EXECUTIVE SUMMARY
The document is based on consensus among the experts and best available evidence pertaining to Indian population and is meant for practice in India.
All postcholecystectomy gallbladder specimens should be opened and examined carefully by the operating surgeon and be sent for histopathological examination.
All “incidental” gall bladder cancers (GBCs) picked up on histopathological examination should have an expert opinion.
Evaluation of a patient with early GBC should include essential tests: A computed tomography (CT) scan (multi-detector or helical) of the abdomen and pelvis for staging with a CT chest or chest X-ray, and complete blood counts, renal and liver function tests. magnetic resonance imaging/positron emission tomography (PET)-CT are not recommended for all patients.
For early stage disease (up to Stage IVA), surgery is recommended. The need for adjuvant treatment would be guided by the histopathological analysis of the resected specimen.
Patients with Stage IVB/metastatic disease must be assessed for palliative e.g. endoscopic or radiological intervention, chemotherapy versus best supportive care on an individual basis. These patients do not require extensive workup outside of a clinical trial setting.
There is an urgent need for multicenter trials from India covering various aspects of epidemiology (viz., identification of population at high-risk, organized follow-up), clinical management (viz., bile spill during surgery, excision of all port sites, adjuvant/neoadjuvant therapy) and basic research (viz., what causes GBC).
Keywords: Gall bladder cancer, ICMR, guidelines, treatmemt
INCIDENCE
Gall bladder cancer is a common cancer in the northern and Northeastern States of India.[1] The six cancer registries of the Indian Council of Medical Research (ICMR) (1990-1996) show a 10 times lower incidence of GBC per 1 00 000 in South India compared with the North, the age-adjusted incidence rate for females being 0.8 in Chennai in the South and 8.9 in Delhi in the North. GBC ranks among the first 10 cancers in the ICMR registries (2006-2008) of Delhi, Dibrugarh, Kolkata, Bhopal and Mumbai. The incidence of GBC increases after the age of 45 years and is maximum at the age of 65 years. The 5-year survival rate for surgically resected patients was reported to be 5%.[1,2]
PURPOSE
Several international consensus guidelines are available for the management of GBC.[3,4] However, it is not feasible to apply these guidelines to the Indian population owing to differences in the incidence of the disease in different parts of India, as compared to the rest of the world, socio-economic factors, and availability of resources. Therefore, it is essential to analyze the evidence pertaining to GBC from India and the rest of the world with an aim to formulate reliable, evidence-based guidelines that could be applicable to Indian patients bearing in mind the socio-cultural diversity, the distribution of resources and the availability and accessibility to health-care. Taking into consideration peripheral oncology centers, regional cancer centers and tertiary cancer centers in major cities, the set of recommendations includes two categories, viz.
Desirable/ideal: Tests and treatments that may not be available at all centers but the centers should aspire to have them in the near future; and
Essential: The minimum should be offered to all patients by all the centers treating cancer patients.
DIAGNOSIS AND STAGING
Most patients with GBC present in advanced stages as the symptoms are nonspecific and many present after cholecystectomy with GBC diagnosed on histopathology. Clinicians must be aware of the symptoms and signs of GBC such as constant pain in right hypochondrium, and unexplained weight loss. Patients with advanced GBC can present with jaundice, gastric outlet obstruction, cachexia, anorexia, ascites, left supraclavicular lymphadenopathy and hard lump in right hypochondrium. These should prompt the clinician to investigate the patient to confirm or rule out GBC.[4,5]
Evaluation of a patient presenting with a GBC should be aimed at accurate staging and assessing resectability of the disease.[3,4]
Diagnosis of gall bladder cancer
Histology or cytology is essential for the diagnosis of GBC, this could be in the form of fine needle aspiration cytology or biopsy or histopathology from the resected specimen. However, in an apparently resectable gallbladder mass on a good quality contrast-enhanced CT (CECT), preoperative confirmation by a FNAC is not warranted. Tumor markers such as serum carcinoembryonic antigen and carbohydrate antigen 19-9 are not recommended in the routine management of GBC (Level 3B) but they may be useful during patient follow-up if they are raised at the time of initial diagnosis (Level 5B).[6]
Staging of gall bladder cancer
Routine investigations to be performed include complete blood counts, renal function tests and liver function tests and coagulation profile in a jaundiced patient. A CECT scan (multi-detector or helical) of the abdomen and pelvis for staging with a CT chest or chest X-ray is regarded as essential for the appropriate staging of GBC.[7,8] Optional investigations available but not routinely recommended except in specific clinical scenarios include a magnetic resonance cholangio pancreaticography, endoscopic retrograde cholangio pancreaticography/percutaneous transhepatic cholangiography if a therapeutic intervention (biliary stenting) is planned.[9]
Staging laparoscopy is recommended for all patients undergoing primary surgery with intention to resect and may also be of use in patients with a diagnosis of incidental GBC prior to going ahead with radical re-surgery.[10] While performance of 2-(18F) fluoro 2-deoxyD-glucose PET or PET-CT is not routinely recommended, it may be used if available to stage patients prior to radical surgery.[11]
In patients with metastatic disease, it is essential to have histological confirmation prior to commencing palliative chemotherapy. Extensive investigations are discouraged in patients presenting with features suggestive of metastatic GBC such as poor performance status, ascites left supraclavicular lymph node enlargement and multiple liver metastases.
Staging should be performed as per the American Joint Committee on Cancer staging manual[7] (7th edition, updated in 2010) and patients should be assigned a TNM stage.[12]
TREATMENT PLAN
Treatment of each patient should ideally be undertaken by a multidisciplinary team based on the stage of GBC. The intent of treatment is “curative” for patients with Stage I to IVA and “palliative” for patients with Stage IVB disease. In patients with locally advanced disease, surgical resection can be considered following neoadjuvant therapy only if a complete/R0 (microscopically negative margins) resection is feasible.[13,14,15] Participation in clinical trials should be encouraged.
Early gall bladder cancer
Early GBC may be discovered as a surprise finding on cholecystectomy specimen after histopathological examination (incidental GBC) of a cholecystectomy specimen or this may be suspected preoperatively (suspected GBC) on imaging - wall thickening, polypoidal lesion, porcelain gallbladder or per-operatively.[13,15]
Incidental gall bladder cancer
Re-resection is advised in patients with pT1b and beyond GBC with the aim to resect all possible residual disease.[16,17,18] In patients undergoing re-resection for incidental GBC, the chances of residual disease in liver and regional lymph nodes increase with increasing T stage; positive cystic duct margin predicts residual disease in common bile duct (CBD).[17]
Further surgery following detection of incidental GBC should be undertaken as soon as possible. The interval between cholecystectomy and subsequent radical resection are not significant factors influencing survival although it is difficult to interpret this due to selection bias.[18]
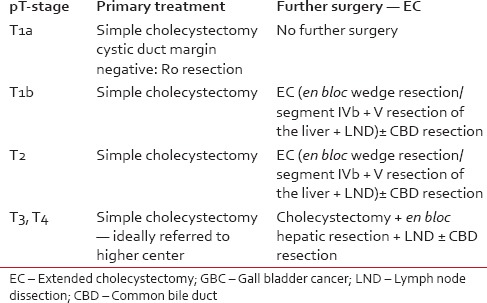
Management of pT1b disease varies from simple cholecystectomy to extended cholecystectomy (EC). EC should include cholecystectomy with en bloc limited hepatic resection (2-3 cm wedge resection or segment IVb + V) and lymphadenectomy with or without bile duct excision. Lymph node dissection should include portal, gastrohepatic and retroduodenal regions. A minimum lymph node count of 6 or more is considered optimum.[19,20] Patients pT1b disease treated with simple cholecystectomy have a recurrence rate of in 60%, hence EC is treatment of choice.[20] Table 1 shows recommendations as per the pathological stage.[20,21,22]
Table 1.
Surgical recommendations based on pathology of an incidental GBC
Suspected gall bladder cancer
If a resectable GBC is detected on-table and expertise for EC is not available, no attempt should be made to take an open biopsy from the gallbladder. An omental/lymph node biopsy may however be taken and the patient should be referred to a specialist with a detailed note of intra-operative findings.
Issues in management of early gall bladder cancer
Laparoscopic port site excision
Evidence in support of routine excision of all port-sites during re-resection for incidental GBC is lacking. Routine port site resection as part of radical re-surgery, has not been shown to improve outcomes, hence is not recommended.[23,24]
Extent of liver resection
Factors influencing extent of liver resection are:
Location of tumor in gallbladder,
Morphological pattern of liver involvement,
Possibility of achieving R0 resection.
The extent of liver resection varies from nonanatomical 2-3 cm wedge resection, to anatomical parenchyma sparing Segment IVb/V resection up to extended right hepatectomy. For tumors located at the fundus or body of the gallbladder, with no or minimal liver infiltration, a nonanatomical wedge or Segment IVb and V resection usually suffices. For tumors with extensive liver infiltration or tumors infiltrating the hepatic hilum and right portal pedicle, an extended right hepatectomy is required for complete resection. This may necessitate preoperative biliary decompression and portal vein embolization to ensure adequate liver remnant volume. No randomized trials exist on the subject stating a superiority of one over the other and most follow center specific practice. Extensive liver resections improve results of advanced GBC at a cost of high morbidity (50%) and mortality (18%).[25,26]
Lymphadenectomy
Lymphadenectomy is an integral component of radical surgery for GBC. The extent of local resection and lymph node dissection is based on T stage and evaluation of important nodes by frozen section. A positive interaortocaval node upon frozen section at laparotomy indicates metastatic and incurable GBC. A “standard” lymph node dissection in which the cystic (12c), peri-choledochal (12b), hilar (12h), proper hepatic artery (12a), peri-portal (12p), postero-superior pancreatico-duodenal (13a) and common hepatic artery lymph nodes are removed with skeletonization of hepatic artery, portal vein and bile duct is recommended.[22,27]
Bile duct resection
Extra hepatic bile duct may be involved by direct infiltration by GBC or by permeation from lymph nodes of the hepatoduodenal ligament and was reported as 54.2%, 67.7% respectively in one study with only 15% free of invasion in advanced GBC.[28] Curative resection is possible in 75% patients without bile duct infiltration but in only <30% with bile duct infiltration.[29] Thus, routine bile duct resection does not give further survival advantage. CBD resection is recommended in tumors of gallbladder neck or cystic duct.
In resections for IGBC when the cystic duct margin is positive or on occasions where it is difficult to distinguish between tumor and scar in the high-density lipoprotein (HDL).
Bulky nodal disease in the HDL where CBD excision facilitates more complete nodal clearance.
Adjacent organ resection
For patients with involvement of the adjacent colon, antro-pyloric region of stomach or duodenum, complete resection may be achieved by addition of segmental colectomy, distal gastrectomy or sleeve resection of the first part of duodenum as required. Addition of a pancreaticoduodenectomy, however, should be considered very carefully and selectively because of the high morbidity and mortality associated with it.[30]
Role of chemotherapy and chemoradiotherapy in nonmetastatic gall bladder cancer
Data on adjuvant therapy in GBC is very limited. Most of the recommendations represent extrapolation of studies on patients with advanced GBC and biliary tract cancer. The two large retrospective studies have shown no benefit of adjuvant chemotherapy.[31,32] However, the number of patients who received adjuvant therapy in these studies was small with a heterogeneous group of patients. A phase III trial from Japan evaluated the role of adjuvant chemotherapy in pancreatobiliary cancers and showed that the 5-year overall survival in GBC patients was significantly better in the chemotherapy group (26.0%) compared with the control group (14.4%) (P = 0.0367).[33]
In a retrospective study of 2325 patients by Mojica et al., adjuvant chemoradiation has shown a better median survival (14 months vs. 8 months; P < 0.0001) in the chemoradiation group and this benefit was even more in node positive patients.[34] A multivariate Cox proportional hazards model on SEER database of 4180 patients also showed better survival in patients with node positive and pT2 or higher GBC.[35] A recently published retrospective study from India on 32 patients who received adjuvant chemoradiotherapy after EC showed that adjuvant chemoradiotherapy, followed by adjuvant chemotherapy improves outcomes in patients with R1 resection and node-positive disease.[36]
At present no data from phase III trials is available on the best adjuvant therapy after R0 resection of GBC. Institutional policies vary from adjuvant gemcitabine/fluoropyrimidine based chemotherapy to fluoropyrimidine chemoradiotherapy. Given the poor prognosis of these patients, it can be recommended that for patients pT2 and above stages (Level 2A).
There is a report of the use of neoadjuvant chemotherapy from a tertiary center in 37 Indian patients with locally advanced GBC and has shown that 17 patients who were able to undergo (46%) R0 resection had a significantly better overall survival — median not reached versus 9.5 months and progression free survival of 25.8 versus 5.6 months respectively.[37] Participation in clinical trials is encouraged for the use of neoadjuvant therapy.
Metastatic gall bladder cancer
Role of chemotherapy in recurrent/metastatic gall bladder cancer
Several phase II trials have shown benefit with gemcitabine, cisplatin, oxaliplatin, capecitabine and 5-FU based chemotherapy in biliary tract cancer.[38,39,40,41] Pooled analysis of 104 trials involving 1368 patients revealed that gemcitabine combined with platinum based chemotherapy shows maximum benefit in advanced biliary tract cancer.[42]
The superiority of gemcitabine plus cisplatin over gemcitabine alone was shown in the multicenter ABC-02 trial, in which 410 patients with locally advanced (25%) or metastatic bile duct (n = 242), gallbladder (n = 148) or ampullary (n = 20) cancer were randomly assigned to six courses of cisplatin (25 mg/m2) followed by gemcitabine (1000 mg/m2) on days 1 and 8, every 21 days, or gemcitabine alone (1000 mg/m2 days 1, 8, 15, every 28 days).[43] At a median follow-up of 8.2 months, median overall survival was significantly greater with combination therapy (11.7 vs. 8.1 months).[43] A study on 210 Indian patients with advanced GBC treated at a tertiary center with gemcitabine-platinum based chemotherapy shows that the median progression free survival and overall survival of 5 and 10 months respectively is comparable to other studies. For the use of second-line therapy on progression (75 patients), this study showed response rates of 16.1%, clinical benefit rate of 34% and an overall survival benefit of 6 months reinforcing the fact that though response rates are low for second line therapy, there is survival benefit and this should be considered in select patients.[44]
Gemcitabine/cisplatin combination has not been directly compared to other gemcitabine combinations (e.g., with capecitabine, leucovorin-modulated 5-FU, or oxaliplatin) or capecitabine plus oxaliplatin in randomized trials but this is the current standard of care in patients with advanced GBC (Level 1A). There is currently no standard recommendation for second line therapy for patients with advanced GBC but there are clinical trials ongoing comparing FOLFOX regimen to best supportive care alone (UK National Cancer Research Institute ABC-06 clinical trial NCT 01926236, opened in January 2014).
Role of radiotherapy in metastatic gall bladder cancer
Radiotherapy may be useful in the metastatic setting of GBC in those patients who develop painful bony metastases.
Role of targeted therapy
The French BINGO trial randomly assigned 101 patients to receive gemcitabine plus oxaliplatin with or without cetuximab and showed that the addition of cetuximab to chemotherapy did not enhance the activity of chemotherapy in patients with advanced biliary cancer.[45] Currently, there is no evidence of efficacy of targeted agents like cetuximab, bevacizumab, small TKIs or multi kinase inhibitors in GBC. Hence, these should be offered only as a part of well-designed clinical trials.
FOLLOW-UP AND REHABILITATION
Patients should be encouraged to maintain lead a healthy lifestyle and abstain from tobacco and alcohol. The aim of follow-up is to detect recurrences early as well as to assess any complication due to surgery/radiotherapy. Follow-up is done every 6 months for the first 2 years with each visit comprising of clinical examination (including history and physical examination). There is no robust data to support aggressive surveillance postresection. Patients may be followed up by imaging. Re-staging according to initial workup should be considered in the event of disease relapse or progression.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Nandakumar A, Gupta PC, Gangadharan P, Visweswara RN, Parkin DM. Geographic pathology revisited: Development of an atlas of cancer in India. Int J Cancer. 2005;116:740–54. doi: 10.1002/ijc.21109. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Registry Programme, Indian Council of Medical Research: Three Year Report of Population Based Cancer Registries; 2009-2011. ICMR, Three Year Report of the PBCRs: 2006-2008. 2008. [Last accessed on 2015 May 6]. Available from: http://www.pbcrindia.org/
- 3.Eckel F, Brunner T, Jelic S. ESMO Guidelines Working Group. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi40–4. doi: 10.1093/annonc/mdr375. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers. Version 2. 2014. [Last accessed 2015 May 8]. Available from: http://www.nccn.org.
- 5.Regimbeau JM, Fuks D, Bachellier P, Le Treut YP, Pruvot FR, Navarro F, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur J Surg Oncol. 2011;37:505–12. doi: 10.1016/j.ejso.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett DL, Fong Y. Tumours of the gall bladder. In: Blumgart LH, Fong Y, editors. Surgery of the Liver and Biliary Tract. 3rd ed. London: WB Saunders; 2000. pp. 993–1015. [Google Scholar]
- 7.Ohtani T, Shirai Y, Tsukada K, Hatakeyama K, Muto T. Carcinoma of the gallbladder: CT evaluation of lymphatic spread. Radiology. 1993;189:875–80. doi: 10.1148/radiology.189.3.8234719. [DOI] [PubMed] [Google Scholar]
- 8.Efremidis SC, Vougiouklis N, Zafiriadou E, Sofianou F, Sbarounis C, Fardellas Y, et al. Pathways of lymph node involvement in upper abdominal malignancies: Evaluation with high-resolution CT. Eur Radiol. 1999;9:868–74. doi: 10.1007/s003300050757. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, et al. Preoperative evaluation of gallbladder carcinoma: Efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16:676–84. doi: 10.1002/jmri.10212. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer — An analysis of 409 patients: A prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258:318–23. doi: 10.1097/SLA.0b013e318271497e. [DOI] [PubMed] [Google Scholar]
- 11.Shukla PJ, Barreto SG, Arya S, Shrikhande SV, Hawaldar R, Purandare N, et al. Does PET-CT scan have a role prior to radical re-resection for incidental gallbladder cancer? HPB (Oxford) 2008;10:439–45. doi: 10.1080/13651820802286910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th ed. New York, NY: Springer; 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 13.de Aretxabala XA, Roa IS, Burgos LA, Araya JC, Villaseca MA, Silva JA. Curative resection in potentially resectable tumours of the gallbladder. Eur J Surg. 1997;163:419–26. [PubMed] [Google Scholar]
- 14.Miyazaki M, Itoh H, Ambiru S, Shimizu H, Togawa A, Gohchi E, et al. Radical surgery for advanced gallbladder carcinoma. Br J Surg. 1996;83:478–81. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- 15.Behari A, Sikora SS, Wagholikar GD, Kumar A, Saxena R, Kapoor VK. Longterm survival after extended resections in patients with gallbladder cancer. J Am Coll Surg. 2003;196:82–8. doi: 10.1016/s1072-7515(02)01611-3. [DOI] [PubMed] [Google Scholar]
- 16.Toyonaga T, Chijiiwa K, Nakano K, Noshiro H, Yamaguchi K, Sada M, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg. 2003;27:266–71. doi: 10.1007/s00268-002-6609-9. [DOI] [PubMed] [Google Scholar]
- 17.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: Implications for re-resection. J Gastrointest Surg. 2007;11:1478–86. doi: 10.1007/s11605-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 18.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: Comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–69. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito H, Ito K, D’Angelica M, Gonen M, Klimstra D, Allen P, et al. Accurate staging for gallbladder cancer: Implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–5. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 20.Wagholikar GD, Behari A, Krishnani N, Kumar A, Sikora SS, Saxena R, et al. Early gallbladder cancer. J Am Coll Surg. 2002;194:137–41. doi: 10.1016/s1072-7515(01)01136-x. [DOI] [PubMed] [Google Scholar]
- 21.Wakai T, Shirai Y, Yokoyama N, Ajioka Y, Watanabe H, Hatakeyama K. Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol. 2003;10:447–54. doi: 10.1245/aso.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Barreto SG, Pawar S, Shah S, Talole S, Goel M, Shrikhande SV. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg. 2014;38:484–9. doi: 10.1007/s00268-013-2266-4. [DOI] [PubMed] [Google Scholar]
- 23.Fuks D, Regimbeau JM, Pessaux P, Bachellier P, Raventos A, Mantion G, et al. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg. 2013;150:277–84. doi: 10.1016/j.jviscsurg.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg O, Kristoffersson A. Port site metastases from gallbladder cancer after laparoscopic cholecystectomy. Results of a Swedish survey and review of published reports. Eur J Surg. 1999;165:215–22. doi: 10.1080/110241599750007072. [DOI] [PubMed] [Google Scholar]
- 25.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–22. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi A, Miyakawa S, Ishihara S, Miyazaki M, Ohtsuka M, Shimizu H, et al. Gallbladder bed resection or hepatectomy of segments 4a and 5 for pT2 gallbladder carcinoma: Analysis of Japanese registration cases by the study group for biliary surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:518–24. doi: 10.1007/s00534-012-0584-9. [DOI] [PubMed] [Google Scholar]
- 27.Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: Location, count or ratio? J Gastrointest Surg. 2011;15:1017–25. doi: 10.1007/s11605-011-1528-4. [DOI] [PubMed] [Google Scholar]
- 28.Kaneoka Y, Yamaguchi A, Isogai M, Harada T, Suzuki M. Hepatoduodenal ligament invasion by gallbladder carcinoma: Histologic patterns and surgical recommendation. World J Surg. 2003;27:260–5. doi: 10.1007/s00268-002-6702-0. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012–7. doi: 10.1016/j.surg.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal AK, Mandal S, Singh S, Sakhuja P, Puri S. Gallbladder cancer with duodenal infiltration: Is it still resectable? J Gastrointest Surg. 2007;11:1722–7. doi: 10.1007/s11605-007-0320-y. [DOI] [PubMed] [Google Scholar]
- 31.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Oncol. 2008;98:485–9. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 32.Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: An analysis of 4,770 patients. Cancer. 2007;110:572–80. doi: 10.1002/cncr.22815. [DOI] [PubMed] [Google Scholar]
- 33.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma. A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma? Cancer. 2002;95:1685–95. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 34.Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol. 2007;96:8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]
- 35.Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR, Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–7. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal S, Gupta PK, Rastogi N, Lawrence A, Kumari N, Das KJ, et al. Outcomes of adjuvant chemoradiation and predictors of survival after extended cholecystectomy in gall bladder carcinoma: A single institution experience from an endemic region. J Gastrointest Cancer. 2015;46:48–53. doi: 10.1007/s12029-014-9676-x. [DOI] [PubMed] [Google Scholar]
- 37.Sirohi B, Mitra A, Jagannath P, Singh A, Ramadvar M, Kulkarni S, et al. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol. 2015;11:1501–9. doi: 10.2217/fon.14.308. [DOI] [PubMed] [Google Scholar]
- 38.Sirohi B, Singh A, Jagannath P, Shrikhande SV. Chemotherapy and targeted therapy for gall bladder cancer. Indian J Surg Oncol. 2014;5:134–41. doi: 10.1007/s13193-014-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A, Mohanti B, Raina V, Shukla N, Pal S, Dwary A, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol. 2010;65:497–502. doi: 10.1007/s00280-009-1055-0. [DOI] [PubMed] [Google Scholar]
- 40.Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516–20. doi: 10.1038/sj.bjc.6601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: A randomized controlled study. J Clin Oncol. 2010;28:4581–6. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
- 42.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 44.Sirohi B, Rastogi S, Singh A, Sheth V, Dawood S, Talole S, et al. Use of gemcitabine-platinum in Indian patients with advanced gall bladder cancer. Future Oncol. 2015;11:1191–200. doi: 10.2217/fon.14.295. [DOI] [PubMed] [Google Scholar]
- 45.Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–28. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


