Abstract
Objective
To determine whether periodontitis is associated with human papillomavirus (HPV) status of head and neck squamous cell carcinoma (HNSCC).
Design and Setting
Hospital-based case-control study in a comprehensive cancer center.
Patients
Evaluation included all patients diagnosed with incident primary squamous cell carcinoma of the oral cavity, oropharynx, and larynx between 1999 and 2007 for whom tissue samples and dental records were available (N = 124). Patients younger than 21 years and those with a history of cancer were excluded. Periodontitis history was assessed by alveolar bone loss in millimeters from panoramic radiographs by one examiner blinded to cancer status.
Main Outcome Measure
The presence of HPV-16 DNA in paraffin-embedded tumor samples was identified by polymerase chain reaction.
Results
The prevalence of HPV-positive HNSCC was 50 of 124 patients (40.3%). A higher proportion of oropharyngeal cancers were HPV-positive (32 of 49 [65.3%]) compared with oral cavity (9 of 31 [29.0%]) and laryngeal (9 of 44 [20.5%]) cancers. Each millimeter of alveolar bone loss was associated with 2.6 times increased odds (odds ratio [OR], 2.61; 95% CI, 1.58-4.30) of HPV-positive tumor status after adjustment for age at diagnosis, sex, and smoking status. The strength of the association was greater among patients with oropharyngeal SCC (OR, 11.70; 95% CI, 2.09-65.53) compared with those with oral cavity SCC (OR,2.32; 95% 0,0.65-8.27) and laryngeal SCC (OR, 3.89; 95% CI, 0.95-15.99).
Conclusions
A history of chronic inflammatory disease in the oral cavity may be associated with tumor HPV status in patients with HNSCC. This association seems to be stronger among palienLs with oropharyngeal cancer compared with those who have oral cavity or laryngeal SCC.
The National Cancer Institute’s Surveillance, Epidemiology and End Results Program has reported a steady increase in the incidence of oropharyngeal cancers in the United States since 1973 despite the significant decline in tobacco use since 1965.1 Similar trends are observed in other parts of the world, and the underlying reason for this increase is attributed mainly to oral human papillomavirus (HPV) infection.2, 3
A highly effective vaccine to prevent cervical HPV infection is recommended for females aged 9 to 26 years and males aged 9 to 21 years before potential exposure.4 However, oral HPV infection can be transmitted at or any time after birth, and the target population for the vaccine to prevent oral HPV infection has not been defined.2,3 A large percentage of the general population who have been exposed to the virus, as well as those who have developed an HPV-related disease, do not benefit from the vaccine. The HPV is commonly transmitted and most of the infections are cleared rapidly by the immune system. Rather than the mere presence of the virus at one time point, its persistence is critical for the development of HPV-related diseases.5 Therefore, targeting factors associated not only with the acquisition but also with the persistence of the infection may contribute to its prevention and treatment.
Epidemiologic studies6,7 suggest that chronic inflammation of the cervix increases the risk for cervical HPV infection and cervical cancer. In addition, molecular studies8,9 have shown that inflammatory cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor, modulate proliferation of HPV and expression of its oncogenes E6 and E7 in cervical epithelial cells. Furthermore, a nonsteroidal anti-inflammatory drug has been shown10 to induce degradation of HPV oncoproteins, leading to growth arrest and apoptosis of cervical carcinoma cells. The oral cavity and cervix are lined with similar types of mucosa, and the same HPV types cause cervical cancer and head and neck squamous cell carcinoma (HNSCC). In the oral cavity, periodontitis is a chronic inflammation of structures around teeth (periodontium) associated with gram-negative anaerobic bacteria in dental biofilm.11 The ensuing chronic inilammation leads to local pathologic anatomic changes, namely, periodontal pocket formation and alveolar bone loss (ABL) that is usually irreversible. The inflamed periodontium continuously releases inflammatory cytokines into saliva. The level of these cytokines in saliva is proportional to the extent and severity of periodontitis.12
Previous studies have suggested that chronic periodontitis is associated with increased risks of oral premalignant lesions13 and HNSCC.14 In addition, a history of periodontitis predicted poorly differentiated tumor status in the oral cavity.14 A small study15 among patients with base-of-tongue cancer suggested a synergy between periodontitis and tumor HPV status. The present study extends this work to assess the association of periodontitis and other dental factors with the presence of HPV-16 in oral cavity, oropharyngeal, and laryngeal cancers.
METHODS
STUDY DESIGN AND POPULATION
We performed a hospital-based case-control study. The outcome of interest was the tumor HPV status. Therefore, cases were patients with HPV-positive tumors and controls were patients with HPV-negative tumors. The study sample was derived from the patient population of the Department of Dentistry and Maxillofacial Prosthetics (DMFP), Roswell Park Cancer Institute, Buffalo, New York. All patients with newly diagnosed primary SCC of the oral cavity, oropharynx, and larynx between June 15,1999, and September 14,2007, who had tissue samples available from the pathology archives were included. Patients who were younger than 21 years and those with a history of cancer were excluded. In addition, patients hospitalized with other conditions that might be associated with periodontitis were excluded. These conditions included immunodeficiency, congenital anomalies, and trauma, gunshot, and motor vehicle accidents that involve the periodontium. The DMFP department is located within the same area as the Department of Head and Neck Surgery and provides dental services to patients with cancer as well as to healthy individuals. All patients seen in the Head and Neck Surgery department who receive radiotherapy and/or chemotherapy are admitted to the DMFP department before initiation of their cancer treatment. During the study period, 247 patients who met the inclusion criteria were seen in the Head and Neck Surgery department and were tested for tumor HPV status. From those, 219 patients (88.7%) had pathology samples of sufficient quality to determine tumor HPV status and 124 patients (50.2%) were seen in the DMFP department and composed the study population. Most patients came from surrounding Erie, Niagara, and Chautauqua counties. The institutional review boards of Roswell Park Cancer Institute and the State University of New York at Buffalo approved the study protocol.
The following sites, coded according to the International Classification of Diseases for Oncology, third edition,16 were included: oral cavity (oral tongue [C02.0-C02.9], gum [C03.0-C03.9], floor of the mouth [C04.0-C04.9], hard palate [C05.0], buccal mucosa [C06.01, vestibule 1C06.11, and retromolar area [C06.2]), oropharynx (base of tongue [COLO], soft palate [C05.1], tonsil (C09.0-C09.9], and oropharynx [C10.0-C10.9]), and larynx (glottis [C32.0], supraglottis [C32.1]), and subglottis [C32.2]). The remaining head and neck cancer sites were excluded to increase the homogeneity of the study population.
ASSESSMENT OF DENTAL VARIABLES
The severity of ABL, as well as the presence of dental caries, fillings, and missing teeth, was assessed from oral panoramic radiographs obtained at the time of admission before the initiation of cancer treatment. Alveolar bone loss was measured in millimeters, using an operator-interactive program from digitized radiographic images, as described previously.15 The measurements were performed at the mesial and distal sites of all natural teeth, except the third molars, by a trained and calibrated periodontist (M.T.) who was blinded to the patients’HPV status. Alveolar bone loss is an established measure of periodontitis and its accuracy and reliability have been established,17 To establish intraexaminer reliability in the present study, duplicate measurements on 5 study patients (207 sites) were made within a 3-day interval. The mean (SD) difference of duplicate measurements was 0.22(0.41) mm.
DETECTION OF TUMOR HPV DNA
The DNA was extracted from three 10-μm paraffin-embedded tumor sections identified by the pathology department. Microtome blades were changed between each embedded block to prevent cross-contamination between specimens. The presence of HPV-16 DNA was determined by polymerase chain reaction (PCR), as described previously.15 The DNA extraction and PCR amplification were performed in separate laboratories to reduce contamination. All reactions were assembled in a dedicated laminar-flow sterile cabinet that had been decontaminated by exposing all surfaces to a 10% bleach solution for 30 minutes before and after PCR setup. The PCR was performed in triplicate in a 50-μL cocktail containing 0.25mM forward and reverse type-specific primers for the E6 regions of HPV-16, 1,5mM magnesium chloride, 2.0mM dNTPs (deoxynucleotide triphosphates), and 1 U of Taq polymerase (Invitrogen) in a 1× amplification buffer supplied by the manufacturer. The presence of a 109–base pair fragment when 5-μL aliquots of the PCR were resolved by electrophoresis on a 3% low-electroendosmosis agarose gel containing 1 mg/mL of ethidium bromide was indicative of the presence of HPV-16 in the tumor. Assays were performed in triplicate, and tumor DNAs from which the 109–base pair HPV-16–specific fragment was visible in at least 2 of the 3 reactions were considered positive for the presence of the virus. To minimize false-negative findings, an additional 40 cycles of PCR were performed on 5-μL aliquots of the previous PCR products of apparently HPV-16–negative tumors, for a total of 80 cycles. Amplification of β-actin or β-globin genes served as a PCR control, and 28 of 247 samples (11.3%) were excluded because of lack of amplification. Mock reactions containing no DNA were, used as negative controls for each group of samples amplified. The HPV assays were performed without the technician’s knowledge of periodontal status.
COVARIATES
Selection and definition of covariates were limited with the information available in existing patient records. The following variables were available from the Roswell Park Cancer Institute Hospital Information System: age at diagnosis (in years), sex; race/ethnicity (non-Hispanic white, other), marital status (married, other), smoking status (never, former, current), alcohol use (never, ever), TNM stage (I-IV), tumor differentiation (poor, moderate, well), and tumor site (oral cavity, oropharynx, larynx). The Hospital Information System data were obtained on admission by interview and were recorded electronically.
STATISTICAL ANALYSIS
The distribution (mean [SD], frequency, and percentage) of available relevant variables in the study population was described by tumor HPV status, and the differences were tested by 2-tailed, unpaired t lest for continuous and χ1 test for categorical variables. The box and whisker plot was used to show the distribution of ABL in HPV-negative and HPV-positive tumors with the 5-point summary, that is, minimum, lower quartile, median, upper quartile, and maximum. Whenever possible, variables were defined continuously. To estimate the effect of dental variables on tumor HPV status, crude odds ratios (ORs) and their 95% CIs were obtained from 2×2 cross tabulations. Independent effects of dental variables on tumor HPV status were estimated from multiple logistic regression analysis after adjusting the effects of potential confounders. Because of collinearity, the effect of each dental variable on tumor HPV status was evaluated in a separate model. Variable selection for multivariate analysis was based on change in estimate. We adjusted only for variables that caused the OR for the oral variables to change in either direction by more than 10%. Potential multiplicative interactions of dental variables with smoking status and tumor site were evaluated by including an interaction term in the multivariate models. A 2-sided P ≤ .05 was considered statistically significant. Commercial software (SPSS Statistics, version 20.0, 2011; IBM Corp) was used for all data analyses.
RESULTS
A total of 124 incident primary HNSCC cases were included in the study. From those, 31 cases (25.0%) were located in the oral cavity, 49 (39.5%) in the oropharynx, and 44 (35.5%) in the larynx. Fifty of 124 tumor samples (40.3%) were positive for HPV-16 DNA. A higher proportion of oropharyngeal cancers were HPV-positive (65.3%) compared with oral cavity (29.0%) and laryngeal (20.5%) cancers. The prevalence of HPV-positive tumors was significantly lower in patients with moderately orwell-differentiated tumors compared with those with poorly differentiated tumors (34.9% vs 55.6%; P = .01) and in current smokers compared with never smokers (34.9% vs 70.0%; P = .047). Patients with HPV-positive and HPV-negative tumors were not significantly different with regard to tumor stage, age at diagnosis, sex, race/ethnicity, marital status, and alcohol use (Table 1).
Table 1.
Patient Characteristics
| NO. (%) |
||||
|---|---|---|---|---|
| Characteristic | All Patients (N = 124) |
HPV-Negative HNSCC (n = 74)a |
HPV-Positive HNSCC (n = 50) |
P valueb |
| Tumor site | ||||
| Oral cavity | 31 (25.0) | 22 (71.0) | 9 (29.0) | |
| Oropharynx | 49 (39.5) | 17 (34.7) | 32 (65.3) | .002 |
| Larynx | 44 (35.5) | 35 (79.5) | 9 (20.5) | .39 |
| TNM stage | ||||
| I or II | 23 (18.5) | 10 (43.5) |

|
|
| III or IV | 89 (71.8) | 57 (64.0) | .01 | |
| Unknown | 12 (9.7) | 7 (58.3) | ||
| Tumor differentiation | ||||
| Moderate/well | 83 (66.9) | 54 (65.1) |
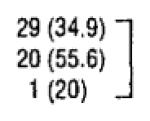
|
|
| Poor | 36 (29.0) | 16 (44.4) | .01 | |
| Unknown | 5 (4.0) | 4 (80) | ||
| Age at diagnosis, mean (SD), y Sex |
58.5 (10.16) | 59.34 (10.51) | 57.18 (9.57) | .23 |
| Women | 31 (25.0) | 21 (87.7) |

|
29 |
| Men | 93 (75.0) | 53 (57.0) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 109 (87.9) | 65 (59.6) |

|
.98 |
| Other | 15 (12.1) | 9 (60.0) | ||
| Marital status | ||||
| Married | 67 (54.0) | 40 (59.7) |

|
.99 |
| Other | 57 (46.0) | 34 (59.6) | ||
| Smoking status | ||||
| Never | 10 (8.1) | 3 (30.0) | 7 (70.0) | |
| Former | 51 (41.1) | 30 (58.8) | 21 (41.2) | .11 |
| Current | 63 (50.8) | 41 (65.1) | 22 (34.9) | .047 |
| Alcohol use | ||||
| No | 49 (39.5) | 30 (61.2) |
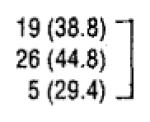
|
|
| Yes | 58 (46.8) | 32 (55.2) | .53 | |
| Unknown | 17 (13.7) | 12 (76) | ||
Abbreviations: HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus.
Incident primary HNSCC.
P values were derived from t tests for continuous variables and χ2 tests for categorical variables.
Patients with HPV-positive tumors had significantly higher ABL (4.57 mm vs 3.78 mm; P = .001) and fewer missing teeth (7.38 vs 11.02; P = .02) compared with those with HPV-negative tumors. The prevalence of dental caries, fillings, and edentulous state were not significantly associated with tumor HPV status (Table 2). Use of the box and whisker plot showed differences in ABL between the HPV-negative and HPV-positive groups in the minimum (1.43 mm vs 2.75 mm), lower quartile (2.97 mm vs 3.85 mm), median (3.45 mm vs 4.71 mm), upper quartile (4.86 mm vs 5.09 mm), and maximum (6.23 mm vs 6.79 mm) values. The differences in median values (3.44 mm vs 4.71 mm) and tn interquartile ranges (2.97-4.86 mm vs 3.85-5.09 mm) demonstrate that the distributions of ABL in the 2 groups are significantly different (Figure).
Table 2.
Distribution of Dental Variables in the Study Population
| Mean (SD) |
||||
|---|---|---|---|---|
| Characteristic | All Patients (N = 124) |
HPV-Negative HNSCC (n = 74)a |
KPV-Positive HNSCC (n = 50) |
P Valueb |
| Alveolar bone loss, mm | 4.14 (1.19) | 3.78 (1.22) | 4.57 (0.98) | .001 |
| Caries | 1.58 (2.60) | 1.73 (3.01) | 1.40 (2.00) | .55 |
| Fillings | 5.32 (4.16) | 5.25 (4.27) | 5.40 (4.08) | .86 |
| Missing teeth | 9.39 (7.42) | 11.02 (7.46) | 7.38 (6.93) | .02 |
| Edentulous, No. (%)c | ||||
| No | 94 (75.8) | 52 (55.3) | 42 (44.7) | |
| Yes | 30 (24.2) | 22 (73.3) | 8 (26.7) | .08 |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus.
Incident primary HNSCC.
P values were derived from t tests for continuous variables and χ2 tests for categorical variables.
No teeth present.
Figure.
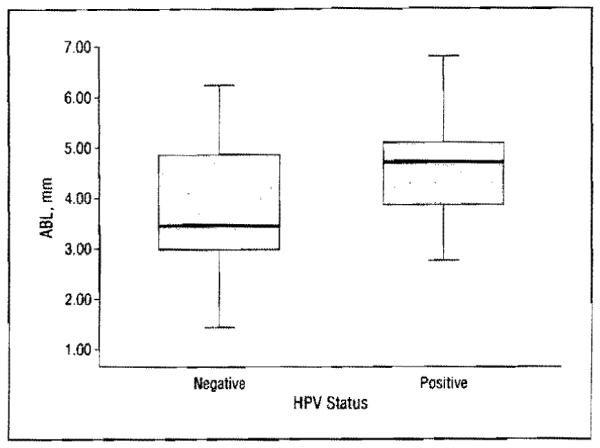
Distribution of alveolar bone loss (ABL) in the human papillomavirus (HPV)-negative and HPV-positive groups. Boxes represent lower to upper quartiles. with bars indicating the median values and whiskers indicating minimum and maximum values.
When included in the multivariate models containing each dental variable, only smoking status caused the ORs for ABL and edentulous status to change more than 10%. We also included age at diagnosis and sex in the models. Each millimeter of ABL increased the odds of HPV-positive tumor status 2.6 times (OR, 2.61; 95%, 1.58-4.30) after adjustment forage at diagnosis, sex, and smoking status. The remaining dental variables—caries, fillings, missing teeth, and edentulous state—were not significantly associated with tumor HPV status (Table 3).
Table 3.
Associations of Dental Variables With Tumor HPV Status
| Characteristic | Crude OR (95% CI) | P Value | Adjusted OR (95% Cl)a | P Value |
|---|---|---|---|---|
| Alveolar bone loss, per mm | 1.85 (1.25-2.75) | .002 | 2.61 (1.58-4.30) | <.001 |
| Caries, per tooth | 0.95 (0.81-1.12) | .55 | 0.92 (0.78-1.10) | .38 |
| Fillings, per tooth | 1.01 (0.92-1.11) | .86 | 1.00 (0.90-1.11) | .96 |
| Missing teeth, per tooth | 0.93 (0.88-0.99) | .02 | 0.94 (0.87-1.01) | .07 |
| Edentulous b | 0.45 (0.18-1.11) | .08 | 0.53 (0.21-1.37) | .19 |
Abbreviations; HPV, human papillomavirus; OR, odds ratio.
0dds ratios were derived from multiple logistic regression models including each of the oral variables, age at diagnosis, sex, and smoking status.
No teeth present.
There was a significant interaction between tumor site and ABL (P =.048). Stratification of the multiple logistic-regression model showed that the strength of the association between ABL and tumor HPV status was stronger in patients with oropharyngeal SCC (OR, 11.70; 95% CI, 2.09-65.53) compared with those with oral cavity SCC (OR, 2.32; 95% CI, 0.65-8.27) and larvngeal SCC (OR, 3.89; 95% CI, 0.95-15.99) (Table 4). There was no significant interaction between smoking status and any of the dental variables.
Table 4.
Periodontitis-Tumor HPV Association Stratified by Tumor Site
| Tumor Site | No. of Patients | Crude OR (95% CI) | P Value | Adjusted OR (95% Cl)a | P Value |
|---|---|---|---|---|---|
| Oral cavity | 30 | 1.41 (0.63-3.16) | .41 | 2.32 (0.65-8.27) | .19 |
| Oropharynx | 49 | 7.74 (2.18-27.40) | .002 | 11.70 (2.09-65.53) | .005 |
| Larynx | 45 | 3.17 (1.09-9.24) | .04 | 3.89 (0.95-15.99) | .06 |
Abbreviations: ABL, alveolar bone loss; HPV, human papillomavirus; OR, odds ratio.
Odds ratios were derived from multiple logistic regression models including alveolar bone loss, age at diagnosis, sex, and smoking status.
COMMENT
The results of this study suggest that periodontitis, a chronic inflammatory disease, may be associated with the HPV status of HNSCC. The strength of this association was greater among patients with oropharyngeal SCC compared with those with laryngeal and oral cavity SCC. The other dental variables—caries, fillings, and missing teeth—were not significantly associated with tumor HPV status.
An association between chronic inflammation and HPV infection is biologically plausible. The HPV infects basal cells of the epithelium exclusively and gains access through breaks in the mucosa. In addition, replication of the virus is closely associated with basal cell proliferation.5 Mucosal damage, microulcerations, and consequent epidielial proliferation mediated by inflammatory cytokines released from periodontitis sites provide an ideal environment for initial HPV infection and its persistence.18 In this inflammatory environment, HPV is also shed in greater amounts, leading to increased risk of viral transmission.19 The validity of the association between chronic inflammation and HPV infection is supported by multiple epidemiologic and molecular studies of cervical HPV infection.6-9
The observed association between periodontitis and oral HPV infection may be alternatively explained through the direct effects of bacteria. Concurrent infection with Chlamydia trachomatis was shown20 to increase the persistence of cervical HPV infection and the risk of cervical cancer. Bacteria that infect periodontitis sites successfully colonize and persist in the oral mucosa.21 Whether the association between periodontitis and oral HPV infection is through direct effects of bacteria or through stimulation of inflammation is yet to be determined. It is possible that both mechanisms are involved, but substantial evidence suggests that the periodontal bacteria can travel from affected tissues to distant sites via saliva and the bloodstream and cause tissue injury through inflammatory reactions.22
This study suggests that the association between periodontitis and tumor HPV statusisstrongerin patients with oropharyngeal SCC compared with those with oral cavity and laryngeal SCC. Although periodontitis is localized to the structures around teeth, periodontal pocket contents including inflammatory cytokines, bacteria, viruses, enzymes, and toxins are continuously shed into saliva. On the other hand, saliva has no access into periodontal pockets because of continuous Hushing of gingival crevicular fluid, which is an inflammatory infiltrate.11,12 In addition to its role as a means of transport, saliva provides a means of interaction between different carcinogens. Furthermore, salivary enzymes metabolize tobacco, alcohol, and dietary components into carcinogens.23 The fact that most HNSCC occurs at saliva-draining areas supports the critical role of saliva for HNSCC. Therefore, periodontal pockets, rather than being the sites of carcinogenesis, are probably a source of inflammatory cytokines, bacteria, and viruses in saliva.19,21
It is interesting that besides periodontitis, other dental variables—caries, fillings, and missing teeth—were not significantly associated with tumor HPV status. Although the number of missing teeth was significantly associated with tumor HPV status in univariate analysis, it lost statistical significance after adjustment for explanatory variables. Missing teeth is a surrogate measure for previous disease, with more than half of the missing teeth attributable to dental caries. Dental caries did not have a significant association with tumor HPV status in this study. Many previous studies combined periodontitis and dental caries in a single index variable as an indicator of poor oral health; however, these are 2 very different oral diseases. Periodontitis is caused by gramnegative anaerobic bacteria and is a significant source of inflammatory markers in saliva12,24; dental caries is simply the demineralization of tooth structures by lactic acid formed by gram-positive facultative bacteria and is not a source of inflammatory markers in saliva.25 Cariogenic bacteria, such as streptococci and lactobacilli, have been associated with periodontal health.24,25 The fact that only periodontitis was associated with tumor HPV status points to the potential association of inflammation with tumor HPV status.
This was a retrospective study using existing data, and information on certain explanatory variables, including duration of tobacco and alcohol use, smokeless tobacco, diet, and sexual history, was not available. However, reliable information on the pertinent demographic and lifestyle variables, histologic confirmation of tumor diagnoses, sensitive HPV assays, and quantitative and objective measures of periodontitis history was available. We used an E6 gene-specific PCR to detect HPV-16. Although this method is more sensitive than others (eg, in situ hybridization or immunohistochemical assays) in its ability to detect the presence of HPV, it docs not provide information on viral titer. Information on viral titer is potentially important to understand the natural history of the oral HPV infection.5 Latent HPV is present in basal cells in low copy numbers.26 Factors that influence the proliferation of basal cells (eg, inflammation) may be critical to explain activation of the virus and molecular and clinical characteristics of HPV-positive HNSCC.19 Longitudinal studies using quantitative methods for evaluation of inflammation and HPV infection will help test these hypotheses.
Alveolar bone loss is a widely used diagnostic measure of periodontitis and is often used as the standard against which new diagnostic tests are compared.27-32 The close association between periodontal inflammation and ABL, as well as the rate of bone loss, has been well documented.28,29 Studies12 have also established a direct association between the severity of ABL and salivary levels of inflammatory markers. The conventional periodontal treatment stops further alveolar bone loss but does not result in bone gain. Therefore, ABL represents the histoty of periodontitis accurately, regardless of treatment.27 There are 2 alternative clinical measures of periodontitis. The first one, clinical attachment loss, is highly correlated with ABL.27,30 The other one, pocket or probing depth, does not reflect the history of periodontitis accurately because it can be reduced with treatment.27,30 Biomarkers document only current inflammation and do not give any information on history.27,28 In addition, inflammatory biomarkers are not specific to periodontitis and do not identify the source of inflammation. For example, the tumor and its environment also generate significant amounts of inflammatory cytokines. In the absence of a robust clinical variable such as ABL, it would be difficult to establish the association between periodontitis and tumor HPV status. Therefore, ABL is an appropriate measure for this study, documenting longstanding periodontitis history. We hope that this study will stimulate future investigations using both clinical variables and biomarkers to confirm this association.
Alveolar bone loss associated with periodontal inflammation is a slow, chronic process that is usually irreversible.28,29 In this study, the radiographs were obtained at admission, documenting the history of periodontitis before cancer diagnosis. The normal ABL in healthy young adults is generally accepted to be 2 mm or less,17 and the mean annual ABL varies between 0.07 and 0.14 mm.29 Therefore, the mean difference in ABL (0.8 mm) between patients with HPV-positive and HPV-negative HNSCC observed in this study is clinically significant, representing 6 to 11 years of periodontitis history. In a previous study,14 periodontitis was also an independent risk factor for HNSCC, and ABL in patients with HNSCC was significantly higher compared with ABL in healthy controls (4.00 vs 2.44 mm; P < .001). In the present study, since the entire population consisted of patients with HNSCC, the ABL levels in both HPV-positive and HPV-negative groups were high, but the difference was still statistically significant (4.57 mm vs 3.78 mm; P = .001). This suggests that periodontitis/inflammation has independent effects for HNSCC and HPV infection. It is also possible that at least part of the effect of inflammation on HNSCC is mediated through its effect on HPV infection. Because of the retrospective design of this study, we cannot determine whether periodontitis preceded oral HPV infection. These 2 oral infections may have occurred concurrently or HPV infection may have preceded periodontitis. The temporal relationship between periodontitis and oral HPV infection thus needs to be ascertained by prospective studies including individuals without cancer.
The accuracy and reliability of the method used in this study to measure ABL on digitized radiographic images are supported by extensive literature.17,31,32 The average deviation between the observed bone height and the true bone height is usually less than 5%.32 In addition, ABL measurements from panoramic radiographs have been shown to be comparable to clinical measurements.30,32 The concordance between the clinical attachment loss and ABL measured at the same sites is usually higher than 80%.17,30 However, it is well known that radiographs underestimate the true amount of bone loss.27-32 Nevertheless, since the same method was used to measure ABL for both cases and controls by a single examiner blinded to HPV status, all factors related to accuracy or reliability would only lead to nondifferential misclassification, which always results in underestimation of the true effect. In other words, the true effect of periodontitis on tumor HPV status may be stronger than the one observed in the present study,33
Perhaps the most significant strength of this study is that patients with both HPV-positive and HPV-negalive tumors were selected from the same source population. All patients with newly diagnosed HNSCC seen in the DMFP department during the study period who met the inclusion criteria were included. The panoramic radiographs (from which ABL measurements were performed) of all patients were obtained at baseline before treatment was initiated. In addition, ABL measurements were performed without knowledge of the tumor HPV status. Therefore, potential selection or measurement bias in our study is not expected to be significant.
If prospective studies in cancer-free populations confirm that chronic local inflammation plays a significant role in the natural history of oral HPV infection, the public health implications would be important. Periodontitis is easy to detect and may represent a clinical high-risk profile for oral HPV infection. Prevention or treatment of sources of inflammation in the oral cavity may be a simple yet effective way to reduce the acquisition and persistence of oral HPV infection.
Acknowledgments
Funding/Support: This study was supported by grants 1R03CA119262 from the National Cancer Institute and T32-DE07034 from the National Institute of Dental and Craniofacial Research.
Footnotes
Author Contributions: Drs Tezal and Hyland had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Tezal and Marshall Acquisition of data: Tezal, Marshall, and Stoler. Analysis and interpretation of data: Tezal, Scannapieco, Wactawski-Wende, Hyland, and Rigual, Drafting of the manuscript: Tezal, Marshall, and Stoler. Critical revision of the manuscript for important intellectual content: Tezal, Scannapieco, Wactawski-Wende, Hyland, Marshall, and Rigual. Statistical analysis: Hyland and Marshall. Obtained funding: Tezal and Scannapieco. Administrative, technical, and material support: Tezal, Marshall, and Stoler. Study supervision: Wactawski-Wende.
Financial Disclosure: None reported.
REFERENCES
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 2.Ernster JA, Sciotto CG, O'Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 3.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States. 2009-2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh WK. Human papillomavirus infection: a concise review of natural history. Obstet Gynecol. 2009;114(1):139–143. doi: 10.1097/AOG.0b013e3181ab6878. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, Hillier SL, Rabe LK, et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papilomavirus (HPV) Cancer Epidemiol Biomarkers Prev. 2001;10(10):1021–1027. [PubMed] [Google Scholar]
- 7.Koutslcy LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intrapithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias M, Plowman GD, Woodworth CD. Interleukin-6 and interleukin 6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995;146(4):944–952. [PMC free article] [PubMed] [Google Scholar]
- 9.Galotti D, Chung J, Iglesias M, et al. Tumor necrosis factor-α promotes human papillomavirus (HPV) E6/E7 RNA expression and cyclin-dependent kinase activily in HPV-immortalized keratinocyres by ras-dependent pathway. Mol Carcinog. 2000;27(2):97–109. doi: 10.1002/(sici)1098-2744(200002)27:2<97::aid-mc5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Karl T, Seibert N, Stöhr M, Osswald H, Rösl F, Finzer P. Suhndac induces specific degradation of the HPV oncoprotein E7 and causes growth arrest and apoptosis in cervical carcinoma cells. Cancer Lett. 2007;245(1-2):103–111. doi: 10.1016/j.canlet.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Genco RJ. Clinical innovations in managing inflammation and penodontat diseases: the Workshop on Inflammation and Periodontal Diseases. J Periodontol. 2008;79(8):1609–1611. doi: 10.1902/jop.2008.080305. (suppl) [DOI] [PubMed] [Google Scholar]
- 12.Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontal. 2005;76(3):406–410. doi: 10.1902/jop.2005.76.3.406. [DOI] [PubMed] [Google Scholar]
- 14.Tezal M, Sullivan MA, Hyland A, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Culcer Epidemiol Biomarkers Prev. 2009;18(9):2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 15.Tezal M, Sullivan Nasca M, Stoler DL, et al. Chronic periodontitis-human papillomavirus synergy in base or tongue cancers. Arch Otolaryngol Head Neck Surg. 2009;135(4):391–396. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 16.Percy C, van Holten V, Muir C. International Classification of Diseases for Oncology. 3rd World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 17.Hausmann E, Allen K, Dunford R, Christersson L. A reliable computerized method to determine the level of the radiographic alveolar crest. J Periodontal Res. 1989;24(6):368–369. doi: 10.1111/j.1600-0765.1989.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams DA. Inflammatory cytokines and mucosal injury. J Natl Cancer Inst Monogr. 2001;29(29):26–30. doi: 10.1093/oxfordjournals.jncimonographs.a003435. [DOI] [PubMed] [Google Scholar]
- 19.Hormia M, Willberg J, Ruokonen H, Syrjänen S. Marginal períodontium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol. 2005;76(3):358–363. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 20.Samoff E, Koumans EH, Markowitz LE, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162(7):668–675. doi: 10.1093/aje/kwi262. [DOI] [PubMed] [Google Scholar]
- 21.Yimaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral eolthelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. pt 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scannapieco FA, Wano B, Shiau HJ. Oral bacteria and respiratory Infection: effects on respiratory pathogen adhesion and epithelial cell proinflammatory cytokine production. Ann Periodontol. 2001;6(1):78–86. doi: 10.1902/annals.2001.6.1.78. [DOI] [PubMed] [Google Scholar]
- 23.Reznick AZ, Hershkovich O, Nagler RM. Saliva—a pivotal player in the pathogenesis of oropharyngeal cancer. Br J Cancer. 2004;91(1):111–118. doi: 10.1038/sj.bjc.6601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Socransky SS, Haffajee AD. Periodontal microbial ecology. Ptriodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental carles in humans. J Dent Educ. 2001;65(10):1028–1037. [PubMed] [Google Scholar]
- 26.Stubenrauch F, Laimins LA. Human papillomavirus life cycle:active and latent phases. Semin Cancer Biol. 1999;9(6):379–386. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- 27.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 28.Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90(2):143–153. doi: 10.1177/0022034510385236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papapanou PN, Wennström JL, Grōndahl K. A 10-year retrospective study of periodontal disease progression. J Clin Periodontal. 1989;16(7):403–411. doi: 10.1111/j.1600-051x.1989.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 30.Papapanou PN, Wennstrorn JL. Radiographic and clinical assessments of destructlve periodontal disease. J Clin Periodontal. 1989;16(9):609–612. doi: 10.1111/j.1600-051x.1989.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 31.Benn DK. A review of the reliability of radiographic measurements in estimating alveolar bone changes. J Clin Periodontal. 1990;17(1):14–21. doi: 10.1111/j.1600-051x.1990.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 32.Walsh TF, al-Hokail OS, Fosam EB. The relationship of bone loss ob rved on panoramic radiographs with clinical periodontal screening. J Clin Periodontol. 1997;24(3):153–157. doi: 10.1111/j.1600-051x.1997.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 33.Hennekens CH, Buring JE. Epidemiology in Medicine. LittleBrown & Co; Boston. MA: 1987. Analysis of epidemiologic studies: evaluating the role of bias; pp. 272–286. [Google Scholar]


