Abstract
Background
Any form of surgery or tissue damage causes release of tissue factor into the circulation. This may lead to the accelerated consumption of coagulation factors, resulting in severe consumptive coagulopathy. In this study, we compared the molecular markers involved in coagulation activation during cardiopulmonary bypass (CPB) between patients who underwent aortic replacement surgery and those who underwent valve surgery.
Methods
This prospective observational study was performed in each 14 patients who underwent aortic replacement surgery or valve surgery. We evaluated the differences in the levels of fibrinogen, activated factor VII (FVIIa), thrombin–antithrombin complex (TAT), and soluble fibrin monomer complex (SFMC) during surgery between these two groups.
Results
The change in fibrinogen levels showed no difference between the groups. The magnitude of increase in TAT was much larger in patients who underwent aortic replacement surgery than in those who underwent valve surgery (173.6 vs. 49.4 ng/mL; p = 0.0001). More importantly, the elevation of FVIIa was significantly higher in patients who underwent aortic replacement (28.5 vs. 19.0 mU/mL; p = 0.0122). The magnitude of increase in SFMC was also larger in the aortic replacement surgery.
Conclusions
The activation of coagulation during CPB was dramatically higher in the aortic replacement surgery compared with the valve surgery, probably owing to the activation of the extrinsic coagulation pathway in the former. This could potentially exacerbate consumptive coagulopathy after CPB termination in patients who underwent aortic replacement, possibly resulting in massive hemorrhage due to impaired hemostasis.
Keywords: Tissue factor, Activated factor VII, Cardiopulmonary bypass, Aortic replacement surgery, Extrinsic coagulation pathway
Background
Cardiovascular surgery is frequently accompanied by a bleeding tendency, probably resulting from the impairment of platelet activation and coagulation caused by cardiopulmonary bypass (CPB). Aortic replacement surgery using CPB is frequently complicated by massive hemorrhage that in turn, is most commonly aggravated by severe hypofibrinogenemia due to dilutional coagulopathy. It has been reported that not only dilutional coagulopathy but also consumptive coagulopathy by continuous thrombin generation progresses during CPB despite full heparinization of blood [1, 2]. The activation of the intrinsic coagulation pathway including the contact phase has been regarded to be more important in thrombin generation during CPB because of the direct contact of circulating blood with CPB. However, the activation of the extrinsic coagulation pathway by tissue factor (TF) released into the circulation may be a primary origin of the thrombin generation during CPB [3]. TF, which is abundantly expressed in the vascular wall, epicardium, fat, bone, lungs, brain, and muscles [4, 5], activates factor VII (FVII) and forms the TF/activated factor VII (FVIIa) complex. This complex may then generate thrombin massively during CPB despite full heparinization because of the limited thrombin-inhibiting effect and the subsequent soluble fibrin formation of heparin with antithrombin on the extrinsic coagulation pathway.
In general, aortic replacement surgery is so invasive that it can potentially cause widespread tissue damage and increase the release of TF into the circulation. So far, some studies have shown the activation of extrinsic coagulation pathway during CPB in coronary artery bypass grafting (CABG) or valve replacement surgery. However, the pathology and mechanisms of consumptive coagulopathy during CPB in aortic replacement surgery have not been elucidated [6]. In this study, we analyzed and compared some molecular markers of coagulation activation {e.g., thrombin-antithrombin complex (TAT), soluble fibrin monomer complex (SFMC), FVIIa} during CPB between patients undergoing aortic replacement surgery and those with valve surgery. Significantly higher levels of TAT and FVIIa during CPB were observed in patients who underwent aortic replacement surgery compared with those who underwent valve surgery. This observation suggests the accelerated activation of coagulation and consumptive coagulopathy, through the activation of the extrinsic coagulation pathway in patients who underwent aortic replacement, possibly resulting in the impaired hemostasis frequently observed in aortic replacement surgery.
Methods
The Ethics Committee of the Teikyo University School of Medicine approved this study. We enrolled patients older than 20 years who were scheduled to undergo elective aortic replacement surgery or valve replacement surgery, including those undergoing surgery combined with CABG. With a significant level of 5 % and a power of 80 %, a total of 20 patients were needed to detect a 200 ng/ml difference in TAT level with a standard deviation of 150 ng/ml. We estimated that 30 patients should be included in the trial, 15 patients in each group, because of exclusion criteria and complications during surgery. Written Informed consent was obtained from all patients. From June 2013 until April 2014, 14 patients who underwent aortic replacement surgery and 14 patients who underwent valve replacement surgery were registered for this prospective observational study. Patients with congenital bleeding tendency or low platelet count (i.e., <100 × 103/μL) were excluded. Patients who were administered anticoagulant or antiplatelet agents were enrolled if these medications were stopped before surgery. Patients with significant liver and/or renal dysfunction were excluded as well.
Anesthesia was induced with fentanyl (4–6 μg/kg), midazolam (2–5 mg/body), and rocuronium (0.6–1.0 mg/kg), and then, sevofulrane was used for maintenance. The patients received artificial respiration, with oxygen concentration at 40 % and end tidal carbon dioxide maintained at 30–40 mmHg. A peripheral intravenous line, a radial or a brachial arterial line, a central venous catheter, and the Swan-Ganz catheter were used to monitor the patients. Heparin at 300 U/kg was injected before the start of CPB, and additional heparin was suitably administered so that the activated clotting time would be more than 400 s throughout the procedure. Heparin was neutralized with protamine (3 mg/kg) at the end of CPB. A heparin coating or macromolecule polymer coating was used in all CPB circuits. Blood from the pleural and pericardial cavities were recirculated through the reservoir by suction. The transfusion in each case was decided in charge by the anesthesiologist because we do not have a standard protocol.
Blood samples were taken through arterial catheterization at the induction of anesthesia, at the start of CPB, 1 h from the start of CPB, and at the end of CPB (i.e., after protamine administration). We measured fibrinogen, SFMC, and FVIIa by using STA-R Evolution (Diagnostica STAGO, Asnières sur Seine, France), while TAT was evaluated by using STACIA (LSI Medience, Tokyo, Japan). As a reagent, STA® Fibrinogen (Diagnostica STAGO) was used for fibrinogen, STACIA CLEAIR TAT (LSI Medience) for TAT, Auto LIA®FM (Roche Diagnostics K.K., Tokyo, Japan) for SFMC, and STACLOT® VIIa-rTF (Diagnostica STAGO) for FVIIa.
We reported the data as the mean ± standard deviation or as the median (first quartile – third quartile) value. We used the unpaired t-test or Mann-Whitney U test to identify differences in the demographic data of patients. The Mann-Whitney U test was used to determine significant differences in the levels of the indicated markers during CPB between the groups. A p-value of <0.05 was considered statistically significant. Microsoft Excel Statistics 2012 was used for the statistical analysis.
Results
The patient characteristics and preoperative laboratory data showed no significant differences between the groups, except that the TAT and SFMC levels were significantly higher in the aortic replacement surgery group (Table 1). No significant differences were also observed between the groups except the duration of CPB time, time required for hemostasis, bleeding and transfusion volumes during surgery (Table 2).
Table 1.
Demographic data of patients
| Aortic Replacement Group (n =14) | Valve Replacement Group (n = 14) | p value | |||
|---|---|---|---|---|---|
| Age (years) | 68.4 ± 6.5 | 70.8 ± 7.3 | 0.1809 | ||
| Sex (M/F) | 9/5 | 10/4 | |||
| Height (cm) | 164.3 ± 10.6 | 158.9 ± 10.3 | 0.0946 | ||
| Weight (kg) | 64.8 ± 16.8 | 57.6 ± 11.3 | 0.0961 | ||
| BSA (m2) | 1.70 ± 0.24 | 1.59 ± 0.19 | 0.0892 | ||
| Cases | Ascending Aorta Replacement + AVR | 2 | MVP | 1 | |
| AVR | 2 | ||||
| Descending Aorta Replacement + CABG | 1 | AVR + TAP | 1 | ||
| Descending Aorta Replacement | 2 | AVR + MVP | 2 | ||
| TAR + AVR | 1 | AVR + MVP + CABG | 1 | ||
| TAR + CABG | 3 | AVR + MVR + TAP | 1 | ||
| TAR | 4 | AVR + CABG | 5 | ||
| Ascending Aorta Replacement + AVR + CABG | 1 | MVR + TAP | 1 | ||
| Fibrinogen (mg/dL) | 339 (274–386) | 314 (287–356) | 0.4762 | ||
| TAT (ng/mL) | 7.7 (4–14.4) | 2.1 (1.2–3.2) | 0.0009* | ||
| SFMC (μg/mL) | 20.8 (4.1–109.2) | 3.9 (2.4–4.8) | 0.0038* | ||
| FVIIa (mU/mL) | 35.0 (26.3–42.3) | 29.5 (21.3–34.8) | 0.4616 | ||
| Hemoglobin (g/dL) | 11.6 (10.5–12.9) | 11.1 (10.1–13.2) | 0.5812 | ||
| Platelet (×103/mL) | 168 (138–194) | 169 (133–190) | 0.7304 | ||
| PT-INR | 1.17 (1.11–1.24) | 1.17 (1.14–1.21) | 0.9084 | ||
| APTT (s) | 33.6 (29.8–35.1) | 34.0 (29.9–37.0) | 0.6458 | ||
AVR aortic valve replacement, MVP mitral valve plasty, MVR mitral valve replacement
TAP tricuspid annuloplasty, CABG coronary artery bypass grafting, TAR total arch replacement
*Significant difference between the two groups
Table 2.
Intraoperative and postoperative data
| Aortic replacement group (n = 14) | Valve replacement group (n = 14) | p value | |
|---|---|---|---|
| Heparin dose for CPB (×103 IU) | 18.0 ± 5.2 | 17.6 ± 3.8 | 0.4182 |
| CPB time (min) | 216 (181–232) | 142 (112–179) | 0.0024* |
| Duration of aortic crossclamp (min) | 136 (110–182) | 120 (86–153) | 0.4479 |
| Time required for hemostasis | 77 (61–147) | 42 (36–68) | 0.0082* |
| Bleeding volume during surgery (mL) | 3080 (1888–4539) | 1535 (1204–1799) | 0.0009* |
| Postoperative blood loss-24 h (mL) | 1228 (611–1648) | 746 (459–1519) | 0.4622 |
| Transfusion of red cell concentrates during surgery (U) | 14 (10.5–17.5) | 9 (4.5–14) | 0.0421* |
| Transfusion of fresh frozen plasma during CPB (U) | 6 (4–6) | 0 (0–4) | 0.0012* |
| Transfusion of fresh frozen plasma during surgery (U) | 10 (7–20) | 2 (0–7.5) | 0.0023* |
| Transfusion of platelet concentrates during surgery (U) | 20 (20–20) | 0 (0–20) | 0.0014* |
| Postoperative hemoglobin (g/dL) | 10.9 (10.1–11.6) | 11.1 (9.8–12.2) | 0.8721 |
*Significant difference between the two groups
As it has been suggested that the bleeding tendency and impaired hemostasis during surgery depends upon the decrease in the plasma level of fibrinogen, we measured the fibrinogen level during CPB in each case to evaluate the progression of coagulopathy. Although we observed the tendency for hypofibrinogenemia progression during CPB in the aortic replacement surgery group, the magnitude of decrease in the fibrinogen level during CPB was not significantly different between the groups (Fig. 1). We transfused fresh frozen plasma (FFP) before termination of CPB as a conventional therapy to prevent severe hypofibrinogenemia. A median of 6 [3–5] units was transfused in 12 patients who underwent aortic replacement, and a median of 0 (0–4) units was transfused in 5 patients who underwent valve surgery, showing a significant difference between the groups (p = 0.0012).
Fig. 1.
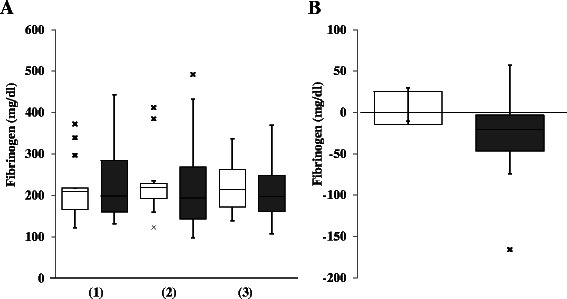
Comparison of fibrinogen level between the groups. a: Fibrinogen level at the start of cardiopulmonary bypass (CPB) (1), at 1 h from the start of CPB (2), and at the end of CPB (3). b: The change of fibrinogen level during CPB. □ (open column): valve surgery; ■ (closed column): aortic replacement surgery; x: outlier. Data are showed as box and whisker plots (25 and 75 percentiles, outliers). Outliers are showed as cases with values more than 1.5 times of box lengths from either end of the box. No significant difference in the change of fibrinogen level during CPB was detected between the groups
To examine the activation of coagulation during surgery, the levels of TAT and FVIIa during CPB were measured in each case. Although the TAT level at the start of CPB showed no difference between the groups, it dramatically increased during CPB in the aortic replacement group (Fig. 2a). The TAT levels at 1 h from the start of CPB and at the end of CPB were significantly higher in the aortic replacement group than those in the valve surgery group (i.e., 54.4 [40.8–76.9] ng/mL vs. 24.3 [14.9–33.6] ng/mL, p = 0.0051 at 1 h of CPB; 178.8 [144.2–262.5] ng/mL vs. 70.3 [50.6–80.4] ng/mL, p = 0.0001 at the end of CPB). Therefore, a significantly larger increase in the TAT level during CPB was demonstrated in the aortic replacement group than that in the valve surgery group (i.e., 173.6 [125.6–221.5] ng/mL vs. 49.4 [42.7–69.2] ng/mL, p = 0.0001) (Fig. 2b). Meanwhile, both the FVIIa level at the end of CPB and the increase in the FVIIa during CPB were significantly higher in the aortic replacement group than in the valve surgery group (i.e., 35 [24.5–43.8] mU/mL vs. 25 [16.5–26.8] mU/mL, p = 0.0289 at the end of CPB; 28.5 [22.3–36.5] mU/mL vs. 19.0 [15.3–24.3] mU/mL; p = 0.0122 during CPB) (Fig. 3). Thus, accelerated coagulation activation progressed via the extrinsic pathway during CPB in the aortic replacement surgery group compared with the valve surgery group.
Fig. 2.
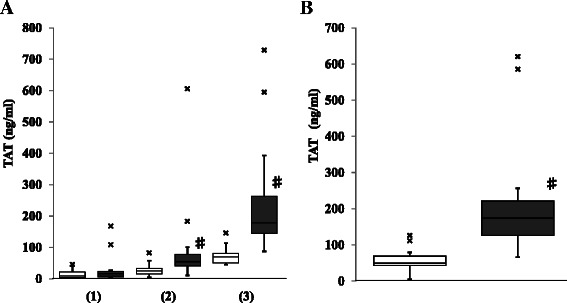
Comparison of thrombin–antithrombin complex (TAT) between the groups. a: TAT at the start of cardiopulmonary bypass (CPB) (1), at 1 h from the start of CPB (2), and at the end of CPB (3). b: The increase in TAT during CPB. □ (open column): valve surgery; ■ (closed column): aortic replacement surgery; x: outlier; Data are showed as box and whisker plots (25 and 75 percentiles, outliers). Outliers are showed as cases with values more than 1.5 times of box lengths from either end of the box. #: p < 0.05
Fig. 3.

Comparison of activated factor VII (FVIIa) between the groups. a: FVIIa at the start of cardiopulmonary bypass (CPB) (1), at 1 h from the start of CPB (2), and at the end of CPB (3). b: The increase in FVIIa during CPB. □ (open column): valve surgery; ■ (closed column): aortic replacement surgery; x: outlier; Data are showed as box and whisker plots (25 and 75 percentiles, outliers). Outliers are showed as cases with values more than 1.5 times of box lengths from either end of the box. #: p < 0.05
Finally, we analyzed the level of SFMC, which shows the degree of fibrin generation and consumption of fibrinogen, during CPB in each case. We observed significantly higher levels of SFMC at all measurement time points in the aortic replacement group than those in the valve surgery group (i.e., 15.0 [4.2–42.5] μg/mL vs. 4.1 [3.0–4.8] μg/mL, p = 0.0179 at the start of CPB; 24.5 [3.9–69.1] μg/mL vs. 3.9 [3.5–5.2] μg/mL, p = 0.0344 at 1 h of CPB; 78 [20–122.6] μg/mL vs. 19.8 [13.5–33.6] μg/mL, p = 0.0216 at the end of CPB) (Fig. 4a). Although the magnitude of increase in SFMC levels during CPB was larger in the aortic replacement surgery group, no significant difference was observed between the groups (Fig. 4b). To demonstrate the typical accelerated activation of coagulation in aortic replacement surgery compared with valve surgery, the time courses of fibrinogen, TAT, and SFMC in the representative case for each group are shown in Fig. 5. The aortic replacement surgery case showed a dramatic elevation of TAT and SFMC during CPB. A total of 720 mL of fresh frozen plasma was transfused to prevent progressing hypofibrinogenemia, resulting in diminished reduction of the fibrinogen level (Fig. 5b).
Fig. 4.
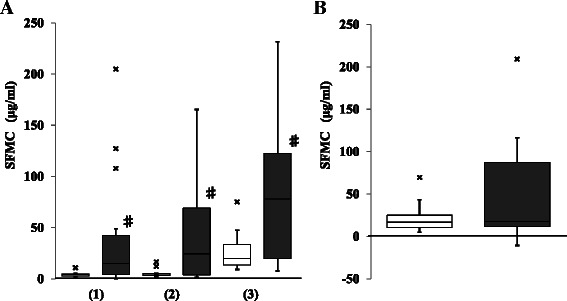
Comparison of soluble fibrin monomer complex (SFMC) between the groups. a: SFMC at the start of cardiopulmonary bypass (CPB) (1), at 1 h from the start of CPB (2), and at the end of CPB (3). b: The increase in SFMC during CPB. □ (open column): valve surgery; ■ (closed column): aortic replacement surgery; x: outlier; Data are showed as box and whisker plots (25 and 75 percentiles, outliers). Outliers are showed as cases with values more than 1.5 times of box lengths from either end of the box. #: p < 0.05. No significant difference in the increase in SFMC during CPB was detected between the groups
Fig. 5.
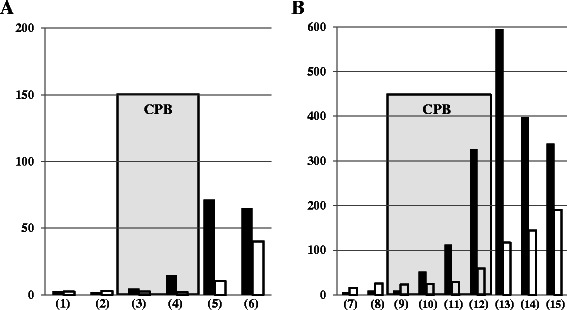
Time courses of coagulation markers during surgery in the representative case of each group. a: valve surgery; b: aortic replacement surgery. ■ (closed column): thrombin–antithrombin complex (ng/mL); □ (open column): soluble fibrin monomer complex (μg/mL). CPB time is shown by the gray zone. (1) and (7): pre-operation; (2) and (8): pre-CPB; (3) and (9): the start of CPB; (4) and (10): 1 h after the start of CPB; (5) and (13): the end of CPB; (6) and (15): post-operation; (11): 2 h after the start of CPB; (12): 3 h after the start of CPB; (14): 1 h after the end of CPB
Discussion
Cardiovascular surgery using CPB decreases the plasma concentration of coagulation factors, including fibrinogen, primarily by hemodilution with CPB priming and intravenous fluids [7]. Thoracic aortic replacement surgery is frequently accompanied by massive hemorrhage due to severe hypofibrinogenemia [8]. A recent report showed that fibrinogen concentration frequently fell below 150 mg/dL during CPB in patients who underwent thoracic aortic repair surgery [8]. We also observed the tendency for hypofibrinogenemia progression during CPB in the aortic replacement group, but not in the valve surgery group (Fig. 1). In order to avoid massive bleeding due to severe hypofibrinogenemia, we usually transfuse FFP before termination of CPB in aortic replacement surgery. Indeed, significantly more number of units of FFP was transfused during CPB in the aortic replacement group, implying that the fibrinogen levels tended to decrease more during CPB in aortic replacement surgery compared with valve surgery. Although there were no significant differences in the decrease in plasma fibrinogen level during CPB between the groups (Fig. 1), consumptive coagulopathy as well as dilutional coagulopathy may underlie the development of hypofibrinogenemia during CPB in aortic replacement surgery.
There have been several reports of increased coagulation activation markers (e.g., prothrombin fragment 1 + 2, TAT, fibrinopeptide A, fibrin monomer) during CPB in CABG surgery [9]. In this study, significantly higher TAT levels during CPB were demonstrated in the aortic replacement group compared with the valve surgery group (Fig. 2). The TAT level, which reflects thrombin generation, was extremely high (i.e., the mean value was 180 ng/mL) at the end of CPB in the aortic replacement surgery group, as demonstrated by the representative case (Fig. 5b). This observation indicates the dramatic activation of coagulation during CPB in aortic replacement surgery because of the TAT level of approximately 40 ng/mL that is typical of disseminated intravascular coagulation caused by sepsis or hematological malignancy [10]. The significant elevation of TAT during CPB in aortic replacement surgery may reflect the CPB time. However, the significant difference in TAT levels were already observed at 1 h from the start of CPB in spite of no difference at the start of CPB between the groups, indicating that the elevation of TAT did not correlate with the CPB time in each group. Thus, thrombin generation was strikingly elevated in the aortic replacement group, independent of the CPB time. The TAT will reflect the generation of thrombin through the prothrombinase complex, and this reflects both extrinsic and intrinsic pathways, more correctly tenase pathways, with a complexity of positive and negative feedback pathways and regulatory processes which are no doubt highly activated during cardiac surgery. Thrombin generation during CPB might be influenced by the concentration of heparin, recirculation of blood aspirated from surgical field, and the coating of the extracorporeal circuit [11–14]. Previous analyses of thrombin generation and extrinsic coagulation pathway activation in suctioned blood from the pericardium and pleural space [15, 16] have demonstrated that the TAT level from the pleural cavity was much higher than that in the plasma. Thus, TAT elevation during CPB in aortic repair surgery may be primarily attributed to the recirculation of blood aspirated from the surgical field.
More importantly, the elevation of FVIIa was also significantly higher in the aortic replacement surgery group compared with the valve surgery group (Fig. 3), indicating a highly activated extrinsic coagulation pathway in the latter group despite full heparinization during CPB in both groups. Although heparin can strongly inhibit the intrinsic coagulation pathway as well as thrombin, its inhibitory effect in the extrinsic coagulation pathway may be relatively weak. Several reports, including the analysis on time course of intraoperative coagulation activation in elective CABG surgery [6], have demonstrated the activation of extrinsic coagulation pathway during CPB [12]. FVII is activated by TF that is released from tissues and/or the vascular injury site. As aortic replacement surgery is highly invasive, leaked blood into the pleural cavity in the surgical field may contain high amounts of TF. This leaked blood is usually recirculated into the CPB through suction, resulting in the accelerated activation of the FVII in the patients’ blood. Taken together, the consumptive coagulopathy due to activation of extrinsic coagulation pathway, may progress continuously during CPB in aortic replacement surgery. It has been suggested that reducing thrombin generation in CPB may result in decreased blood loss and transfusion volumes [12, 14, 17]. Managing the heparin concentration during CPB may contribute to the inhibition of coagulation activation during CPB [18, 19]. Heparin induces the release of the tissue factor pathway inhibitor (TFPI), which binds to TF and inactivates it [10], from vascular endothelial cells [20]. The increase in TFPI in heparinized blood during CPB [21] may suppress TF/FVIIa activity to some extent. In any case, consumptive coagulopathy may progress during CPB in aortic replacement surgery as shown in this study, and thus, it is useful to evaluate the change of the activation markers of coagulation during CPB.
Although we observed the tendency for larger increases in the SFMC during CPB in the aortic replacement group compared with the valve surgery group, the difference was not significant between the groups (Fig. 4). SFMC includes soluble fibrin and consists of one-molecule of fibrin monomer and two-molecules of fibrinogen, fibrin degradation products, and fibrinogen. This explains how the level of SFMC is influenced by fibrin degradation products and fibrinogen, but it does not necessarily reflect an increase in TAT. Moreover, the half-life of SFMC (i.e., a couple of hours) is much longer than that of TAT (i.e., less than half hour). In some cases of thoracic aortic aneurysm, considerably high levels of SFMC (e.g., >50 μg/mL) were detected before surgery. The SFMC values were influenced by not only the activation of coagulation pathway but also fibrinolytic pathway. Moreover, the size of aneurysm may influence the data of SFMC due to the activation of fibrinolytic system, resulting in the variable data of SFMC before surgery. This resulted in no significance in the SFMC elevation during CPB between the groups. Thus, elevation of SFMC in aortic replacement surgery may be influenced not only by intraoperative tissue injury, but also by preoperative progression of vascular atherosclerosis. Sustained elevation of SFMC after CPB suggests intravascular fibrin or microthrombi formation after CPB, contributing to organ dysfunction in patients who underwent aortic replacement surgery [22, 23].
Conclusions
The activation of coagulation cascade as indicated by increases in FVIIa and increased thrombin generation in TAT during CPB was dramatically higher in the aortic replacement surgery group compared with the valve surgery group. This could potentially exacerbate consumptive coagulopathy during and after CPB in patients who underwent aortic replacement, possibly resulting in perioperative massive hemorrhage due to impaired hemostasis.
Acknowledgement
The authors thank Moe Fujimoto for technical assistance.
Funding
Internal sources.
Abbreviations
- APTT
Activated partial thromboplastin time
- AVR
Aortic valve replacement
- CABG
Coronary artery bypass grafting
- CPB
Cardiopulmonary bypass
- FFP
Fresh frozen plasma
- FVIIa
Activated factor VII
- MVP
Mitral valve plasty
- MVR
Mitral valve replacement
- PC
Platelet concentrate
- PT
Prothrombin time
- RBC
Red blood cell
- SFMC
Soluble fibrin monomer complex
- TAA
Thoracic aortic aneurysm
- TAP
Tricuspid annuloplasty
- TAR
Total arch replacement
- TAT
Thrombin–antithrombin complex
- TF
Tissue factor
- TFPI
Tissue factor pathway inhibitor
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HS collected samples, analyzed the data, and wrote the manuscript. KY designed the study and helped to write the manuscript. AK collected the data. YN statistically analyzed the data, SS reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hideo Sato, Phone: 03-3964-1211, Email: hideos3150@yahoo.co.jp.
Koji Yamamoto, Email: kojiy@saitama-med.ac.jp.
Akihito Kakinuma, Email: icg02177@nifty.com.
Yoshinori Nakata, Email: ynakata@med-teikyo-u.ac.jp.
Shigehito Sawamura, Email: sawamura@med.teikyo-u.ac.jp.
References
- 1.Brister SJ, Ofosu FA, Buchanan MR. Thrombin generation during cardiac surgery: is heparin the ideal anticoagulant? Thromb Haemost. 1993;70:259–262. [PubMed] [Google Scholar]
- 2.Boisclair MD, Lane DA, Philippou H, Sheikh S, Hunt B. Thrombin production, inactivation and expression during open heart surgery measured by assays for activation fragments including a new ELISA for prothrombin Fragment F1 + 2. Thromb Haemost. 1993;70:253–258. [PubMed] [Google Scholar]
- 3.Boisclair MD, Lane DA, Helen P, Esnouf MP, Sheikh S, Hunt B, Smith KJ. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood. 1993;82:3350–3357. [PubMed] [Google Scholar]
- 4.Yamamoto K, Loskutoff DJ. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samad F, Pandey M, Loskutoff DJ. Regulation of tissue factor gene expression in obesity. Blood. 2001;98:3353–3358. doi: 10.1182/blood.V98.12.3353. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter TF, LeBleu TH, Douglas JM, Jr, Leslie JB, Parker JK, Greenberg CS. Characterization of prothombin activation during cardiac surgery by hemostatic molecular markers. Anesthesiology. 1994;80:520–526. doi: 10.1097/00000542-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rahe-Meyer N, Pichlmaier M, Haverich A, Solomon C, Winterhalter M, Piepenbrock S, Tanaka KA. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102:785–792. doi: 10.1093/bja/aep089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto K, Usui A, Takamatsu J. Fibrinogen concentrate administration attributes to significant reductions of blood loss and transfusion requirements in thoracic aneurysm repair. J Cardiothorac Surg. 2014;9:90. doi: 10.1186/1749-8090-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood. 2003;101:4355–4362. doi: 10.1182/blood-2002-08-2400. [DOI] [PubMed] [Google Scholar]
- 10.Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, Ohmori T, Mimuro J, Sakata Y. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis:a prospective observational study. Crit Care. 2014;18:R13. doi: 10.1186/cc13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Despotis GJ, Joist JH, Hogue CW, Jr, Alsoufiev A, Joiner-Maier D, Santoro SA, Spitznagel E, Jl W. More effective suppression of hemostatic system activation in patients undergoing cardiac surgery by heparin dosing based on heparin blood concentration rather than ACT. Thromb Haemost. 1996;76:902–908. [PubMed] [Google Scholar]
- 12.De Somer F, Van Belleghem Y, Caes F, Francois K, Van Overbeke H, Arnout J, Taeymans Y, Van Nooten G. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2002;123:951–958. doi: 10.1067/mtc.2002.120334. [DOI] [PubMed] [Google Scholar]
- 13.Aldea GS, Soltow LO, Chandler WL, Triggs CM, Vocelka CR, Crockett GI, Shin YT, Curtis WE, Verrier ED. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg. 2002;123:742–755. doi: 10.1067/mtc.2002.120347. [DOI] [PubMed] [Google Scholar]
- 14.Aldea GS, O’Gara P, Shapira OM, Treanor P, Osman A, Patalis E, Arkin C, Diamond R, Babikian V, Lazar HL, Shemin RJ. Effect of anticoagulation protocol on outcome in patients undergoing CABG with heparin-bonded cardiopulmonary bypass circuits. Ann Thorac Surg. 1998;65:425–433. doi: 10.1016/S0003-4975(97)01347-7. [DOI] [PubMed] [Google Scholar]
- 15.Philippou H, Adami A, Davidson SJ, Pepper JR, Burman JF, Lane DA. Tissue factor is rapidly elevated in plasma collected from the pericardial cavity during cardiopulmonary bypass. Thromb Haemost. 2000;84:124–128. [PubMed] [Google Scholar]
- 16.Weerwind PW, Lindhout T, Caberg NE, De Jong DS. Thrombin generation during cardiopulmonary bypass the possible role of retransfusion of blood aspiration from surgical field. Thromb J. 2003;1:3. doi: 10.1186/1477-9560-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Despotis GJ, Joist JH, Hogue CW, Alsoufiev A, Kater K, Goodnough LT, Santoro SA, Spitznagel E, Rosenblum M, Lappas DG. The impact of heparin concentration and activated clotting time monitoring on blood conservation: a prospective, randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;110:46–54. doi: 10.1016/S0022-5223(05)80008-X. [DOI] [PubMed] [Google Scholar]
- 18.Despotis GJ, Joist JH. Anticoagulation and anticoagulation reversal with cardiac surgery involving cardiopulmonary bypass: an update. J Cardiothorac Vasc Anesth. 1999;13:18–29. [PubMed] [Google Scholar]
- 19.Koster A, Fischer T, Praus M, Haberzettl H, Kuebler WM, Hetzer R, Kuppe H. Hemostatic activation and inflammatory response during cardiopulmonary bypass: impact of heparin management. Anesthesiology. 2002;97:837–841. doi: 10.1097/00000542-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Broze GJ, Jr, Girard TJ. Tissue factor pathway inhibitor: structure-function. Front Biosci. 2013;17:262–280. doi: 10.2741/3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima T, Gando S, Kenmotsu O, Masio H, Goda Y, Kawahigashi H, Watanabe N. Another point of view on the mechanism of thrombin generation during cardiopulmonary bypass: Role of tissue factor pathway inhibitor. J Cardiothorac Vasc Anesth. 2001;15:60–64. doi: 10.1053/jcan.2001.20278. [DOI] [PubMed] [Google Scholar]
- 22.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335:1857–1863. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 23.Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD. On-pump versus off-pump coronary revascularization: evaluation of renal function. Ann Thorac Surg. 1999;68:493–498. doi: 10.1016/S0003-4975(99)00566-4. [DOI] [PubMed] [Google Scholar]


