Abstract
CONTEXT:
Adipose tissue-derived mesenchymal stem cells (AT-MSCs) are less invasive than bone marrow mesenchymal stem cells to obtain for cell therapy.
AIMS:
The aims of this study were to evaluate the germinal cells characteristics and repairs in seminiferous tubules of busulfan-induced azoospermic rats after AT-MSCs transplantation.
SETTINGS AND DESIGN:
Experimental case-control study.
MATERIALS AND METHODS:
In the present experimental study, donors AT-MSCs were isolated from subcutaneous adipose tissue of two Sprague-Dawley rats. The recipients (n = 5) were received two doses of 10 mg/kg of busulfan with 21 days interval to stop endogenous spermatogenesis. After induction of azoospermia by busulfan, rats were injected with the AT-MSCs into the efferent duct of right testes. After 60 days, the right testes were injected AT-MSCs were compared to left azoospermic testes. Five untreated male rats served as negative control.
STATISTICAL ANALYSIS USED:
Stereological indices were analyzed by one-way ANOVA and LSD post-hoc test. The spermatogenesis index was compared using Mann–Whitney U test.
RESULTS:
After stereological analyses, the seminiferous tubules treated with AT-MSCs had normal morphology. The untreated seminiferous tubules were empty. Spermatogenesis was observed in most cell-treated seminiferous tubules.
CONCLUSIONS:
The testis of busulfan-induced azoospermic rats accepted transplanted AT-MSCs. The transplanted AT-MSCs could induce spermatogenesis in seminiferous tubules of the rat.
KEY WORDS: Adipose tissue, azoospermia, busulfan, cell therapy, mesenchymal stem cell, rat
INTRODUCTION
Busulfan is a chemotherapeutic agent that most often use as low dose in a long time manner to treat chronic myeloid leukemia.[1] Before allogeneic transplantation of hematopoietic cell, busulfan is used as a myeloablative agent.[2] Despite its beneficial effects, cells with high division activities such as germ cells are more susceptible to busulfan side-effects. Spermatogonial stem cells of different species can be killed by busulfan,[3,4] but it has no effects on DNA synthesis. However, when busulfan intoxicates the cells in the G1 phase, inhibits the next mitosis.[5,6] It is clear that cytotoxic therapy such as certain chemotherapy drugs temporarily or even permanently influences spermatogenesis. For instance, busulfan-treated mice exhibited a marked increase in apoptosis,[7] a decrease in testis weight[7] and long-term morphological damage to sperm production.[8]
The negative effects of cytotoxic drugs which use in cancer therapy on spermatogenesis had important value[9] and more efforts were done and focused to diminish these side effects or finding new methods to compensate their harmful effects. Stem cell transplantation has become a new therapeutic strategy for restoring organ or tissue structure and function.[10,11,12] In 1994, spermatogonial stem cell transplantation was first reported by Brinster and Zimmermann[13] in the treatment of male infertility, and after that, this method were used for treatment of male infertility in other animals. Furthermore, using of Sertoli cell transplantation can restore spermatogenesis and fertility in infertile mice.[14]
Mesenchymal stem cells (MSCs) have been isolated from virtually every organ including fat, liver, spleen, pancreas, kidney, lung, muscle, and brain,[15] However, in recent years, bone marrow and adipose tissues MSCs (BM-MSCs and AT-MSCs, respectively) have greater potential for tissue repair, because of their ability to differentiate into bone, fat, cartilage, muscle, neurons, hepatocytes, insulin-producing cells and skin in the appropriate conditions in vivo.[12] Adipose tissue is less invasive and less expensive than bone marrow to obtain and contain more stem cell in compared to the aspirated bone marrow.[16] Therefore, the aims of this study were to evaluate the germinal cells characteristics and repairs in seminiferous tubules of busulfan-induced azoospermic rats after AT-MSCs transplantation.
MATERIALS AND METHODS
Animals
Twelve male Sprague-Dawley rats weighing 250–300 g were housed in the Laboratory Animal Center, Shiraz University of Medical Sciences, Shiraz, Iran in temperature-controlled rooms (20°C–22°C) under 12 h light/dark cycle. The rats were fed with standard commercial chow diet ad libitum. This work was approved by the Ethical Committee of Shiraz University of Medical Sciences (Shiraz, Iran). All efforts were made to minimize suffering during the experimental period.
Isolation and culture of rat adipose tissue-derived mesenchymal stem cells
Rats (n = 2) were anesthetized by ketamine (intraperitoneal injection, 100 mg/kg, Woerden, Netherlands) and xylazine (intraperitoneal injection, 7 mg/kg, Alfazyne, Woerden, Netherlands). The area between the shoulders on the back was shaved and disinfected. A 5 cm incision was made on the skin, and 1 g of subcutaneous adipose tissue was collected and then, the area was sutured, and the postsurgical region was disinfected. Tissues were washed 3 times with phosphate buffered saline (PBS; GIBCO cat. no. 18912-014) containing 1% penicillin and streptomycin (Sigma cat. no. P-4687 and S-1277, St. Louis, USA) to remove the blood cells. The tissue samples were chapped with a scalpel in small pieces and were digested in 0.2% collagenase Type II at 37°C on a shaker for 40 min.
It was then filtered and centrifuged (5 min, 1500 rpm) and the supernatant was removed, and the pelleted stromal connective tissue fraction was resuspended in 5 ml Dulbecco's Modified Eagles Medium (DMEM; Gibco cat. no. 12800-116). The suspension were transferred into 88% DMEM containing 10% fetal bovine serum (FBS; Gibco, cat. no. 10270-106), and 1% penicillin and streptomycin, and plated in 25 ml flasks for culture and expansion at 37°C in an incubator with 5% CO2 and saturated humidity. This initial passage of the primary cell culture was referred to as “passage 0”. After 48 h of incubation, the cultures were washed with PBS and kept in the stromal medium. Nonadherent cells with the medium were removed after 24 h and fresh medium was added again. The culture medium was changed every 3 days. When the cells reached 80–90% confluence, the cells were harvested using 0.25% trypsin (Gibco cat. no. 15090-046), counted and passaged into new flasks.
To obtain a sufficient number of cells, they were sub-cultured 2 times using standard methods of trypsinization. The cells achieved confluence after about 10 days. Cells were collected in the second passage and were counted using a hemocytometer. They were resuspended in frozen solution including 10% dimethyl sulfoxide (MP Bio cat. no. 196055) and 90% FBS, at a density of 2 × 106 viable cells/ml and were aliquoted into sterile plastic cryovials that were labeled with the sex, freezing serial number, and the date. The vials were kept at −20°C for 60 min and then they were transferred to −70°C for 24 h, and finally transferred to liquid nitrogen for long-term storage.
Before surgical treatment, the cryovials were removed from the liquid nitrogen and quickly thawed in a 37°C water bath. When the ice clump was almost thawed, 1 ml of cell culture medium (89% DMEM, 10% FBS, and 1% penicillin and streptomycin) was added, centrifuged at 1500 rpm. The AT-MSCs were transferred into a flask with gently blown into uniform single cell suspension, and cultured at 37°C and 5% CO2. AT-MSCs subcultured up to passage 4.
Adipose tissue-derived mesenchymal stem cells osteogenic and adipogenic differentiations
For in-vitro osteogenic differentiation, cells at 90% confluence were cultivated in DMEM, 15% FBS, 200 μM L-ascorbic acid, 10 mM glycerolphosphate, and 100 nM dexamethasone. The medium was changed twice a week for 3 weeks. After 21 days, osteogenic differentiation was evaluated using Alzarin Red staining. In brief, AT-MSCs cultures were fixed with 4% paraformaldehyde for 10 min. Then cells were incubated for 20 min at room temperature in 1% Alizarin Red S and 1% ammonium hydroxide. Following incubation, cultures were washed 4 times, 5 min each time with 1 ml dH2O replacing the water at each 5 min interval and air-dried. Alizarin Red S dye binds to calcium ions present in mineralized deposits resulting in a brilliant red staining (all reagents from Sigma-Aldrich, USA).
For adipogenic differentiation, cells at 90% confluence were cultivated in DMEM, 15% FBS, 0.2 mM L-glutamine, 100 μM L-ascorbic acid, 200 μM indomethacin, and 100 nM dexamethasone. The medium was changed twice a week for 3 weeks. After 21 days, adipogenic differentiation was evaluated using Oil Red O staining, which shows the presence of triglyceride deposits. In brief, for evaluating the generation of oil droplets, the En-MSCs were fixed in 10% formalin for 10 min at room temperature and washed twice with water. Oil Red O working solution was prepared by adding 6 mL of stock solution (0.5 g Oil Red in 100 mL isopropanol) to 4 mL distilled water, mixed, and filtered through Whatman filter paper. Next, Oil Red O stain was added and incubated for 1 h at room temperature. Finally, the cells were rinsed several times with water and observed under an inverted microscope (all reagents from Sigma-Aldrich, USA).
Characterization of adipose tissue-derived mesenchymal stem cells by reverse transcription-polymerase chain reaction
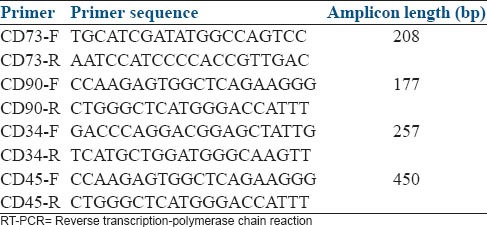
To quantify the expression of AT-MSCs specific markers (CD73 and CD90) and absence of hematopoietic stem cells specific markers (CD34 and CD45), polymerase chain reaction (PCR) was performed. First of all, total RNA was extracted using the Column RNA Isolation Kit (Denazist-Asia, Iran) in accordance with the manufacturer's instructions. Total RNA concentration was evaluated by spectrophotometer. After that, complementary DNA (cDNA) synthesis from RNA samples was done by using AccuPower Cycle Script RT PreMix Kit (Bioneer, Korea) according to the manufacturer's instructions. Briefly, 15 μL of total RNA was used for each reaction, and the volume had been reached up to 20 μL with the DEPC water. Twelve thermal cycles was performed with the following way: 30 s at 20°C for primer annealing, 4 min at 42°C for cDNA synthesis, 30 s at 55°C for melting secondary structure and cDNA synthesis and 5 min at 95°C in order to heat inactivation. In the third step, 1 μL of template (cDNA) was mixed with other reagents consisting of PCR buffer, MgCl2, H2O, dNTPs, Taq DNA polymerase, forward and reverse primers (CD73, CD90, CD34 and CD45) [Table 1]. Then, microfuge tubes containing 20 μL above mixing were put in Thermocycler (Eppendorf Mastercycler Gradient, Eppendorf, Hamburg, Germany). Thirty amplification cycles were run, consisting of 30 s denaturation at 95°C, 30 s annealing at 64°C (for CD45 and CD73) and 62°C (for CD34 and CD90) and 30 s extension at 72°C with the 2 min at 95°C for primary denaturation and 5 min at 72°C for final extension. PCR products were evaluated for the presence of considered bands by gel electrophoresis with the aid of DNA safe stain in 1.5% agarose gel medium. Produced bands were visualized under UV radiation by Gel documentation system (UVtec, Cambridge, UK).
Table 1.
Sequences of RT-PCR primers used to quantify the expression of adipose tissue-derived mesenchymal stem cells specific markers (CD73 and CD90) and hematopoietic stem cells specific markers (CD34 anCD45) in rat
Busulfan treatment of rats and adipose tissue-derived mesenchymal stem cells transplantation
Five untreated male rats served as negative control (normal group). The other male rats (n = 5) were induced azoospermia with injection of two doses of busulfan (10 mg/kg body weight, intraperitoneally, Busilvex®, Pierre Fabre Medicament, Boulogne, France) with 21 days interval. Thirty-five days after the last busulfan injection, male rats were anesthetized by ketamine and xylazine. Their abdominal area was surgically prepared in dorsal recumbency. A 1 cm midline abdominal incision was made to expose the peritoneal cavity. Under a stereomicroscope (Model SZN, Optika, Italy) the fat pad attached to the right epididymis and testis was gently pulled using an iris forceps. The testis was exteriorized, and the testicular artery and epididymis were clearly visible. A thin sterile plastic card with a 30° V-like cut was put underneath the testis as a holder. Suspension of AT-MSCs was mixed with sterile trypan blue (1:1, v/v) and was loaded into the polyethylene tube connected to a 1 ml syringe. A pulled pipette was attached into the tube. By applying pressure to the syringe, the cell suspension was gently forced into the pipette. The efferent duct that connects the testis to the epididymis was identified and fat tissue was gently removed around the ducts using a sharp needle. To make a good contrast between the translucent duct and the fat tissue and membrane around it, a triangular hard black plastic card was inserted underneath the duct [Figure 1a]. The pipette was carefully inserted into a duct in the bundle of the efferent duct and gently threaded a few mm toward the testis [Figure 1b]. While avoiding moving the injection pipette, the plunger of the syringe was gently depressed and the blue suspension flowed into the rete testis. Then, almost all surface seminiferous tubules were filled with suspension [Figure 1c]. AT-MSCs’ mixture (106 cells in 100 μL) was completely injected. The testis was returned to the abdominal cavity. The abdominal wall and the skin were sutured. The untreated left testis was served as positive control (azoospermia group).
Figure 1.
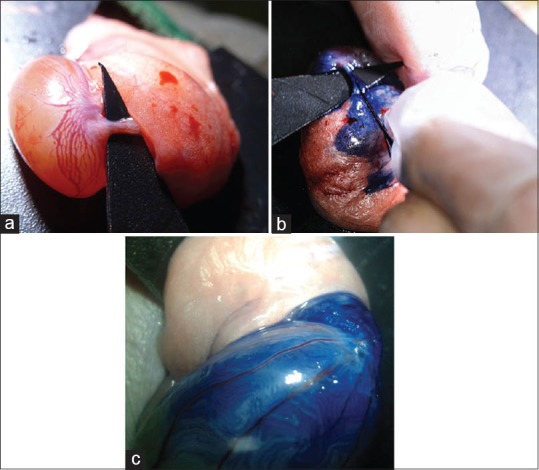
(a) To make a good contrast between the translucent efferent duct and the fat tissue and the membrane around it, a triangular hard black plastic card was inserted underneath the duct. (b) Adipose tissue-derived mesenchymal stem cells (AT-MSCs) transplantation (106 cells were mixed with trypan blue) into efferent duct of rat testis. (c) Seminiferous tubules were filled with mixture of AT-MSCs and dye. Te: Testis; Ep: Head of epididymis
Stereological analysis of testes
Sixty days after cell injection, animals were euthanized with ether and their testes were collected and fixed in a 10% formalin buffer solution, dehydrated, immersed in xylene and embedded in paraffin. Histological sections were made from each block. The 5 μm thickness Hematoxylin and Eosin stained sections were examined under a light microscope for any spermatogenic activity.
For each testis, five vertical sections from the polar and the equatorial regions were sampled. In one cross-section per animal, all tubules were evaluated for the presence of spermatogonia, spermatocytes, and spermatids. In 10 circular transverse sections of tubules, each from a different region of the testis and each homogeneous throughout with respect to cell associations and spermiogenesis, we measured inner, outer and total diameters, calculated areas of the cellular and luminal regions and cross-sectional area of the tubule, and counted the number of late spermatids present. The mean seminiferous tubule diameter (D) was derived by taking the average of two diameters, D1 and D2 at right angles. The cross-sectional area (Ac) of the seminiferous tubules was determined using the equation Ac = πD2/4, where π is equivalent to 3.142 and D the mean diameter of seminiferous tubules.[3]
A testis was rated for its spermatogenic potential (modified spermatogenic index) on a modified scale of 0–5.[3] The index was based on the appearance of the spermatogenic cells throughout the testis and included number of cell layers, types of cells, and the presence of late spermatids in the seminiferous tubules. The index and criteria were as follows: 0, no spermatogenic cells; 1, only spermatogonia present; 2, spermatogonia and spermatocytes present; 3, spermatogonia, spermatocytes and round (early) spermatids present with <50 late spermatids per tubule; 4, spermatogonia, spermatocytes, and round spermatids present; and up to 50–100 late spermatids per tubule; and 5, all cell types present and >100 late spermatids per tubule.
Statistical analysis
Means and standard error (SE) of the data of stereological indices of seminiferous tubules were subjected to Kolmogorov-Smirnov test of normality and analyzed by one-way ANOVA (SPSS for Windows, version 11.5, SPSS Inc., Chicago, Illinois, USA), and post-hoc test was performed by LSD test. The spermatogenesis index of seminiferous tubules was compared using Mann–Whitney U test. P < 0.05 was considered to be statistically significant. Group means and their SE were reported in the text and graphs (GraphPad Prism version 5.01 for Windows, GraphPad software Inc., San Diego, CA, USA).
RESULTS
Culture of adipose tissue-derived mesenchymal stem cells
AT-MSCs showed a fibroblast-like, spindle-shaped morphology after they attached to the culture flasks. AT-MSCs proliferation started 3–4 days after incubation and a monolayer 80% confluence was obtained after 12–15 days [Figure 2].
Figure 2.
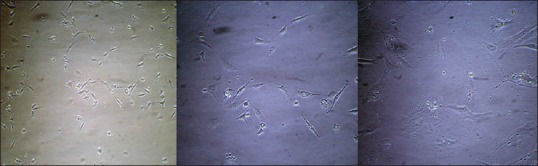
Morphological and phenotypic characteristics of rat adipose tissue-derived mesenchymal stem cells. Stem cells exhibited large, flattened or fibroblast-like morphology
Adipose tissue-derived mesenchymal stem cells osteogenic and adipogenic differentiations
To further confirm the differentiation capacity of AT-MSCs, osteogenic and adipogenic differentiations was induced. After culture of AT-MSCs in differentiation osteogenic medium, the cells differentiated toward osteoblasts as verified by positive staining with Alzarin Red staining [Figure 3a]. AT-MSCs treated by the adipogenic differentiation protocol showed the presence of intracellular lipid droplets, which were confirmed by Oil Red O staining [Figure 3b]. The AT-MSCs grown in culture medium alone (undifferentiation) did not show any lipid droplets at any of the time points examined, and maintained their typical fibroblast-like shape.
Figure 3.
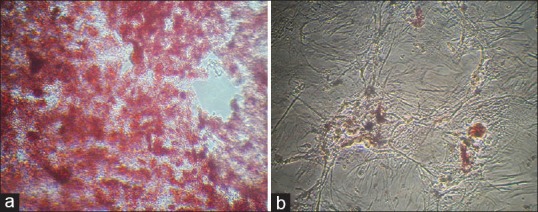
Adipose tissue-derived stem cells cultivated in (a) osteogenic medium and stained with Alizarin Red (×100) and (b) in adipogenic medium and were stained with Oil Red O at day 21 after induction (×150)
Characterization by reverse transcription-polymerase chain reaction
According to the proof of the mesenchymal property of AT-MSCs, these cells were expanded up to passage 4 and then they were analyzed using a PCR assay. Figure 4 displays positive expression for CD90 (mesenchymal cell marker) and negative expression for CD45 (hematopoietic cell marker).
Figure 4.
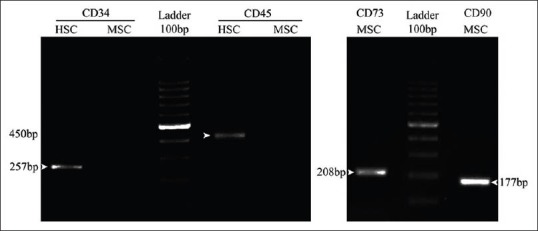
Activation of specific mesenchymal marker (CD73 and CD90) compared with deactivation of specific hematopoietic marker (CD34 and CD45) of rat adipose tissue-derived stem cells. mesenchymal stem cell (MSC), adipose tissue-derived MSC; HSC, hematopoietic stem cell
Histological assessment of spermatogenesis
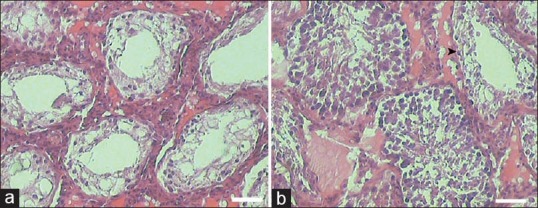
After induction, the azoospermia in the rats with a double injection of 10 mg/kg of busulfan, the testes of the rats were microscopically examined for any spontaneous spermatogenesis recovery and no sign was observed. After the treatment with busulfan, the seminiferous tubules of the testes were not treated with AT-MSCs were empty and their spermatogenesis process was disrupted [Figure 5a]. The examination of sections showed only Sertoli cell appearance for samples without AT-MSCs therapy [Figure 5a]. Histological examinations of testes in busulfan treatment group after 2 months revealed some degenerative changes such as seminiferous tubular atrophy and germinal epitheliums degenerations in the most of tubules. The large vacuolated lumen occupied seminiferous tubules and the atrophic germinal epithelium covered peripheral zone of seminiferous tubules as a thin band [Figure 6b].
Figure 5.
(a) Sections of seminiferous tubules of busulfan treated rat. The seminiferous tubules were empty and without germinal layer cells indicating the absence of spermatogenesis. (b) Sections of seminiferous tubules of treated rat with adipose tissue-derived mesenchymal stem cells. Most of tubules appeared to be filled with spermatogenic cells up. Some seminiferous tubules were not treated (arrow). White line indices are equal to 10 μm. H and E
Figure 6.
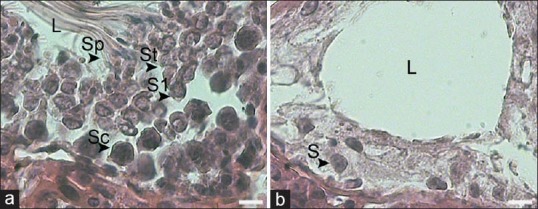
(a) Sections of seminiferous tubule of treated rat with adipose tissue-derived mesenchymal stem cells (AT-MSCs). L: Lumen; Sc: Spermatogonia; S1: Spermatocytes; St: Spermatid and Sp: Spermatozoa. (b) Sections of seminiferous tubule of busulfan treated rat which is not transplanted with AT-MSCs, demonstrate empty seminiferous tubules that indicate no spermatogenic activity. L: Lumen and Se: Sertoli cell. White line indices are equal to 50 μm. H and E staining
However, the presence of spermatogonia in the seminiferous tubules with AT-MSCs transplantation was observed [Figure 5b]. Moreover, the tubules appeared to be filled up with germinal cells (spermatogonia, primary spermatocytes, spermatids, and sperms) in the sections of AT-MSCs-treated testis [Figure 6a].
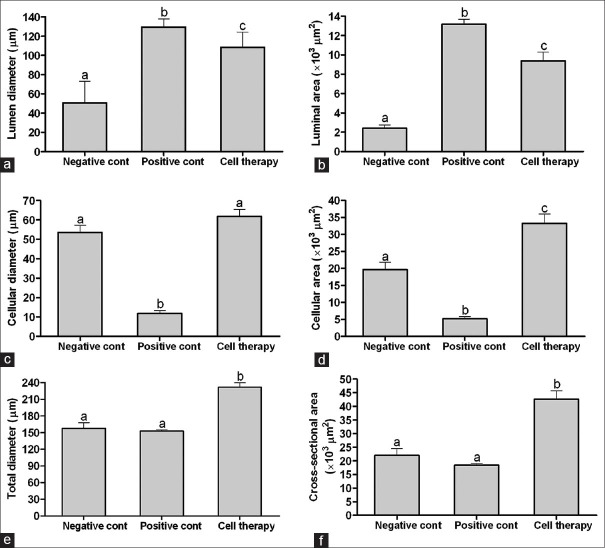
Stereological measurements indicated the lumen diameter and luminal area of the seminiferous tubules in rats with stem cell transplantation were more than the normal rats (P = 0.001 and P = 0.001, respectively) and less than azoospermia rat (P = 0.05 and P = 0.001, respectively; Figure 7a and b). Cellular diameter and cellular area of the seminiferous tubules in rats with stem cell transplantation did not have difference with normal rats (P = 0.38) and both groups were more than the azoospermia rats (P = 0.001 and P = 0.001; Figure 7c and d).
Figure 7.
Mean and standard error of stereological indices of seminiferous tubules in busulfan-induced azoospermic testis treated with adipose tissue-derived mesenchymal stem cells (cell therapy) in comparison with busulfan treated testes (azoospermia) and intact testis (normal) in rat. (a) Lumen diameter (μm), (b) Luminal area (μm2), (c) Cellular diameter (μm), (d) Cellular area (μm2), (e) Total diameters (μm), (f) Cross-sectional area of the tubule (μm2) a, b, c different superscript letters show significant differences between groups (P < 0.05)
Total diameter and cross-sectional area of the tubules of the seminiferous tubules in rats with stem cell transplantation was more than the normal rats (P = 0.001 and P = 0.001, respectively) and azoospermia rats (P = 0.001 and P = 0.001, respectively; Figure 7e and f).
Spermatogenesis index of seminiferous tubules in rats with stem cell transpantation did not have difference with normal rats (P = 0.001) and both groups were more than the azoospermia rats (P = 0.001; Figure 8).
Figure 8.
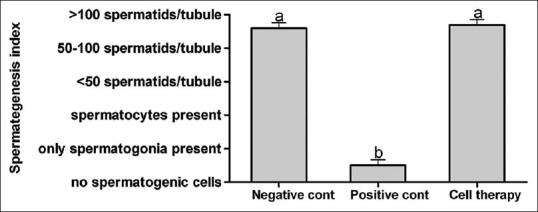
Mean and standard error of spermatogenesis index of seminiferous tubules in busulfan-induced azoospermic testis treated with adipose tissue-derived mesenchymal stem cells (cell therapy) in comparison with busulfan treated testes (azoospermia) and intact testis (normal) in rat a, b Different superscript letters show significant differences between groups (P < 0.05)
DISCUSSION
In the present study, AT-MSCs interactions in the rat testis were evaluated functionally by transplantation of busulfan-induced azoospermic rat testis. Our results demonstrated that injected AT-MSCs differentiated to testicular germinal cells in their new niche and this confirms that cell therapy could help the fast repair of pathological changes in testicular seminiferous tubules. Our results also clearly showed that AT-MSCs can reconstitute the tubular microenvironment by producing germinal cells within the host seminiferous tubules.
Approximately 1% of all men in the general population at 2013 suffer from azoospermia in forms of obstructive or nonobstructive, and azoospermic men constitute approximately 10–15% of all infertile men.[17] Many efforts were done to treat these infertile men, especially nonobstructive azoospermic ones because their treatment is most difficult.[12] In spite of hormone or surgical treatment, in recent years, more attention has been centralized on the cell and stem cell therapy. However, one of the currently major challenges of medical research is the development of stem cell therapy.[18] The particular microenvironments in which AT-MSCs are implanted plays a vital role in the course of their differentiation. In some studies, combination of in-vitro differentiation and in vivo transplantation was performed to obtain advanced differentiated spermatozoa.[19,20] However, the damaged somatic environment of the testis is one of the most problem and limiting step in this method of infertility treatment. The damaged testis is not receptive to spermatogonial stem cells transplantation and thereby not able to treat patient azoospermia.[21] This may be the reason that primary efforts about stem cell therapy in azoospermic and infertile animal model were not successful.
Lassalle et al.[22] reported that adult male BM-MSCs cannot transdifferentiate into germ cells to produce postmeiotic haploid cells after transplantation into the testis, and that male bone marrow does not appear to contain germinal stem cells. In similar findings, mouse and human adult BM-MSCs, grown in-vitro in the presence of retinoic acid, were found to express germ cell markers, but they failed to undergo spermatogenesis after transplantation into testes.[23,24]
However, more recently, Lue et al.[25] showed that transplanting of BM-MSCs into the testis of a busulfan-treated infertile mouse, differentiated BM-MSCs into germ cells, Sertoli cells, and Leydig cells. Moreover, Cakici et al.[18] reported that the treatment of testes of busulfan-treated infertile rats with AT-MSCs injection appeared morphologically normal with spermatogenesis in some tubules of cell-treated after 12 weeks. Consistent with our findings, Monsefi et al.[26] showed that the transplanted BM-MSCs could differentiate into germinal cells in seminiferous tubules of Wistar rats. It seems both BM- and AT-MSCs were found effective in treating animal model of azoospermia. However on the point of clinical view, bone marrow could only be obtained in limited quantity but the adipose tissue is usually available in abundance. Therefore, there is a major difference in the potential of clinical application of AT-MSCs and BM-MSCs.
CONCLUSION
The fertile status of busulfan-induced azoospermic rats was recovered by transplantation of AT-MSCs. This finding raises the possibility of using AT-MSCs to treat azoospermia in human. However, more similar studies are needed in different animal models to approve using of this technique in the treatment of azoospermia and infertility in human.
ACKNOWLEDGMENTS
The authors would like to appreciate the kind of Stem Cell and Transgenic Technology Research Center for laboratory cooperation.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
REFERENCES
- 1.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program 2010. 2010:368–76. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 2.Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: Maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–53. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Panahi M, Keshavarz S, Rahmanifar F, Tamadon A, Mehrabani D, Karimaghai N, et al. Busulfan induced azoospermia: Stereological evaluation of testes in rat. Vet Res Forum. 2014 [In press] [PMC free article] [PubMed] [Google Scholar]
- 4.Panahi M, Karimaghai N, Rahmanifar F, Tamadon A, Vahdati AA, Mehrabani D, et al. Stereological evaluation of testes after the induction of infertility using busulfan in hamster. Comp Clin Pathol. 2014 [In press] [Google Scholar]
- 5.de Rooij DG, Vergouwen RP. The estimation of damage to testicular cell lineages. Prog Clin Biol Res. 1991;372:467–80. [PubMed] [Google Scholar]
- 6.Kramer MF, de Rooij DG. The effect of three alkylating agents on the seminiferous epithelium of rodents. Virchows Arch B. 1969;4:276–82. doi: 10.1007/BF02906083. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, Yeo SM, et al. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 2004;575:41–51. doi: 10.1016/j.febslet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: Cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987;176:259–68. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 9.Dohle GR. Male infertility in cancer patients: Review of the literature. Int J Urol. 2010;17:327–31. doi: 10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 10.Pfister O, Della Verde G, Liao R, Kuster GM. Regenerative therapy for cardiovascular disease. Transl Res. 2014;163:307–20. doi: 10.1016/j.trsl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Leatherman J. Stem cells supporting other stem cells. Front Genet. 2013;4:257. doi: 10.3389/fgene.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, et al. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int J Mol Sci. 2014;15:13151–65. doi: 10.3390/ijms150813151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68:1064–71. doi: 10.1095/biolreprod.102.009977. [DOI] [PubMed] [Google Scholar]
- 15.Lin F. Adipose tissue-derived mesenchymal stem cells: A fat chance of curing kidney disease? Kidney Int. 2012;82:731–3. doi: 10.1038/ki.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs. bone marrow: In vitro comparison of their tropism towards gliomas. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058198. e58198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo) 2013;68(Suppl 1):27–34. doi: 10.6061/clinics/2013(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isik A, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed Res Int 2013. 2013 doi: 10.1155/2013/529589. 529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Hu HL, Li P, Yang S, Zhang W, Ding H, et al. Generation of male germ cells from induced pluripotent stem cells (iPS cells): An in vitro and in vivo study. Asian J Androl. 2012;14:574–9. doi: 10.1038/aja.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 21.Easley CA, 4 th, Simerly CR, Schatten G. Stem cell therapeutic possibilities: Future therapeutic options for male-factor and female-factor infertility? Reprod Biomed Online. 2013;27:75–80. doi: 10.1016/j.rbmo.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassalle B, Mouthon MA, Riou L, Barroca V, Coureuil M, Boussin F, et al. Bone marrow-derived stem cells do not reconstitute spermatogenesis in vivo. Stem Cells. 2008;26:1385–6. doi: 10.1634/stemcells.2007-0767. [DOI] [PubMed] [Google Scholar]
- 23.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–63. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 24.Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, et al. Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007;63:69–76. [PubMed] [Google Scholar]
- 25.Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, et al. Fate of bone marrow stem cells transplanted into the testis: Potential implication for men with testicular failure. Am J Pathol. 2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11:537–44. [PMC free article] [PubMed] [Google Scholar]