Abstract
Purpose
Nuclear factor kappa-B (NF-κB), a transcriptional factor that has been shown to be constitutively active in cervical cancer, is part of an important pathway leading to treatment resistance in many tumor types. The purpose of our study was to determine whether expression of NF-κB in pre-treatment specimens and specimens taken shortly after treatment initiation correlated with outcome in cervical cancer patients treated with definitive chemoradiation.
Experimental Design
Eighteen patients with locally advanced cervical cancer were enrolled on a study in which cervical biopsies were obtained before radiation therapy and 48 hours after treatment initiation. Matched biopsies from 16 of these patients were available and evaluated for the nuclear expression of NF-κB protein using immunohistochemical staining.
Results
After median follow-up of 43 months, there were 9 total treatment failures. Nuclear staining for NF-κB was positive in 3 of 16 (19%) pre-treatment biopsies and 5 of 16 (31%) post-radiation biopsies. Pre-treatment expression of NF-κB nuclear staining correlated with increased rates of local-regional failure (100% vs. 23%, p = .01), distant failure (100% vs. 38%, p = .055), disease-specific mortality (100% vs. 31%, p = .03) and overall mortality (100% vs. 38%, p = .055). Best rates of local-regional failure (0%) and highest rates of overall survival (80%) were seen in the 5 patients where the expression of NF-κB was negative in pre-treatment samples but changed to positive in the samples obtained 48 hours after treatment initiation.
Conclusion
Our data suggest that pre-treatment nuclear expression of NF-κB may be associated with a poor outcome for cervical cancer patients treated with chemoradiation. Although these data require validation in a larger group of patients, the results support the continued study of the relationship between NF-κB and outcome in patients treated for carcinoma of the cervix.
Keywords: radiation, chemotherapy, cervical cancer, nuclear transcription factor kappa b
Introduction
Cervical cancer continues to be a major cause of morbidity and mortality worldwide, with an estimated incidence of over 11,000 new cases in the United States alone(1). Most patients with locally-advanced disease (IB2—IVA) achieve a durable response to definitive concurrent cisplatin-based chemoradiation therapy (2–4), however a majority of those who recur have a poor prognosis despite improving salvage therapies (5). Being able to predict which patients are likely to recur after standard therapy through tumor molecular profiling and biomarker studies is likely to improve overall outcome in these patients by helping to select those who may benefit from individualized targeted therapies.
NF-κB is a transcription factor whose role in the pathogenesis of a wide variety of tumor types including cervical cancer has been well described (6–8). Cumulative evidence suggests that this protein functions as a mediator of cellular survival, inflammation, angiogenesis, and treatment resistance through regulating the transcription of over 200 target genes. NF-κB has recently become a major target for cancer drug development as demonstrated by the many non-specific natural and synthetic compounds shown to exert their therapeutic effects at least in part through the inhibition of NF-κB (9, 10). Furthermore recent pre-clinical and clinical studies that combine these agents with radiation and chemotherapy have shown promising results (9–11).
The role of NF-κB as a potential prognostic biomarker has only recently been explored. In several cancers including breast, prostate, skin, lung, and pancreas, NF-κB nuclear expression has correlated with poor clinical outcome (12–16). To date, no studies have evaluated the clinical prognostic significance of NF-κB nuclear expression in human cervical cancer.
Our goal in the current study was to evaluate the predictive potential of NF-κB through immunohistochemistry evaluation of prospectively collected paired cervical tumor biopsy samples obtained before and shortly after the initiation of definitive chemoradiation in patients with locally advanced cervical cancer.
Methods
This study was conducted as part of a prospective pilot study that aimed to evaluate gene expression profiling and potential molecular biomarkers in patients with locally advanced cervical cancer who were treated with definitive chemoradiation. Data from the gene expression profiling investigations have been published recently (17). The protocol was approved by the institutional review board at M. D. Anderson Cancer Center and all enrolled patients provided written informed consent to be a part of the protocol.
Patients with FIGO stage IB2 or IIA (with tumors > 4cm) and stage IIB or IIIB squamous cell carcinoma of the cervix were included. Patients with any previous treatment for cervical cancer were excluded. Treatment consisted of radiation therapy to a dose of 45 Gy to the pelvis in 180 cGy fractions using AP/PA fields and 15- or 18-MV photons, delivered concurrently with weekly cisplatin chemotherapy (usually 40 mg/m2 with or without 5-fluorouracil). Patients with suspicious para-aortic lymph nodes on CT were biopsied and if positive, patients received extended field radiotherapy to encompass the para-aortic lymph nodes. No patients underwent para-aortic lymphadenectomy. This was followed by 2 low-dose rate intracavitary brachytherapy treatments (after-loading tandem and ovoid system) spaced 2 weeks apart. During the second intracavitary treatment, the final (usually the sixth) chemotherapy dose was given.
For the purpose of this study, biopsies of the cervical cancer were performed before and after approximately 48 hours from the start of treatment. At the time of the second biopsy, all patients had received 2 or 3 external-beam treatments and the first dose of chemotherapy.
Immunohistochemistry
Slides for immunohistochemistry staining were made from four-nm tissue sections taken from paraffin blocks that were mounted on charged slides. Slides were then deparaffinized and rehydrated with descending grades of ethyl alcohol. Immunohistochemistry staining was performed using the NF-κβ (Santa Cruz cat # sc109, diluted 1:100) antibody. All biopsy specimens taken before treatment and 48 hours after treatment initiation were analyzed.
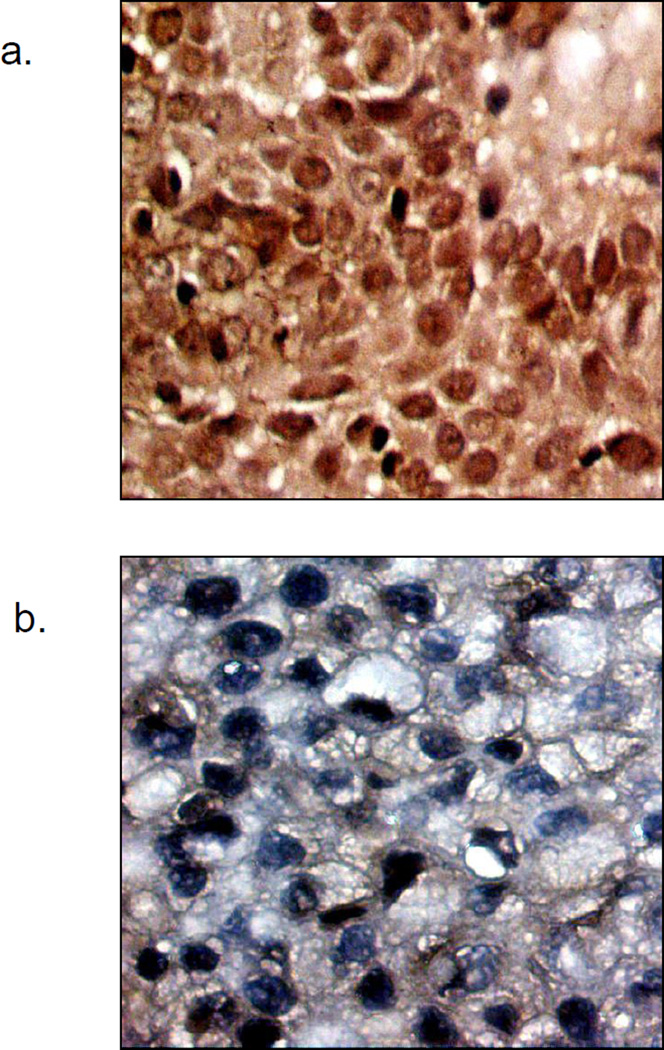
A gynecologic pathologist (R.B.), who was blinded to treatment outcomes, scored the specimens according to the intensity of nuclear staining as compared to cytoplasmic staining (absent [0%], weak [< 20%], moderate [>20% < 50%], and strong [>50%]). This method is consistent with previously published reports that categorize NF-κB staining according to percentage of positive staining (18–20). Positive and negative controls were used for each set of stained slides. Cases with absent or weak nuclear staining were labeled negative and those with moderate or strong staining were considered positive. Figure 1 shows an example of positive nuclear staining (a) and negative nuclear staining (b).
Figure 1.
a. Example of positive nuclear staining of NF-κB.
b. Example of negative nuclear staining of NF-κB.
Statistical Analyses
A Chi-square or Fisher’s exact test was used to analyze the relationships of nuclear expression of NF-κB in the pre-treatment and post-treatment groups with clinical outcomes. All statistical analyses were performed using SPSS software version 12. Local-regional failure was defined as central, pelvic, or para-aortic recurrence.
Results
Clinical characteristics of patients in study
A total of 18 patients with cervical cancer were enrolled in the study and treated with chemoradiation between April 2001 and August 2002. Tumor specimens were inadequate in 2 patients therefore the analysis included 16 patients. The clinical characteristics and outcomes of these 16 patients are presented in Table 1. At a median follow-up of 43 months (range 9–79 months), 9 of 16 patients (56%) had experienced either local or distant recurrence. Of these 9 patients with recurrent disease, 3 had pelvic recurrences; 2 with an isolated central recurrence and 1 with an isolated pelvic nodal recurrence. Four patients developed para-aortic recurrences outside their radiation fields (1 of whom developed central failure first) and 3 patients’ recurrences were distant only. Six of the 7 patients with pelvic or para-aortic recurrences developed subsequent distant metastases. Patients with CT evidence of pelvic lymphadenopathy at presentation were more likely to develop subsequent local or distant failure than patients with negative initial pelvic scans (88% versus 25%, p = 0.01). Median time to first failure was 21 months (range 3–38 months). By last follow-up, 8 out of 16 patients (50%) were alive including 2 patients with disease (13%). Seven of the remaining 8 patients died of their disease at a median of 34 months after diagnosis.
Table 1.
Clinical characteristics and outcomes
| Patient | Age | Follow−up (mo) | FIGO stage | LN status1 | Grade | Relapse2 | Survival | NF− κB Pre3 | NF− κB Post4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | 59 | IIIA | Pelvic | 3 | none | Alive | − | − |
| 2 | 56 | 16 | IIB | Pelvic + PA | 2 | distant | Alive | − | − |
| 3 | 40 | 74 | IB2 | none | 2 | none | Alive | − | − |
| 4 | 36 | 50 | IIB | none | 2 | none | Dead | − | − |
| 5 | 53 | 43 | IB2 | Pelvic | pelvic | Dead | − | − | |
| 6 | 54 | 56 | IIB | none | 2 | distant | Alive | − | + |
| 7 | 49 | 9 | IIB | none | 3 | paraaortic | Dead | + | − |
| 8 | 44 | 76 | IIB | none | 2 | none | Alive | − | + |
| 9 | 54 | 79 | IIB | none | none | Alive | − | + | |
| 10 | 61 | 41 | IIIB | Pelvic | 3 | central | Dead | + | − |
| 11 | 66 | 34 | IIIB | Pelvic + PA | 2 | distant | Dead | − | + |
| 12 | 46 | 32 | IIB | Pelvic | 3 | paraaortic | Dead | − | − |
| 13 | 30 | 19 | IB2 | Pelvic | 3 | paraaortic | Dead | + | − |
| 14 | 33 | 73 | IB2 | none | 2 | none | Alive | − | − |
| 15 | 40 | 43 | IIB | none | none | Alive | − | + | |
| 16 | 28 | 34 | IB2 | Pelvic | 3 | central | Dead | − | − |
FIGO, International Federation of Gynecology and Obstetrics
CT evidence of pelvic and/or para-aortic (PA) lymph node involvement prior to chemoradiation
Site of first relapse
NF-κB expression before treatment initiation
NF-κB expression 2 days after treatment initiation
Eight patients (50%) received concurrent weekly cisplatin and 8 (50%) received cisplatin/5-FU. Four patients (all who received 5-FU along with cisplatin) received less than the prescribed dose of chemotherapy due to toxicity (3 patients received only 3 cycles and 1 patient received 5 cycles with a 50% dose reduction). The median primary tumor size was 6.3 cm (range 3–8 cm). The type of chemotherapy regimen and tumor size did not correlate with any clinical endpoint. The median dose to Point A was 88.5 Gy (range 84.1–96.1 Gy).
Correlation of NF-κB expression with clinical outcome
In the pre-treatment biopsy specimens, nuclear staining for NF-κB was negative in 13 patients (81%) and positive in 3 patients (19%). In the post-treatment specimens, 11 (69%) were considered negative and 5 (31%) were positive. Selected characteristics as they relate to NF-κB staining are presented in Table 2. In the pre-treatment specimens, NF-κB positivity correlated with increased rates of distant metastases (100% vs. 31%, p = .03), local-regional-failure (100% vs. 23%, p = .02), and overall relapse (local and distant, 100% vs. 38%, p = .055). In the post-treatment specimens, local-regional failure occurred more often in patients with negative nuclear staining for NF-κB (45% vs. 0%, p = .07). Neither pre-treatment nor post-treatment expression correlated with other variables including lymph node status at presentation or stage.
Table 2.
NF-κB staining and clinical outcomes
| Pre-treatment | Post-treatment initiation | |||||
|---|---|---|---|---|---|---|
| Characteristic | NF-κB positive1 | NF-κB negative2 | p-value | NF-κB positive1 | NF-κB negative2 | p-value |
| Overall Survival | ||||||
| Alive | 0 | 8 | .06 | 4 | 4 | .11 |
| Dead | 3 | 5 | 1 | 7 | ||
| Disease-specific Survival | ||||||
| Alive | 0 | 9 | .03 | 4 | 5 | .20 |
| Dead | 3 | 4 | 1 | 6 | ||
| Distant Metastases | ||||||
| Yes | 3 | 5 | .06 | 2 | 6 | .59 |
| No | 0 | 8 | 3 | 5 | ||
| Local-regional Failure3 | ||||||
| Yes | 3 | 3 | .01 | 0 | 6 | .04 |
| No | 0 | 10 | 5 | 5 | ||
positive includes moderate or strong nuclear staining
negative includes absent or weak nuclear staining
local-regional includes central, pelvic, and para-aortic relapses
When comparing pre-treatment and post-treatment specimens, 5 patients (31%) had a change to positive nuclear staining after initial negative staining, 3 patients (19%) had a change to negative nuclear staining after an initial positive nuclear stain, and 8 patients (50%) had no change in nuclear staining category. Changes in these expression patterns as they correlate with clinical outcomes are presented in Table 3. In the 5 patients who had a change to positive nuclear staining, there was a significantly lower rate of local-regional failure (0%) than in those without a change in staining (38%) or in those who had a change to negative nuclear staining (100%) ( p = .01). Non-significant improvements in rates of distant metastases and relapse of any kind favored these 5 patients as well.
Table 3.
NF-κB staining pattern changes and clinical outcome
| Characteristic | Change to positive1 | Change to negative2 | No change3 | p-value |
|---|---|---|---|---|
| Survival | ||||
| Alive | 4 | 0 | 4 | .09 |
| Dead | 1 | 3 | 4 | |
| Disease-specific Survival | ||||
| Alive | 4 | 0 | 5 | .08 |
| Dead | 1 | 3 | 3 | |
| Distant Metastases | ||||
| Yes | 2 | 3 | 3 | .16 |
| No | 3 | 0 | 5 | |
| Local-regional failure4 | ||||
| Yes | 0 | 3 | 3 | .02 |
| No | 5 | 0 | 5 |
negative in pre-radiation specimen and subsequently positive in specimen taken 48 hours after treatment initiation
positive in pre-radiation specimen and subsequently negative in specimen taken 48 hours after treatment initiation
no change in positivity or negativity between the patient specimens
local-regional includes central, pelvic, and para-aortic relapses
Discussion
To our knowledge this is the first report evaluating NF-κB nuclear expression as a potential prognostic marker in locally advanced cervical cancer patients treated with definitive chemoradiation therapy. Our results suggest that pre-treatment expression of NF-κB may correlate with more aggressive tumor behavior as evidenced by significantly increased local-regional (100% vs. 23%) and distant failure rates (100% vs. 38%) in those with positive nuclear staining. Interestingly, the lowest rates of local and distant failure (0% and 20%, respectively) and highest rate of survival (100%) were seen in the subgroup of patients whose matched biopsy specimens demonstrated a change in NF-κB expression from negative in the pre-treatment sample to positive in the specimen taken 48 hours after treatment initiation.
Finding molecular markers that are able to effectively predict treatment failure after chemoradiation will allow a more individualized approach to cervical cancer by selecting patients who may benefit from targeted therapies. NF-κB is a necessary component for normal cellular response to stress and immunity, but abnormally elevated NF-κB signaling in tumors has been shown to be an important contributor to cellular resistance to chemotherapy and radiation (6, 9, 10, 21). It does so in part through the transcription of target genes that lead to increased cellular survival (c-Myc, Cyclin D1), inhibition of apoptosis (cIAP, Bcl-2) and overall reduction in cell death in response to treatment. This is further evidenced by the potentiation of chemotherapy- and radiation-induced apoptosis and treatment effect by the targeted inhibition of NF-κB (6, 9, 10, 22).
The aberrant constitutive activation of NF-κB in cervical cancer has been shown by two independent reports (7, 8). One group used 106 paraffin-embedded cervical tissue specimens of different histologic grades to show that NF-κB expression increased progressively with more invasive tumors (7). A subsequent report confirmed the progressive DNA-binding activity of NF-κB with higher grade cervical lesions (8). Taken together, this data supports the hypothesis that NF-κB may be a valuable target in cervical cancer as in other tumor types.
The role of NF-κB as a potential prognostic biomarker in tumors is less clear. Only a handful of reports have demonstrated that NF-κB expression correlates with poor clinical outcome in a variety of other tumor types. This has been postulated to be the result of enhanced NF-κB-mediated transcription of genes related to tumor progression and treatment resistance. In a previous study of breast cancer specimens at our institution, the nuclear expression of NF-κB as assessed by immunohistochemistry correlated with the expression of Bcl-2 and Bax, leading to the conclusion that the NF-κB/Bcl-2 pathway may be associated with a poor response to neoadjuvant chemotherapy (12). In 42 prostate cancer patients with a positive margin after radical prostatectomy, NF-κB nuclear expression predicted strongly for biochemical recurrence on multivariate analysis (13). Furthermore, the in vitro and in vivo study of melanoma demonstrated that NF-κB nuclear expression inversely correlated with disease-specific survival and was an independent adverse prognostic factor (14). Finally, two recent reports in non-small cell lung cancer and pancreatic cancer have demonstrated that NF-κB nuclear expression independently predicts for poor outcome (15, 16).
Results from the current study require validation in a larger sample size. While enhanced expression of NF-κB in tumors prior to the start of chemoradiation may indicate aggressive tumor biology and greater resistance to treatment, our finding of significantly worse clinical outcomes among those with increased nuclear expression of NF-κB in pre-treatment samples were based on relatively few patients and total events. Furthermore, the technique of NF-κB staining and the subsequent scoring of individual slides are subject to uncertainty. For example, the finding of worse outcomes in patients whose tumors changed from positive NF-κB expression in pre-treatment specimens to negative expression in specimens taken 2 days after treatment initiation is somewhat counterintuitive (see table 3) given that the targeted inhibition of NF-κB expression in cervical cancer (among other tumor types) leads to enhanced radiosensitivity and increased cancer cell death (23). A mechanism for this interesting finding may be related to the pulsatile and temporal nature of NF-κB activation in response to stimuli such as radiation (24), the effects of which may be best illustrated by evaluating additional biopsies taken at defined intervals throughout the course of treatment. Finally, while our report did not report HPV status, future series evaluating the role of NF-κB in cervical cancer would benefit from such correlation in light of recent data supporting a major role of NF-κB in HPV-mediated carcinogenesis (25, 26).
In conclusion, our data suggests that the pre-treatment expression of NF-κB may be associated with a poor outcome in locally advanced cervical cancer patients treated with definitive chemoradiation therapy and additional studies aimed at validating this finding in independent data sets are warranted. Furthermore, changes in nuclear expression of NF-κB shortly after treatment initiation may hold prognostic value. Taken together, our results support the continued study of NF-κB as a potential prognostic biomarker in cervical cancer.
Footnotes
Conflict of interest statement: No authors have any conflict of interest with regard to the work submitted in this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 3.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 4.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–972. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]
- 5.Leitao MM, Jr, Chi DS. Recurrent cervical cancer. Curr Treat Options Oncol. 2002;3:105–111. doi: 10.1007/s11864-002-0056-6. [DOI] [PubMed] [Google Scholar]
- 6.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 7.Nair A, Venkatraman M, Maliekal TT, et al. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- 8.Prusty BK, Husain SA, Das BC. Constitutive activation of nuclear factor -kB: preferntial homodimerization of p50 subunits in cervical carcinoma. Front Biosci. 2005;10:1510–1519. doi: 10.2741/1635. [DOI] [PubMed] [Google Scholar]
- 9.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 10.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Campo JM, Prat A, Gil-Moreno A, et al. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008;110:S72–S76. doi: 10.1016/j.ygyno.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Buchholz TA, Garg AK, Chakravarti N, et al. The nuclear transcription factor kappaB/bcl-2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Clin Cancer Res. 2005;11:8398–8402. doi: 10.1158/1078-0432.CCR-05-0885. [DOI] [PubMed] [Google Scholar]
- 13.Fradet V, Lessard L, Begin LR, et al. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 14.Gao K, Dai DL, Martinka M, et al. Prognostic significance of nuclear factor-kappaB p105/p50 in human melanoma and its role in cell migration. Cancer Res. 2006;66:8382–8388. doi: 10.1158/0008-5472.CAN-05-4402. [DOI] [PubMed] [Google Scholar]
- 15.Jin X, Wang Z, Qiu L, et al. Potential biomarkers involving IKK/RelA signal in early stage non-small cell lung cancer. Cancer Sci. 2008;99:582–589. doi: 10.1111/j.1349-7006.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97:523–530. doi: 10.1038/sj.bjc.6603878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klopp AH, Jhingran A, Ramdas L, et al. Gene expression changes in cervical squamous cell carcinoma after initiation of chemoradiation and correlation with clinical outcome. Int J Radiat Oncol Biol Phys. 2008;71:226–236. doi: 10.1016/j.ijrobp.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 18.Charalambous MP, Lightfoot T, Speirs V, et al. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009;101:106–115. doi: 10.1038/sj.bjc.6605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espinosa I, Briones J, Bordes R, et al. Activation of the NF-kappaB signalling pathway in diffuse large B-cell lymphoma: clinical implications. Histopathology. 2008;53:441–449. doi: 10.1111/j.1365-2559.2008.03139.x. [DOI] [PubMed] [Google Scholar]
- 20.Long YM, Ye S, Rong J, et al. Nuclear factor kappa B: a marker of chemotherapy for human stage IV gastric carcinoma. World J Gastroenterol. 2008;14:4739–4744. doi: 10.3748/wjg.14.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed KM, Cao N, Li JJ. HER-2 and NF-kappaB as the targets for therapy-resistant breast cancer. Anticancer Res. 2006;26:4235–4243. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 23.Kamer S, Ren Q, Dicker AP. Differential radiation sensitization of human cervical cancer cell lines by the proteasome inhibitor velcade (bortezomib, PS-341) Arch Gynecol Obstet. 2009;279:41–46. doi: 10.1007/s00404-008-0667-7. [DOI] [PubMed] [Google Scholar]
- 24.Ashall L, Horton CA, Nelson DE, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branca M, Giorgi C, Ciotti M, et al. Upregulation of nuclear factor-kappaB (NF-kappaB) is related to the grade of cervical intraepithelial neoplasia, but is not an independent predictor of high-risk human papillomavirus or disease outcome in cervical cancer. Diagn Cytopathol. 2006;34:555–563. doi: 10.1002/dc.20514. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Oh JM, No JH, et al. Involvement of NF-kappaB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis. 2009;30:753–757. doi: 10.1093/carcin/bgp066. [DOI] [PubMed] [Google Scholar]