4).
Figure 4.
Exercise training–induced adipokines have an endocrine effect and improve whole-body metabolism. We propose a model whereby exercise causes WAT to release adipokines, which can act in an endocrine manner to improve metabolism in skeletal muscle, liver, and BAT or in an autocrine or paracrine manner to improve WAT function.
Conclusions and Future Directions
Exercise training results in profound changes to WAT, including increased expression of genes involved in mitochondrial biogenesis, increased mitochondrial activity, increased beiging of scWAT, and an altered adipokine profile of WAT. An emerging concept from these studies is that exercise training–induced adaptations to scWAT contribute to the improved systemic metabolic homeostasis that occurs with regular exercise. These effects could be due to novel training-induced adipokines originating from the trained adipose tissue. It will be important to define these putative adipokines and understand their function in tissues throughout the body. Future studies are also needed to determine whether these dramatic effects of exercise training on adipose tissue, which have been performed primarily in rodent models, occur in human subjects. Given the alarming prevalence of obesity and type 2 diabetes and the vast negative ramifications of both a sedentary lifestyle and type 2 diabetes on population health, human exercise studies will be critical to gain further insight into the function of novel adipokines and define their role in glucose metabolism and their impact on human health. Exercise-induced adipokines may have additional benefits on overall health beyond glucose metabolism and could be interesting novel therapeutic targets for obesity, type 2 diabetes, and other diseases.
Article Information
Funding. This work was supported by National Institutes of Health grants F32-DK-091048 (to K.I.S.), T32-DK-07260-038 (to R.J.W.M.), R01-DK-099511 and R01-AR-042238 (to L.J.G.), and 5P30-DK-36836 (to Diabetes Research Center at Joslin Diabetes Center). K.I.S. was also supported by the Mary K. Iacocca Fellowship and an American Heart Association Scientist Development Grant (15SDG22990000).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I.S., R.J.W.M., and L.J.G. wrote and edited the manuscript. L.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
References
- 1.Bonadonna RC, Saccomani MP, Seely L, et al. Glucose transport in human skeletal muscle. The in vivo response to insulin. Diabetes 1993;42:191–198 [DOI] [PubMed] [Google Scholar]
- 2.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 2010;6:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997;145:614–619 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005;81:555–563 [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008;117:1658–1667 [DOI] [PubMed] [Google Scholar]
- 6.Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res 1997;5:93–99 [DOI] [PubMed] [Google Scholar]
- 7.Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr 2003;77:1192–1197 [DOI] [PubMed] [Google Scholar]
- 8.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008;7:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab 2007;292:E298–E307 [DOI] [PubMed] [Google Scholar]
- 10.Gesta S, Blüher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 2006;103:6676–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atzmon G, Yang XM, Muzumdar R, Ma XH, Gabriely I, Barzilai N. Differential gene expression between visceral and subcutaneous fat depots. Horm Metab Res 2002;34:622–628 [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollisch KS, Brandauer J, Jessen N, et al. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab 2009;297:E495–E504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig BW, Hammons GT, Garthwaite SM, Jarett L, Holloszy JO. Adaptation of fat cells to exercise: response of glucose uptake and oxidation to insulin. J Appl Physiol 1981;51:1500–1506 [DOI] [PubMed] [Google Scholar]
- 16.Stanford KI, Middelbeek RJW, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015;64:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol 1991;261:E410–E414 [DOI] [PubMed] [Google Scholar]
- 18.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1alpha mRNA expression in rat adipose tissue. J Physiol 2009;587:1607–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirshman MF, Wardzala LJ, Goodyear LJ, Fuller SP, Horton ED, Horton ES. Exercise training increases the number of glucose transporters in rat adipose cells. Am J Physiol 1989;257:E520–E530 [DOI] [PubMed] [Google Scholar]
- 20.Enerbäck S. The origins of brown adipose tissue. N Engl J Med 2009;360:2021–2023 [DOI] [PubMed] [Google Scholar]
- 21.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science 2010;328:1113–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevellin E, Scorzeto M, Olivieri M, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014;63:2800–2811 [DOI] [PubMed] [Google Scholar]
- 25.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao L, Choi EY, Liu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011;14:324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisani DF, Djedaini M, Beranger GE, et al. Differentiation of human adipose-derived stem cells into “brite” (brown-in-white) adipocytes. Front Endocrinol (Lausanne) 2011;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elabd C, Chiellini C, Carmona M, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009;27:2753–2760 [DOI] [PubMed] [Google Scholar]
- 29.Cinti S. The adipose organ at a glance. Dis Model Mech 2012;5:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 2009;297:E977–E986 [DOI] [PubMed] [Google Scholar]
- 31.Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab 2013;24:408–420 [DOI] [PubMed] [Google Scholar]
- 32.Long JZ, Svensson KJ, Tsai L, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014;19:810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 2014;20:396–407 [DOI] [PubMed] [Google Scholar]
- 34.Hirata M, Suzuki M, Ishii R, et al. Genetic defect in phospholipase Cδ1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes 2011;60:1926–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranallo RF, Rhodes EC. Lipid metabolism during exercise. Sports Med 1998;26:29–42 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–465 [DOI] [PubMed] [Google Scholar]
- 37.Rao RR, Long JZ, White JP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014;157:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman BJ, Streeper RS, Farese RV Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A 2006;103:15675–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts LD, Boström P, O’Sullivan JF, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014;19:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vernochet C, Mourier A, Bezy O, et al. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab 2012;16:765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernlohr DA. Exercise and mitochondrial function in adipose biology: all roads lead to NO. Diabetes 2014;63:2606–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 2005;19:1498–1500 [DOI] [PubMed] [Google Scholar]
- 43.Fu L, Liu X, Niu Y, Yuan H, Zhang N, Lavi E. Effects of high-fat diet and regular aerobic exercise on global gene expression in skeletal muscle of C57BL/6 mice. Metabolism 2012;61:146–152 [DOI] [PubMed] [Google Scholar]
- 44.Keller P, Vollaard NB, Gustafsson T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol (1985) 2011;110:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlou MP, Diamandis EP. The cancer cell secretome: a good source for discovering biomarkers? J Proteomics 2010;73:1896–1906 [DOI] [PubMed] [Google Scholar]
- 46.Skalnikova H, Motlik J, Gadher SJ, Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics 2011;11:691–708 [DOI] [PubMed] [Google Scholar]
- 47.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med 2008;14:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 49.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc 2005;64:163–169 [DOI] [PubMed] [Google Scholar]
- 50.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am 2008;37:753–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes 1997;46:1159–1166 [DOI] [PubMed] [Google Scholar]
- 52.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 2008;295:E586–E594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraemer RR, Johnson LG, Haltom R, et al. Serum leptin concentrations in response to acute exercise in postmenopausal women with and without hormone replacement therapy. Proc Soc Exp Biol Med 1999;221:171–177 [DOI] [PubMed] [Google Scholar]
- 54.Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med 2010;44:620–630 [DOI] [PubMed] [Google Scholar]
- 55.Kanaley JA, Fenicchia LM, Miller CS, et al. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int J Obes Relat Metab Disord 2001;25:1474–1480 [DOI] [PubMed] [Google Scholar]
- 56.Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res 2014;2014:726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 2002;13:84–89 [DOI] [PubMed] [Google Scholar]
- 58.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blüher M, Williams CJ, Klöting N, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care 2007;30:3110–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab 2010;95:911–919 [DOI] [PubMed] [Google Scholar]
- 61.Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism 2006;55:1375–1381 [DOI] [PubMed] [Google Scholar]
- 62.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab 2006;91:5107–5112 [DOI] [PubMed] [Google Scholar]