Acbstract
Background
Inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) has been intensively studied to lower low-density lipoprotein cholesterol (LDL-C) levels. The purpose of this meta-analysis was to evaluate the safety and efficacy of anti-PCSK9 antibodies in randomized, controlled trials (RCTs).
Methods
PubMed, EMBASE, CENTRAL databases, and recent conferences were searched. Safety outcomes were rates of common adverse events. Efficacy outcomes included percentages of LDL-C lowering and other lipid changes compared with placebo and ezetimibe, respectively.
Results
Twenty-five RCTs encompassing 12,200 patients were included. The rates of common adverse events were firstly reported in our study by pooling together all evidence in RCTs, showing largely no significant difference between anti-PCSK9 antibodies and placebo (or ezetimibe), except that alirocumab was associated with reduced rates of death (relative risk (RR): 0.43, 95 % confidence interval (CI): 0.19 to 0.96, P = 0.04) and an increased rate of injection-site reactions (RR: 1.48, 95 % CI: 1.05 to 2.09, P = 0.02); evolocumab reduced the rate of abnormal liver function (RR: 0.43, 95 % CI: 0.20 to 0.93, P = 0.03), both compared with placebo. No significant difference in safety outcomes was detected between monthly 420 mg and biweekly 140 mg evolocumab treatments. Monthly 420 mg evolocumab treatment significantly reduced LDL-C by −54.6 % (95 % CI: −58.7 to −50.5 %) and by absolute −78.9 mg/dl (95 % CI: −88.9 to −68.9 mg/dl) versus placebo, and by −36.3 % (95 % CI: −38.8 to −33.9 %) versus ezetimibe, and increased high-density lipoprotein cholesterol (HDL-C) by 7.6 % (95 % CI: 5.7 to 9.5 %) versus placebo and 6.4 % (95 % CI: 4.3 to 8.4 %) versus ezetimibe. An equal or even greater change was observed following biweekly 140 mg administration. Significant and favorable changes were also detected in other lipids following evolocumab treatment. Biweekly 50 to 150 mg alirocumab lowered LDL-C by −52.6 % (95 % CI: −58.2 to −47.0 %) versus placebo, by −29.9 % (95 % CI: −32.9 to −26.9 %) versus ezetimibe, and increased HDL-C by 8.0 % (95 % CI: 4.2 to 11.7 %) versus placebo.
Conclusions
Evolocumab and alirocumab were safe and well-tolerated from our most-powered analyses. Both antibodies substantially reduced the LDL-C level by over 50 %, increased the HDL-C level, and resulted in favorable changes in other lipids.
Electronic supplementary material
The online version of this article (doi:10.1186/s12916-015-0358-8) contains supplementary material, which is available to authorized users.
Keywords: PCSK9, Monoclonal antibody, Evolocumab, Alirocumab, Safety, Efficacy, LDL-C, Meta-analysis, Randomized controlled trials
Background
Hypercholesterolemia is a major risk factor for cardiovascular disease (CVD) [1]. The introduction of statins has substantially reduced CVD events around the world and is recommended as first-line therapy for CVD management [2]. However, a necessity for other lipid-lowering (especially low-density lipoprotein cholesterol (LDL-C) lowering) agents still exists because some patients cannot tolerate statins due to adverse events, or cannot achieve intensive LDL-C lowering because of extremely high baseline LDL-C levels, or patients with very high risk of CVD events need more intensive lowering therapy [3].
The role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in cholesterol regulation has been established since PCSK9 mutations were first discovered in autosomal dominant hypercholesterolemia (ADH) in 2003 [4]. PCSK9 binds to LDL receptors (LDLR) and facilitates the degradation of LDLRs [5] and thus leads to LDL-C increase, indicating great therapeutic potential. Therefore, inhibiting PCSK9 by monoclonal antibodies [6, 7], small interfering RNA [8], and small molecule inhibitors [9] has been evaluated to lower LDL-C levels in human studies during the last few years. However, a comprehensive analysis of the safety of anti-PCSK9 antibodies is absent, and efficacy outcomes on lipid profiles are not uniformly consistent. Therefore, we performed a comprehensive review of the current available evidence to address the safety (to provide the exact rates of common adverse events) and the efficacy (to determine the exact extent of lipid changing effect) of anti-PCSK9 antibodies.
Methods
Literature search
We sought to identify all randomized, controlled trials (RCTs) evaluating the safety and efficacy of PCSK9 monoclonal antibodies. We searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to 6 October 2014, using the following search terms and key words: ‘AMG 145’, ‘evolocumab’, ‘SAR236553’, ‘REGN727x’, ‘SAR236553/REGN727’, ‘alirocumab’ and ‘PCSK9’. Reference lists of the identified reports and relevant reviews were manually checked. Major conference proceedings were searched to retrieve unpublished studies until the end of the American Heart Association (AHA) scientific sessions on 20 November 2014. We did not apply any restriction on languages.
Study selection
Eligibility assessment was performed by two investigators (XZ and QZ). Studies were included if they: 1) were RCTs; 2) involved human subjects; 3) evaluated the safety and efficacy of an anti-PCSK9 antibody (evolocumab or alirocumab); and 4) reported mean differences with corresponding confidence intervals (CIs) or provided data necessary to calculate such. We did not restrict the type of study populations. We excluded animal studies, studies which were not randomized, and studies using other anti-PCSK9 antibodies, such as bococizumab, or PCSK9 inhibitors such as small interfering RNA because of the limited number of trials published regarding these PCSK9 inhibitors.
Outcomes
The safety outcomes were rates of common adverse events, and the primary efficacy endpoints were percent and absolute reductions in LDL-C following anti-PCSK9 antibody treatment. Secondary outcomes included: 1) LDL-C reduction at 52 weeks follow-up for evolocumab; 2) other lipid profile changes stratified by treatment dosages and durations of follow-up.
Data collection
Data were abstracted independently by two reviewers (XZ and QZ) using a standardized data extraction form. When there were disagreements, a third reviewer (LZ) checked the data. The following information was extracted: trial name/first author, year of publication, number of patients, duration of follow-up, age, gender, race, diabetes mellitus, coronary heart disease (CHD), PCSK9 level and all lipid profiles at baseline. Patient profile and background lipid-lowering therapy, treatments and doses in each study were also recorded. For safety endpoints, we extracted the number of events of interest and total number of patients in each group. For efficacy outcomes, as a priority, we extracted the mean differences and their corresponding 95 % CIs or standard errors (SEs) of anti-PCSK9 antibody versus placebo or ezetimibe for each lipid items. Alternatively, mean changes and 95 % CIs (or SEs) from baseline after either anti-PCSK9 antibody or placebo (or ezetimibe) treatments were extracted, thereafter mean differences of anti-PCSK9 antibody versus controls were calculated.
Quality assessment
We followed the Cochrane Collaboration’s tool to assess the risk of bias of included trials. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other sources of bias were included in the assessment independently performed by two reviewers (QZ and LZ).
Statistical analysis
For all efficacy outcomes, the mean differences following anti-PCSK9 treatment versus placebo or ezetimibe were pooled across studies using the DerSimonian-Laird random-effects models. Comparisons of anti-PCSK9 antibodies with placebo or ezetimibe were performed separately and stratified by dosages of antibodies. Adverse event rates were also pooled with random-effects models. Trials in which the endpoint was not detected in any of the treatment groups were excluded in the analysis of that endpoint. For studies in which only one of the groups had no event of interest, the estimate of treatment effect and its confidence interval were calculated after adding 0.5 to each cell of the 2 × 2 table for the trial [10, 11]. We used the I2 statistic to assess the consistency across studies, with 25 %, 50 %, and 75 % indicating low, moderate, and high degrees of heterogeneity respectively. Meanwhile, the χ2-based Q test was applied, and a P >0.10 suggests significant heterogeneity. Begg’s test and Egger’s test were performed to assess publication bias. Sensitivity analyses were carried out by omitting one study at one time to evaluate the consistency of the results.
In the LAPLACE-2 trial [7], efficacy data comparing evolocumab and ezetimibe were only reported in five subgroups stratified by background lipid-lowering therapies. We combined the results from these subgroups into a single group using the formulae recommended by the Cochrane Collaboration [12]. All analyses were conducted with the STATA version 11.0 software (STATA Corporation, College Station, TX, USA). The meta-analysis was in line with recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Additional file 1).
Results
Study selection and characteristics
Our systematic literature search yielded 273 studies. After excluding duplicate publications and studies which clearly did not meet the inclusion criteria based on titles and abstracts, 22 studies were retrieved for full-text review. Six studies were further excluded, in which one study was not RCT [13] and two were phase 1 trials with either non-constant dosage of anti-PCSK9 antibodies or with too few participants [14,15]. Nine additional studies were identified in the recent conference of the European Society of Cardiology (ESC) and AHA, and were included in the meta-analysis [16-22] (Fig. 1). During the revision process of this paper, two of the trials (ODYSSEY LONG TERM and ODYSSEY COMB II trials) included in our analysis as conference presentations [16, 18] from 2014 AHA and ESC scientific sessions were published [23, 24]. Thus, 25 studies were included, encompassing a total of 12,200 patients. Twelve trials were conducted using anti-PCSK9 antibody evolocumab (AMG 145) [7, 25–35], and 13 were on alirocumab (SAR236553/REGN727) [16–22, 36–39]. The OSLER study was carried out based on participants from four parent trials (GAUSS, MENDEL, LAPLACE-TIMI 57, and RUTHERFORD) and was followed up for 52 weeks [30].
Fig. 1.
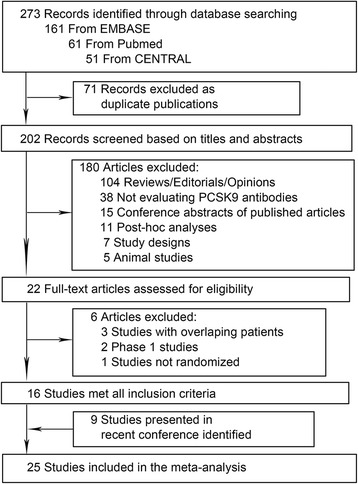
Flow diagram for study selection
Baseline characteristics of individual trials are shown in Table 1 and Table S1 and S2 (in Additional file 2). Several studies did not report age, lipids or PCSK9 level in the overall population. Therefore, we presented these characteristics in control populations (placebo or ezetimibe) in these studies given the significantly similar baseline values between the anti-PCSK9 treatment group and controls. All randomized trials included were published or presented in major conferences between 2012 and 2014. The mean age ranged from 31 to 62 years, and the percentage of women from 37 to 74; over 80 % of the patients were white. Regarding evolocumab, all trials were followed up for 12 weeks, except the OSLER and DESCARTES trials [25, 30], which were followed up for 52 weeks. With regard to alirocumab, most trials were followed up for 24 weeks except three phase 2 trials which were followed-up for 8 to 12 weeks [36, 37, 39]. All included RCTs had a low risk of bias, as detailed in Table S3 (in Additional file 2).
Table 1.
Baseline characteristics of included randomized trials
| Trial/first author | Year | Number | Follow-up, weeks | Age, years | Women, number (%) | LDL-C, mmol/L | Total-C, mmol/L | HDL-C, mmol/L | Free PCSK9, nmol/L | Statin use, number (%) | Ezetimibe use, number (%) | CHD, number (%) | DM, number (%) | Patient profile and background lipid-lowering therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RUTHERFORD | 2012 | 167 | 12 | 50 (13) | 79 (47) | 4.0 (1.1) | 6.1 (1.3) | 1.3 (0.4) | 8.3 (2.4) | 150 (89) | 108 (64) | 35 (21) | NA | HeFH with LDL-C ≥2.6 mmol/L. Statin ± ezetimibe |
| LAPLACE-TIMI 57 | 2012 | 629 | 12 | 62 (55, 67) | 320 (51) | 3.2 (0.7) | 5.2 (0.9) | 1.4 (0.4) | 6.2 (1.7) | 627 (99) | 57 (9) | 187 (30) | 103 (16) | LDL-C ≥2.2 mmol/L and triglycerides ≤4.5 mmol/L. Statin ± ezetimibe |
| GAUSS | 2012 | 157 | 12 | 62 (8) | 100 (64) | 5.0 (1.3) | 7.3 (1.4) | 1.5 (0.5) | 5.3 (1.4) | 0 | 64 (40) | 21 (13.4) | NA | LDL-C ≥2.6 mmol/L with diagnosed CHD or risk equivalent; ≥3.4 mmol/L without CHD or risk equivalent and 2 or more risk factors, or ≥4.1 mmol/L without CHD or risk equivalent and with 1 or 0 risk factors. No/low-dose statin or statin-intolerance |
| MENDEL | 2012 | 406 | 12 | 51 (12) | 267 (66) | 3.7 (0.6) | 5.7 (0.8) | 1.4 (0.4) | 4.8 (1.2) | 0 | 45 (11) | 0 | 1 (0.2) | LDL-C ≥2.6 and <4.9 mmol/L and triglycerides ≤4.5 mmol/L, and a 10 year Framingham risk score for coronary heart disease of up to 10 %. No background anti-lipid therapy |
| YUKAWA | 2014 | 307 | 12 | 62 (10) | 114 (37) | 3.7 (0.5) | 5.8 (0.6) | 1.4 (0.3) | 5.6 (1.8) | 307 (100) | NA | 77 (25) | 117 (38) | LDL-C ≥3.0 mmol/L and triglycerides ≤4.5 mmol/L high risk for cardiovascular events. Statin ± ezetimibe |
| MENDEL-2 | 2014 | 614 | 12 | 54 (10) | 423 (69) | 3.6 (0.5) | NA | 1.5 (1.1, 2.0) | 3.9 (1.2) | 0 | 154 (25) | 0 | 1 (0.1) | LDL-C ≥2.6 and <4.9 mmol/L, triglycerides ≤4.5 mmol/L, and 10-year Framingham coronary heart disease risk scores ≤ 10 %. No lipid regulating drugs within 3 months |
| LAPLACE-2 | 2014 | 1896 | 12 | 60 (10) | 868 (46) | 2.8 (1.0) | 4.9 (1.1) | 1.4 (0.4) | 4.9 (1.6) | 1327 (70) | NA | 427 (23) | 293 (16) | LDL-C ≥3.9 mmol/L (no statin at screening), ≥2.6 mmol/L (nonintensive statin at screening), or ≥2.1 mmol/L (intensive statin at screening) and triglyceride ≤4.5 mmol/L |
| GAUSS-2 | 2014 | 307 | 12 | 62 (10) | 141 (46) | 5.0 (1.5) | NA | 1.3 (0.5) | 4.4 (1.7) | 55 (18) | NA | NA | 62 (20) | LDL-C ≥ 2.6 mmol/L and triglycerides ≤4.5 mmol/L. No/low-dose statin or statin-intolerance |
| DESCARTES | 2014 | 901 | 52 | 57 (10) | 471 (52) | 2.7 (0.6) | 4.6 (0.7) | 1.4 (0.4) | 6.7 (2.2) | 790 (88) | 189 (21) | 136 (15) | 104 (12) | LDL-C ≥1.9 mmol/L and triglycerides ≤4.5 mmol/L. Statin ± ezetimibe |
| OSLER | 2014 | 1104 | 52 | 57 (12) | 610 (55) | 3.7 (1.0) | 5.8 (1.2) | 1.4 (0.4) | 5.8 (2.1) | 691 (63) | NA | 210 (19) | 109 (10) | From parent studies (RUTHERFORD, LAPLACE-TIMI 57, GAUSS, MENDEL) |
| TESLA | 2014 | 49 | 12 | 31 (13) | 24 (49) | 9.0 (3.5) | NA | 1.0 (0.3) | 9.0 (2.7) | 49 (100) | 45 (92) | 21 (43) | NA | Homozygous familial hypercholesterolaemia, LDL-C ≥3.4 mmol/L. Statin ± ezetimibe |
| RUTHERFORD-2 | 2014 | 329 | 12 | 51 (14) | 139 (42) | 3.9 (1.0) | NA | 1.4 (0.4) | 6.0 (1.7) | 329 (100) | 204 (62) | 103 (31) | NA | HeFH patients ≥2.6 mmol/L. Statin ± ezetimibe |
| McKenney | 2012 | 183 | 12 | 57 (10) | 96 (53) | 3.4 (0.7) | 5.4 (0.7) | 1.3 (0.3) | NA | NA | NA | 10 (6) | 22 (12) | LDL-C ≥2.6 mmol/L on stable-dose atorvastatin for ≥6 weeks |
| Stein | 2012 | 77 | 12 | 53 (10) | 30 (39) | 3.9 (0.9) | 6.1 (1.0) | 1.4 (0.3) | NA | 77 (100) | 55 (71) | 32 (42) | 3 (4) | HeFH and LDL-C ≥2.6 mmol/L. Statin ± ezetimibe |
| Roth | 2012 | 92 | 8 | 57 (10) | 55 (60) | 3.2 (0.5) | 5.2 (0.7) | 1.4 (0.4) | NA | 92 (100) | NA | 3 (3) | 14 (15) | LDL-C ≥2.6 mmol/L on stable-dose atorvastatin for ≥7 weeks |
| ODYSSEY COMBO II | 2014 | 720 | 24 | 61 (9) | 530 (74) | 2.7 (0.9) | NA | NA | NA | 719 (99.9) | NA | 580 (81) | 221 (31) | LDL-C ≥1.8 mmol/L (history of CVD) or ≥2.6 mmol/L (no history of CVD) High CV-risk patients on max-tolerated statin |
| ODYSSEY FH I | 2014 | 486 | 24 | 52 (12) | 212 (55) | 3.7 (1.2) | NA | NA | NA | 486 (100) | 277 (57) | 225 (46) | 56 (12) | HeFH, inadequately controlled on maximally tolerated stable statin therapy with or without other LLT |
| ODYSSEY FH II | 2014 | 249 | 24 | 53 (13) | 118 (47) | 3.5 (1.1) | NA | NA | NA | 249 (100) | 165 (66) | 88 (35) | 10 (4) | HeFH, inadequately controlled on maximally tolerated stable statin therapy with or without other LLT |
| ODYSSEY LONG TERM | 2014 | 2341 | 24 | 61 (10) | 884 (38) | 3.2 (1.1) | NA | NA | NA | 2339 (99.9) | 334 (14) | 1607 (69) | 809 (35) | HeFH or High-CV risk patients LDL-C ≥1.8 mmol/L on max-tolerated statin therapy with or without other LLT |
| ODYSSEY MONO | 2014 | 103 | 24 | 60 (5) | 48 (47) | 3.6 (0.6) | 5.8 (0.8) | 1.6 (0.5) | NA | 0 | 0 | NA | 4 (4) | LDL-C ≥2.6 and <4.9 mmol/L, 10-year risk of fatal cardiovascular events ≥1 % and ≤5 % |
| ODYSSEY ALTERNATIVE | 2014 | 251 | 24 | 63 (10) | 114 (45) | 5.0 (1.8) | NA | 1.3 (0.4) | NA | NA | NA | 118 (47) | 60 (24) | Statin intolerant patients (by medical history) with LDL-C ≥70 mg/dl (very-high CV risk) or ≥ 100 mg/dl (moderate/high risk) |
| ODYSSEY COMBO I | 2014 | 316 | 24 | 63 (9) | 108 (34) | 2.7 (0.9) | NA | 1.3 (0.3) | NA | 315 (100) | 26 (8) | 247 (78) | 136 (43) | High CV risk on maximally tolerated statin with or without other LLT (LDL-C ≥70 mg/dl manifest CVD; or LDL-C ≥100 mg/dl with DM and other risk factors or CKD) |
| ODYSSEY HIGH FH | 2014 | 107 | 24 | 52 (11) | 50 (47) | 5.2 (1.1) | NA | NA | NA | 107 (100) | 26 (24) | 53 (50) | 15 (14) | HeFH inadequately controlled on maximally tolerated stable statin therapy with or without other LLT (LDL-C ≥160 mg/dl) |
| ODYSSEY OPTION I | 2014 | 205 | 24 | 66 (9) | 75 (36) | 2.6 (0.8) | NA | NA | NA | 182 (100) | NA | NA | NA | Patients with prior CVD + LDL-C ≥70 mg/dl, or CV risk factors + LDL-C ≥100 mg/dl |
| ODYSSEY OPTION II | 2014 | 204 | 24 | 60 (10) | 88 (43) | 2.7 (1.1) | NA | NA | NA | 175 (100) | NA | NA | NA | Patients with prior CVD + LDL-C ≥70 mg/dl, or CV risk factors + LDL-C ≥100 mg/dl |
Data are mean (SD), mean (SE), number (%), or median (IQR); lipid profiles are mean (SE) if not indicated; age is mean (SD). DESCARTES, the Durable Effect of PCSK9 Antibody Compared with Placebo Study trial; GAUSS, the Goal Achievement after Utilizing an anti-PCSK9 antibody in Statin Intolerant Subjects trial; LAPLACE-TIMI 57, the LDL-C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy (LAPLACE)–Thrombolysis in Myocardial Infarction (TIMI) 57 trial; MENDEL, Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels trial; OSLER, the Open Label Study of Long Term Evaluation Against LDL-C trial; RUTHERFORD, The Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder trial; TESLA, The Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities; YUKAWA, the StudY of LDL-Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk trial
CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; HDL-C, high-density lipoprotein (HDL) cholesterol; HeFH, heterozygous familial hypercholesterolemia IQR, interquartile range; LDL-C, low-density lipoprotein (LDL) cholesterol; LLT, lipid-lowering therapy; NA, not applicable; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation; SE, standard error; Total-C, total cholesterol
Safety outcomes of evolocumab
The pooled estimate for overall incidence of any treatment emergent adverse events (TEAEs) was 52.2 % (95 % CI: 44.8 to 59.7 %) at 12 weeks follow-up, which was not significantly different from placebo (pooled rate: 45.2 %; 95 % CI: 40.6 to 49.8 %) (relative risk (RR): 1.07, 95 % CI: 0.95 to 1.21) or ezetimibe (pooled rate: 54.7 %; 95 % CI: 41.3 to 68.0 %) (RR: 0.92, 95 % CI: 0.84 to 1.01, Table 2). Serious TEAE occurred in 1.9 % patients, TEAEs leading to discontinuation in 1.6% patients at 12 weeks following evolocumab treatment. Only 1 in 3,068 patients died at 12 weeks follow-up and 3 in 1,335 patients at 52 weeks follow-up, which were all similar to control groups (Table 2). Sixteen in 2,797, 12 in 2,797, and 20 in 2,287 patients developed creatine kinase (CK) elevations greater than five times the upper limit of normal (ULN), elevations in aspartate aminotransferase/alanine aminotransferase (AST/ALT) levels greater than three times the ULN, and adjudicated cardiovascular events respectively. Patients receiving evolocumab had a lower risk of developing abnormal liver function (AST/ALT greater than three times ULN) than those receiving placebo at 12-week follow-up (RR: 0.43, 95 % CI: 0.20 to 0.93, P = 0.03), but the difference did not maintain at 52-week follow-up. The pooled incidence of musculoskeletal and connective-tissue disorders was 9.8 % (95 % CI: 4.1 to 15.4 %), which was not significantly different with placebo (pooled rate: 7.1 %; 95 % CI: 1.6 to 12.6 %) (RR 1.08, 95 % CI: 0.70 to 1.67) or ezetimibe (pooled rate: 6.1 %; 95% CI: 0.7 to 11.5 %) (RR 1.10, 95 % CI: 0.61 to 2.00). Injection-site reactions occurred in 2.2 % of patients. No significant difference in any reported adverse event was found between monthly 420 mg and biweekly 140 mg administration at 12 weeks follow-up (Table 3). The event rates at 52-week follow-up following evolocumab are also reported in Table 2.
Table 2.
Adverse event rates at 12- and 52-week follow-up following evolocumab, placebo or ezetimibe treatments
| Safety endpoints | Evolocumab (12 Week) | Placebo (12 Week) | Evolocumab versus Placebo (12 Week) | Ezetimibe (12 Week) | Evolocumab versus Ezetimibe (12 Week) | Evolocumab (52 Week) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled event rate (95 % CI) | Event/Total | Pooled event rate (95 % CI) | Event/Total | RR (95 % CI) | P value | Pooled event rate (95 % CI) | Event/Total | RR (95 % CI) | P value | Event/Total | |
| TEAE (Any) | 52.2 (44.8, 59.7) | 1472/3068 | 45.2 (40.6, 49.8) | 534/1240 | 1.07 (0.95, 1.21) | 0.260 | 54.7 (41.3, 68.0) | 278/554 | 0.92 (0.84, 1.01) | 0.074 | 78.4 (1047/1335) |
| TEAE (Serious) | 1.9 (1.4, 2.4) | 64/3068 | 1.2 (0.5, 1.9) | 23/1240 | 0.96 (0.60, 1.55) | 0.876 | 0.9 ( 0.3, 1.6) | 7/554 | 1.35 (0.61, 3.00) | 0.458 | 6.4 (85/1335) |
| Leading to discontinuation | 1.6 (0.9, 2.4) | 56/3068 | 1.1 (0.4, 1.8) | 21/1240 | 0.78 (0.46, 1.32) | 0.354 | 3.5 (1.0, 6.0) | 24/554 | 0.68 (0.42, 1.11) | 0.127 | 3.0 (40/1335) |
| Death | NA | 1/3068 | NA | 1/1240 | NA | NA | NA | 0/554 | NA | NA | 0.2 (3/1335) |
| CK >5 ULN | 0.5 (0.1, 0.8) | 16/2797 | 0.5 (0.2, 0.8) | 8/1150 | 0.57 (0.21, 1.51) | 0.258 | 0.5 (0, 0.8) | 4/509 | 0.55 (0.17, 1.81) | 0.325 | 1.0 (14/1335) |
| ALT or AST > 3 ULN | 0.2 (0.1, 0.4) | 12/2797 | 0.8 (0.3, 1.2) | 13/1150 | 0.43 (0.20, 0.93) | 0.033 | 0.7 (0.1, 1.3) | 4/509 | 0.43 (0.14, 1.34) | 0.147 | 1.3 (18/1335) |
| Adjudicated cardiovascular events | 0.6 (0.2, 1.1) | 20/2287 | 0.5 (0.1, 0.9) | 7/1014 | 1.07 (0.41, 2.76) | 0.892 | 0.9 (0, 1.9) | 2/266 | 0.50 (0.12, 2.13) | 0.346 | 1.1 (15/1335) |
| Musculoskeletal and connective-tissue disorders | 9.8 (4.1, 15.4) | 144/1397 | 7.1 (1.6, 12.6) | 39/508 | 1.08 (0.70, 1.67) | 0.738 | 6.1 (0.7, 11.5) | 13/231 | 1.10 (0.61, 2.00) | 0.751 | 9.2 (68/736) |
| Back pain | 2.6 (1.7, 3.4) | 56/2208 | 1.8 (0.7, 2.8) | 21/912 | 1.05 (0.53, 2.11) | 0.883 | 2.5 (0.9, 4.1) | 8/298 | 0.72 (0.34, 1.54) | 0.4 | 6.4 (85/1335) |
| Arthralgia | 1.7 (1.0, 2.5) | 35/1862 | 1.7 (0.9, 2.6) | 14/803 | 1.04 (0.56, 1.93) | 0.912 | 1.5 (0.2, 2.9) | 4/266 | 0.97 (0.35, 2.64) | 0.945 | 5.7 (76/1335) |
| Muscle spasms | 1.9 (0.7, 3.2) | 45/2193 | 1.3 (0.5, 2.0) | 11/803 | 1.02 (0.42, 2.49) | 0.963 | 2.5 (0.7, 4.3) | 13/400 | 0.67 (0.30, 1.50) | 0.335 | 2.3 (14/599) |
| Myalgia | 3.5 (1.5, 5.6) | 48/1382 | 1.0 (0.2, 1.8) | 5/399 | 1.13 (0.37, 3.43) | 0.833 | 5.0 (0.6, 9.4) | 23/333 | 0.68 (0.30, 1.56) | 0.364 | 4.0 (24/599) |
| Headache | 3.4 (2.2, 4.6) | 86/2830 | 2.6 (1.5, 3.7) | 34/1122 | 0.81 (0.53, 1.24) | 0.331 | 2.8 (1.2, 4.4) | 20/554 | 0.94 (0.57, 1.55) | 0.798 | 4.0 (24/599) |
| Injection-site reactions | 2.2 (1.3, 3.1) | 64/2831 | 1.7 (0.9, 2.5) | 26/1184 | 1.06 (0.67, 1.67) | 0.816 | 2.0 (0.4, 3.6) | 13/522 | 1.02 (0.54, 1.93) | 0.955 | 5.4 (72/1335) |
| Gastrointestinal disorders | 5.6 (2.7, 8.4) | 118/1620 | 5.3 (1.9, 8.7) | 33/580 | 1.09 (0.68, 1.75) | 0.73 | 6.8 (0.1, 13.4) | 18/301 | 0.81 (0.47, 1.39) | 0.441 | 6.3 (38/599) |
| Nasopharyngitis | 6.2 (3.6, 8.8) | 115/1746 | 4.2 (2.1, 6.3) | 28/580 | 1.39 (0.93, 2.08) | 0.11 | 4.8 (1.6, 8.0) | 18/333 | 0.54 (0.30, 1.15) | 0.113 | 11.5 (153/1335) |
| Influenza | 1.7 (0.5, 2.8) | 27/1220 | 2.0 (0, 4.3) | 9/317 | 0.89 (0.38, 2.07) | 0.792 | 2.1 (0.1, 4.0) | 5/179 | 0.34 (0.10, 1.18) | 0.090 | 7.3 (97/1335) |
| Upper respiratory tract infection | 4.2 (2.5, 5.9) | 43/1015 | 2.9 (0.3, 5.6) | 12/317 | 1.01 (0.54, 1.90) | 0.964 | 5.3 (0, 14.4) | 5/77 | 0.74 (0.22, 2.50) | 0.624 | 8.5 (113/1335) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CK, creatine kinase; NA, not applicable; TEAE, treatment emergent adverse event; ULN, upper limit of normal
Table 3.
Adverse event rates at 12-week follow-up following different dosages of evolocumab treatments
| Safety endpoints | 420 mg Q4W (12 Week) | 140 mg Q2W (12 Week) | 420 mg Q4W versus 140 mg Q2W (12 Week) | |||
|---|---|---|---|---|---|---|
| Pooled event rate (95 % CI) | Event/Total | Pooled event rate (95 % CI) | Event/Total | RR (95 % CI) | P value | |
| TEAE (Any) | 52.1 (42.9, 61.3) | 565/1228 | 52.0 (43.1, 60.9) | 496/1095 | 1.01 (0.92, 1.10) | 0.873 |
| TEAE (Serious) | 1.4 (0.8, 1.9) | 23/1228 | 2.5 (1.6, 3.3) | 30/1095 | 0.69 (0.39, 1.23) | 0.214 |
| Leading to discontinuation | 1.4 (0.6, 2.2) | 26/1228 | 1.8 (0.8, 2.9) | 26/1095 | 0.94 (0.54, 1.64) | 0.835 |
| Death | NA | 0/1228 | NA | 1/1095 | NA | NA |
| CK >5 ULN | 0.5 (0.1, 0.9) | 8/1183 | 0.1 (0, 0.3) | 2/1050 | 1.58 (0.44, 5.75) | 0.484 |
| ALT or AST >3 ULN | 0.4 (0.2, 0.7) | 5/1183 | 0.5 (0.1, 0.8) | 5/1050 | 0.70 (0.20, 2.44) | 0.573 |
| Adjudicated cardiovascular events | 1.1 (0.2, 2.0) | 3/288 | 1.5 (0.3, 2.6) | 6/285 | 0.56 (0.17, 1.92) | 0.36 |
| Musculoskeletal and connective-tissue disorders | 3.9 (1.0, 6.9) | 21/421 | 8.0 (3.4, 12.5) | 30/386 | 0.63 (0.29, 1.34) | 0.227 |
| Back pain | 3.6 (1.0, 6.3) | 20/830 | 2.4 (1.4, 3.5) | 20/788 | 1.09 (0.42, 2.87) | 0.86 |
| Arthralgia | 1.8 (0.9, 2.7) | 13/687 | 1.5 (0.6, 2.3) | 10/678 | 1.27 (0.56, 2.87) | 0.568 |
| Muscle spasms | 2.0 (0.5, 3.5) | 18/823 | 1.4 (0.4, 2.5) | 15/780 | 1.10 (0.56, 2.20) | 0.776 |
| Myalgia | 1.3 (0, 2.7) | 11/414 | 2.3 (0.4, 4.2) | 12/378 | 0.91 (0.40, 2.06) | 0.82 |
| Headache | 3.1 (1.7, 4.4) | 40/1142 | 2.7 (1.3, 4.2) | 26/1043 | 1.40 (0.84, 2.33) | 0.202 |
| Injection-site reactions | 2.0 (0.9, 3.1) | 18/577 | 2.4 (0.8, 4.0) | 19/540 | 0.94 (0.50, 1.78) | 0.853 |
| Gastrointestinal disorders | 4.9 (2.3, 7.5) | 35/580 | 5.4 (2.5, 8.2) | 28/488 | 1.13 (0.68, 1.87) | 0.637 |
| Nasopharyngitis | 4.9 (2.5, 7.4) | 36/613 | 4.1 (1.7, 6.6) | 24/488 | 1.10 (0.63, 1.89) | 0.744 |
| Influenza | 1.2 (0.1, 2.4) | 9/350 | 2.5 (0.4, 5.4) | 8/225 | 0.19 (0.03, 1.10) | 0.064 |
| Upper respiratory tract infection | 4.6 (2.2, 6.9) | 14/247 | 4.8 (1.2, 8.4) | 6/123 | 1.30 (0.46, 3.70) | 0.621 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CK, creatine kinase; NA, not applicable; RR, relative risk; TEAE, treatment emergent adverse event; ULN, upper limit of normal
Safety outcomes of alirocumab
Three phase 2 studies reported safety outcomes at 8 to 12 weeks, while other phase 3 studies reported either at 24-week or 52-week follow-up. Safety profiles were pooled together in all trials. Any TEATs happened in 71.7 % (95 % CI: 67.7 to 75.6 %) patients following alirocumab treatment, mirrored to those with placebo (68.4 %, 95 % CI: 58.7 to 76.2 %) (RR: 1.00, 95 % CI: 0.92 to 1.10) or ezetimibe treatment (70.1 %, 95 % CI: 62.9 to 77.4 %) (RR: 1.01, 95 % CI: 0.96 to 1.07, Table 4). TEAEs which were serious or led to discontinuation occurred in 8.6 % and 4.8 % of patients, respectively. Fifteen in 3,363, 11 in 992, and 7 in 862 died following alirocumab, placebo or ezetimibe treatments, respectively, showing a lower rate in alirocumab compared with placebo (RR: 0.43, 95 % CI: 0.19 to 0.96, P = 0.04), but not ezetimibe (RR: 0.48, 95 % CI: 0.16 to 1.45, P = 0.19). CK greater than three times ULN, ALT/AST greater than three times ULN, and adjudicated cardiovascular events were detected in 2.0 %, 0.9 %, and 2.6 % of patients, respectively (Table 4). A trend toward a lower rate of serum CK level elevation was observed in alirocumab group than placebo group (RR: 0.72, 95 % CI: 0.52 to 1.01, P = 0.06). Musculoskeletal and connective-tissue disorders occurred in 16.7 % patients. A higher rate of injection-site reactions was detected following alirocumab administration (pooled rate: 6.0 %, 95 % CI: 3.8 to 8.2 %) than placebo (pooled rate: 3.7 %, 95 % CI: 2.5 to 4.8 %) (RR: 1.48, 95 % CI: 1.05 to 2.09, P = 0.02). Neurocognitive disorders were observed in 0.6 % alirocumab-treated patients. Still, all other reported adverse events rates did not differ significantly between alirocumab and placebo/ezetimibe treatments.
Table 4.
Adverse event rates following alirocumab, placebo or ezetimibe treatments
| Safety endpoint | Alirocumab | Placebo | Alirocumab versus Placebo | Ezetimibe | Alirocumab versus Ezetimibe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled event rate (95 % CI) | Event/Total | Pooled event rate (95 % CI) | Event/Total | RR (95 % CI) | P value | Pooled event rate (95 % CI) | Event/Total | RR (95 % CI) | P value | |
| TEAE (Any) | 71.7 (67.7, 75.6) | 2561/3425 | 68.4 (58.7, 78.2) | 783/1007 | 1.00 (0.92, 1.10) | 0.938 | 70.1 (62.9, 77.4) | 605/862 | 1.01 (0.96, 1.07) | 0.615 |
| TEAE (Serious) | 8.6 (4.5, 12.8) | 455/3363 | 9.3 (1.2, 17.4) | 158/992 | 0.94 (0.79, 1.12) | 0.47 | 8.5 (4.1, 12.8) | 91/862 | 1.03 (0.81, 1.31) | 0.815 |
| Leading to discontinuation | 4.8 (2.7, 6.9) | 187/3363 | 4.6 (2.1, 7.1) | 56/992 | 1.07 (0.78, 1.47) | 0.667 | 7.9 (3.9, 12.0) | 69/862 | 0.83 (0.38, 1.83) | 0.645 |
| Death | 0.5 (0.3, 0.7) | 15/3363 | 1.2 (0.5, 1.8) | 11/992 | 0.43 (0.19, 0.96) | 0.04 | 0.5 (0.1, 1.0) | 7/862 | 0.48 (0.16, 1.45) | 0.192 |
| CK >3 ULN | 2.0 (1.0, 3.1) | 114/3415 | 3.9 (2.0, 5.8) | 54/1003 | 0.72 (0.52, 1.01) | 0.059 | 2.4 (1.1, 3.7) | 28/855 | 0.75 (0.46, 1.24) | 0.261 |
| ALT or AST >3 ULN | 0.9 (0.5, 1.3) | 25/1869 | 1.3 (0.2, 2.4) | 2/218 | 0.95 (0.26, 3.47) | 0.94 | 0.5 (0.2, 0.9) | 4/858 | 1.91 (0.75, 4.88) | 0.176 |
| Musculoskeletal and connective-tissue disorders | 16.7 (5.9, 27.6) | 536/2450 | 17.3 (3.8, 30.7) | 235/865 | 1.00 (0.87, 1.14) | 0.967 | 22.3 (0, 46.5) | 74/416 | 0.80 (0.60, 1.05) | 0.108 |
| Injection-site reactions | 6.0 (3.8, 8.2) | 225/3425 | 3.7 (2.5, 4.8) | 42/1007 | 1.48 (1.05, 2.09) | 0.024 | 3.0 (1.1, 4.9) | 35/862 | 1.30 (0.88, 1.92) | 0.194 |
| Adjudicated cardiovascular events | 2.6 (1.3, 3.9) | 109/3130 | 3.2 (1.3, 5.0) | 38/930 | 0.94 (0.64, 1.39) | 0.768 | 1.2 (0.5, 1.9) | 15/811 | 1.29 (0.71, 2.36) | 0.405 |
| Nervous system disorders | 9.3 (4.2, 14.5) | 338/2813 | 6.6 (0.0, 15.9) | 145/865 | 0.97 (0.81, 1.17) | 0.776 | 6.2 (4.2, 8.2) | 34/536 | 0.85 (0.56, 1.30) | 0.461 |
| Gastrointestinal disorders | 16.4 (9.4, 23.4) | 332/1845 | 13.3 (5.1, 21.5) | 158/865 | 1.01 (0.57, 1.80) | 0.964 | 9.8 (1.6, 8.0) | 5/51 | 1.77 (0.63, 4.91) | 0.276 |
| Neurocognitive disorders | 0.6 (0.2, 1.1) | 27/2923 | 0.6 (0.1, 1.0) | 6/930 | 1.03 (0.23, 4.60) | 0.97 | 1.3 (0.5, 2.1) | 8/609 | 0.65 (0.22, 1.91) | 0.431 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CK, creatine kinase; RR, relative risk; TEAE, treatment emergent adverse event; ULN, upper limit of normal
Primary efficacy outcomes of evolocumab
All six dosages of evolocumab significantly decreased LDL-C level at 12 weeks follow-up, with the greatest reductions achieved in monthly 420 mg evolocumab (mean reduction: −54.6 %, 95 % CI: −58.7 to −50.5 %) and biweekly 140 mg evolocumab (mean reduction: −60.4 %, 95 % CI: −68.8 to −52.0 %) versus placebo (Fig. 2 and Additional file 2: Table S4). There was significant heterogeneity in both comparisons (I2 = 80.4 % and 93.9 %, respectively). Biweekly administration of 140 mg evolocumab led to even greater reduction than 420 mg monthly treatment, both of which reduced the LDL-C level by over 50 %. The effect is likely dose-dependent with the same frequency of administration. Likewise, in absolute level changes, 420 mg monthly and 140 mg biweekly dosing lowered LDL-C by −78.9 mg/dl (95 % CI: −88.9 to −68.9 mg/dl) and −81.6 mg/dl (95 % CI: −92.0 to −71.1 mg/dl), respectively (Additional file 2: Figure S1 and Table S4).
Fig. 2.
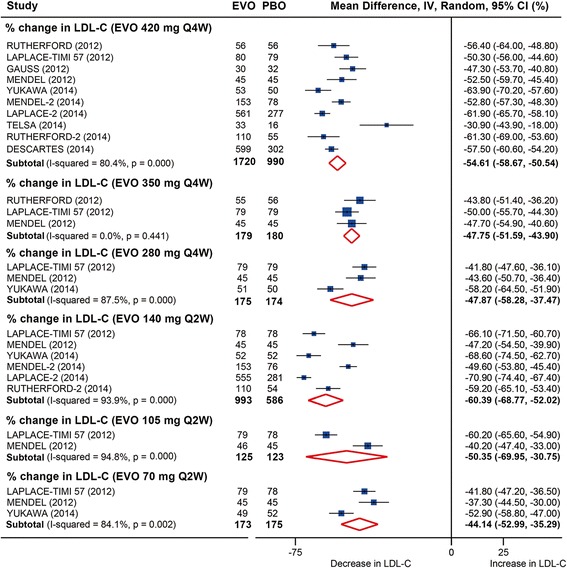
Pooled analysis for percent changes in LDL-C following evolocumab treatments stratified by dosages versus placebo at 12 weeks follow-up. EVO, evolocumab; PBO, placebo. LDL-C, low-density lipoprotein cholesterol
Compared with ezetimibe, significant lowering of LDL-C also occurred in all evolocumab dosages at week 12. Monthly 420 mg and biweekly 140 mg evolocumab administration reduced LDL-C level by −36.3 % (95 % CI: −38.8 to −33.9 %) and −38.2 % (95 % CI: −41.5 to −34.5 %), respectively, versus ezetimibe (Fig. 3 and Additional file 2: Table S4). No significant heterogeneity was detected in the comparisons (I2 = 0 and 28.4 %, respectively). Fewer studies reported absolute changes of LDL-C level versus ezetimibe; meta-analyses of these studies demonstrated largely similar but less remarkable results compared with those versus placebo.
Fig. 3.
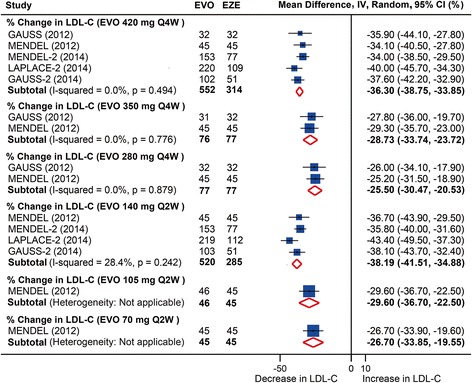
Pooled analysis for percent changes in LDL-C following evolocumab treatments stratified by dosages versus ezetimibe at 12 weeks follow-up. EVO, evolocumab; EZE, ezetimibe. LDL-C, low-density lipoprotein cholesterol
LDL-C percent and absolute changes at the mean of weeks 10 and 12 following evolocumab treatments versus placebo or ezetimibe were all significant and similar to those changes at week 12 (Additional file 2: Table S5).
Other efficacy outcomes of evolocumab
All dosages except monthly 280 mg evolocumab treatment significantly increased high-density lipoprotein cholesterol (HDL-C) levels at week 12 compared with placebo. The HDL-C level was increased by 7.6 % (95 % CI: 5.7 to 9.5 %) and 6.9 % (95 % CI: 5.4 to 8.4 %) by monthly 420 mg and biweekly 140 mg evolocumab treatment, respectively (Fig. 4 and Additional file 2: Table S6). No significant heterogeneity was detected in the comparisons (I2 = 23.3 % and 0, respectively). These two dosages of evolocumab also increased the HDL-C level compared with ezetimibe by 6.4 % (95 % CI: 4.3 to 8.4 %) and 7.2 % (95 % CI: 4.4 to 10.0 %), with no significant heterogeneity (I2 = 0 and 32.2 %, respectively).
Fig. 4.
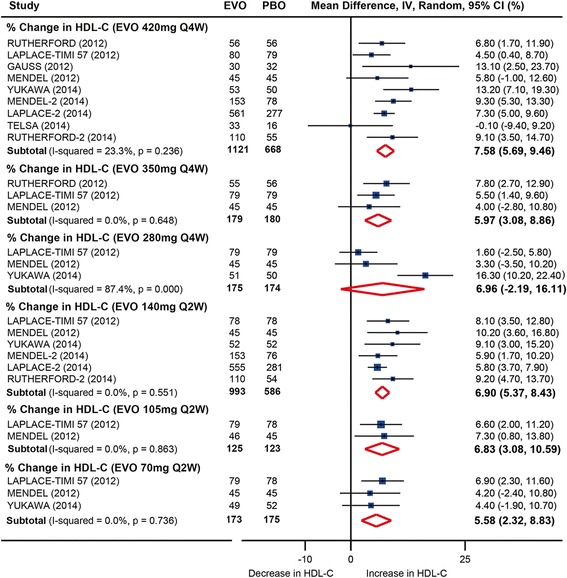
Pooled analysis for percent changes in HDL-C following evolocumab treatments stratified by dosages versus placebo at 12 weeks follow-up. EVO, evolocumab; PBO, placebo. HDL-C, high-density lipoprotein cholesterol
Compared with placebo, all dosages of evolocumab generated significant reductions of total cholesterol (TC), TC/HDL-C, non-HDL-C and very low-density lipoprotein cholesterol (VLDL-C), which were decreased by monthly 420 mg evolocumab by −36.7 % (95 % CI: −38.9 to −34.4 %), −41.3 % (95 % CI: −45.7 to −36.9 %), −52.1 % (95 % CI: −55.1 to −49.1 %), and −22.8 % (95 % CI: −27.5 to −18.0 %), respectively at week 12, with low to modest levels of heterogeneity (I2 = 38.0 %, 64.7 %, 57.9 % and 6.6 %, respectively) (Table 5 and Additional file 2: Figures S2 to S5, Tables S7 to S10). Similar results were detected following biweekly 140 mg evolocumab treatment.
Table 5.
Additional lipid efficiency outcomes following evolocumab treatments stratified by dosages versus placebo at 12-week follow-up
| Endpoints | Evolocumab dosages | Mean Difference, % (95 % CI) | Test for overall effect | Number of studies | Number of individuals | Heterogeneity | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | P value | Evolocumab | Placebo | I 2 | P value | P_Begg | P_Egger | ||||
| TC | 420 mg Q4W | −36.66 (−38.93, −34.39) | 31.60 | 0.000 | 6 | 539 | 825 | 38.0 % | 0.153 | 1.000 | 0.980 |
| 140 mg Q2W | −40.48 (−45.33, −35.62) | 16.35 | 0.000 | 4 | 456 | 730 | 85.3 % | 0.000 | 0.734 | 0.552 | |
| HDL-C | 420 mg Q4W | 7.58 (5.69, 9.46) | 7.89 | 0.000 | 9 | 688 | 1121 | 23.3 % | 0.236 | 1.000 | 0.843 |
| 140 mg Q2W | 6.90 (5.37, 8.43) | 8.84 | 0.000 | 6 | 586 | 993 | 0.0 % | 0.551 | 0.133 | 0.030 | |
| Non-HDL-C | 420 mg Q4W | −52.11 (−55.07, −49.14) | 34.42 | 0.000 | 8 | 672 | 1088 | 57.9 % | 0.020 | 0.618 | 0.499 |
| 140 mg Q2W | −56.07 (−61.67, −50.47) | 19.62 | 0.000 | 6 | 586 | 993 | 89.0 % | 0.000 | 0.452 | 0.616 | |
| TC/HDL-C | 420 mg Q4W | −41.26 (−45.65, −36.87) | 18.43 | 0.000 | 6 | 317 | 374 | 64.7 % | 0.015 | 0.566 | 0.302 |
| 140 mg Q2W | −44.85 (−49.11, −40.59) | 20.64 | 0.000 | 4 | 229 | 285 | 63.6 % | 0.041 | 0.089 | 0.126 | |
| VLDL-C | 420 mg Q4W | −22.75 (−27.46, −18.04) | 9.47 | 0.000 | 6 | 567 | 925 | 6.6 % | 0.374 | 0.452 | 0.335 |
| 140 mg Q2W | −24.83 (−38.29, −11.38) | 3.62 | 0.000 | 4 | 480 | 831 | 82.3 % | 0.001 | 0.734 | 0.462 | |
| ApoB | 420 mg Q4W | −45.14 (−49.16, −41.12) | 22.00 | 0.000 | 9 | 688 | 1121 | 78.8 % | 0.000 | 0.076 | 0.027 |
| 140 mg Q2W | −52.69 (−57.40, −47.98) | 21.91 | 0.000 | 6 | 586 | 993 | 85.6 % | 0.000 | 0.707 | 0.450 | |
| ApoA1 | 420 mg Q4W | 5.17 (2.60, 7.73) | 3.95 | 0.000 | 6 | 317 | 374 | 40.6 % | 0.135 | 0.566 | 0.517 |
| 140 mg Q2W | 6.26 (1.71, 10.82) | 2.69 | 0.007 | 4 | 230 | 285 | 74.5 % | 0.008 | 0.308 | 0.129 | |
| ApoB/ApoA1 | 420 mg Q4W | −48.06 (−52.70, −43.43) | 20.32 | 0.000 | 7 | 395 | 527 | 72.4 % | 0.001 | 0.649 | 0.351 |
| 140 mg Q2W | −53.68 (−57.77, −49.59) | 25.74 | 0.000 | 5 | 305 | 438 | 65.8 % | 0.020 | 0.806 | 0.500 | |
| TG | 420 mg Q4W | −15.70 (−20.35, −11.05) | 6.62 | 0.000 | 9 | 688 | 1121 | 42.5 % | 0.084 | 0.118 | 0.030 |
| 140 mg Q2W | −17.35 (−23.50, −11.20) | 5.53 | 0.000 | 6 | 586 | 993 | 59.8 % | 0.029 | 1.000 | 0.039 | |
| Lp(a) | 420 mg Q4W | −25.40 (−29.09, −21.70) | 13.47 | 0.000 | 9 | 688 | 1121 | 47.1 % | 0.057 | 1.000 | 0.626 |
| 140 mg Q2W | −32.39 (−38.92, −25.87) | 9.73 | 0.000 | 6 | 586 | 993 | 79.3 % | 0.000 | 1.000 | 0.819 | |
| PCSK9 | 420 mg Q4W | −44.04 (−53.90, −34.17) | 8.75 | 0.000 | 6 | 540 | 908 | 85.2 % | 0.000 | 0.452 | 0.473 |
| 140 mg Q2W | −60.92 (−83.94, −37.89) | 5.18 | 0.000 | 2 | 132 | 188 | 92.9 % | 0.000 | 1.000 | NA | |
ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/ApoA1, ratio of ApoB/ApoA1; CI, confidence interval; HDL-C, high-density lipoprotein (HDL) cholesterol; Lp(a), lipoprotein(a); NA, not applicable; Non-HDL-C, non-HDL cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; TC, total cholesterol; TC/HDL-C, ratio of total cholesterol/HDL cholesterol; TG, triglycerides; VLDL-C, very low-density lipoprotein (VLDL) cholesterol
A significant increase in apolipoprotein A1 (ApoA1) was found at week 12 in all dosages of evolocumab except biweekly 105 mg administration. Monthly 420 mg and biweekly 140 mg treatment increased the ApoA1 level by 5.2 % (95 % CI: 2.6 to 7.7 %) and 6.3 % (95 % CI: 1.7 to 10.8 %) versus placebo, respectively (Table 5 and Additional file 2: Figure S6 and Table S11).
All dosages of evolocumab significantly lowered apolipoprotein B (ApoB), ApoB/ApoA1, and lipoprotein(a) (Lp(a)) at week 12, with monthly 420 mg treatment reducing levels by −45.1 % (95 % CI: −49.2 to −41.1 %), −48.1 % (95 % CI: −52.7 to −43.4 %), and −25.4 % (95 % CI: −29.1 to −21.7 %), respectively, versus placebo (Table 5 and Additional file 2: Figure S7 to S9, Tables S12 to S14). Modest to high levels of heterogeneity were found in both comparisons (I2 = 78.9 %, 72.4 %, and 47.1 %, respectively).
A significant decrease in triglycerides (TG) was found at week 12 in all dosages of evolocumab except biweekly 105 mg administration. Monthly 420 mg and biweekly 140 mg treatments lowered the TG level by −15.7 % (95 % CI: −20.4 to −11.1 %) and −17.4 % (95 % CI: −23.5 to −11.2 %) versus placebo, respectively (Table 5 and Additional file 2: Figure S10 and Table S15). A modest level of heterogeneity was detected.
The free PCSK9 level was decreased by any dosage of evolocumab treatment. At week 12, monthly 420 mg and biweekly 140 mg treatments lowered the PCSK9 level by −44.0 % (95 % CI: −53.9 to −34.2 %) and −60.9 % (95 % CI: −83.9 to −37.9 %) versus placebo, respectively (Table 5 and Additional file 2: Table S16). Significant heterogeneity was detected.
Similar results were obtained at the mean of weeks 10 and 12, and largely similar but less remarkable results were achieved when compared with ezetimibe (Additional file 2). Two RCTs reported efficacy outcomes of monthly 420 mg treatment at 52 weeks follow-up. Likewise, all the comparisons were significant (Additional file 2: Figure S11).
Efficacy outcomes of alirocumab
Both monthly and biweekly administration of alirocumab significantly lowered LDL-C levels, with biweekly 50 to 150 mg treatment reduced by over −50 % (mean reduction: −52.6 %, 95 % CI: −58.2 to −47.0 %) versus placebo, a less marked reduction was achieved when compared with ezetimibe (mean reduction: −29.9 %, 95 % CI: −32.9 to −26.9 %) and by monthly 150 to 300 mg treatment versus placebo (mean reduction: −32.2 %, 95 % CI: −48.7 to −15.6 %). Significant heterogeneity was detected in comparisons with placebo (Fig. 5A).
Fig. 5.

Pooled analysis for percent changes in LDL-C (a) and HDL-C (b) following alirocumab treatments stratified by dosages versus placebo or ezetimibe. ALIR, alirocumab; EZE, ezetimibe; PBO, placebo. HDL-C, high-density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol
HDL-C level was increased by 8.0 % (95 % CI: 4.2 to 11.7 %) following biweekly 50 to 150 mg treatment and by 7.4 % (95 % CI: 3.8 to 11.1 %) after monthly 150 to 300 mg administration. No significant heterogeneity was found (I2 = 0 for both comparisons) (Fig. 5B). Meta-analyses of other efficiency outcomes demonstrated reduction in TC, non-HDL-C, ApoB, and Lp(a), and an increase in ApoA1 following alirocumab treatment, which are shown in Table S17 (in Additional file 2).
No significant publication bias was found in most analyses, detailed in each table in Additional file 2. Sensitivity analyses did not generated inconsistent results.
Discussion
For the first time we provide in our study the rates of common adverse events following PCSK-9 antibody treatments by enrolling the largest sample size of patients and thus offering the most robust power, and detected largely no significant difference in major adverse events rates between antibody administration and control treatment, and no difference between different dosages of evolocumab. Notably, evolocumab reduced the rate of abnormal liver function, and alirocumab was associated with reduced rates of death and abnormal kidney function. Meanwhile, we determined the extent of LDL-C lowering of anti-PCSK9 antibodies: LDL-C level was reduced by over 50 % even though patients were on stable statin treatment. The extents of other favorable lipids changes were also documented in our meta-analyses.
It is worth noting that the favorable effects of anti-PCSK9 antibodies were largely achieved in populations who were already on stable statin treatments, indicating an additive, or even synergistic effect to statin in lowering LDL-C levels. This is not a surprise because statin therapy has been well documented to increase PCSK9 levels [40]; thus, inhibiting PCSK9 might enhance the LDL-C lowering effects of statins. Indeed, missense mutations in PCSK9 increased response to statin therapy in unrelated hypocholesterolemic subjects and familial hypercholesterolemia patients [41]. Likewise, in our meta-analysis, the combination of anti-PCSK9 antibody and statin resulted in a very high-intensity LDL-lowering effect, which is recommended by the 2013 American College of Cardiology (ACC)/AHA guideline suggesting no LDL-C goals. Meta-analyses of RCTs on statins also demonstrated that further reductions in LDL-C produce definite further reductions in CVD events [42], even in people at low risk of CVDs [43].
Two fundamental elements could lead to safety concerns: firstly those due to monoclonal antibody administration and secondly due to achieving very low LDL-C levels. We were unable to obtain the mean LDL-C level at the end of antibody administration due to lack of patient-level data in our study. However, estimated lowest LDL-C levels from observations of each study were less than 50 mg/dl, which was more remarkable than those achieved in the recently presented IMPROVE-IT trial (Improved reduction of outcomes: vytorin efficacy international trial) [44]. A combination of simvastatin and ezetimibe led to a mean LDL-C level as low as 53.2 mg/dl at one-year follow-up in high-risk patients with acute coronary syndrome, but showed good safety outcomes, indicating that an even lower level of LDL-C might not result in safety concerns. This notion was further confirmed by two recently published trials with regard to both evolocumab and alirocumab, with a longer follow-up of approximately 12 months [23, 45]. The OSLER trial, an extension trial of several phase 2 and phase 3 parent trials (most of which were included in our meta-analysis), showed similar rates of adverse events in patients with LDL-C levels less than 40 mg/dl or less than 25 mg/dl as in those with higher LDL-C levels following evolocumab treatment [45]. The full-term follow-up of the ODYSSEY LONG TERM trial also revealed similar frequency of adverse events among patients who had a LDL-C level less than 25 mg/dl and those who did not [23]. More straightforwardly in our meta-analysis, administration of both anti-PCSK9 antibodies showed promising safety profiles, except that administration of alirocumab was associated with a higher rate of injection-site reactions.
Whether anti-PCSK9 antibody treatments could translate into improved cardiovascular outcomes remains to be confirmed. The ongoing FOURIER (NCT01764633) and ODYSSEY OUTCOMES (NCT01663402) trials will answer this question by assessing the effect of evolocumab and alirocumab on major CVD events with about five years follow-up. However, the probable clinical benefits could be preliminarily inferred based on current evidence: 1) anti-PCSK9 antibodies substantially reduced LDL-C, non-HDL-C and ApoB levels, all of which are positively associated with CVD events [46], and ‘a lower LDL-C, a better outcome’ has been indicated not only in the era of statins but also following combined use of statins and ezetimibe [44]; 2) anti-PCSK9 antibodies significantly increased HDL-C and ApoA1 levels, which are strongly associated with reduced CVD risk, even in patients achieving very low LDL-C [47]; 3) in the ARIC study, loss-of-function PCSK9 mutations resulted in 28 % (15 %) reduction in LDL-C, and 88 % (47 %) reduction in CHD risk in African-Americans (white people) [48]; combined analyses in other cohort studies also generated 30 % reduction in ischemic heart disease risk [49]; and 4) more direct evidence from the longer-term follow-up results of the OSLER and ODYSSEY LONG TERM trials, although with a limitation of exploratory analysis, both of these trials suggested that patients receiving anti-PCSK9 antibodies had a significantly lower risk of major adverse cardiovascular events, which is consistent with our study showing alirocumab reduced the rates of death. Notably, both trials demonstrated that the cumulative incidence curves diverged progressively over time; therefore, a more remarkable benefit might be expected given a longer-term follow-up. Provided the exploratory nature of these trials, the limited follow-up length and the small number of cardiovascular events, results from ongoing FOURIER (over 27,500 high-risk patients with cardiovascular disease) and ODYSSEY OUTCOMES trials (over 18,000 patients who have experienced an acute coronary syndrome event 4 to 52 weeks prior to randomization) are urgently needed to provide definite answers.
Study limitations
First, the meta-analysis was based on study-level instead of patient-level data. Second, a high level of heterogeneity exists in several analyses. Heterogeneities in patient profile (unrelated or familial hypercholesterolemia) and background lipid-lowering therapy (maximum-tolerated statin, statin-intolerance, or no background anti-lipid therapy) are likely to account for part of this heterogeneity. We performed subgroup analyses based on the type of study population and heterogeneity still existed (data not show). Therefore, we pooled these data with random-effects models. Third, additional ongoing trials evaluating the efficacy and safety of alirocumab are to be published in a few years. However, with respect to primary efficacy endpoint, dramatic upregulating-LDL effects needed to be reported to balance the lowering-LDL effects demonstrated in our study given the number of patients known to have participated in these ongoing trials (ODYSSEY CHOICE I, ODYSSEY OLE, and so on), which is unlikely. Fourth, with respect to analysis on safety profiles, wide-range 95 % CIs were observed in several endpoints, which made precise estimation of the incidences of these endpoints impossible. Meanwhile, several composite endpoints were included in our study, such as adjudicated cardiovascular events, which might lower the ability of detecting each individual endpoint. Fifth, most trials included in our study had a relatively short-term follow-up (12 and 52 weeks for evolocumab, and mostly 24 weeks for alirocumab), thus rare events could not be fully revealed. Sixth, we could not rule out bias of selective reporting on several safety outcomes; to minimize this bias, we reviewed all the materials (including supplementary materials and relevant publications in other papers) provided by these studies and extracted and analyzed all these data. Notably, no obvious selective reporting bias was detected in major safety endpoints, such as any TEAEs, serious TEAEs, abnormal liver function, abnormal kidney function, injection-site reactions, musculoskeletal disorders and so on. Seventh, most patients enrolled are white; therefore, caution should be taken to interpret in other populations.
Conclusions
Evolocumab and alirocumab were safe and well-tolerated, largely showing no significant differences in rates of common adverse events with placebo or ezetimibe controls. No difference was detected following different dosages of evolocumab treatments regarding safety profiles. Both anti-PCSK9 antibodies substantially reduced LDL-C by over 50 %, increased HDL-C levels, and resulted in favorable changes in other lipids. We await the results of ongoing trials evaluating their effects on CVD events.
Acknowledgements
BX was supported by the National Natural Science Foundation of China (NO. 81070195, 81270281, and 81200148). The funders had no role in the study design, data collection and analysis, writing of the report, and decision to submit the article for publication.
Abbreviations
- ADH
autosomal dominant hypercholesterolemia
- AST/ALT
aspartate aminotransferase/alanine aminotransferase
- CHD
coronary heart disease
- CI
confidence interval
- CK
creatine kinase
- CVD
cardiovascular disease
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- LDLR
LDL receptor
- PCSK9
proprotein convertase subtilisin/kexin type 9
- RCT
randomized controlled trial
- RR
relative risk
- TC
total cholesterol
- TEAE
treatment emergent adverse event
- TG
triglycerides
- ULN
upper limit of normal
- VLDL-C
very low-density lipoprotein cholesterol
Additional files
PRISMA 2009 Checklist.
Additional baseline characteristics of included randomized trials. Table S2. Safety endpoints and monitoring methods (mainly visiting periods) of included randomized trials. Table S3. Risk of bias analysis for included randomized trials. Figure S1. Forest plot demonstrating absolute changes in LDL cholesterol (LDL-C) stratified by dosages following evolocumab treatments versus placebo at 12 weeks follow-up. Table S4. Percent and absolute changes of LDL cholesterol (LDL-C) at week 12 after evolocumab treatment versus placebo or ezetimibe. Table S5. Percent and absolute changes of LDL cholesterol at mean of weeks 10 and 12 after evolocumab treatment versus placebo or ezetimibe. Table S6 to S15. Percent change of HDL cholesterol (Table S6), total cholesterol (Table S7), total cholesterol/HDL-C ratio (Table S8), non-HDL cholesterol (Table S9), VLDL cholesterol (VLDL-C, Table S10), apolipoprotein A1 (ApoA1, Table S11), apolipoprotein B (ApoB, Table S12), ApoB/ApoA1 ratio (Table S13), lipoprotein(a) (Lp(a), Table S14), and triglycerides (TG, Table S15) at week 12 and at mean of weeks 10 and 12 after evolocumab treatment versus placebo or ezetimibe. Figure S2 to S10. Forest plot demonstrating changes in total cholesterol (TC, Figure S2), total cholesterol/HDL-C ratio (Figure S3), non-HDL cholesterol (non-HDL-C, Figure S4), VLDL cholesterol (VLDL-C, Figure S5), apolipoprotein A1 (ApoA1, Figure S6), apolipoprotein B (ApoB, Figure S7), ApoB/ApoA1 ratio (Figure S8), lipoprotein(a) (Lp(a), Figure S9), and triglycerides (TG, Figure S10) stratified by dosages following evolocumab treatments versus placebo at 12 weeks follow-up. Table S16. Percent change of PCSK9 at week 12 after evolocumab treatment versus placebo. Figure S11. Forest plot demonstrating changes in lipid profiles following monthly 420 mg evolocumab treatments versus placebo at 52 weeks follow-up. Table S17. Percent changes of other endpoints following alirocumab treatment versus placebo or ezetimibe.
Footnotes
Xin-Lin Zhang, Qing-Qing Zhu and Li Zhu contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XZ, QZ, and LZ conceived the study, selected studies and extracted the data, analyzed and interpreted the data, wrote the first draft of the manuscript, and revised the manuscript. JC, QC, GL, JX, and LK contributed to the study protocol and analyzed and interpreted the data. BX conceived the study, interpreted the data, wrote the first draft of the manuscript, and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xin-Lin Zhang, Email: xinlzhang0807@gmail.com.
Qing-Qing Zhu, Email: athena_qqz@qq.com.
Li Zhu, Email: med_zhuli@163.com.
Jian-Zhou Chen, Email: 290601427@qq.com.
Qin-Hua Chen, Email: qh_chen87@163.com.
Guan-Nan Li, Email: lgnnju88@163.com.
Jun Xie, Email: hsp70xj@163.com.
Li-Na Kang, Email: lina_kang80@126.com.
Biao Xu, Email: xubiao@medmail.com.cn.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, Bairey MC, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–75. doi: 10.1038/nrcardio.2014.84. [DOI] [PubMed] [Google Scholar]
- 4.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–82. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong WJ, Wei J, Zuo ZY, Wang YM, Song DQ, You XF, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029–37. doi: 10.1016/j.metabol.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Schomig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:894–904. doi: 10.1016/j.jacc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, Bradburn MJ, Egger M. Meta-analysis in Stata. In: Egger M, Smith GD, Altman D, editors. Systematic reviews in health care. London: Blackwell BMJ Books; 2001. p. 357. [Google Scholar]
- 12.Higgins JP, Deeks JJ. Selecting studies and collecting data. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. 510. Chichester: The Cochrane Collaboration; 2011. p. 7:1. [Google Scholar]
- 13.Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–20. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 14.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 15.Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98. doi: 10.1016/j.jacc.2012.08.986. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JG. Long-term safety, tolerability and efficacy of alirocumab versus placebo in high cardiovascular risk patients: first results from the ODYSSEY LONG TERM study in 2,341 patients. Barcelona: Paper presented at 2014 Scientific Sessions of European Society of Cardiology; 2014. [Google Scholar]
- 17.Luscher TF. Efficacy and safety of alirocumab: results from the ODYSSEY COMBO II study and results of ODYSSEY FH I and FH II studies. Barcelona: Paper presented at 2014 Scientific Sessions of European Society of Cardiology; 2014. [Google Scholar]
- 18.Cannon CP. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study. Barcelona: Paper presented at 2014 Scientific Sessions of European Society of Cardiology; 2014. [Google Scholar]
- 19.Moriarty PM. ODYSSEY ALTERNATIVE: efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody, alirocumab, versus ezetimibe, in patients with statin intolerance as defined by a placebo run-in and statin rechallenge arm. Chicago: Paper presented at 2014 Scientific Sessions of American Heart Association; 2014. [Google Scholar]
- 20.Kereiakes DJ. Efficacy and safety of alirocumab in high cardiovascular risk patients with suboptimally controlled hypercholesterolemia on maximally tolerated doses of statins: the ODYSSEY COMBO I Study. Chicago: Paper presented at 2014 Scientific Sessions of American Heart Association; 2014. [Google Scholar]
- 21.Ginsberg HN. ODYSSEY HIGH FH: efficacy and safety of alirocumab in patients with severe heterozygous familial hypercholesterolemia. Chicago: Paper presented at 2014 Scientific Sessions of American Heart Association; 2014. [Google Scholar]
- 22.Bays H. Efficacy and safety of combining alirocumab with atorvastatin or rosuvastatin versus statin intensification or adding ezetimibe in high cardiovascular risk patients: ODYSSEY OPTIONS I and II. Chicago: Paper presented at 2014 Scientific Sessions of American Heart Association; 2014. [Google Scholar]
- 23.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of Alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–19. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 26.Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–40. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 29.Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–82. doi: 10.1253/circj.CJ-14-0130. [DOI] [PubMed] [Google Scholar]
- 30.Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129:234–43. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 31.Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 32.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–17. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 34.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 35.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 36.Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 37.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 38.Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 39.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Awan Z, Seidah NG, MacFadyen JG, Benjannet S, Chasman DI, Ridker PM, et al. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem. 2011;58:183–9. doi: 10.1373/clinchem.2011.172932. [DOI] [PubMed] [Google Scholar]
- 41.Berge KE. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol. 2006;26:1094–100. doi: 10.1161/01.ATV.0000204337.81286.1c. [DOI] [PubMed] [Google Scholar]
- 42.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)62027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannon CP. IMPROVE-IT trial: a comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndromes. Chicago: Paper presented at 2014 Scientific Sessions of American Heart Association; 2014. [Google Scholar]
- 45.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 46.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–12. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen JC, Boerwinkle E, Mosley TJ, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 49.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol. 2010;55:2833–42. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]


