Abstract
The epidemic of obesity and type 2 diabetes has increased interest in pathways that affect energy balance in mammalian systems. Brown fat, in all of its dimensions, can increase energy expenditure through the dissipation of chemical energy in the form of heat, using mitochondrial uncoupling and perhaps other pathways. We discuss here some of the thermodynamic and cellular aspects of recent progress in brown fat research. This includes studies of developmental lineages of UCP1+ adipocytes, including the discovery of beige fat cells, a new thermogenic cell type. We also discuss the physiology and transcriptional control of brown and beige cells in rodents and the state of current knowledge about human brown fat.
Role of Brown Fat From a Physiological Perspective: Thermodynamics
Thermoregulation was a very important aspect of mammalian evolution. The earliest mammals were shrew-like creatures who could search for food and hide in places that were too cool and dark for many of their lizard contemporaries (1). In addition to being warm blooded, mammals also were adept at performing adaptive thermogenesis, giving them the ability to further increase their metabolic rates when conditions would otherwise result in hypothermia. From the perspective of humans in 2015, it is easy to forget that thermoregulation, in all of its dimensions, was a critical part of mammalian history.
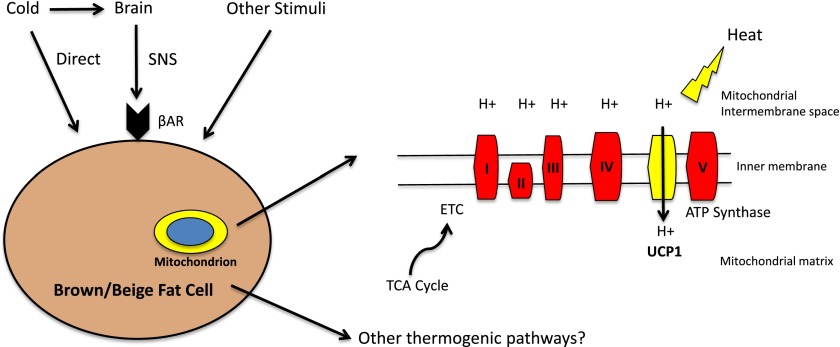
Chemical energy can be used to perform work and generate heat. Thermogenesis comes from chemical reactions in which the liberated free energy is not captured in other molecules (e.g., ATP or creatine phosphate) or used for work. The best known example of a heat-generating pathway is the futile cycle of proton pumping in brown fat and beige fat through the actions of uncoupling protein 1 (UCP1) (2,3). In cells expressing UCP1, the oxidation of lipids and carbohydrates results in the extraction of high-energy electrons, which flow down the electron transport chain (ETC) as protons are pumped across the inner mitochondrial membrane (Fig. 1). The resulting electrochemical gradient across the inner mitochondrial membrane, which is usually coupled to ATP synthesis via complex V of the ETC, is dissipated by a “leak” of protons back across the inner membrane by UCP1. Thus, much of the chemical energy generated by fuel oxidation in brown fat cells powers a futile proton cycle, which does no work and is instead liberated as heat.
Figure 1.
Schematic of adaptive thermogenesis in brown and beige adipocytes. This process is typically thought of as being indirectly activated by cold, via the sympathetic nervous system (SNS). Catecholamines stimulate β-adrenergic receptors (βAR), ultimately activating UCP1-dependent thermogenesis. Adaptive thermogenesis can also be activated directly by cold in beige adipocytes and by other stimuli that may signal independently from the β-adrenergic receptors. The reducing equivalents generated by the tricarboxylic acid (TCA) cycle enter the ETC. This generates a proton gradient across the inner mitochondrial membrane. Instead of linking this gradient to ATP synthesis via complex V, UCP1 is able to uncouple this gradient with the chemical energy converted to heat.
It is also worth noting that, from a thermodynamic perspective, there is nothing special about proton cycling, and such a thermogenic machine could, in theory, be built from other components of cellular metabolism. Indeed, some deep-diving fish have a “heater organ,” which is a specialized muscle that entirely lacks a myofibrillar apparatus. This organ, found between the eye and brain in deep-diving marlin and certain tuna, uses a futile cycle of calcium leaking from the sarcoplasmic reticulum and ATP-dependent calcium uptake to raise the local temperature near the brain of the fish (4). In this case, the physiological goal is not to warm the whole body of the marlin, but to just make it a bit more astute than the other fish around it!
Is UCP1 the only thermogenic pathway of importance in mammals? While no other pathway of thermogenesis except shivering has been convincingly demonstrated, suggestive data from the scientific literature hints that such pathways probably exist. If mice lacking UCP1 are abruptly switched from ambient temperatures to cold (4°C), they develop life-threatening hypothermia (5). On the other hand, if they are gradually exposed to lower temperatures, they can survive quite normally at 4°C (6). This suggests that there are compensatory thermogenic programs that can be activated as long as thermal stress is applied more gradually. In addition, there are examples of experiments in mice where certain mutations have caused very robust increases in energy expenditure and thermogenesis, but where the levels of UCP1 mRNA and protein are unchanged. For example, deletion of Par-1b/MARK2 in mice results in increased energy expenditure and brown fat activation, although UCP1 protein levels were unchanged (7). Thus, alternative pathways of thermogenesis seem important and are worthy of further study.
Discovery of Beige Fat and Its Physiology
UCP1+ brown fat has long been known to occur in two distinct anatomical locations in rodents. Developmentally formed depots, best typified by the interscapular and perirenal regions, are composed of tightly packed brown fat cells of relatively uniform appearance. These depots exist under most physiological conditions, although they may change in color and lipid content. On the other hand, UCP1+ cells can accumulate in small pockets in white fat depots, especially in the subcutaneous adipose tissues, particularly when mice are exposed to long-term cold or stimuli with hormones such as catecholamines and other β-adrenergic agonists (8). This pathway of adaptive thermogenesis is robustly activated by cold via an indirect pathway mediated by the sympathetic nervous system. However, fat cells are also able to directly sense cold, though the mechanism is not well understood (9).
In both anatomical situations, brown fat cells have multiple small lipid droplets, numerous mitochondria, and rich innervation and vascularization. Despite these similarities, it is now clear that the “classical” brown fat cells and the inducible “beige” fat cells come from different developmental lineages and are, in fact, distinct cell types. The key finding in this regard was the observation that the classical developmentally formed brown fat arises from a myf5+ lineage shared with skeletal muscle (10). Consistent with this, primary cultures of classical brown fat express low levels of certain genes characteristic of skeletal muscle (11). Subsequent work indicated that the brown fat/skeletal muscle decision occurs between 9.5 and 12.5 days of gestation in mice (12). Conversely, the UCP1+ cells induced in subcutaneous white fat depots by cold or β-adrenergic agonists come from a myf5− lineage (10). These beige fat cells have now been cloned and express a relatively low level of thermogenic genes, such as UCP1 and type 2 deiodinase in the basal state, but can induce these genes to levels essentially equivalent to classical brown fat cells when given hormonal stimuli (13). Moreover, the beige and brown fat cells have gene signatures that allow for distinction of these cells types; however, their comparative physiological properties and overall roles in metabolism are not completely understood.
It is now clear that thermogenic adipocytes, taken as a whole, contribute very importantly to metabolic homeostasis in rodents. The partial ablation of UCP1+ cells through the transgenic expression of a toxigene led to these animals being more susceptible to obesity and diabetes (14). Similarly, mice lacking UCP1 through targeted mutation had an increased body weight and fat content, at least when raised at thermoneutrality (15). Excessive shivering apparently prevented obesity when these experiments were performed at ambient temperatures. The first transgenic model with increased brown and beige fat was the adipose-selective expression of FOXC2, a transcription factor that activated cAMP metabolism in recipient cells (16). These mice, which were resistant to diet-induced obesity and diabetes, had expansions of both the classical brown and beige fat. Deciphering the individual roles of brown and beige fat cells has been difficult until appropriate molecular tools were recently developed. PRD1-BF-1-RIZ1 homologous domain-containing protein-16 (PRDM16) is an important transcriptional coregulator in both brown and beige fat, but when its expression was elevated through a promoter expressed in all fat depots (aP2), phenotypic changes were observed in the erstwhile white fat depots, which developed copious pockets of beige fat cells (17,18). The classical brown fat, which expresses very high levels of PRDM16 in the basal state, showed few or no changes with a further elevation in PRDM16 expression. Interestingly, visceral fat depots also showed more “browning,” but this occurred only when the transgenic mice also received stimulation with a β-adrenergic agonist.
While studies in cultured cells had shown that classical brown fat cells require PRDM16 to develop and maintain a thermogenic gene program, ablation of PRDM16 from all fat cells in vivo was not sufficient to significantly alter the function of the classical brown fat. On the other hand, the development of beige fat cells was severely reduced, at both the histological and molecular level (19). This allowed the first critical analysis of the physiological role of the beige fat. These mice developed a moderate obesity, compared with control animals, with an unusual expansion of the subcutaneous fat depots. Visceral fat was apparently unaltered in amounts. These beige fat–deficient mice also showed a rather profound hepatic insulin resistance that was associated with liver steatosis. One last point of interest is that the subcutaneous fat from the PRDM16-ablated mice showed the presence of more inflammatory macrophages and increased the expression of proinflammatory genes. The latter aspect was also observed with cultured subcutaneous cells lacking PRDM16, indicating that this transcriptional coregulator plays a role in the relative resistance of subcutaneous fat to inflammation and is an important determinant of subcutaneous versus visceral fat phenotype.
The role of PRDM16 in the classical brown fat in vivo is interesting and somewhat more complex. Ablation of this factor with a myf5-driven Cre recombinase causes a loss of brown fat function and gene expression, but only at 6 months of age (20). Prior to that, the brown fat is relatively normal. However, ablation of PRDM16 along with its closest homolog, PRDM3, causes an early and severe loss of brown fat thermogenic gene expression and normal histology. Thus, it appears that both thermogenic cell types depend on PRDM16, but, at least in early life, the classical brown fat has a second factor that can support development, PRDM3.
Transcriptional Control
Harnessing the therapeutic potential of brown and beige fat requires a detailed understanding of the molecular mechanisms responsible for the determination and maintenance of each cell type. The nuclear hormone receptor peroxisome proliferator–activated receptor γ (PPARγ) is necessary and sufficient for the development of all fat cells (21). However, white and brown adipocytes have drastically different phenotypes, indicating that other transcriptional regulators must be involved. A yeast two-hybrid screen identified PPARγ coactivator-1α (PGC-1α) as a cold-inducible binding partner of PPARγ (22). In brown fat, PGC-1α induces mitochondrial biogenesis, oxidative metabolism, and thermogenesis. PGC-1α is now appreciated to regulate numerous physiological processes in a variety of metabolically important tissues. While brown and beige fat cells lacking PGC-1α have significantly blunted thermogenic gene expression, these cells still retain the molecular signature of brown fat cells (23,24). This motivated the search for brown adipocyte identity factors. The transcriptional regulator PRDM16 was found to be highly enriched in brown fat, whereas it is virtually undetectable in visceral white fat cells. Forced expression of PRDM16 in cultured white fat cells induces PGC-1α and thermogenic genes, as well as mitochondrial genes and brown fat identity genes (17). In addition to activating these pathways, PRDM16 also binds CCAAT/enhancer binding protein β (c/EBPβ) and recruits the corepressor proteins CtBP1 and CtBP2 to repress white fat or muscle gene expression (25). Importantly, the expression of PRDM16 and its binding partner c/EBPβ in fibroblasts is sufficient to promote differentiation into functional brown fat cells, which can be detected by [18F]-2-fluoro-2-deoxy-d-glucose ([18F]-FDG) positron emission tomography (PET) when transplanted into mouse models (26).
An increasing number of transcriptional regulators has been identified as important in brown and beige fat biology. The transcription factor early B-cell factor-2 (Ebf2) is enriched in brown relative to beige adipocytes. It functions upstream of PRDM16 to promote binding of PPARγ to the promoters of brown-selective genes (27). EHMT1 is a histone lysine methyltransferase that purifies with the PRDM16 transcriptional complex in brown fat. EHMT1 is required for brown fat lineage specification and for thermogenesis (28). Presumably, there are also beige-selective factors upstream of PRDM16 and beige-selective epigenetic regulators that modulate the phenotype of these cells. TLE3 is a cofactor that can repress thermogenesis in both brown and beige adipocytes. It is able to compete with PRDM16 for binding to PPARγ, and thereby modulates white versus brown/beige phenotype (29,30). Forced expression of TLE3 in adipocytes results in impaired thermogenesis, while deletion enhances thermogenesis in brown and beige fat. A number of other factors have also been shown to play roles in this biology, but a comprehensive review is beyond the scope of this article.
Rediscovery of Human Brown Fat and Its Brown Versus Beige Nature
Until fairly recently, brown fat was thought to be present in meaningful amounts only in human infants and small mammals. The combination of insufficient hair, fur, and insulation, along with a high body surface area-to-mass ratio make babies and small mammals particularly susceptible to hypothermia. As a result, they have developed significant interscapular brown fat, which defends body temperature by adaptive thermogenesis. This brown fat was thought to regress by adulthood, unless exposed to catecholamine excess (as in pheochromocytoma) or long-term cold exposure (as in outdoor workers in cold climates). In 2009, several groups (31–33) “rediscovered” brown fat in adult humans. These studies made use of [18F]-FDG PET to confirm that adults have glucose-avid tissue with imaging characteristics of adipose in deposits in the supraclavicular and spinal regions. As assayed by [18F]-FDG PET, the amount of “active” tissue is inversely associated with BMI, and this activity is increased by cold exposure. Follow-up studies (34) have shown that this tissue has the histological appearance of adipose tissue and expresses several molecular markers of brown fat including UCP1. Current efforts are focused on developing optimal imaging modalities to detect this tissue and to quantify its absolute amounts and activity. This is particularly important since brown and beige fat also oxidize lipids, which would not be detectable by [18F]-FDG PET technology.
Research has increasingly suggested that adult human brown fat shares features of the inducible beige fat described in rodents. Brown and beige fat cell lines have now been cloned from mice, allowing for the identification of unique molecular markers of each cell type. Multiple studies (13,35,36) have shown that beige-selective markers are expressed more in human brown fat samples than are markers of the classical brown fat of rodents. A very recent study (37) characterized a human brown fat cell line and showed that it actually shares more molecular features with beige than brown adipocytes. However, the brown fat in human infants, located in the interscapular region, seems most similar to the interscapular classical brown fat in rodents. In some adult humans, cells with both brown and beige characteristics have been identified, with the depth in the neck seeming to be a determinant of relative brown versus beige character (36,38,39). While further studies will clarify this question, this tissue may well contain both brown and beige cells in varying amounts in each individual.
Therapeutic Potential
As a deeper understanding of brown and beige fat has emerged, researchers have turned to strategies to induce the activity of these tissues as a possible therapy for obesity and metabolic diseases. Calculations have suggested that maximal cold-induced brown fat thermogenesis would be between 25 and 400 kcal/day in lean healthy volunteers (40). Given the inducibility of beige fat, activating these cells could result in even more substantial effects. However, the greatest benefits may not relate to increased energy expenditure per se, but rather might result from enhanced glucose and lipid disposal. These properties suggest promise as a treatment for diabetes and hyperlipidemia. In addition to taking up and consuming glucose (31–33), brown adipose tissue takes up free fatty acids from triglyceride-rich lipoproteins. Cold exposure in mice upregulates this pathway, resulting in accelerated plasma triglyceride clearance (41).
Chemical uncouplers have been used for weight loss for over 75 years. While compounds like dinitrophenol can result in impressive weight loss, they have also been associated with complications, including fatal hyperthermia. While this has understandably resulted in caution, it is possible that more specific uncouplers or targeted activators of UCP1 or other futile cycles may provide metabolic benefit with an acceptable safety profile. In that regard, a recent publication (42) described the development of controlled-release mitochondrial protonophores that can apparently safely uncouple in the liver, and in rats these compounds can improve insulin resistance, diabetes, hypertriglyceridemia, and hepatic steatosis. Brown and beige fat activity is robustly induced by thyroid hormone and catecholamines, but side effects would almost certainly limit their use. The β3-selective adrenergic agonist CL 316,243 potently activates brown and beige fat in rodents (43). Human trials have been disappointing, perhaps due to limited oral bioavailability. Mirabegron is a clinically approved β3-adrenergic agonist that is used to treat overactive bladder. This drug was recently shown to activate human brown fat, which may renew interest in this pathway (44).
Since cold robustly activates brown and beige fat, some investigators have suggested that moderate cold exposure could be used as a therapeutic approach. At least in healthy subjects, daily exposure to 19°C for 2 h was sufficient to activate brown fat, resulting in weight loss (45). In another small human study (46), alternating cycles of cold exposure were shown to result in improved insulin sensitivity. While these studies suggest the potential of cold as a treatment modality, our societal preference for thermal comfort may make this unfeasible as a broad approach.
Thiazolidinediones (TZDs) cause browning of the white fat and may do so by stabilizing PRDM16, resulting in its accumulation (47). However, these drugs have been associated with weight gain, fluid retention, and cardiovascular events, which have diminished enthusiasm for their use. Deacetylation of PPARγ by SirT1 promotes browning of the white fat, suggesting the possibility of developing compounds that selectively modulate this pathway (48). Other regulators of interest include bone morphogenetic protein 7 (BMP7) and BMP8b, cyclooxygenase-2 (COX-2), and natriuretic peptides, though their pleiotropic actions might limit their potential as drug targets. Fibroblast growth factor 21 (FGF21) is being examined as a therapeutic agent, but it also has diverse actions and may be associated with bone loss.
Translating these discoveries into drug candidates will ultimately require a more complete understanding of brown/beige fat biology in humans. Mouse studies suggest that PRDM16 is a key control point in brown and beige fat phenotype. Data suggest that PRDM16 has substantial regulation posttranslationally. An increasing understanding of the relevant modifications of PRDM16 and the upstream signaling pathways involved may provide new opportunities for therapeutic targeting of brown and beige fat.
Article Information
Acknowledgments. The authors thank laboratory members for helpful discussions. Due to limitations in space, it was not possible to discuss all of the important contributions made in this area.
Funding. Support for the studies described here was provided by the American Heart Association (P.C.), National Institutes of Health grant DK-31495 (B.M.S.), and The JPB Foundation (B.M.S.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.C. and B.M.S. wrote the manuscript. B.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
References
- 1.Grady JM, Enquist BJ, Dettweiler-Robinson E, Wright NA, Smith FA. Dinosaur physiology. Evidence for mesothermy in dinosaurs. Science 2014;344:1268–1272 [DOI] [PubMed] [Google Scholar]
- 2.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013;5:1196–1203 [DOI] [PubMed] [Google Scholar]
- 3.Jacobsson A, Stadler U, Glotzer MA, Kozak LP. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem 1985;260:16250–16254 [PubMed] [Google Scholar]
- 4.da Costa DC, Landeira-Fernandez AM. Thermogenic activity of the Ca2+-ATPase from blue marlin heater organ: regulation by KCl and temperature. Am J Physiol Regul Integr Comp Physiol 2009;297:R1460–R1468 [DOI] [PubMed] [Google Scholar]
- 5.Enerbäck S, Jacobsson A, Simpson EM, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997;387:90–94 [DOI] [PubMed] [Google Scholar]
- 6.Meyer CW, Willershäuser M, Jastroch M, et al. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol 2010;299:R1396–R1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurov JB, Huang M, White LS, et al. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc Natl Acad Sci U S A 2007;104:5680–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013;27:234–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye L, Wu J, Cohen P, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 2013;110:12480–12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007;104:4401–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010;48:424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowell BB, S-Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366:740–742 [DOI] [PubMed] [Google Scholar]
- 15.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–209 [DOI] [PubMed] [Google Scholar]
- 16.Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001;106:563–573 [DOI] [PubMed] [Google Scholar]
- 17.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007;6:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms MJ, Ishibashi J, Wang W, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab 2014;19:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994;79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 22.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–839 [DOI] [PubMed] [Google Scholar]
- 23.Kleiner S, Mepani RJ, Laznik D, et al. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci U S A 2012;109:9635–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 2006;3:333–341 [DOI] [PubMed] [Google Scholar]
- 25.Kajimura S, Seale P, Tomaru T, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 2008;22:1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009;460:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajakumari S, Wu J, Ishibashi J, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab 2013;17:562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013;504:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villanueva CJ, Waki H, Godio C, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab 2011;13:413–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villanueva CJ, Vergnes L, Wang J, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARγ specifies lipid storage versus thermogenic gene programs. Cell Metab 2013;17:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 33.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 34.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009;23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 35.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013;19:631–634 [DOI] [PubMed] [Google Scholar]
- 37.Shinoda K, Luijten IHN, Hasegawa Y, et al. . Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015;21:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013;17:798–805 [DOI] [PubMed] [Google Scholar]
- 39.Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013;19:635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab 2014;20:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011;17:200–205 [DOI] [PubMed] [Google Scholar]
- 42.Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 2015;347:1253–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998;102:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cypess AM, Weiner LS, Roberts-Toler C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 2015;21:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee P, Smith S, Linderman J, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014;63:3686–3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012;15:395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012;150:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]