Abstract
Cellular senescence is a fundamental aging mechanism that has been implicated in many age-related diseases and is a significant cause of tissue dysfunction. Accumulation of senescent cells occurs during aging and is also seen in the context of obesity and diabetes. Senescent cells may play a role in type 2 diabetes pathogenesis through direct impact on pancreatic β-cell function, senescence-associated secretory phenotype (SASP)-mediated tissue damage, and involvement in adipose tissue dysfunction. In turn, metabolic and signaling changes seen in diabetes, such as high circulating glucose, altered lipid metabolism, and growth hormone axis perturbations, can promote senescent cell formation. Thus, senescent cells might be part of a pathogenic loop in diabetes, as both a cause and consequence of metabolic changes and tissue damage. Therapeutic targeting of a basic aging mechanism such as cellular senescence may have a large impact on disease pathogenesis and could be more effective in preventing the progression of diabetes complications than currently available therapies that have limited impact on already existing tissue damage. Therefore, senescent cells and the SASP represent significant opportunities for advancement in the prevention and treatment of type 2 diabetes and its complications.
Introduction
Type 2 diabetes is an increasing threat to human healthspan, particularly in the face of rising obesity prevalence and a rapidly aging population. Diabetes incidence and prevalence increase with age, with 25.9% of Americans 65 years or older having diabetes compared with 9.3% in the general population (1). Type 2 diabetes can be a consequence of obesity, whose prevalence remains over 30% in adults older than 60 (2). In addition, diabetes is a major risk factor for premature onset of multiple age-related conditions, including renal dysfunction, cardiovascular disease, stroke, impaired wound healing, infection, depression, and cognitive decline (3–6). Despite increased understanding of the pathogenesis of diabetes and its comorbidities, current therapies have limited efficacy with respect to its progression and complications. Novel therapeutic targets and strategies are needed to advance the treatment and prevention of type 2 diabetes, especially its complications. Of note, we and our colleagues recently identified a possibly causal link between cellular senescence and aging phenotypes by showing that the clearance of senescent cells in a mouse progeroid model in vivo can delay age-related tissue dysfunction, including cataract formation, lipodystrophy, and lordokyphosis (7). These points raise the possibility that cellular senescence could be a novel target for clinical interventions for age-related diseases, potentially including type 2 diabetes. In this Perspective, we speculate that senescent cells and the senescence-associated secretory phenotype (SASP) could be transformative therapeutic targets for type 2 diabetes and its complications.
Cellular Senescence: A Basic Aging Mechanism
Like other age-related chronic diseases, diabetes may be caused in part by a convergence of the basic aging mechanisms that underlie age-related tissue dysfunction, including chronic “sterile” (not pathogen-associated) inflammation, macromolecular damage, progenitor cell dysfunction, and cellular senescence (8). In the past decade, cellular senescence has emerged as a possible cause of general tissue dysfunction and aging phenotypes (9,10). Cellular senescence is an essentially irreversible growth arrest that occurs in response to various cellular stressors, such as telomere erosion, DNA damage, oxidative stress, or oncogenic activation, and is thought to have arisen as an antitumor mechanism (11). In addition to growth arrest, senescent cells adopt several unique identifying characteristics, including a flattened morphology in culture, upregulation of cell cycle inhibitors such as p21 and p16, accumulation of DNA damage foci, reactive oxygen species production, and shifted optimum pH of lysosomal β-galactosidase (senescence-associated β-galactosidase [SA-βgal] activity) (11).
Although senescent cells are incapable of dividing, they are metabolically active. This high metabolic activity supports the release of proinflammatory cytokines, chemokines, and growth factors collectively known as the SASP (12,13). Through the SASP, a low absolute number of senescent cells in a tissue (typically <20%) may be able to exert systemic effects (11). For example, obesity-associated senescent cells may promote chronic, low-grade sterile inflammation. In this way, senescent cells might be a link between obesity and inflammation that contributes to the development and progression of type 2 diabetes (Fig. 1) (14,15). Although cellular senescence is normally a defense mechanism against tumor development, presence or persistence of a high number of senescent cells can promote tumor progression because of inflammation, tissue disruption, and growth signals due to the SASP (16). Senescent cells can also initiate a deleterious positive feedback mechanism by promoting the spread of senescence to nearby cells (17–19).
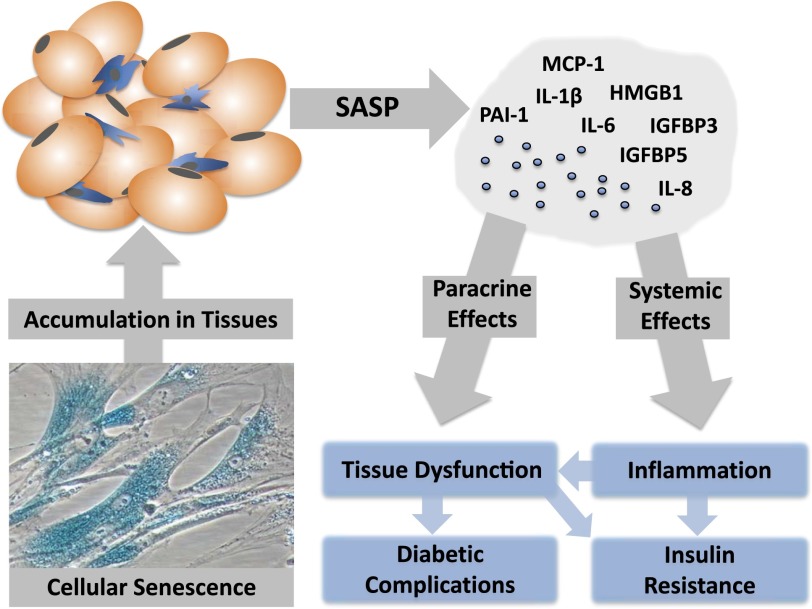
Figure 1.
Senescent cells may play a role in driving insulin resistance and diabetes complications. Senescent cells accumulate in tissues throughout the body with aging and obesity and in disease states. The SASP is a diverse group of proinflammatory cytokines, chemokines, and growth factors released by senescent cells, which may act both locally and systemically. The SASP may affect the function of neighboring cells within a tissue through paracrine mechanisms, contributing to tissue dysfunction and damage that can lead to diabetes complications. In addition, SASP factors may circulate and add to a chronic inflammatory state commonly implicated as a contributor in the development of insulin resistance.
Senescent cell burden is low in young individuals but increases with aging in several tissues including adipose tissue, skeletal muscle, kidney, and skin (20–22). In particular, components of the metabolic syndrome, including abdominal obesity, diabetes, hypertension, and atherosclerosis, are among the many pathologies associated with increased senescent cell burden (20,23–25). The potential roles that cellular senescence may play in chronic disease have been recently reviewed (10,18,26). Senescent cell accumulation can occur due to a variety of factors, such as various age-related chronic diseases, oxidative stress, the hormonal milieu, developmental factors, chronic infection (e.g., HIV), certain medications (e.g., chemotherapy or certain HIV protease inhibitors), and radiation exposure (9,10,27,28).
Several possible “subtypes” of cellular senescence have been identified, including oncogene-induced senescence, stress-induced premature senescence, and the classical replicative senescence (10). The full extent of senescence inducers and the dynamics of senescent cell turnover are not yet fully known. For example, tissue-specific senescent cells that are found in disease states, such as senescent cholangiocytes in primary sclerosing cholangitis, seem to accumulate through mechanisms that are less age-dependent than those in normal chronological aging (29). It is possible that some senescent cells form acutely in response to damage and are quickly cleared, while other senescent cells may evade immune clearance and persist to contribute to a chronic inflammatory state (30,31).
In addition to the heterogeneity in senescence-inducing signals, senescent cells play several different roles once formed, which can be positive or negative. Although cellular senescence plays a negative role in age-related disease by causing tissue dysfunction, it is a crucial mechanism by which potentially tumorigenic cells are neutralized. Senescence is also involved in normal embryogenesis, development, and wound repair (32–34). Thus, cellular senescence is necessary for the health of an organism but can also have detrimental effects, especially when senescent cell abundance increases with aging.
The association between diabetes and senescence may be complex and complementary. On one hand, it is likely that the diabetic microenvironment could be permissive to the development and accumulation of senescent cells. On the other hand, senescent cells may contribute to the tissue dysfunction and comorbidities observed in type 2 diabetes. We speculate that these complex interactions might lead to a “malignant” positive feedback, in which metabolic dysfunction in prediabetes leads to cellular senescence that contributes to the worsening of tissue and metabolic function, which further increases the formation and decreases the clearance of senescent cells (Fig. 2).
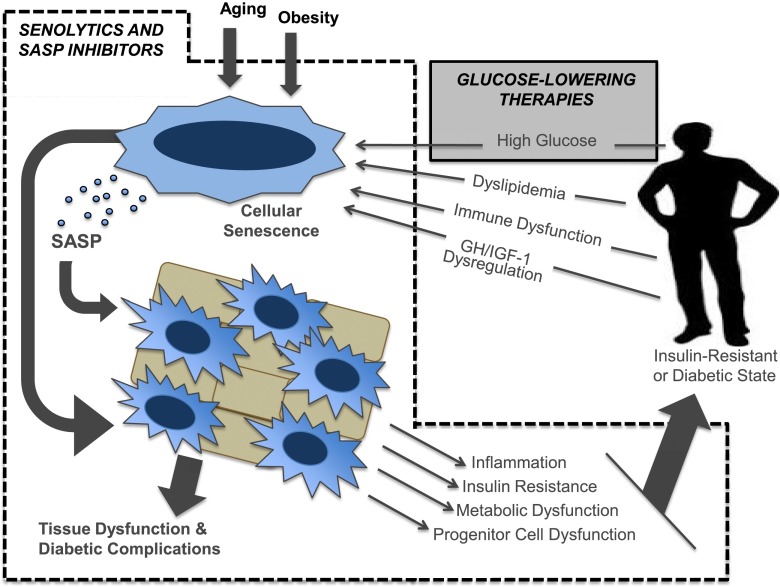
Figure 2.
Senolytic or SASP-inhibiting therapies present new opportunities for targeting type 2 diabetes and its complications. Senescent cells that accumulate during aging and obesity may contribute to inflammation, insulin resistance, metabolic dysfunction, and progenitor cell dysfunction through the SASP and direct effects of senescent cells in tissues. These effects might promote insulin resistance and type 2 diabetes as well as contribute to tissue damage and diabetes complications. Metabolic and immune perturbations in the diabetic state may, in turn, promote the formation of more senescent cells. Current glucose-lowering therapies (gray box) are able to exert a limited effect on this pathogenic loop established by senescent cells. As shown in the dotted box, by targeting senescent cells and their associated SASP, we speculate that the tissue dysfunction that leads to diabetes complications may be slowed or alleviated, and the pathogenic loop between cellular senescence, diabetes, and tissue dysfunction may be broken. GH, growth hormone.
Diabetic Microenvironment Promotes Cellular Senescence
High Glucose
High glucose drives premature senescence in vitro in endothelial cells, renal mesangial cells, adipose-derived stem cells (also known as preadipocytes or fat cell progenitors), and fibroblasts (35–37). The mechanism of glucose-induced senescence is not clear, although potential candidates include mitochondrial dysfunction and increased reactive oxygen species (38). High glucose can also potentiate the formation of advanced glycation end products (AGEs) (39). Increased AGE signaling through their receptors (RAGE), of which the SASP factor HMGB1 is also the agonistic ligand (see below), has been shown to cause premature senescence in renal tubular cells (40).
Growth Hormone/IGF-1 Axis
Alterations in the growth hormone/IGF signaling pathway, as well as differential responses of target tissues in obesity and diabetes, might play a role in promoting cellular senescence. The ability of multiple individual components of this pathway to induce senescence has been recently shown, including growth hormone, IGF-1, and IGF binding protein 5 (IGFBP5) (27,28,41). Other related factors, such as IGFBP3, have been implicated as SASP components that may play a role in the propagation of senescence signals to neighboring cells (42). More research is needed to determine whether, in turn, senescent cells and the SASP affect growth hormone axis signaling, and especially how this might impact insulin resistance and diabetes pathogenesis.
A p53-mediated premature senescence can result from chronic exposure to IGF-1, whose levels may be differentially regulated in diabetes due to hyperinsulinemia and changing levels of IGFBP family members (28,43). Consistent with IGF-1–mediated senescence, the activity of Akt, a major effector of the insulin/IGF-1 signaling pathway, is increased in cellular senescence. Inhibition of Akt has been shown to increase replicative lifespan (i.e., delay senescence) of primary endothelial cells in vitro (20,44).
The levels of IGFBP3, which increase as a result of replication-, doxorubicin-, and other stress-induced senescent cell states, may be modulated by the SASP component PAI-1. PAI-1 is a serine protease inhibitor that inhibits the tissue plasminogen activator, which cleaves IGFBP3 (42). IGFBP3 has been shown to cause insulin resistance independently of IGF binding, as evidenced by decreased GLUT4 translocation to the plasma membrane as well as reduced Akt phosphorylation in response to insulin in adipocytes and omental adipose explants (45). In addition, overexpression of IGFBP3 in mice leads to decreased glucose tolerance, insulin resistance, and hyperglycemia (46). Another IGFBP member, IGFBP5, has been found to increase during endothelial and fibroblast senescence and may induce cellular senescence on its own through a p53-dependent, p16-independent mechanism (41).
Decreased growth hormone axis activity extends lifespan in organisms from C. elegans to mice. Recently, growth hormone activity in mice was shown to correlate positively with senescent cell burden, and this, in turn, was inversely related to lifespan (27). Despite reduced levels of growth hormone signaling, the opposite is seen in obesity and obesity-related type 2 diabetes (i.e., increased senescent cell burden and decreased healthspan). This disparity could be due to the underlying cause of decreased growth hormone signaling and could indicate that glucose or other metabolites may play a larger role in diabetes-associated senescence than the growth hormone axis. The extent and effects of insulin and growth hormone signaling perturbations in obesity and diabetes and especially their impact on aging mechanisms, such as cellular senescence, have yet to be fully determined. The relationship between diabetes, senescence, and the growth hormone/IGF-1 axis are complex and require further study.
Ceramides
Ceramide synthesis is upregulated in obesity and diabetes and is a major contributor to lipotoxicity, which, in turn, causes tissue damage such as pancreatic β-cell apoptosis (47). Ceramides induce senescence markers in fibroblasts and endothelial cells in vitro, suggesting that high circulating or local levels of ceramides could be a driver of cellular senescence (48). Increased intracellular ceramide content has been shown to promote cellular senescence, owing to changes in fatty acid metabolism that occur as a response to environmental stress through the p53 and p38 pathways (49). Notably, the diabetic biomarker 1-deoxysphinganine, when converted to its ceramide metabolite 1-deoxy-dihydroceramide, decreases insulin secretion in Ins-1 β-cells and primary islets and triggers p21-dependent cellular senescence in Ins-1 cells (50). Moreover, inhibition of de novo ceramide synthesis in diet-induced obese and ob/ob mice has been shown to decrease the SASP factors PAI-1 and MCP-1 and improve metabolic phenotypes such as glucose and insulin tolerance (51).
Cellular Senescence in the Pathogenesis of Type 2 Diabetes
SASP: Mediator of Insulin Resistance?
Chronic, sterile, low-grade inflammation is associated with both obesity and aging and is thought to be a major contributing factor for the development of insulin resistance (Fig. 1) (14,52). Components of the SASP, such as interleukin (IL)-6, IL-8, and MCP-1, are increased in obese adults and adolescents and could contribute to such a proinflammatory state, together with effects of macrophages and other mediators of inflammation (31,53,54). Macrophage accumulation may, in turn, be promoted through effects of the chemokine MCP-1, which is produced by senescent cells as part of the SASP (12). Case-control studies have found that elevated IL-6 and the combined elevation of IL-6 and IL-1β, both SASP factors, are independent predictors of diabetes (55,56). Another prominent SASP component, PAI-1, is increased in the circulation and tissues, such as coronary arteries of patients with diabetes (57). HMGB1, recently shown to be involved in inflammatory signaling during p53-dependent cellular senescence (58), signals through RAGE, which plays a role in driving diabetes complications as well (59). We speculate that senescent cells, which accumulate in obesity and aging, may be the source of some of the inflammatory factors that are associated with risk for insulin resistance and diabetes. However, more research is needed to determine to what extent senescent cells might contribute to this inflammatory state and to establish if there is a causal link between senescent cells, the SASP, and insulin resistance.
Cellular Senescence and Adipose Tissue Dysfunction
In many people, adipose tissue is the largest organ, particularly in the context of obesity. Adipose tissue dysfunction related to both obesity and aging is associated with an increase in senescent cell burden (20). Preadipocytes, the precursors to mature adipocytes in fat tissue, are among the cells known to be susceptible to the development of cellular senescence (20,27). Senescent preadipocyte abundance is greater in obese subjects compared with lean age-matched counterparts, even in young individuals (20). Increased senescent preadipocytes could contribute to declines in adipogenic and lipogenic potential, with consequent development of age-related lipodystrophy, leading to lipotoxicity and inflammation (20). The SASP also may interfere with adipogenesis through inflammatory cytokine release. Adipogenesis is crucial for insulin responsiveness, as insulin receptors and GLUT4 increase as preadipocytes differentiate into mature adipocytes (60). Peroxisome proliferator–activated receptor γ and C/EBPα, which increase as preadipocytes differentiate, are required transcription factors for both adipogenesis and acquisition of insulin sensitivity (60). Experiments in progeroid INK-ATTAC;BubR1H/H mice showed that senescent cell clearance could reverse age-related lipodystrophy. The response of adipose tissue to senescent cell clearance in other contexts, such as obesity or diabetes, has not yet been reported (7).
Because the SASP includes macrophage attractant proteins such as MCP-1, it is possible that senescent cells in adipose tissue could be a driver of the macrophage infiltration that has been proposed to play an important role in adipose tissue inflammation leading to insulin resistance in obesity (12,52). However, the temporal relationships among macrophage infiltration, senescent cell development, and insulin resistance are unknown and require further study. Senescent cells and the SASP could influence other processes in adipose tissue that impact insulin resistance, such as autophagy. Autophagy has been implicated in the establishment of oncogene-induced senescence; however, little is known about the role of autophagy in senescence triggered by metabolic stresses such as in obesity (61).
The deleterious effects of cellular senescence on adipose tissue function may lead to decreased capacity for fat accumulation and the development of the “spillover” of toxic free fatty acid that may play a role in development of cellular dysfunction in diabetes (62). Ectopic fat deposition in liver, muscle, and heart, which contributes to insulin resistance in those tissues, could be a result of dysfunctional adipose tissue that cannot store further lipid in both aging and obesity (62). This lipotoxicity may further contribute to pancreatic β-cell dysfunction, atherosclerosis, renal disease, and other accelerated age-related diseases such as mild cognitive impairment or dementia (62).
Pancreatic β-Cell Senescence
Pancreatic β-cell senescence has been implicated as a contributor to type 2 diabetes, at least in a model of high-fat feeding (63). This could be a direct mechanism through which senescence contributes to diabetes, as the decline of β-cell function and mass is a hallmark of type 2 diabetes progression. The cell cycle inhibitor p27, a marker of senescence, increases in pancreatic β-cells in genetic mouse models of type 2 diabetes, and p27 deletion in those mice increased insulin secretion and islet mass through increased proliferation (64). In mice lacking p53-dependent apoptosis, the burden of senescent cells increases rapidly and is associated with dysfunction of pancreatic β-cells, causing an overt diabetic phenotype in 3–4 months (65). This appears to be an accelerated model of age-related diabetes and is consistent with the hypothesis that cellular senescence over the lifespan could be a cause of decreased insulin synthesis and release from pancreatic β-cells. Therefore, drugs that eliminate senescent cells—senolytic agents—may possibly prevent β-cell dysfunction due to cellular senescence when and if they become available (66). Senolytic therapy administered around the first diagnosis of prediabetes might delay or prevent individuals from progressing to overt diabetes. Similarly, senolytic therapies could possibly prevent patients with noninsulin-dependent diabetes from progressing to insulin-dependent diabetes by preserving some β-cell function.
Senescence and Diabetes Complications
Although much work still needs to be done to link cellular senescence to the pathogenesis of diabetes, many studies have indicated that senescent cell burden is increased in tissues that undergo damage in diabetes, such as the skin, pancreas, and kidney (22,63,67). The aging microenvironment limits regenerative potential of young progenitor cells in vivo, and conversely, aged progenitor cells exhibit improved function when exposed to a young systemic microenvironment (68). It is possible that senescent cells are a contributor to the aging microenvironment, which may limit the regenerative potential of cells in every tissue. Without effective cell turnover and tissue repair, phenotypes seen in diabetes, such as retinopathy, islet degeneration, and renal damage, may potentially be initiated or worsened. For example, the accumulation of senescent cells could contribute to infectious complications of diabetes and impede tissue repair in diabetic skin wounds. Also, it is possible that circulating SASP factors originating from fat or other tissues could lead to dysfunction in tissues without a high local burden of senescent cells (Fig. 1).
There are emerging data indicating a role of senescent cells in neurodegeneration and cognitive dysfunction (69). Increased risk of cognitive impairment in diabetes could be tied to senescence in the brain, either of neurons themselves or senescence of infiltrating or glial cells. Neurons can adopt a senescence-like phenotype, including DNA damage foci and SA-βgal activity in dementia and other neurodegenerative diseases (70,71). Alternatively, the SASP generates factors that affect brain function, such as IL-6 (72). Potentially, SASP factors originating from tissues outside the central nervous system could contribute to cognitive dysfunction in diabetes as well (73).
Cardiac progenitor aging is accelerated in diabetes, and heart disease is a major complication of diabetes (74). Perhaps systemic effects of the increased senescent cell burden in diabetes contribute to this, together with the effects of lipotoxicity on endothelial cells and increased prothrombotic SASP factors, such as PAI-1. Senescent cells also accumulate in atherosclerotic plaques (24). Cardiovascular disease risk can even be elevated in patients with prediabetes, suggesting that this might be an opportune time to target senescent cells. Microvascular complications of diabetes, such as retinopathy, neuropathy, and nephropathy, could be linked to cellular senescence as well, as endothelial cells have been shown to be susceptible to glucose-induced senescence (35). Senescent cells not only may be a contributing cause of diabetes, but also may accelerate tissue injury and be a central mechanism underlying complications of diabetes.
Cellular Senescence Establishes Pathogenic Positive Feedback Loops in Diabetes
As discussed above, one of the main characteristics of cellular senescence is that it promotes development and accumulation of more senescent cells in nearby and distant tissues, leading to a “snowball” effect (Fig. 1). This may be accomplished through the SASP, promotion of chronic “sterile” inflammation, reactive oxygen species, impedance of immune clearance of senescent cells, or other mechanisms.
Certain SASP factors originating from senescent cells in particular tissues or types of senescent cells may be able to induce senescence in neighboring cells, propagating senescence signals (19). For example, PAI-1, a SASP factor produced by many cell types, can stabilize IGFBP3 by inhibition of tissue plasminogen activator, which normally cleaves it. IGFBP3, itself a SASP factor in some cell types, is induced during senescence of breast cancer cells caused by a variety of stresses, and, in turn, upregulates PAI-1 by inducing senescence in fibroblasts and endothelial cells (42). Importantly, overexpression of PAI-1 is sufficient to induce replicative senescence as indicated by SA-βgal activity in mouse embryonic fibroblasts (75). Decreased PAI-1, either by small interfering RNA knockdown or SIRT1 repression, results in decreased senescent cells (75,76). Furthermore, PAI-1 knockdown extends lifespan and healthspan in Klotho-deficient mice, which have an accelerated aging-like phenotype (77). Thus, inhibitors targeting certain SASP components, such as PAI-1, could limit the effects of senescent cells as well as decrease their numbers and may be a therapeutic option in diabetes and other cellular senescence-related diseases.
Cellular senescence can be accompanied by mitochondrial dysfunction that, in turn, causes oxidative stress, which has been implicated as a cause of insulin resistance in muscle tissue with aging (78,79). Oxidative species produced by dysfunctional mitochondria may also induce neighboring cells to undergo senescence, amplifying senescent cell burden in fat, the pancreas, and other tissues, potentially establishing another pathogenic loop causing senescent cell accumulation.
Immune cells, which are responsible for clearing senescent cells, are susceptible to developing both age- and metabolic-related dysfunction (80,81). In addition to increasing susceptibility to infection, senescence-induced immune dysfunction may contribute to decreased senescent cell clearance in aging and diabetes, accelerating the increase in senescent cell burden. Senescent cells may interfere with their own clearance locally or systemically by releasing SASP factors that, like tumor cells, impede the ability of immune cells, such as natural killer cells or macrophages, to clear them. For example, the SASP could contribute to a chronic proinflammatory microenvironment in diabetes that causes a decline in natural killer cell function, as is seen in aging and obesity (31,82–84). Also, IL-6, a prominent SASP factor, interferes with macrophage migration (85). Through these means, senescent cells may contribute to their own propagation within tissues, outpacing mechanisms of the immune system that normally clear them.
Therapeutic Targeting of Senescent Cells
Therapeutic clearance of cellular senescence is an active area of research in which there is much opportunity for progress. Senescent cell clearance may prove valuable in slowing progression of general age-related dysfunction and is also worth testing in diseases correlated with increased senescent cell burden, such as diabetes. Our understanding of the causes, disease associations, and cell type– or tissue-specific features of cellular senescence is steadily increasing, including unique features that we may be able to exploit in therapeutic strategies. Therapies could be developed either to target and eliminate senescent cells directly or to alleviate local and systemic effects of the SASP. Because cellular senescence is a basic aging mechanism thought to play a role in numerous age-related diseases, targeting senescent cells could have widespread impact both for individual patients and on a population scale. For example, we speculate that a therapy that reduces senescent cells may prevent or ameliorate diabetes, Alzheimer disease, and cardiovascular disease simultaneously (9). By impacting age-related diseases as a group, therapeutic clearance of senescent cells and their effects could have immense benefits to human healthspan and lifespan (18).
Do Current Diabetes Therapies Affect Senescence?
Existing diabetes therapies, namely metformin and acarbose, extend lifespan in nondiabetic mice (86,87). In human cancer patients with diabetes, metformin was associated with a decrease in 2-year mortality compared with other glucose-lowering medications (88). Surprisingly, metformin was recently shown to confer a slight but significant lifespan benefit to patients with diabetes compared with control subjects without diabetes (89). Metformin may also confer a decreased cancer risk in patients with diabetes (90). A recent study linked this antineoplastic activity of metformin to inhibition of the SASP by interfering with proinflammatory nuclear factor-κB signaling (91). Metformin alleviates diabetes, diabetes complications, and the metabolic syndrome. Among the mechanisms through which it acts may be inhibition of the SASP and related inflammation, a speculation that merits further experimental testing (91). Current therapies for diabetes may have other beneficial effects in addition to solely lowering glucose. It is possible that, in addition to their antidiabetes effects, these agents target basic aging mechanisms, such as cellular senescence and the SASP.
Targeting the SASP
It has yet to be shown whether inhibition of the SASP is as effective in preventing age-related disease progression as senescent cell clearance may be. This issue is complicated by cell-type, tissue-type, and organismal differences in the composition of the SASP, as well as the multiple effects that SASP factors can play in inflammation, immunity, and normal physiology. It is unclear whether senescent cell removal or SASP inhibition would be more beneficial, and this may depend on organ system and disease pathogenesis. Targeting individual SASP factors, such as PAI-1, might even prevent spread of senescence or interfere with senescent cell viability. Particular SASP components may be most responsible for certain pathologies, and if so, they should be directly targeted in certain diseases. Special attention should be paid to side effect profiles during the development of such therapies. While targeting the SASP could inhibit senescent cell formation by disrupting cytokine, RAGE, or serine protease inhibitor signaling pathways, it might also have effects on other cellular processes involving those SASP factors, such as immune function, tumorigenesis, wound healing, or hemostasis (10,34).
Feasibility of Senescence as a Therapeutic Target
Developing strategies to increase lifespan or healthspan, such as strategies that target cellular senescence and the SASP, is challenging due to the impractical nature of the required clinical trials. This has been a barrier for the translation of basic aging biology advances into clinical contexts. However, targeting senescent cells and the SASP in the setting of disease, such as diabetes, could provide proof of principle for therapies that target aging mechanisms. Diabetes complications may be the perfect focus for initial studies of senescence in diabetes. By removing senescent cells or their systemic effects, it may be possible to both halt the progression of diabetic tissue damage as well as to improve insulin sensitivity. For patients with prediabetes, therapy targeting senescent cells may prevent progression to frank diabetes and its complications.
There are currently no ideal systemic markers of senescent cell burden that could be used to assess the efficacy of senescent cell clearing, or senolytic, drugs. For example, the cytokine IL-6 is an important component of the SASP and appears to be an indicator of frailty (92). However, due to the ubiquity of IL-6 in proinflammatory processes, circulating levels may not be specific enough to quantify senescent cell burden. Current methods for assessing senescent cell burden in vivo are at the tissue level, either by quantification of senescent cells through methods such as immunohistochemistry for markers including heterochromatin foci, p16 expression, SA-βgal activity, or screening for transgenic labels such as the p16INK4A promoter-driven GFP in the INK-ATTAC mouse (7).
In vivo studies of senescent cell clearance as a preventative therapy would potentially be difficult because little is known about the time frame of senescent cell formation. Many studies have drawn associations between cellular senescence and disease states, such as renal tubular senescence in diabetes; however, the timeline for senescent cell appearance is unclear (67). Therefore, testing whether senescent cells have a causal role in these pathologies is challenging to delineate. For example, careful studies are necessary to understand whether senescent cell accumulation precedes the development of diabetes complications.
Similarly, the mechanism of senescent cell clearance is largely unidentified. It has been suggested that senescent cells are cleared by the immune system, but the specific immune cell types involved and the kinetics of clearance are unknown (83). As organisms age, immune system function declines, and this could decrease the rate at which organisms are able to clear senescent cells from various tissues (80). This may be one mechanism by which senescent cell burden increases with chronological age. In turn, SASP factors may contribute to immune dysfunction, leading to a vicious positive feedback cycle of increasing senescent cell burden.
The development of transgenic mice from which senescent cells can be selectively cleared allowed the demonstration of senescent cells’ causal role in disease (7). The success of these studies has prompted interest in devising strategies for senescent cell clearance that could be implemented in humans, and efforts are currently under way to do so. However, more research is needed to identify unique features of senescent cells that distinguish them from healthy dividing cells and that can be exploited in therapeutic approaches. For example, novel strategies may be uncovered by studying the mechanisms through which senescent cells are cleared by the innate immune system or by identifying proteins or signaling networks that are upregulated exclusively, or to an extreme extent, in senescent cells. Recently, senescent cells induced by chemotherapy in oncology patients were metabolically targeted by the administration of 2-deoxyglucose to exploit the increased glucose utilization observed in senescent lymphoma cells (93). In another example, PAI-1 inhibitors reduced senescent cell abundance in Klotho-deficient mice (77). The time is ripe for studies like these to leverage the growing knowledge about cellular senescence into translational research opportunities that may eventually impact patients (9).
Conclusions
Senescent cell burden increases in aging and obesity and may play a role in causing or exacerbating type 2 diabetes. In turn, features of diabetes may cause an increase in senescent cell number, which would further promote chronic inflammation and initiate a vicious cycle of senescent cell formation in multiple tissues. This increased senescent cell burden may play a role in the tissue damage that contributes to diabetes complications. It will be crucial to explore the extent and characteristics of metabolic dysfunction in animal models with a high senescent cell burden in order to determine the effect of senescent cells on metabolic dysfunction, including insulin resistance. Shared mechanisms between the development of insulin resistance and cellular senescence indicate that perhaps by clearing senescent cells, both the metabolic components and complications of diabetes could be ameliorated. Experiments to test this possibility, such as in animal models that allow senescent cell clearance, will be extremely important in establishing whether senescent cells are a therapeutic target in diabetes. More research is needed to understand the kinetics of senescent cell formation, especially in the setting of persisting metabolic stimuli in obesity and diabetes or in the setting of SASP factors and chronic inflammation due to senescent cells already present. In addition, whether there are unique features of diabetes-associated senescence that may allow targeting of senescent cells in this context needs to be determined. Diabetes complications, especially cardiovascular disease and microvascular complications including neuropathy, nephropathy, and retinopathy, have been particularly difficult to treat by glucose control alone (94). This may be because much damage occurs before diagnosis and initiation of treatment. Glucose-lowering medications have limited effects on ameliorating existing tissue damage and might not be very effective in reducing senescent cell burden. Senolytics or SASP-protective agents, used alone or in conjunction with current glucose-lowering therapies, may be a way to delay, prevent, alleviate, or even treat hitherto resistant complications of diabetes (66) (Fig. 2). Although many ideas expressed in this Perspective have been at the level of speculation or hypothesis, our intent is to spark discussion and help to generate hypotheses that may link basic aging mechanisms, such as cellular senescence, to diabetes and lead to advances in its treatment and prevention. We hypothesize that clearing senescent cells or targeting the SASP may present opportunities for the development of revolutionary therapies for diabetes and its complications.
Article Information
Acknowledgments. J. Armstrong and M. Jensen of Mayo Clinic provided editorial assistance. A.K.P. thanks the Mayo Medical Scientist Training Program for fostering an outstanding environment for physician-scientist training. Mayo Clinic, J.L.K., T.T., and A.K.P. have a financial interest related to senolytic drug discovery research and therapeutic use.
Funding. This work was supported by National Institutes of Health grants F30 AG046061 (A.K.P.), AG044396 (J.L.K.), and AG013925 (J.L.K.) and the Glenn Foundation for Medical Research and Noaber Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham Study. Diabetes Care 1979;2:120–126 [DOI] [PubMed] [Google Scholar]
- 4.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 5.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 28 October 2015 [Epub ahead of print]. DOI: 10.1016/j.exger.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014;15:482–496 [DOI] [PubMed] [Google Scholar]
- 11.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8:729–740 [DOI] [PubMed] [Google Scholar]
- 12.Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 2009;9:81–94 [DOI] [PubMed] [Google Scholar]
- 14.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141–150 [DOI] [PubMed] [Google Scholar]
- 15.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 16.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120:513–522 [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 2014;17:324–328 [DOI] [PubMed] [Google Scholar]
- 18.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson G, Wordsworth J, Wang C, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 2012;11:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell 2010;9:667–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melk A, Schmidt BMW, Vongwiwatana A, Rayner DC, Halloran PF. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 2005;5:1375–1382 [DOI] [PubMed] [Google Scholar]
- 22.Waaijer ME, Parish WE, Strongitharm BH, et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012;11:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 2009;15:1082–1087 [DOI] [PubMed] [Google Scholar]
- 24.Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res 2007;100:15–26 [DOI] [PubMed] [Google Scholar]
- 25.Westhoff JH, Hilgers KF, Steinbach MP, et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 2008;52:123–129 [DOI] [PubMed] [Google Scholar]
- 26.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol 2013;48:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stout MB, Tchkonia T, Pirtskhalava T, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany, NY Online) 2014;6:575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran D, Bergholz J, Zhang H, et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 2014;13:669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014;59:2263–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 2010;16:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muñoz-Espín D, Cañamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell 2013;155:1104–1118 [DOI] [PubMed] [Google Scholar]
- 33.Meuter A, Rogmann LM, Winterhoff BJ, Tchkonia T, Kirkland JL, Morbeck DE. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J Assist Reprod Genet 2014;31:1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014;31:722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokoi T, Fukuo K, Yasuda O, et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes 2006;55:1660–1665 [DOI] [PubMed] [Google Scholar]
- 36.Cramer C, Freisinger E, Jones RK, et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 2010;19:1875–1884 [DOI] [PubMed] [Google Scholar]
- 37.Blazer S, Khankin E, Segev Y, et al. High glucose-induced replicative senescence: point of no return and effect of telomerase. Biochem Biophys Res Commun 2002;296:93–101 [DOI] [PubMed] [Google Scholar]
- 38.Ksiazek K, Passos JF, Olijslagers S, von Zglinicki T. Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. Biochem Biophys Res Commun 2008;366:793–799 [DOI] [PubMed] [Google Scholar]
- 39.Peppa M, Uribarri J, Vlassara H. Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clinical Diabetes 2003;21:186–187 [Google Scholar]
- 40.Liu J, Huang K, Cai GY, et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 2014;26:110–121 [DOI] [PubMed] [Google Scholar]
- 41.Kim KS, Seu YB, Baek SH, et al. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell 2007;18:4543–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elzi DJ, Lai Y, Song M, Hakala K, Weintraub ST, Shiio Y. Plasminogen activator inhibitor 1—insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc Natl Acad Sci U S A 2012;109:12052–12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frystyk J. Free insulin-like growth factors—measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res 2004;14:337–375 [DOI] [PubMed] [Google Scholar]
- 44.Minamino T, Miyauchi H, Tateno K, Kunieda T, Komuro I. Akt-induced cellular senescence: implication for human disease. Cell Cycle 2004;3:449–451 [PubMed] [Google Scholar]
- 45.Chan SS, Twigg SM, Firth SM, Baxter RC. Insulin-like growth factor binding protein-3 leads to insulin resistance in adipocytes. J Clin Endocrinol Metab 2005;90:6588–6595 [DOI] [PubMed] [Google Scholar]
- 46.Silha JV, Gui Y, Murphy LJ. Impaired glucose homeostasis in insulin-like growth factor-binding protein-3-transgenic mice. Am J Physiol Endocrinol Metab 2002;283:E937–E945 [DOI] [PubMed] [Google Scholar]
- 47.Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mouton RE, Venable ME. Ceramide induces expression of the senescence histochemical marker, beta-galactosidase, in human fibroblasts. Mech Ageing Dev 2000;113:169–181 [DOI] [PubMed] [Google Scholar]
- 49.Ford JH. Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging. Age (Dordr) 2010;32:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuellig RA, Hornemann T, Othman A, et al. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes 2014;63:1326–1339 [DOI] [PubMed] [Google Scholar]
- 51.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab 2009;297:E211–E224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30:1347–1355 [DOI] [PubMed] [Google Scholar]
- 54.Utsal L, Tillmann V, Zilmer M, et al. Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-γ levels in 10- to 11-year-old boys with increased BMI. Horm Res Paediatr 2012;78:31–39 [DOI] [PubMed] [Google Scholar]
- 55.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 56.Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52:812–817 [DOI] [PubMed] [Google Scholar]
- 57.Schneider DJ, Sobel BE. PAI-1 and diabetes: a journey from the bench to the bedside. Diabetes Care 2012;35:1961–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davalos AR, Kawahara M, Malhotra GK, et al. p53-Dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 2013;201:613–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 2002;1:1–15 [DOI] [PubMed] [Google Scholar]
- 60.Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci 1999;892:134–145 [DOI] [PubMed] [Google Scholar]
- 61.Young AR, Narita M, Ferreira M, et al. Autophagy mediates the mitotic senescence transition. Genes Dev 2009;23:798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tchkonia T, Corkey BE, Kirkland JL. Current views of the fat cell as an endocrine cell: lipotoxicity. In Overweight and the Metabolic Syndrome: From Bench to Bedside (Endocrine Updates). Bray GA, Ryan DH, Eds. New York, Springer, 2006, p. 105–118 [Google Scholar]
- 63.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 2005;48:58–67 [DOI] [PubMed] [Google Scholar]
- 64.Uchida T, Nakamura T, Hashimoto N, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 2005;11:175–182 [DOI] [PubMed] [Google Scholar]
- 65.Tavana O, Puebla-Osorio N, Sang M, Zhu C. Absence of p53-dependent apoptosis combined with nonhomologous end-joining deficiency leads to a severe diabetic phenotype in mice. Diabetes 2010;59:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 9 March 2015 [Epub ahead of print]. DOI: 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verzola D, Gandolfo MT, Gaetani G, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 2008;295:F1563–F1573 [DOI] [PubMed] [Google Scholar]
- 68.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764 [DOI] [PubMed] [Google Scholar]
- 69.Golde TE, Miller VM. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimers Res Ther 2009;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease? J Intern Med 2013;273:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 2012;11:996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol 2014;13:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995;2:241–248 [DOI] [PubMed] [Google Scholar]
- 74.Rota M, LeCapitaine N, Hosoda T, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 2006;99:42–52 [DOI] [PubMed] [Google Scholar]
- 75.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 2006;8:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan YZ, Gao P, Zhou S, et al. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell 2014;13:890–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eren M, Boe AE, Murphy SB, et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A 2014;111:7090–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Passos JF, Nelson G, Wang C, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 2010;6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007;120:435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghesquière B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature 2014;511:167–176 [DOI] [PubMed] [Google Scholar]
- 82.Chiu BC, Martin BE, Stolberg VR, Chensue SW. The host environment is responsible for aging-related functional NK cell deficiency. J Immunol 2013;191:4688–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007;445:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany, NY Online) 2012;4:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sebastian C, Lloberas J, Celada A. Molecular and cellular aspects of macrophage aging. In Handbook on Immunosenescence. Fulop T, Ed. New York, Springer, 2009, p. 919–945 [Google Scholar]
- 86.Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 2014;13:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013;4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist 2013;18:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 2014;16:1165–1173 [DOI] [PubMed] [Google Scholar]
- 90.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012;7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moiseeva O, Deschênes-Simard X, St-Germain E, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013;12:489–498 [DOI] [PubMed] [Google Scholar]
- 92.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc 2002;50:1268–1271 [DOI] [PubMed] [Google Scholar]
- 93.Dörr JR, Yu Y, Milanovic M, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 2013;501:421–425 [DOI] [PubMed] [Google Scholar]
- 94.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med 2010;123(Suppl.):S3–S11 [DOI] [PubMed] [Google Scholar]