Abstract
CONTEXT:
Letrozole, a third generation aromatase inhibitor is gaining importance in ovulation induction. Some prefer to use it as a second line agent in women who fail to respond to clomifene citrate. However, our knowledge about the predictors of response to letrozole is limited.
AIMS:
The study was aimed at identifying the factors associated with letrozole resistance among women with World Health Organization (WHO) group II anovulation.
SUBJECTS AND METHODS:
Study was conducted at the infertility clinic at a tertiary care hospital in Sri Lanka. A case–control study design was used and included 50 subjects with WHO group II anovulation (25 clomifene responsive and 25 clomifene resistant). After a treatment cycle of letrozole, the factors were compared between the subjects who responded and those who failed to respond to treatment.
RESULTS:
Ovulation was achieved in 76% (n = 19) of subjects who had responded to clomifene previously and in 24% (n = 6) with clomifene resistance. The factors associated with letrozole resistance included the presence of hirsutism (odds ratio [OR]: 3.89; 95% confidence interval [CI]: 1.2–12.3) and clomifene resistance (OR: 10.03; 95% CI: 2.81–35.7). The early follicular phase mean (standard deviation) luteinizing hormone level was significantly higher among the nonresponders (9.75 [4.78] – 7.28 [2.3]; P = 0.02). Nonresponders showed significantly lower levels of oestradiol on the 5th and 9th days (28.50 [3.39] pg/mL vs. 7.49 [3.62] pg/mL; P = 0.0007 and 142.04 [76.22] pg/mL vs. 28.10 [12.8] pg/mL; P = 0.0001) of the menstrual cycle, respectively.
CONCLUSIONS:
The features associated with resistance to Letrozole at a dose of 2.5 mg show some overlap with those associated with clomifene resistance. However, some features do not show similar association. The effectiveness of letrozole at a dose of 2.5 mg in induction of ovulation among women with clomifene resistance is low and it does not seem to be a suitable treatment at a dose of 2.5 mg for this indication.
KEY WORDS: Anovulation, clomifene resistance, letrozole, letrozole resistance
INTRODUCTION
Ovulatory dysfunction is the commonest cause of female infertility, affecting nearly 20–30% of couples seeking infertility treatment.[1,2] In a study done at the same tertiary hospital in Sri Lanka, a prevalence rate of 38% was noted.[3] The affected individuals are divided in to three categories, termed as World Health Organization (WHO) anovulation categories, according to the underlying pathology and the baseline follicle stimulating hormone (FSH) levels. WHO group II anovulation is anovulation occurring in the presence of normal levels of baseline FSH, thus termed normogonadotropic hypogonadism. Polycystic ovary syndrome (PCOS) makes up nearly 70% of patients in this group.[4]
Women with infertility due to group II anovulation are treated by inducing ovulation by oral agents as first line treatment. The low cost, lesser incidence of side effects such as multiple pregnancy and ovarian hyperstimulation syndrome and need for less intense monitoring makes oral agents the preferred choice. Clomifene citrate, which has been in clinical use since 1960s,[5] is the most common ovulation induction agent used worldwide even today. It is an anti-estrogen which blocks the estrogen receptors at the level of hypothalamus and the pituitary, interfering with the normal negative feedback mechanisms, thus increasing the secretion of FSH from the anterior pituitary.[6,7] It is often started at a lower dose (50 mg/day) and increased in subsequent cycles till ovulation is achieved. If ovulation is not achieved at a maximum dose of 150 mg/day, such women are considered to be resistant to clomifene citrate.
Letrozole is a third generation aromatase inhibitor that is emerging as a new oral agent for induction of ovulation. It increases FSH secretion by reducing the estrogen production by affecting the activity of aromatase, an enzyme that catalyzes the rate-limiting step of estrogen production in many tissues such as breast, adipose tissue, and ovaries.[8] It does not cause down regulation of the estrogen receptors. Furthermore, it has a short half-life of 45 hours compared to 5 days with clomifene, and therefore its effects are not present around the time of ovulation and implantation.[8] Therefore, it does not cause the unwanted anti-estrogenic side effects of clomifene on the endometrium and the cervical mucus. The results of preliminary studies on letrozole as an ovulation induction agent have been promising.[9,10,11] The initial popularity of letrozole was shadowed by some concerns on its safety profile since the findings of one small study was presented as a conference paper, which reported an increased incidence of fetal anomalies in babies resulting from letrozole induced cycles.[12] However, subsequent studies did not support this claim[13] and the pharmacokinetics of the drug assures us that, when used in early part of the cycle, the drug will not be present in significant quantities around the time of fertilization and implantation.[14]
The place of letrozole in induction of ovulation is not clearly defined. Our long-standing experience with clomifene and the limited evidence on letrozole, has prevented its use as an alternative to clomifene in induction of ovulation. Therefore, many still use it as a second line agent in the presence of clomifene resistance. The recognized features associated with resistance to anti-estrogen therapy include advanced age, increased body mass index (BMI), presence of PCOS, a high free androgen index, a high luteinizing hormone (LH) level, a raised LH: FSH ratio and a high mean ovarian volume.[15,16] The predictors of treatment response to letrozole are not known. This study was aimed at assessing the factors associated with resistance to letrozole at a dose of 2.5 mg among a group of women with WHO group II anovulation who underwent induction of ovulation with letrozole.
SUBJECTS AND METHODS
This study was done as part of a large study on anovulation and induction of ovulation with oral agents at the infertility clinic at the North Colombo Teaching Hospital, conducted by the University of Kelaniya, Ragama, Sri Lanka, and was done between 2009 and 2011.
Study population and recruitment
A total of 29 women with clomifene resistance were identified among 128 women with ovulatory dysfunction. Clomifene resistance was diagnosed in women who failed to ovulate even at a dose of 150 mg/day. All of them were invited to participate in the study to assess response to letrozole in induction of ovulation. Four did not consent and hence 25 were included in the study with 25 age-matched controls from among those who responded to clomifene citrate. All consecutive patients were invited and recruited among the controls, till the numbers in the age categories were filled. The exclusion criteria used for the study included women with WHO group I or III anovulation (a FSH level < 2 IU/l or > 20 IU/l), previous ovarian surgery, chemotherapy or pelvic radiation, presence of endocrinopathies except PCOS, and previous ovarian drilling. The sample size was not calculated as per a comparison study since this study was done as part of a larger study assessing anovulation among infertile women. Since there were 25 consenting subjects with clomifene resistance in that total study sample, we recruited another 25 clomifene responders for comparison in this study.
Study interventions
All women included were given a 3-month washout period from the last month of clomifene treatment. They were asked to attend clinic on the 2nd day of a subsequent spontaneous menstruation. A hormone profile for FSH, LH was done on this day and a transvaginal ultrasound scan was done to determine the ovarian morphology. The mean ovarian volume and the number of antral follicles present were noted. All the subjects were given letrozole at a dose of 2.5 mg/day for 5 days starting from the 2nd day of the menstrual cycle. The FSH, LH and estradiol levels were measured on the 5th and 9th days of the menstrual cycle and follicle tracking with transvaginal ultrasound was commenced on the 9th day. In order to detect a mature follicle of more than 18 mm, further scans were scheduled with a maximum time interval of 3 days. Such scanning was continued till confirmation of ovulation or detection of development arrest of the follicles.
Outcome variables and measurement
The age was taken in full years to the number of completed years to the last birthday. Age categories were defined by 3 years interval from 18 up to 36 years for selection of age matched controls. Hirsutism was defined as excessive hair growth along the midline of the body including upper lip, chin, chest, abdominal wall, and around the introitus. A raised BMI was taken as above 25 kg/m2.
All hormone assays were done using a Elycsis® 1010 automated analyzer from Roche Diagnostics, Germany, which uses elctrochemiluminescence immunoassay technology. Pelvic ultrasound scans were done by a single person (TSP) using a GE LOGIQ 3 (GE healthcare, USA) scanner with a transvaginal transducer of 4.0–8.0 MHz. Ovarian volume was calculated by taking measurements in three planes and using the formula for volume calculation for an ellipse; volume = length × height × width × 0.523. All follicles with a mean diameter between 2 and 9 mm were taken as antral follicles and were counted in real time scanning. A follicle with a mean diameter of more than 18 mm noted in follicle tracking was taken as a mature follicle and the ovulation was confirmed by observing the disappearance of the follicle and presence of free fluid around the ovary, following detection of a mature follicle.
Data analysis
Based on the response to ovulation induction with letrozole, the study population was divided in to two groups; responders and nonresponders to letrozole. Known clinical and investigatory features of clomifene resistance were compared between these two groups to assess any significant difference. Student's t-test and Wilcoxon signed-rank tests were used for comparison of means between groups with parametric and nonparametric data, respectively. The dichotomous data were compared using odds ratios with 95% confidence interval (CI) using Chi-square test.
Approval for the study was obtained from the ethics review committee of the Faculty of Medicine, University of Kelaniya prior to study commencement. The trial was registered as a clinical trial with the Clinical trials registry of Sri Lanka Medical Association (SLCTR/2008/015), a WHO affiliated registry.
RESULTS
The study population had a mean age of 29.4 years (95% CI: 28.5–30.2) with only four subjects being 35–40 years. The mean BMI was 24.1 kg/m2 (95% CI: 23.4–24.7). PCOS was diagnosed in 33 (66%) subjects. The subjects who responded to clomifene citrate included 11 subjects in whom ovulation was achieved with 50 mg of clomifene per day, 8 with 100 mg and 6 with 150 mg/day. The baseline characteristics of the total study population are shown in Table 1.
Table 1.
The baseline characteristics of the total study population
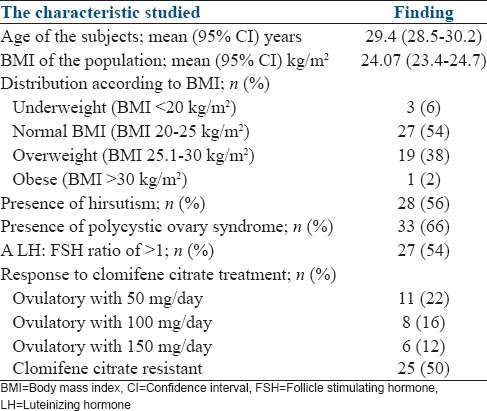
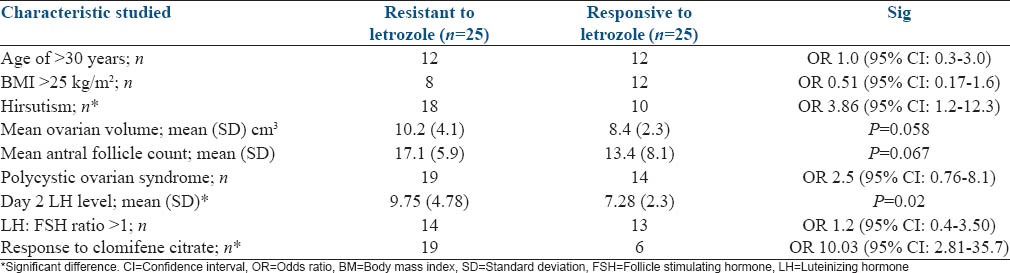
Induction of ovulation was achieved with letrozole at a dose of 2.5 mg/day in 25 (50%) subjects. This included 19 (76%) responders to clomifene and 6 (24%) subjects with clomifene resistance. A comparison of known factors associated with clomifene resistance between subjects who responded to letrozole and those who failed to respond are shown in Table 2.
Table 2.
Comparison of clinical and investigatory findings in letrozole responders and nonresponder's groups
The mean estradiol levels on the 5th and 9th days of the cycle in subjects who responded to letrozole were significantly higher than those who failed to respond (28.50 [3.39] pg/mL vs. 7.49 [3.62] pg/mL; P = 0.0007 and 142.04 [76.22] pg/mL vs. 28.10 [12.8] pg/mL; P = 0.0001, respectively).
DISCUSSION
Though letrozole is gaining popularity as a therapeutic agent for ovulation induction, its place has not been clearly defined. Whether it is effective as a second line agent among those with clomifene resistance is not clearly understood.
This study demonstrated the factors associated with nonresponse to letrozole show a considerable overlap with factors associated with clomifene resistance such as hirsutism, a raised LH level on 2nd day and a LH: FSH ratio >1. When used as a second line agent for ovulation induction among women with clomifene resistance, ovulation can be achieved only in about one in four patients. However, it is able to achieve ovulation in a large proportion of women who would respond to clomifene citrate. Being resistance to clomifene citrate at a dose of 150 mg/day has a 10 times likelihood of nonresponse to letrozole at a dose of 2.5 mg.
The hormone fluctuation in early follicular phase of the cycle showed a significant difference between the two groups of letrozole responders and nonresponders. Women who did not respond to letrozole showed a higher FSH level on the 5th day and the 9th day of the cycle. The levels of serum estradiol on the other hand were lower during the same period. This explains that those subjects who did not respond to letrozole, did exert a response with an elevated FSH, likely owing to the lack of estradiol which is responsible for the negative feedback signaling, but yet failed to achieve follicle development. This study adds to the knowledgebase we have on letrozole as a therapeutic agent for anovulation. While it is effective in some patients with clomifene resistance, its effectiveness at a dose of 2.5 mg in this indication seems to be low.
There are few limitations present in this study. Firstly, we used only a small dose of letrozole (2.5 mg/day) in this study as that was the recommended dose in many literature at the time the study was designed. Since then, higher doses have been used up to 12.5 mg/day in clinical trials with no significant incidence of any adverse events.[17] Secondly, we have not looked at the pregnancy rate as an outcome measure in this study since detection of ovulation was the study end point. Therefore, any discrepancy between ovulation rates and pregnancy rates were not measured. The study population was comprised of a relatively young population with only 8% of the subjects being over 35 years of age. Therefore, any effects on age on the response to treatment could not be assessed in this study.
A recent randomized multicenter double blind trial by Legro et al. was able to demonstrate higher ovulation and pregnancy rates with letrozole in comparison to clomifene in women with PCOS.[13] More women had ovulation with letrozole (88%) in this study compared to clomifene (76%). This corresponds with our findings that the predictors of success in ovulation induction by letrozole, in patients with WHO group II anovulation, is different to those of clomifene citrate, though some overlap exists. These factors should be further studied to identify patient subgroups that are suitable for either medication. The treatment success of letrozole, at a dose of 2.5 mg/day, in women with clomifene resistance is not satisfactory enough to recommend this as a second line treatment agent for this indication.
ACKNOWLEDGMENTS
This study was funded through a research grant from the National Science Foundation of Sri Lanka (Grant No RG/2007/HS/08).
Footnotes
Source of Support: This study was funded through a research grant from the National Science Foundation of Sri Lanka (Grant No RG/2007/HS/08)
Conflict of Interest: None declared.
REFERENCES
- 1.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–7. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton-Fairley D, Taylor A. Anovulation. BMJ. 2003;327:546–9. doi: 10.1136/bmj.327.7414.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palihawadana TS, Wijesinghe PS, Seneviratne HR. Aetiology of infertility among females seeking treatment at a tertiary care hospital in Sri Lanka. Ceylon Med J. 2012;57:79–83. doi: 10.4038/cmj.v57i2.4461. [DOI] [PubMed] [Google Scholar]
- 4.Adamson GD, Baker VL. Subfertility: Causes, treatment and outcome. Best Pract Res Clin Obstet Gynaecol. 2003;17:169–85. doi: 10.1016/s1521-6934(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 5.Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961;178:101–4. doi: 10.1001/jama.1961.03040410001001. [DOI] [PubMed] [Google Scholar]
- 6.Messinis IE. Ovulation induction: A mini review. Hum Reprod. 2005;20:2688–97. doi: 10.1093/humrep/dei128. [DOI] [PubMed] [Google Scholar]
- 7.van Santbrink EJ, Fauser BC. Ovulation induction in normogonadotropic anovulation (PCOS) Best Pract Res Clin Endocrinol Metab. 2006;20:261–70. doi: 10.1016/j.beem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Casper RF, Mitwally MF. Review: Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760–71. doi: 10.1210/jc.2005-1923. [DOI] [PubMed] [Google Scholar]
- 9.Casper RF. Aromatase inhibitors in ovarian stimulation. J Steroid Biochem Mol Biol. 2007;106:71–5. doi: 10.1016/j.jsbmb.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Gregoriou O, Vlahos NF, Konidaris S, Papadias K, Botsis D, Creatsas GK. Randomized controlled trial comparing superovulation with letrozole versus recombinant follicle-stimulating hormone combined with intrauterine insemination for couples with unexplained infertility who had failed clomiphene citrate stimulation and intrauterine insemination. Fertil Steril. 2008;90:678–83. doi: 10.1016/j.fertnstert.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 11.Quintero RB, Urban R, Lathi RB, Westphal LM, Dahan MH. A comparison of letrozole to gonadotropins for ovulation induction, in subjects who failed to conceive with clomiphene citrate. Fertil Steril. 2007;88:879–85. doi: 10.1016/j.fertnstert.2006.11.166. [DOI] [PubMed] [Google Scholar]
- 12.Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertil Steril. 2005;84:S95. [Google Scholar]
- 13.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–29. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casper RF, Mitwally MF. A historical perspective of aromatase inhibitors for ovulation induction. Fertil Steril. 2012;98:1352–5. doi: 10.1016/j.fertnstert.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617–22. doi: 10.1210/jcem.84.5.5705. [DOI] [PubMed] [Google Scholar]
- 16.Madani T, Zafarani F, Eshrati B. Factors influencing ovarian response to tamoxifen in anovulatory patients. Int J Gynaecol Obstet. 2006;95:173–4. doi: 10.1016/j.ijgo.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Pritts EA, Yuen AK, Sharma S, Genisot R, Olive DL. The use of high dose letrozole in ovulation induction and controlled ovarian hyperstimulation. ISRN Obstet Gynecol 2011. 2011 doi: 10.5402/2011/242864. 242864. [DOI] [PMC free article] [PubMed] [Google Scholar]


