Abstract
Despite treatment advances, diabetic eye disease remains a leading cause of visual acuity (VA) loss worldwide. No methods to prospectively determine which patients will gain or lose vision exist, limiting individualized risk assessment and management. We investigated whether noninvasive, readily obtainable spectral domain optical coherence tomography parameters were correlated with VA in eyes with current or resolved center-involved diabetic macular edema (DME). Images were evaluated for disorganization of the retinal inner layers (DRIL), cysts, epiretinal membranes, microaneurysms, subretinal fluid, and outer layer disruption/reflectivity. DRIL affecting ≥50% of the 1-mm central retinal zone was associated with worse VA in all eyes, eyes with current edema, and eyes with resolved edema. Furthermore, early 4-month change in DRIL extent predicted VA change from baseline to 1 year. These data suggest that DRIL is a robust predictor of VA in eyes with present or previous DME and more highly correlated with VA than other widely used measures, such as retinal thickness. If further studies confirm DRIL as a predictive biomarker of future VA, physicians would gain a new tool of substantial clinical and investigative importance that could significantly change the approach to ophthalmic counseling and therapeutic management in patients with diabetes.
Introduction
Ocular complications from diabetes are a leading etiology of vision loss in most developed countries. A common cause of vision loss in patients with diabetes is diabetic macular edema (DME), which is characterized by retinal vasculature leakage resulting in edema of the macula. DME affects nearly 30% of all individuals with diabetes for ≥20 years (1). Despite advances in treatment, there are no reliable methods to determine which individuals with DME will gain or lose vision. This deficit limits individualized risk assessment, therapeutic management decisions, and speed of new drug development.
The identification of reliable markers of current and future visual acuity (VA) in patients with DME is of major medical importance (2). Although modestly associated with visual outcomes, commonly evaluated parameters such as glycemic control, retinopathy severity, presence of DME, and extent of retinal thickness are inadequate predictors of current or future VA in an individual eye (2). The ability to prospectively determine which individuals will or will not respond to available treatments would assist therapeutic selection and reduce the impact of invasive and burdensome treatment programs for patients in whom these programs are likely to be ineffective. The ability to predict when visual recovery will be limited despite ongoing therapies would assist in patient counseling and early visual disability support. The identification of predictive biomarkers of diabetic eye disease would also speed initial identification of lead drug candidates to move into subsequent clinical trials evaluating long-term disease progression. Finally, identification of retinal changes that precede VA loss might lend greater clarity to our understanding of the pathobiology of diabetic retinopathy and provide novel insights into pathogenic mechanisms possibly shared with other microvascular complications, such as nephropathy and neuropathy.
It has long been appreciated that neural changes occur in the diabetic retina that both accompany and precede clinically visible vascular lesions. In the 1960s, histopathology studies documented ganglion cell loss and neuronal degeneration in the diabetic eye (3,4). Additional studies explored electrophysiologic (5) and functional visual abnormalities (6) that reflect early diabetic neural pathology. Because retinal neural pathways conduct visual signals to the brain for processing, damage to neural pathways might adversely affect vision, and structural features of the neural retina, therefore, might provide useful biomarkers of vision in diabetes.
Spectral domain optical coherence tomography (SDOCT) provides high-resolution, noninvasive visualization of the neural retinal architecture and is widely used to quantitatively evaluate retinal thickness. Although optical coherence tomography (OCT)–derived central retinal thickness is commonly used in the evaluation and management of DME, center point thickness explains no more than 27% of the variation in VA (7). Paradoxical improvement in vision when retinal thickness is increasing occurs in up to 17% of patients, and conversely, up to 26% may have worse vision associated with reductions in retinal thickness. Other OCT-derived parameters evaluated as possible surrogates for VA are integrity of the external limiting membrane (ELM) (8,9), integrity of the ellipsoid zone (EZ) (formerly described as the inner segment/outer segment photoreceptor junction) (10), thickness of the photoreceptor outer segment (11), status of the cone outer segment tips (COST) (9), presence of hyperreflective foci (12–15), and subretinal fluid (16). However, none of these measures has been consistently demonstrated to account for visual outcomes in patients with DME.
In this study, we evaluated SDOCT parameters to identify a novel surrogate marker we call disorganization of the retinal inner layers (DRIL). DRIL is highly correlated with VA in eyes with current DME, but more importantly, it reflects VA in eyes where previous DME has resolved. Furthermore, DRIL correlates well with vision, even in eyes in which retinal thickness and VA behave paradoxically. Longitudinal data demonstrate that early changes in foveal DRIL extent are associated with long-term visual outcomes over 1 year in eyes with baseline DME.
Research Design and Methods
This single-site, cross-sectional study was conducted at the Beetham Eye Institute of the Joslin Diabetes Center, a tertiary referral center for diabetes care. The research adhered to the tenets of the Declaration of Helsinki and was approved by the Joslin Diabetes Center Institutional Review Board. Chart review was performed for all individuals who had undergone Spectralis SDOCT imaging (Heidelberg Engineering, Heidelberg, Germany) between November 2011 and June 2012. All images were acquired by a study-certified imager who obtained 49 B-scans spanning a 20 × 20° frame centered on the fovea with 16 automatic real-time mean/scan in high-resolution mode.
Eligible study participants were aged ≥18 years with a history of diabetes (type 1 or 2). Eligible eyes had either current center-involved DME or a history of center-involved DME that had resolved. Eyes were classified as having current DME if they had SDOCT central subfield thickness (CST) ≥320 μm for men or ≥305 μm for women, thresholds established by previous Diabetic Retinopathy Clinical Research Network studies (17). Eyes were classified as having resolved DME if current SDOCT CST was <320 μm for men or <305 μm for women, with documented prior SDOCT CST ≥320 μm for men or ≥305 μm for women, time domain OCT CST ≥250 μm, or previous documentation of center-involved DME necessitating macular laser treatment. Of the 37 eyes with resolved DME, 33 had prior OCT measurements demonstrating edema, and 4 eyes without prior OCT had documented center-involved DME for which macular laser treatment had been performed. Study eyes were categorized as having good VA if the logarithm of the minimum angle of resolution (logMAR) VA was ≤0.14 (Snellen equivalent ≥20/25) and reduced VA if the logMAR VA was >0.14 (Snellen equivalent <20/25).
Exclusion criteria were significant media opacity precluding adequate image quality, cataract surgery within the previous 6 months, and history of uveitis, retinal vein occlusion, or other nondiabetic retinal pathology that might substantially affect VA. Data were recorded on standardized forms and included diabetic retinopathy and DME severity determined by dilated funduscopic examination, age, sex, duration of diabetes, and hemoglobin A1c (HbA1c) recorded from the most recent testing that preceded OCT imaging.
Image Analysis
For each study eye, the central 1-mm diameter (foveal) area and four macular quadrants were evaluated (Supplementary Fig. 1). In the foveal area, seven B-scans were analyzed (including three B-scans above and three below the scan passing through the foveal center). A 1-mm-diameter central overlay centered on the foveal depression on the central scan was placed on each B-scan to define the foveal area. For the quadrants, we assessed seven B-scans beginning 13 line scans above and 13 below the central scan line. The retinal areas nasal and temporal to the central 1-mm section were evaluated, yielding superotemporal, superonasal, inferonasal, and inferotemporal quadrants.
Image analysis was performed by two experienced graders masked to all clinically relevant information, including VA and edema status. The following lesions were evaluated for presence, location, and extent in the foveal area and four macular quadrants: intraretinal cysts (small cysts <250 μm, medium cysts ≥250 μm but <500 μm, and large cysts ≥500 μm in diameter), hyperreflective foci consistent with hard exudates, microaneurysms, microaneurysm ring sign (18), subretinal fluid, and epiretinal membranes (ERMs). Only the foveal area was evaluated for DRIL (see next section). Within the foveal area, COST visibility was also assessed as present or absent. If the COST were visible throughout the 1-mm foveal area, the COST layer was graded as intact. If the COST layer was disrupted within this area, it was graded as not intact. Disruption of the ELM and EZ was graded as present or absent in a manner similar to COST. Reflectivity of the ELM and EZ was assessed by drawing a segmented line across the 1-mm zone overlaying the layer of interest and calculating the average pixel intensity of that line (ImageJ software; National Institutes of Health, Bethesda, MD). Both raw and normalized reflectivities were generated, with normalized reflectivity calculated relative to the reflectivity of the retinal pigment epithelium layer.
Disorganization of the Retinal Inner Layers
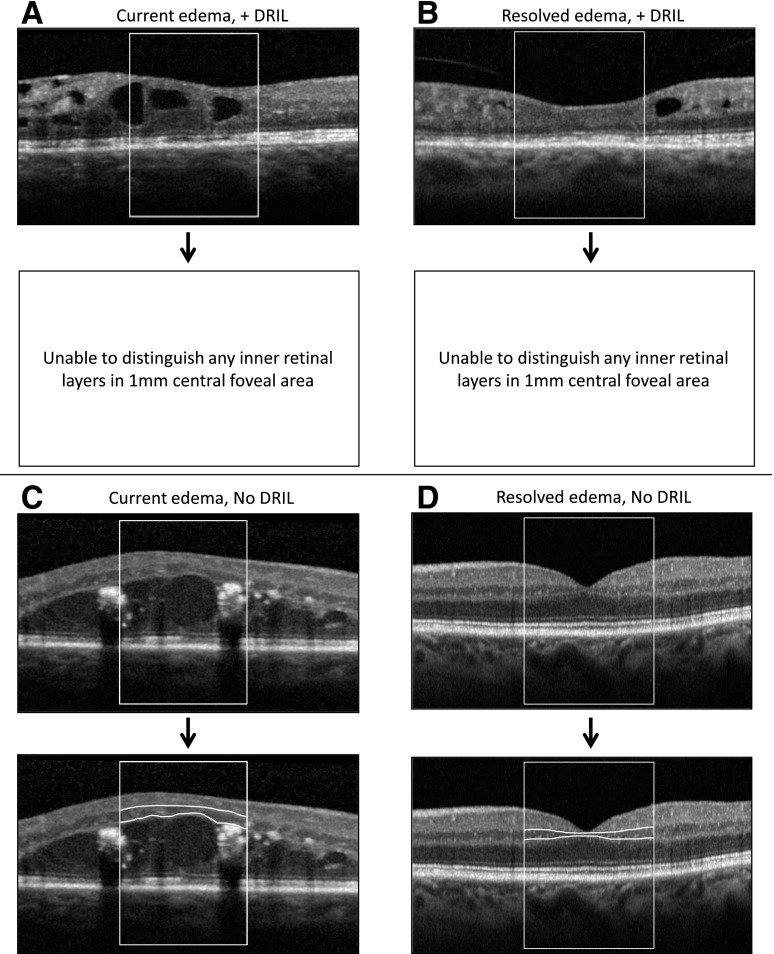
For each of the central seven B-scans, the central 1-mm-wide foveal area was assessed by image graders for whether the boundaries of the ganglion cell/inner plexiform layer complex (evaluated as a single layer complex due to difficulty in distinguishing between these two layers in scans of normal retinas without pathology), inner nuclear layer, and outer plexiform layer could be identified and demarcated. Foveal DRIL was defined as the inability to distinguish boundaries between any two of these inner retinal layers in >50% of the foveal 1-mm zone (Fig. 1). Foveal DRIL could be present with or without center-involved DME, and loss of the normal macular contour did not constitute DRIL by itself unless there was also concurrent loss of retinal layer boundaries. For example, intraretinal cysts were commonly seen in the outer nuclear layer, resulting in overall retinal thickening; however, if the inner retinal layers could still be demarcated, then DRIL was not considered present (Fig. 1C). Reproducibility of DRIL grading was assessed by three independent, masked graders, each evaluating 63 images. Agreement for presence of DRIL affecting ≥50% of the foveal 1-mm zone was 87.3–90.4%, with pairwise κ-statistics ranging from 0.69 to 0.77, indicating substantial agreement (L.B. Aiello, J. Lammer, M.M. Lin, unpublished data). Pearson correlation coefficients for agreement when DRIL extent was graded as a continuous variable ranged from 0.80 to 0.86.
Figure 1.
Representative OCT images showing combinations of presence or absence of DRIL and DME. The central 1-mm area for analysis is enclosed in the box. Lower images of each set show segmentation of inner retinal layers, with white lines demarcating, wherever evident, the boundaries between inner plexiform and inner nuclear as well as between inner nuclear and outer plexiform retinal layers. A: Completely indistinguishable inner retinal layers in the presence of edema. B: Completely indistinguishable inner retinal layers after complete resolution of prior DME. C: Fully distinguishable inner retinal layers despite central DME. D: Fully distinguishable inner retinal layers after complete resolution of prior DME.
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC). The relationship of VA to baseline variables and each OCT parameter of interest were evaluated by nonparametric Wilcoxon rank sum analysis. To adjust for possible confounding, statistically significant parameters in these analyses were included in logistic multivariate regression models to determine the strength of the relationship between each variable and VA. These multivariable models used repeated measures to account for correlations between eyes from individual participants in the study. P < 0.05 was considered significant in these exploratory analyses.
Results
Eighty eyes of 58 individuals were studied. Participant characteristics are reported in Table 1. Participants had mean ± SD age of 62 ± 12 years, diabetes duration of 24 ± 11 years, and most recent HbA1c of 7.7 ± 1.3% (mean HbA1c 56 mmol/mol); 33% were female, and 36% had type 1 diabetes; and racial and ethnic distribution was 79% Caucasian, 10% African American, 5% Hispanic, 2% Asian, and 4% other.
Table 1.
Cross-sectional study population and ocular characteristics
| Study population characteristics | |
| Participants (n) | 58 |
| Age (years) | 61.7 ± 12.3 |
| Sex | |
| Male | 67.2 (39) |
| Female | 32.8 (19) |
| Race/ethnicity | |
| White | 79.3 (46) |
| African American | 10.3 (6) |
| Hispanic/Latino | 5.2 (3) |
| Asian | 1.7 (1) |
| Other/unspecified | 3.5 (2) |
| Type of diabetes | |
| Type 1 | 36.2 (21) |
| Type 2 | 63.8 (37) |
| Duration of diabetes (years) | 23.5 ± 11.2 |
| HbA1c [% (mmol/mol)]* | 7.7 ± 1.3 (61) |
| Ocular characteristics | |
| Eyes (n) | 80 |
| VA (logMAR) | 0.24 ± 0.26 |
| Eye | |
| Right | 43.8 (35) |
| Left | 56.3 (45) |
| DR severity | |
| No apparent retinopathy | 0 (0) |
| Mild NPDR | 27.5 (22) |
| Moderate NPDR | 26.3 (21) |
| Severe NPDR | 20.0 (16) |
| Proliferative DR | 26.3 (21) |
| Groups | |
| Current edema,† good VA‡ | 27.5 (22) |
| Current edema, reduced VA** | 26.3 (21) |
| Resolved edema,‡‡ good VA | 21.3 (17) |
| Resolved edema, reduced VA | 25.0 (20) |
Data are mean ± SD or % (n) unless otherwise indicated. DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy.
*n = 51.
†Current edema: central subfield thickness ≥305 μm for women or ≥320 μm for men (17).
‡Good VA: Snellen equivalent of logMAR VA ≥20/25.
**Reduced VA: Snellen equivalent of logMAR VA <20/25.
‡‡Resolved edema: history of central subfield thickness ≥305 μm for women or ≥320 μm for men with current central subfield thickness below these thresholds.
For study eyes, mean logMAR VA was 0.24 ± 0.26 (∼20/32−2). Forty-three (53.7%) eyes had current edema, and 37 (46.3%) had resolved edema. Of those eyes with current edema, 22 (51.2%) had good VA ≥20/25, and 21 (48.8%) had reduced VA <20/25. Of those eyes with resolved edema, 17 (45.9%) had good VA, and 20 (54.1%) had reduced VA. The participant and ocular characteristics of each subgroup are shown in Table 2.
Table 2.
Study population and ocular characteristics by edema and VA status
| Resolved edema* |
Current edema† |
|||
|---|---|---|---|---|
| Reduced VA‡ (n = 20) | Good VA** (n = 17) | Reduced VA (n = 21) | Good VA (n = 22) | |
| Study population characteristics | ||||
| Age (years) | 60.5 ± 11.0 | 57.4 ± 15.2 | 64.1 ± 8.9 | 60.4 ± 13.8 |
| Sex | ||||
| Male | 50.0 (10) | 88.2 (15) | 57.1 (12) | 59.1 (13) |
| Female | 50.0 (10) | 11.8 (2) | 42.9 (9) | 40.9 (9) |
| Race/ethnicity | ||||
| White | 75.0 (15) | 70.6 (12) | 85.7 (18) | 86.4 (19) |
| African American | 15.0 (3) | 5.9 (1) | 9.5 (2) | 9.1 (2) |
| Hispanic/Latino | 5.0 (1) | 17.7 (3) | 0.0 (0) | 0.0 (0) |
| Asian | 5.0 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Other/unspecified | 0.0 (0) | 5.9 (1) | 4.8 (1) | 4.6 (1) |
| Type of diabetes | ||||
| Type 1 | 50.0 (10) | 41.2 (7) | 23.8 (5) | 36.4 (8) |
| Type 2 | 50.0 (10) | 58.8 (10) | 76.2 (16) | 63.6 (14) |
| Duration of diabetes (years) | 27.4 ± 12.2 | 23.1 ± 10.9 | 24.4 ± 12.7 | 20.4 ± 8.3 |
| HbA1c [% (mean mmol/mol)] | 7.9 ± 1.3 (63)†† | 7.5 ± 1.0 (58)‡‡ | 7.7 ± 1.4 (61)*** | 8.0 ± 1.4 (64)††† |
| Ocular characteristics | ||||
| VA (logMAR) | 0.44 ± 0.19 | 0.02 ± 0.08 | 0.42 ± 0.24 | 0.05 ± 0.07 |
| Eye | ||||
| Right (OD) | 45.0 (9) | 64.7 (11) | 28.6 (6) | 40.9 (9) |
| Left (OS) | 55.0 (11) | 35.3 (6) | 71.4 (15) | 59.1 (13) |
| Diabetic retinopathy severity | ||||
| No DR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mild NPDR | 20.0 (4) | 35.3 (6) | 23.8 (5) | 31.8 (7) |
| Moderate NPDR | 10.0 (2) | 29.4 (5) | 38.1 (8) | 27.3 (6) |
| Severe NPDR | 30.0 (6) | 29.4 (5) | 4.8 (1) | 18.2 (4) |
| Proliferative DR | 40.0 (8) | 5.9 (1) | 33.3 (7) | 22.7 (5) |
Data are mean ± SD or % (n) unless otherwise indicated. DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; OD, oculus dexter; OS, oculus sinister.
*Resolved edema: history of central subfield thickness ≥305 μm for women or ≥320 μm for men with current central subfield thickness below these thresholds (17).
†Current edema: central subfield thickness ≥305 μm for women or ≥320 μm for men (17).
‡Reduced VA: Snellen equivalent of logMAR VA <20/25.
**Good VA: Snellen equivalent of logMAR VA ≥20/25.
††n = 15.
‡‡n = 15.
***n = 19.
†††n = 18.
Within the seven B-scans graded per eye in all eyes, unadjusted bivariate analyses demonstrated a statistically significant relationship between better VA and fewer foveal scans with DRIL (mean ± SD 1.7 ± 2.4 vs. 5.8 ± 2.2 scans, P < 0.0001), any cysts (3.6 ± 2.5 vs. 4.9 ± 2.9 scans, P = 0.011), large cysts (1.2 ± 2.0 vs. 2.9 ± 2.7 scans, P = 0.006), and ERMs (0.3 ± 1.3 vs. 1.8 ± 2.9 scans, P = 0.005) as well as less EZ disruption per scan (43.3 ± 106.0 vs. 128.6 ± 193.4 μm, P = 0.003) (Table 3). No relationship was found between VA and HbA1c, CST, small- or medium-sized cysts, hyperreflective foci consistent with hard exudates, subretinal fluid, the presence of microaneurysms with or without a ring sign, extent of ELM disruption, COST visibility, or EZ or ELM reflectivity.
Table 3.
Unadjusted analyses of relationship between VA and SDOCT parameters in all study eyes
| Reduced VA* | Good VA† | P value | |
|---|---|---|---|
| CST | 373.7 ± 118.2 | 325.0 ± 55.5 | 0.3245 |
| Foveal DRIL (# scans of 7) | 5.8 ± 2.2 | 1.7 ± 2.4 | <0.0001 |
| Foveal cysts (# scans of 7) | |||
| Any | 4.9 ± 2.9 | 3.6 ± 2.5 | 0.0111 |
| Small | 2.1 ± 2.1 | 1.8 ± 2.0 | 0.4918 |
| Medium | 1.3 ± 1.5 | 1.1 ± 1.5 | 0.6629 |
| Large | 2.9 ± 2.7 | 1.2 ± 2.0 | 0.0057 |
| Foveal ERMs (# scans of 7) | 1.8 ± 2.9 | 0.3 ± 1.3 | 0.0047 |
| Foveal hard exudates (# scans of 7) | 5.6 ± 5.6 | 3.1 ± 3.5 | 0.0999 |
| Foveal subretinal fluid (# scans of 7) | 0.3 ± 0.9 | 0.0 ± 0.0 | 0.0517 |
| Foveal microaneurysms (# scans of 7) | 0.5 ± 1.1 | 0.3 ± 0.6 | 0.7271 |
| Any ring sign (# scans of 7) | 0.8 ± 0.8 | 1.0 ± 0.8 | 0.5159 |
| Foveal ELM disruption (μm/B-scan) | 74.6 ± 181.0 | 26.2 ± 83.1 | 0.0632 |
| Foveal ELM reflectivity (arbitrary units/B-scan) | 94.8 ± 15.5 | 92.2 ± 13.6 | 0.4032 |
| COST visible (# scans of 7) | 3.2 ± 3.5 | 4.2 ± 3.9 | 0.2666 |
| Foveal EZ disruption (μm/B-scan) | 128.6 ± 193.4 | 43.3 ± 106.0 | 0.0031 |
| Foveal EZ reflectivity (arbitrary units/B-scan) | 158.0 ± 30.6 | 166.8 ± 27.1 | 0.1220 |
| Superotemporal cysts (# scans of 7) | 1.9 ± 2.6 | 0.5 ± 1.6 | 0.0018 |
Data are mean ± SD. Boldface indicates significance at P < 0.05.
*Reduced VA: Snellen equivalent of logMAR VA <20/25.
†Good VA: Snellen equivalent of logMAR VA ≥20/25 or better.
In eyes with current edema, unadjusted analyses demonstrated a statistically significant relationship between good VA and thinner CST (356.4 ± 50.7 vs. 464.1 ± 97.7 μm, P < 0.001). Good VA was also significantly related to fewer foveal scans with DRIL (2.6 ± 2.8 vs. 6.2 ± 1.8 scans, P < 0.001), any cysts (4.5 ± 2.5 vs. 6.0 ± 1.9 scans, P = 0.014), large cysts (2.0 ± 2.4 vs. 4.0 ± 2.7 scans, P = 0.023), ERMs (0.5 ± 1.7 vs. 2.8 ± 3.3 scans, P = 0.008), and hard exudates (3.3 ± 3.4 vs. 7.9 ± 6.4 units, P = 0.026) as well as to foveal scans with higher EZ reflectivity (166.0 ± 25.0 vs. 148.0 ± 30.3 units, P = 0.020) (Table 4). In eyes with resolved edema, good VA was significantly related in unadjusted analyses only to fewer foveal scans with DRIL (0.5 ± 0.9 vs. 5.4 ± 2.6 scans, P < 0.0001), large cysts (0.2 ± 0.5 vs. 1.7 ± 2.1 scans, P = 0.023), and foveal scans with less EZ disruption (42.7 ± 139.2 vs. 109.7 ± 153.1 μm per scan, P = 0.018) (Table 5).
Table 4.
Unadjusted analyses of relationship between VA and SDOCT parameters in eyes with current edema
| Reduced VA* | Good VA† | P value | |
|---|---|---|---|
| CST | 464.1 ± 97.7 | 356.4 ± 50.7 | 0.0004 |
| Foveal DRIL (# scans of 7) | 6.2 ± 1.8 | 2.6 ± 2.8 | 0.0002 |
| Foveal cysts (# scans of 7) | |||
| Any | 6.0 ± 1.9 | 4.5 ± 2.5 | 0.0135 |
| Small | 2.3 ± 2.0 | 1.9 ± 2.1 | 0.3903 |
| Medium | 1.4 ± 1.8 | 1.5 ± 1.7 | 0.8192 |
| Large | 4.0 ± 2.7 | 2.0 ± 2.4 | 0.0230 |
| Foveal ERMs (# scans of 7) | 2.8 ± 3.3 | 0.5 ± 1.7 | 0.0081 |
| Foveal hard exudates (# scans of 7) | 7.9 ± 6.4 | 3.3 ± 3.4 | 0.0264 |
| Foveal subretinal fluid (# scans of 7) | 0.5 ± 1.3 | 0.0 ± 0.0 | 0.0810 |
| Foveal microaneurysms (# scans of 7) | 0.6 ± 1.4 | 0.4 ± 0.7 | 0.9243 |
| Any ring sign (# scans of 7) | 0.8 ± 0.8 | 1.2 ± 1.0 | 0.6257 |
| Foveal ELM disruption (μm/B-scan) | 91.2 ± 226.3 | 13.7 ± 46.9 | 0.2065 |
| Foveal ELM reflectivity (arbitrary units/B-scan) | 89.3 ± 17.3 | 91.0 ± 14.1 | 0.5950 |
| COST visible (# scans of 7) | 3.9 ± 3.5 | 4.5 ± 3.4 | 0.5475 |
| Foveal EZ disruption (μm/B-scan) | 146.6 ± 227.8 | 43.8 ± 74.8 | 0.0603 |
| Foveal EZ reflectivity (arbitrary units/B-scan) | 148.0 ± 30.3 | 166.0 ± 25.0 | 0.0202 |
| Superotemporal cysts (# scans of 7) | 2.2 ± 2.9 | 0.4 ± 1.5 | 0.0066 |
Data are mean ± SD. Boldface indicates significance at P < 0.05.
*Reduced VA: Snellen equivalent of logMAR VA <20/25.
†Good VA: Snellen equivalent of logMAR VA ≥20/25.
Table 5.
Unadjusted analyses of relationship between VA and SDOCT parameters in eyes with resolved edema
| Reduced VA* | Good VA† | P value | |
|---|---|---|---|
| CST | 278.7 ± 28.5 | 284.2 ± 28.7 | 0.5067 |
| Foveal DRIL (# scans of 7) | 5.4 ± 2.6 | 0.5 ± 0.9 | <0.0001 |
| Foveal cysts (# scans of 7) | |||
| Any | 3.7 ± 3.3 | 2.4 ± 2.2 | 0.2808 |
| Small | 2.0 ± 2.2 | 1.8 ± 2.0 | 0.9252 |
| Medium | 1.1 ± 1.3 | 0.6 ± 0.9 | 0.2238 |
| Large | 1.7 ± 2.1 | 0.2 ± 0.5 | 0.0229 |
| Foveal ERMs (# scans of 7) | 0.9 ± 2.2 | 0.1 ± 0.5 | 0.2263 |
| Foveal hard exudates (# scans of 7) | 3.2 ± 3.5 | 2.8 ± 3.7 | 0.8521 |
| Foveal subretinal fluid (# scans of 7) | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.3913 |
| Foveal microaneurysms (# scans of 7) | 0.5 ± 0.8 | 0.2 ± 0.4 | 0.5219 |
| Any ring sign (# scans of 7) | 0.7 ± 0.8 | 0.8 ± 0.5 | 0.9177 |
| Foveal ELM disruption (μm/B-scan) | 57.2 ± 120.2 | 42.4 ± 114.1 | 0.2147 |
| Foveal ELM reflectivity (arbitrary units/B-scan) | 100.3 ± 11.3 | 93.9 ± 13.2 | 0.1627 |
| COST visible (# scans of 7) | 2.5 ± 3.4 | 3.9 ± 4.6 | 0.3781 |
| Foveal EZ disruption (μm/B-scan) | 109.7 ± 153.1 | 42.7 ± 139.2 | 0.0175 |
| Foveal EZ reflectivity (arbitrary units/B-scan) | 168.0 ± 28.1 | 167.8 ± 30.5 | 0.9498 |
| Superotemporal cysts (# scans of 7) | 1.6 ± 2.4 | 0.6 ± 1.7 | 0.1072 |
Data are mean ± SD. Boldface indicates significance at P < 0.05.
*Reduced VA: Snellen equivalent of logMAR VA <20/25.
†Good VA: Snellen equivalent of logMAR VA ≥20/25.
Bivariate analyses were performed for eyes with apparent paradoxical findings, namely current edema and good VA compared with eyes with resolved edema and reduced VA. Eyes with good VA despite current edema were more likely to have fewer scans with foveal DRIL and lower ELM reflectivity than eyes with resolved edema and reduced VA (2.6 ± 2.8 vs. 5.4 ± 2.6 scans, P = 0.005; 91.0 ± 14.1 vs. 100.3 ± 11.3 units, P = 0.029) (Supplementary Table 1).
The only variable consistently related to VA outcomes in the macular quadrants was the presence of superotemporal cysts. In unadjusted bivariate analyses including all eyes, superotemporal cysts were more frequent in eyes with reduced VA compared with eyes with good VA (0.5 ± 1.6 vs. 1.9 ± 2.6 scans, P = 0.002). This relationship was also statistically significant for eyes with current edema (0.4 ± 1.5 vs. 2.2 ± 2.9 scans, P = 0.007) and in eyes with current edema and good VA compared with resolved edema and reduced VA (0.4 ± 1.5 vs. 1.6 ± 2.4 scans, P = 0.021).
Multivariable Analyses
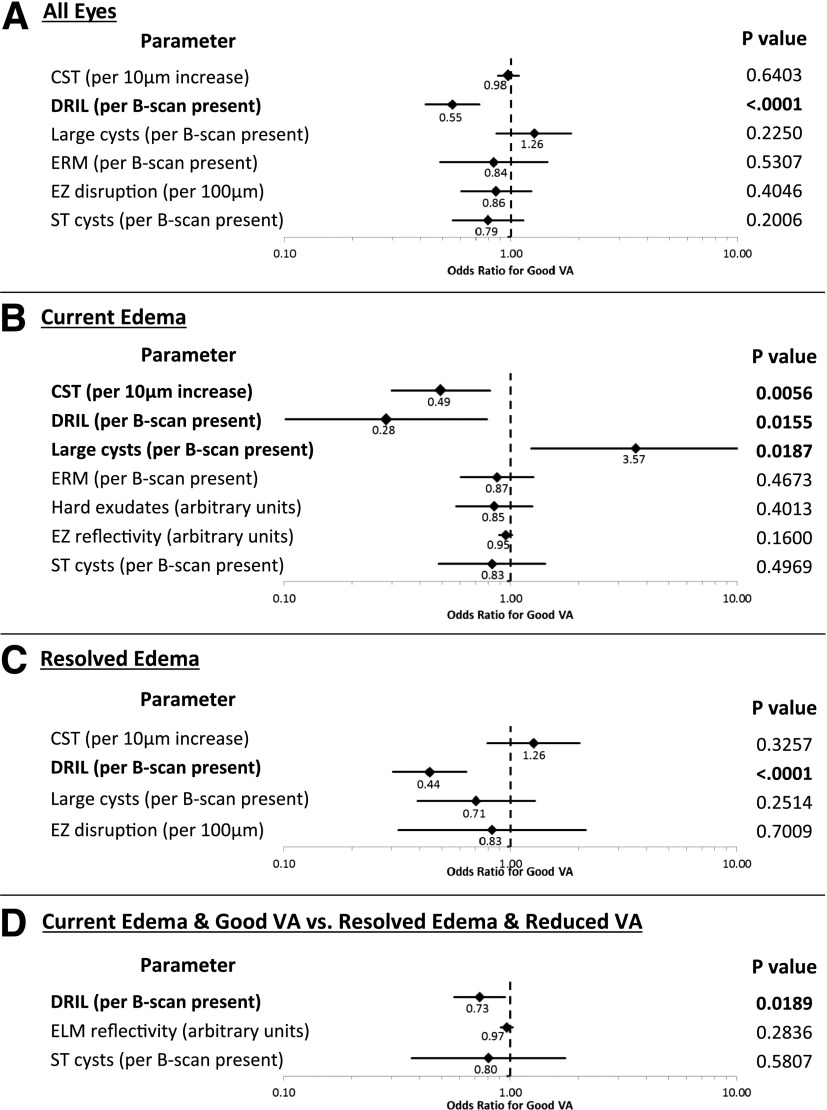
Multivariable modeling adjusting for all characteristics that were statistically significant for VA in unadjusted analyses as well as for correlations between eyes from the same individual was performed. CST was included in all multivariable models as a parameter of a priori interest with regard to VA but not in analyses comparing eyes with current edema and good VA versus resolved edema and reduced VA because CST was used to define these comparison groups. After multivariable analysis, only foveal DRIL remained associated with VA outcomes in all eyes (odds ratio [95% CI] of good VA per B-scan with DRIL present: 0.55 [0.42, 0.72], P < 0.0001) (Fig. 2A). In eyes with current edema (Fig. 2B), multivariate analysis adjusting for all previously statistically significant parameters also showed significant association of VA with presence of foveal DRIL (0.28 [0.10, 0.79] per B-scan, P = 0.016), the presence of large foveal cysts (3.57 [1.24, 10.28] per B-scan, P = 0.019), and, as expected, CST (0.49 [0.30, 0.81] per 10-μm increase, P = 0.006). In eyes with resolved edema (Fig. 2C), VA was significantly related only to foveal DRIL (0.44 [0.30, 0.64], P < 0.0001). Similarly, in eyes with current edema and good VA versus resolved edema and reduced VA, multivariate analysis yielded a statistically significant difference only in foveal DRIL (0.73 [0.57, 0.95], P = 0.019).
Figure 2.
Forest plots demonstrating odds ratios and 95% CIs for good VA from multivariable models comparing eyes with good vs. reduced VA for all eyes (A), eyes with current edema (B), eyes with resolved edema (C), and eyes with current edema and good VA vs. resolved edema and reduced VA (D). All variables significantly associated with VA in the unadjusted analyses were included in creating each model. In addition, CST was included in models 2A, 2B, and 2C as a variable of a priori interest in relation to VA but not in model 2D because the comparison groups for this analysis were defined based on retinal thickness. Boldface indicates significance at P < 0.05. ST, superotemporal.
The odds ratios and 95% CIs for DRIL and additional SDOCT variables included in the models are presented in Fig. 2. Even after adjusting for these variables, the presence of foveal DRIL remained significantly associated with worse VA in all eyes, eyes with current edema, and eyes with resolved edema and when comparing eyes with current edema and good VA to eyes with resolved edema and reduced VA. These DRIL findings remained statistically significant even when the small subgroup of eyes (n = 4) with resolved edema but without baseline OCT scans was excluded from the analyses. In all analyses, when backward elimination was used to create pared-down models, only DRIL was consistently associated with VA at the 99% CI.
Of eyes evaluated for this study, 42.5% (n = 34) had previously received anti-vascular endothelial growth factor (VEGF) treatment, and 10% (8) had undergone macular laser treatment before the study SDOCT and VA assessments. No eyes had undergone previous therapy with intravitreous steroid. When the presence or absence of prior anti-VEGF treatment was controlled for as a possible confounder in the multivariable models presented in Fig. 2, DRIL extent remained significantly and consistently associated with VA in each model (all eyes: P = 0.0002; eyes with current edema only: P = 0.0311; eyes with resolved edema only: P = 0.0002; comparing eyes with current edema and good VA vs. resolved edema and reduced VA: P = 0.0271). When eyes were stratified into subgroups that had received or not received prior intravitreous anti-VEGF treatment, the relationship between DRIL and VA remained statistically significant in each group (point estimate [95% CI] for no prior anti-VEGF: −0.62 [−0.95, −0.30], P = 0.0001; with prior anti-VEGF: −0.55 [−1.00, −0.10], P = 0.0171).
DRIL as a Predictive Biomarker of Visual Outcomes in Eyes With DME
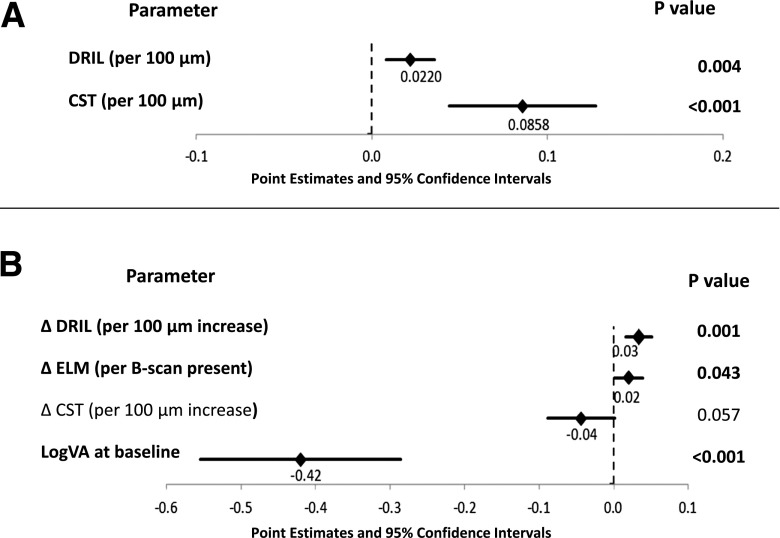
To evaluate the potential for DRIL as a predictive biomarker of long-term VA, longitudinal data were obtained from 96 patients with baseline DME on Spectralis SDOCT imaging who had VA assessed at baseline and 4 and 12 months and repeat SDOCT scans at 4 and 12 months. Baseline demographics of this cohort are presented in Supplementary Table 2. Rather than grading the presence or absence of DRIL affecting ≥50% of the central 1-mm zone, graders used a customized Matlab program for SDOCT analysis to manually ascertain the horizontal extent of DRIL in micrometers that affected the 1-mm-wide area. SDOCT grading was otherwise performed as done for the cross-sectional study. In this cohort of eyes, unadjusted bivariate analyses demonstrated that worse VA at baseline was significantly associated with greater DRIL extent (point estimate [95% CI] 0.03 [0.02, 0.05], P = <0.0001), increasing CST (0.02 [0.01, 0.23], P = <0.0001), greater cyst area (0.004 [0.0002, 0.005], P = 0.001), greater extent of ELM (0.04 [0.01, 0.06], P = 0.004) or EZ (0.04 [0.02, 0.07], P = 0.001) disruption, and decreased visibility of the COST (−0.02 [−0.03, −0.007], P = 0.004). Backward elimination resulted in a multivariable model in which only DRIL (P = 0.004) and CST (P = 0.0004) remained significantly related to baseline VA (Fig. 3).
Figure 3.
Forest plots demonstrating point estimates and 95% CIs for association between baseline VA and baseline average DRIL extent and CST (A) and association between change in VA between baseline and month 12 and change in OCT parameters between baseline and month 4 (B). Baseline VA is included in this multivariable model as a variable of a priori interest in relation to change in VA over time. Boldface indicates significance at P < 0.05.
When changes in SDOCT parameters over 4 months were assessed, unadjusted analyses revealed a significant relationship between VA worsening from baseline to 12 months with increasing DRIL extent (0.04 [0.02, 0.05], P = 0.0001), increasing CST (0.01 [0.005, 0.02], P = 0.01), and increasing ELM disruption (0.03 [0.006, 0.05], P = 0.02). In a multivariable model including these parameters and baseline VA as an a priori variable of interest, 4-month changes in DRIL extent (P = 0.001) and ELM disruption (P = 0.04) but not in CST (P = 0.06) were still significantly associated with 12-month VA (Fig. 3). On average, within the central foveal 1-mm zone, an early 4-month increase in DRIL of 300 μm was associated with a worsening of VA by one line over the 12-month follow-up period.
Discussion
DRIL is a novel, noninvasive parameter that appears to be highly correlated with VA in eyes with either current or resolved DME. Early changes in DRIL extent are also predictive of longer-term VA outcomes in eyes with baseline DME. Although other OCT features evaluated in this study have previously been evaluated in association with visual outcomes in patients with diabetes, DRIL was more robustly and consistently associated with VA than any of these parameters, including central retinal thickness. DRIL was also more highly associated with VA than current glycemic status. These findings are important because a reliable biomarker of VA in patients with DME has yet to be firmly established. If foveal DRIL is validated in future studies as a marker of VA outcomes, its assessment would allow more effective estimation of VA potential, thus directly affecting patient counseling, disease management, and subject selection for DME clinical trials.
A strong association between foveal DRIL and VA in eyes with DME is plausible because the inner plexiform, inner nuclear, and outer plexiform layers contain anatomic structures critical for transmission of visual data from photoreceptors to retinal ganglion cells. The inability to distinguish boundaries between these layers on high-resolution SDOCT imaging may suggest destruction or disorganization of some axons and nuclei of amacrine, bipolar, and/or horizontal cells located in these areas. Pelosini et al. (19) suggested that if edema increases retinal thickness beyond an elastic limit, bipolar axons can snap and cause loss of visual information signaling from photoreceptors to ganglion cells. This bipolar cell destruction may not be completely reversible, potentially accounting for some eyes in which VA does not recover after resolution of DME. Future studies may more fully elucidate histologic changes that accompany the appearance and disappearance of DRIL on SDOCT imaging.
Backward elimination models revealed that DRIL was the only SDOCT parameter consistently associated at the 99% CI with VA in all eyes, eyes with current edema, and eyes with resolved edema. In the current study, retinal anatomic SDOCT parameters other than foveal DRIL, such as large intraretinal cysts, were related to VA in some but not all subgroups of eyes. Other studies have also noted the association between cyst burden and worse VA (20). Soliman et al. (21) found that cystoid spaces, especially in the inner nuclear layer, were associated with worse visual outcome after macular grid laser photocoagulation for DME. Murakami et al. (22) demonstrated that areas beneath outer plexiform layer cystoid spaces had longer spans of disrupted EZ and ELM on OCT. In the current study, the presence of intraretinal cysts was associated with worse VA in unadjusted analyses. However, this relationship was only significant in eyes with current edema in multivariable models when adjusted for the presence of foveal DRIL. These results suggest that VA loss in the presence of retinal cysts may often be primarily occurring from DRIL within the central 1-mm foveal area.
In contrast to other studies, we did not find significant associations between VA and COST visibility or hyperreflective foci suggestive of hard exudates or consistent associations between VA and ELM or EZ disruption or reflectivity. It is possible that a modest association exists between these variables and VA that the current study was underpowered to detect. However, it is clear that in these cohorts, the relationship between foveal DRIL and VA is strong and maintained across multiple analyses despite the relatively modest sample size.
Limitations of this study include the fact that these data are retrospective. However, the study was specifically designed to incorporate approaches that reduce or eliminate some of the classic data acquisition issues generally inherent in retrospective trials. A standardized, highly customized electronic medical record was used for all participants to provide uniform data entry and prevent data acquisition inconsistencies. In addition, all Beetham Eye Institute VA and OCT technicians are study certified for ETDRS (Early Treatment of Diabetic Retinopathy Study) refraction, ETDRS VA measurement, and DRCR.net (Diabetic Retinopathy Clinical Research Network) OCT acquisition, thus maximizing the consistency and accuracy of this key clinically derived information. Patients included in this study had various durations of DME and had undergone various DME treatments, which were not adjusted for in the analyses. Nevertheless, a heterogeneous mix of patients may improve the generalizability of the results from this pilot study. Despite the relatively small sample size, the associations of VA with DRIL were strong enough to be highly statistically significant, even after multivariable regression with several covariates.
These data support the use of DRIL as a surrogate marker of VA and potentially as a predictive biomarker for future VA outcomes in eyes with DME. The results are consistent with another study recently published by our group that assessed shorter-term visual outcomes over 8 months in eyes with baseline DME (23). Of eyes included in the current 12-month study, 52% had baseline and 4-month SDOCT scans that were evaluated in the 8-month study, whereas 48% of the participants in the current study were new. Despite the added participants and longer evaluation time, the associations between DRIL and VA were highly similar between these two studies. However, because these findings are derived from exploratory, single-center, pilot studies, validating the use of DRIL as a predictive biomarker of VA in future larger, multicenter longitudinal studies that prospectively collect data on the evolution of visual outcomes in eyes with DME is critical. Given the association of DRIL with future VA in this and other studies (23), clinicians might use early changes in DRIL extent as a prognostic marker for vision in untreated eyes with DME as well as a predictive marker to assess visual potential in eyes undergoing treatment, thus helping them to identify optimal timing for onset, modification, termination, and reinstitution of DME therapy. In addition, the ability to automate SDOCT grading would greatly facilitate the use of algorithms that use DRIL to predict vision in both clinical and research settings for patients with diabetes worldwide.
In conclusion, DRIL within the 1-mm foveal area identified using noninvasive, high-resolution retinal imaging appears to be a robust, highly correlated, and easily obtained surrogate and potentially predictive marker of VA in patients with current or resolved DME. The results suggest that the association of central DRIL with VA is more consistent than that observed with other previously measured OCT parameters, making foveal DRIL a potentially highly valuable tool of substantial clinical and investigative importance that could significantly change the approach to ophthalmic counseling and therapeutic management in patients with diabetes.
Article Information
Funding. Support was provided by the Eleanor Chesterman Beatson Childcare Ambassador Program Foundation Grant (Tucson, AZ), JDRF International (New York, NY), JDRF 17-2011-359 and 3-SRA-2014-264-M-R, Massachusetts Lions Eye Research Fund (New Bedford, MA), U.S. Department of Health and Human Services National Eye Institute 1-R01-EY-024702-01, and Harvard Medical School Scholars in Medicine Office (Boston, MA).
None of the sources of funding support had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Duality of Interest. J.K.S. has received research support in kind from Optovue. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.K.S. contributed to the study design, analyzed data, and wrote the manuscript. S.H.R. and A.Z.S. contributed to the study design, researched data, and reviewed and edited the manuscript. J.L. and M.M.L. researched data and reviewed and edited the manuscript. S.G.P. and L.B.A. researched data. P.S.S. contributed to the general discussion and reviewed and edited the manuscript. L.P.A. contributed to the study design and general discussion and reviewed and edited the manuscript. J.K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0782/-/DC1.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91:1464–1474 [DOI] [PubMed] [Google Scholar]
- 2.Lasker/IRRF Initiative for Innovation in Vision Science. Summary report on diabetic retinopathy [article online]. 2012. Available from http://www.laskerfoundation.org/programs/irrf.htm. Accessed 13 May 2013
- 3.Wolter JR. Diabetic retinopathy. Am J Ophthalmol 1961;51:1123–1141 [DOI] [PubMed] [Google Scholar]
- 4.Bloodworth JM, Jr. Diabetic retinopathy. Diabetes 1962;11:1–22 [PubMed] [Google Scholar]
- 5.Harrison WW, Bearse MA Jr, Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 2011;52:772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br J Ophthalmol 2012;96:699–703 [DOI] [PubMed] [Google Scholar]
- 7.Browning DJ, Glassman AR, Aiello LP, et al.; Diabetic Retinopathy Clinical Research Network . Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114:525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alasil T, Keane PA, Updike JF, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology 2010;117:2379–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Miyamoto N, Ishida K, Kurimoto Y. Association between external limiting membrane status and visual acuity in diabetic macular oedema. Br J Ophthalmol 2013;97:228–232 [DOI] [PubMed] [Google Scholar]
- 10.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 2010;150:63–67, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forooghian F, Stetson PF, Meyer SA, et al. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina 2010;30:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uji A, Murakami T, Nishijima K, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol 2012;153:710–717, e1 [DOI] [PubMed] [Google Scholar]
- 13.Bolz M, Schmidt-Erfurth U, Deák G, Mylonas G, Kriechbaum K, Scholda C; Diabetic Retinopathy Research Group Vienna . Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 2009;116:914–920 [DOI] [PubMed] [Google Scholar]
- 14.Deák GG, Bolz M, Kriechbaum K, et al.; Diabetic Retinopathy Research Group Vienna . Effect of retinal photocoagulation on intraretinal lipid exudates in diabetic macular edema documented by optical coherence tomography. Ophthalmology 2010;117:773–779 [DOI] [PubMed] [Google Scholar]
- 15.Framme C, Schweizer P, Imesch M, Wolf S, Wolf-Schnurrbusch U. Behavior of SD-OCT-detected hyperreflective foci in the retina of anti-VEGF-treated patients with diabetic macular edema. Invest Ophthalmol Vis Sci 2012;53:5814–5818 [DOI] [PubMed] [Google Scholar]
- 16.Deák GG, Bolz M, Ritter M, Prager S, Benesch T, Schmidt-Erfurth U; Diabetic Retinopathy Research Group Vienna . A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Vis Sci 2010;51:6710–6714 [DOI] [PubMed] [Google Scholar]
- 17.Chalam KV, Bressler SB, Edwards AR, et al.; Diabetic Retinopathy Clinical Research Network . Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:8154–8161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horii T, Murakami T, Nishijima K, Sakamoto A, Ota M, Yoshimura N. Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am J Ophthalmol 2010;150:840–848 [DOI] [PubMed] [Google Scholar]
- 19.Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci 2011;52:2741–2748 [DOI] [PubMed] [Google Scholar]
- 20.Ghazi NG, Scruggs RT, Batchelet AR, et al. Diabetic macular edema. Ophthalmology 2012;119:e1. [DOI] [PubMed] [Google Scholar]
- 21.Soliman W, Sander B, Soliman KA, Yehya S, Rahamn MS, Larsen M. The predictive value of optical coherence tomography after grid laser photocoagulation for diffuse diabetic macular oedema. Acta Ophthalmol (Copenh) 2008;86:284–291 [DOI] [PubMed] [Google Scholar]
- 22.Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol 2011;151:310–317 [DOI] [PubMed] [Google Scholar]
- 23.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol 2014;132:1309–1316 [DOI] [PubMed] [Google Scholar]