Abstract
Purpose
Accurate identification of tobacco use is critical to implement evidence-based cessation treatments in cancer patients. The purpose of this study is to evaluate the accuracy of self-reported tobacco use in newly diagnosed cancer patients.
Methods
Tobacco use questionnaires and blood samples were collected from 233 newly diagnosed cancer patients (77 lung, 77 breast, and 79 prostate cancer). Blood was analyzed for cotinine levels using a commercially available enzyme-linked immunosorbent assay. Patients with cotinine measurements exceeding 10 ng/mL were categorized as current smokers. Smoking status based upon cotinine levels was contrasted with self-report in current smokers, recent quitters (1 or less year since quit), non-recent quitters (>1 year since quit), and never smokers. Multivariate analyses were used to identify potential predictors of discordance between self-reported and biochemically confirmed smoking.
Results
Cotinine confirmed 100 % accuracy in self-reporting of current and never smokers. Discordance in cotinine and smoking status was observed in 26 patients (15.0 %) reporting former tobacco use. Discordance in self-reported smoking was 12 times higher in recent (35.4 %) as compared with non-recent quitters (2.8 %). Combining disease site, pack-year history, and employment status predicted misrepresentation of tobacco use in 82.4 % of recent quitters.
Conclusions
Self-reported tobacco use may not accurately assess smoking status in newly diagnosed cancer patients. Patients who claim to have recently stopped smoking within the year prior to a cancer diagnosis and lung cancer patients may have a higher propensity to misrepresent tobacco use and may benefit from biochemical confirmation.
Keywords: Tobacco, Smoking, Cotinine, Accuracy, Self report (self-report), Cancer, Nicotine
Introduction
Tobacco use is a major risk factor for the development of several cancers, and evidence demonstrates that tobacco adversely impacts cancer treatment outcomes [1–11]. Unfortunately, the majority of studies evaluating the effects of tobacco use on cancer prognosis have relied upon retrospective chart reviews. Studies may also use inaccurate data because most use self-reported assessments obtained during medical consultations that do not use structured tobacco assessments. Retrospective reviews and non-structured assessments increase the risk of misrepresentation and likely underestimate the importance of tobacco use on treatment outcomes [12–15].
Previous studies examining the accuracy of self-reported tobacco use in general patient populations, using nicotine-specific biomarkers such as cotinine, suggest that discordance in self-reported smoking varies by gender, ethnicity, and disease type [16–18]. Very few studies have evaluated biochemical confirmation of self-reported tobacco use in cancer patients; however, data suggest that misrepresentation may occur in substantial proportions of cancer patients [19–21]. In a cohort of head and neck cancer patients evaluated with weekly self-reported assessments and serum cotinine during definitive radiotherapy or chemoradiotherapy, biochemical confirmation was necessary to identify 29.4 % of smokers during treatment [22]. Several factors may contribute to patient misrepresentation such as fear of being judged, aversion to health lectures, and social acceptance [18, 23, 24]. Data suggest that patients suffering from diseases correlated with tobacco use may experience increased pressure for smoking cessation and may be more likely to misrepresent their previous or current tobacco use [25]. Unfortunately, there is very little information on the accuracy of structured assessments in most cancer disease sites.
There is limited data to help establish the potential utility of biochemical confirmation in cancer patients. Studies delineating patients at risk for misrepresentation would be useful to help focus efforts in specific cancer patients rather than subjecting all cancer patients to biochemical confirmation. The purpose of this study was to compare self-reported smoking status with biochemically confirmed tobacco using plasma cotinine analyses among newly diagnosed lung, breast, and prostate cancer patients and to identify potential predictors of misrepresentation.
Methods
The study group included 233 lung, breast, or prostate cancer patients who presented to a National Cancer Institute (NCI) Designated Comprehensive Cancer Center (Roswell Park Cancer Institute, RPCI) with a new cancer diagnosis between 2004 and 2010, and who had received no prior treatment for their newly diagnosed cancer. Patients voluntarily provided a blood specimen and self-reported tobacco use information through a structured epidemiologic questionnaire. All patients consented to voluntary enrollment on an Institutional Review Board-approved epidemiologic and biospecimen study (the RPCI Data Bank and Bio-Repository [DBBR] funded by NCI grant #P30 CA016056) designed to obtain biological samples, standardized epidemiologic information, and clinical information. Newly diagnosed patients voluntarily contributed a blood sample and completed a baseline questionnaire that obtained information including demographic factors, medical history, family history of cancer, physical activity, co-morbidities, food consumption, and tobacco use history. Of patients offered enrollment in the DBBR study, 90 % participated voluntarily. Quit information was based upon age of quitting, thus limiting analyses to quit dates within the past year versus greater than 1 year.
Of the 233 patients, 77 were lung cancer patients, 77 were breast cancer patients, and 79 were prostate cancer patients. These disease sites were chosen to help provide a representation across tobacco-related (lung) as well as traditionally non-tobacco-related (breast, prostate) disease sites. In addition, this allowed for analysis with reasonable gender equity. Within each disease site group, we divided subjects into one of four groups based on self-reported cigarette smoking status: (1) current smoker, (2) never smoker, (3) recent quit (smoking cessation of 1 year or less), and (4) non-recent quit (smoking cessation of greater than 1 year).
The blood samples and data were procured as protected health information and de-identified prior to distribution for analysis. Blood samples were collected and processed to collect serum and plasma. After allowing the biological samples sufficient time for clotting, each specimen was centrifuged at 3,000 rpm for 15 min. Supernatants were automatically aliquoted using a MAPI robot from Cryobiosystem (Division of IMV Technologies, L’Aigle, France). Specimens were then deposited into color-coded 0.5-mL plastic inert straws. Upon completion of processing, each biospecimen was stored in −80C freezers.
Elevated levels of cotinine in biological fluids suggest that nicotine intake through tobacco use or exposure, and is widely recognized as a valid biochemical measure of tobacco smoke [26–28]. Biochemical assessment of cotinine was performed using a commercially available competitive binding enzyme-linked immunosorbent assay (ELISA, Calbiotech, Spring Valley, CA). Subjects whose sample concentrations exceeded 10 ng/mL were categorized as smokers and samples >100 ng/mL were reexamined at a 1:10 dilution according to product guidelines. The product was independently validated by NAM, MR, and GWW with known dose escalated cotinine administration in control plasma samples using procedures according to the product manufacturer.
Descriptive statistics were calculated, and data analyses were conducted using SAS. Descriptive statistics were used to characterize concordance and discordance between self-reported smoking status and cotinine validated nicotine exposure. Univariate and multivariate logistic regression models were used to examine the probability of a true report as a function of gender, race, disease site, age at time of questionnaire, age at time of starting tobacco use, pack years, ever use of other tobacco, ever use of nicotine replacement, secondhand smoke exposure at home, time between completion of questionnaire and collection of blood specimen, and current employment. Exact conditional methods were used to generate the p values in both the univariate and multivariate models. All tests were two-sided at level α = 0.05.
Results
Patient demographics and tobacco use characteristics are reported in Table 1. Women represented all breast cancer cases and 63 % of lung cancer cases. Response rates for age of first smoking, lifetime average cigarettes per day (and pack-year history calculation), lifetime average time to first cigarette, and prior quit attempts were between 96.9 and 100 % in current smokers, recent quitters, and non-recent quitters.
Table 1.
Patient characteristics and tobacco use history
| Tobacco use | Current (n = 30) | Recent quit (n = 65) | Non-recent quit (n = 108) | Never (n = 30) | Total (n = 233) |
|---|---|---|---|---|---|
| Female | 18 (60 %) | 42 (64.6 %) | 48 (44.4 %) | 18 (60 %) | 126 (54 %) |
| Median age (range) | 59 (43–77) | 58 (31–81) | 65 (42–85) | 58 (42–82) | 61 (31–85) |
| Caucasian | 27 (90 %) | 58 (89.2 %) | 107 (99.1 %) | 30 (100 %) | 222 (95 %) |
| Age of first smoking | Response: n = 30 | Response: n = 64 | Response: n = 108 | N/A | Response: n = 202 |
| Median: age 18 | Median: age 17 | Median: age 18 | Median age: 18 | ||
| Range: 9–42 | Range: 11–52 | Range: 12–40 | Range: 9–52 | ||
| Lifetime average cigarettes per day | Response: n = 30 | Response: n = 64 | Response: n = 105 | N/A | Response: n = 199 |
| Median: 20 cigs | Median: 20 cigs | Median: 20 cigs | Median: 20 cigs | ||
| Range: 6–50 | Range: 2–40 | Range: 2–60 | Range: 2–60 | ||
| Pack-year history | Response: n = 30 | Response: n = 63 | Response: n = 105 | N/A | Response: n = 198 |
| Median: 40 | Median: 33 | Median: 22.5 | Median: 31.25 | ||
| Range: 12–102.5 | Range: 1.4–102 | Range: < 1–94 | Range: 0.25–102.5 | ||
| Lifetime how soon after waking for first cigarette | Response: n = 30 | Response: n = 65 | Response: n = 106 | N/A | Response: n = 201 |
| < 5 min: 10 % | < 5 min: 18 % | < 5 min: 27 % | < 5 min: 22 % | ||
| 6–30 min: 13 % | 6–30 min: 20 % | 6–30 min: 18 % | 6–30 min: 18 % | ||
| 31–60: 43 % | 31–60: 31 % | 31–60: 27 % | 31–60 min: 31 % | ||
| > 60 min: 30 % | > 60 min: 28 % | > 60 min: 16 % | > 60 min: 22 % | ||
| Don’t know: 3 % | Don’t know: 3 % | Don’t know: 11 % | Don’t know: 7 % | ||
| Any prior quit attempt using medications | Response: n = 30 | Response: n = 65 | Response: n = 106 | N/A | Response: n = 201 |
| Yes: 69 % | Yes: 46 % | Yes: 31 % | Yes: 42 % | ||
| Any other form of tobacco use (% yes for ever use) | Pipe: 11 % | Pipe: 16 % | Pipe: 26 % | N/A | Pipe: 21 % |
| Cigars: 14 % | Cigars: 11 % | Cigars: 29 % | Cigars: 21 % | ||
| Chewing tob: 4 % | Chewing tob: 2 % | Chewing tob: 3 % | Chewing tob: 3 % | ||
| Snuff: 3 % | Snuff: 2 % | Snuff: 2 % | Snuff: 2 % | ||
| Secondhand smoke exposure (% yes) | At home: 73 % | At home: 72 % | At home: 69 % | At home: 31 % | At home: 66 % |
| At work: 83 % | At work: 68 % | At work: 74 % | At work: 41 % | At work: 69 % | |
| Social: 45 % | Social: 46 % | Social: 24 % | Social: 34 % | Social: 34 % | |
| Currently employed | Yes: 40 % | Yes: 48 % | Yes: 33 % | Yes: 60 % | Yes: 42 % |
Totals for tobacco-related items are relative only to patients reporting a history of tobacco use
Cig cigarette, tob tobacco, N/A not applicable
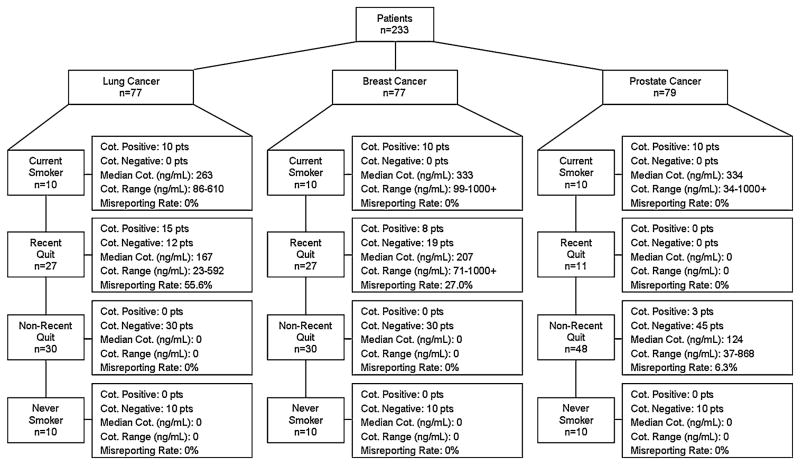
Figure 1 demonstrates false reporting rates according to disease site and tobacco use category. A total of 56 patients tested positive for cotinine including 100 % of self-reported current smokers, 15.0 % of former smokers (recent quit combined with non-recent quit), and 0 % of never smokers. Further analysis of former smokers demonstrated that 35.4 % of self-reported recent quitters and 2.8 % of non-recent quitters tested positive for blood cotinine at levels supporting active smoking. Cotinine values were 100 ng/mL or greater in 85.7 % of patients (range 23–1,000+) with only eight patients less than 100 ng/mL and four patients less than 50 ng/mL (23, 29, 47, and 48 ng/mL). None of the subjects who reported never smoking were found to have cotinine concentrations above 10 ng/mL.
Fig. 1.
Accuracy of self-report according to disease site and tobacco use category. Median and range are expressed for patients with cotinine positive blood. Cot Cotinine
Given the 12-fold increased rate of misrepresentation in recent quitters as compared with non-recent quitters, further variables were analyzed as potential predictors for misrepresentation in recent quitters. Table 2 presents the results of analyses examining the characteristics of recent quitters (n = 65) whose cotinine levels were concordant or discordant with self-reported smoking status. Among recent quitters, discordance with self-reported smoking status was more common in lung cancer patients (55.6 %) compared to those with breast cancer (27.0 %) or prostate cancer (0 %). Univariate analysis demonstrated that disease site, pack-year history, and employment status predicted for misrepresentation of smoking status (p < 0.05). Univariate analysis of time between specimen collection and completion of questionnaire as a continuous variable had no effect on misrepresentation. Further analyses demonstrated that pack-year history (p = 0.059), disease site (p = 0.052), and employment (p = 0.055) were near significant multivariate predictors for misrepresentation of smoking status. Combining disease site, pack-year history, and employment predicted misrepresentation of smoking in 82.4 % of recent quitters.
Table 2.
Univariate predictors of discordance between self-reported and cotinine validated smoking status among recent quitters (n = 65)
| Variables | N | Patients with false report (%) | p value |
|---|---|---|---|
| Gender | 0.288 | ||
| Male | 23 | 6 (26.1 %) | |
| Female | 42 | 17 (40.5 %) | |
| Race | 0.233 | ||
| White | 58 | 19 (32.8 %) | |
| Non-White | 7 | 4 (57.1 %) | |
| Disease site | 0.004 | ||
| Lung | 27 | 15 (55.6 %) | |
| Breast | 27 | 8 (29.6 %) | |
| Prostate | 11 | 0 (0 %) | |
| Age at time of questionnaire | 0.573 | ||
| 50 or under | 18 | 6 (33.3 %) | |
| Over 50 | 47 | 17 (36.2 %) | |
| Age at starting cigarette habit | 0.291 | ||
| Less than 18 | 38 | 16 (42.1 %) | |
| 18 or older | 27 | 7 (25.9 %) | |
| Pack-year history | 0.011 | ||
| Less than 10 | 10 | 9 (0 %) | |
| 10–20 | 7 | 3 (42.8 %) | |
| More than 20 | 44 | 20 (45.5 %) | |
| Ever history of other tobacco use | 0.512 | ||
| No | 52 | 20 (38.5 %) | |
| Yes | 13 | 3 (23.1 %) | |
| Ever use of NRT | 1.000 | ||
| No | 35 | 12 (34.3 %) | |
| Yes | 30 | 11 (36.7 %) | |
| Secondhand smoke exposure at home | 1.000 | ||
| No | 18 | 6 (33.3 %) | |
| Yes | 47 | 17 (36.2 %) | |
| Time between blood collection and return of questionnaire | 0.202 | ||
| Within 2 weeks | 30 | 8 (26.7 %) | |
| More than 2 weeks | 35 | 15 (42.9 %) | |
| Current employment | 0.019 | ||
| Yes | 31 | 6 (19.4 %) | |
| No | 34 | 17 (50.0 %) |
Conclusions
Data suggest that self-reported tobacco use may not accurately assess true smoking status in newly diagnosed cancer patients who are former smokers. Self-reported tobacco use assessments appear to be accurate for current and never smokers with a high degree of accuracy for patients who quit smoking 1 year prior to diagnosis; however, a substantial proportion of patients who claim to have stopped tobacco use within the past year may misrepresent true tobacco use. Discordance between cotinine and self-reported smoking status in recent quitters is observed in both lung and breast cancer patients, suggesting that misreporting is found in cancer sites traditionally associated with tobacco use (lung cancer) as well as disease sites not traditionally associated with tobacco use (breast cancer). However, lung cancer patients had a much higher rate of misrepresenting tobacco use as compared with breast or prostate cancer patients.
The potential effect of misrepresenting tobacco use in cancer patients may significantly alter the interpretation of clinical outcomes. Tobacco use decreases the efficacy of cancer treatment through increased toxicity and complications, poor compliance to cancer treatment, decreased quality of life, increased risk of developing second primary cancers, and increased risk of treatment failure [5–8, 29–34]. Through these effects, tobacco use decreases survival in both tobacco-related and non-tobacco-related disease sites [9, 35, 36]. Importantly, tobacco increases both cancer-related and non-cancer-related mortalities (such as cardiovascular mortality) in cancer patients [5, 36, 37], and data suggest that smoking cessation may improve outcomes [10, 11, 38, 39]. It is well known that inaccurate measurement of exposure to a risk factor, such as smoking, will lead to underestimation of the importance of the exposure to risk or confound assessments for other risk factors [12–15]. Data in non-cancer patients demonstrate discordance between self-reported and biochemically confirmed tobacco use of between 6 and 25 % [17, 18, 23, 24]. Warren et al. further demonstrate that 29.4 % of smokers required biochemical confirmation to accurately identify tobacco use during radiotherapy for head and neck cancer. Notably, a consistent subgroup of patients continued to misrepresent tobacco use throughout treatment, even though patients were aware that biochemical confirmation was being performed [22]. Though several studies demonstrate and adverse effect of tobacco on outcomes in cancer patients [1–11, 29–39], more structured assessments and biochemical confirmation may confer a more substantial adverse effect of smoking on outcome. The importance of biochemical confirmation is exemplified by Marin et al. who demonstrate no correlation between self-reported tobacco use and wound complications from reconstructive surgery in head and neck cancer patients; however, biochemical confirmation with cotinine demonstrated a significant correlation between tobacco use and complications [40]. Collectively, these data suggest that structured smoking assessments potentially combined with biochemical confirmation would improve evaluations of the true associations between tobacco use and outcomes in cancer patients.
An important observation is that misrepresentation occurs in patients from both tobacco-related (lung) and non-tobacco-related (breast) cancer disease sites. Prior observations demonstrate that misrepresentation occurs in other tobacco-related disease sites. Warren et al. demonstrate that 29.4 % of head and neck cancer patients require biochemical confirmation for accurate assessment of tobacco use [22]. Cooley et al. also demonstrate that 18.9 % of smokers misrepresent true tobacco use on follow-up evaluation [41]. The authors are unaware of any studies that have evaluated the accuracy of self-reported tobacco use in breast cancer patients, but our results demonstrate a consistent finding that misrepresentation occurs in patients with tobacco-related or non-tobacco-related cancers. The lack of observed discordance between cotinine levels and self-reported smoking status in recent quitting prostate cancer patients is in part explained by the paucity of recent quitters (11 patients) available for analysis. Additional patients included in the non-recent quit category for prostate patients provide evidence that some smaller proportion of non-recent quitters may also misrepresent true tobacco use.
Patients who stopped tobacco use within the year prior to assessment appear to be at a higher risk for misrepresentation. In a randomized chemoprevention trial to prevent recurrence in head and neck cancer patients, 39.1 % of patients who quit less than 1 year prior to enrollment misrepresented tobacco use as compared with 7.9 % of patients who quit more than 1 year prior to enrollment [42]. Our study demonstrates a similar pattern with higher rates of misrepresentation in cancer patients who quit less than 12 months prior to diagnosis (35.4 %) as compared with patients who quit more than 1 year prior to diagnosis (2.8 %). Collectively, these data support the hypothesis that patients who stopped tobacco use within the past year are at highest risk for misrepresentation.
The potential confounding effect of self-reported secondhand smoke exposure or nicotine replacement therapies was conducted to verify that patients exceeding the non-smoking cut-off point were not misclassified as biochemically confirmed smokers. It is known that exposure to secondhand smoke increases blood cotinine concentrations [26]; however, exposure to secondhand smoke rarely elevates concentrations above 10 ng/mL. Jarvis et al. [28] support cutoff for active tobacco use at cotinine concentrations of 13 ng/mL. The lowest concentration of cotinine detected in this study was 23 ng/mL, suggesting that secondhand smoke exposure was not the primary causal factor for cotinine levels in this cohort of patients. Data demonstrate that NRT can elevate blood cotinine concentration levels to 10–20 ng/mL [27]. To evaluate patients testing between or near this range as potentially cotinine positive due to NRT rather than smoking, all patient samples testing below 30 ng/mL were analyzed for a prior history of NRT. Of the 26 biochemically confirmed smokers who denied current tobacco use, only one patient tested below 30 ng/mL; however, this patient reported no NRT medication use. Notably, the range of cotinine levels in recent quitters was comparable to cotinine ranges in self-reported current smokers. Moreover, the median cotinine levels in recent quitters (167 ng/mL in lung cancer patients and 207 ng/mL in breast cancer patients) supported a moderate active smoking habit. Though longitudinal analyses were not available for these patients, these data suggest that the proportion of recent quitters that misrepresent tobacco use are likely making little concerted effort to reduce tobacco use and data further support active smoking as the primary source of cotinine in blood.
There are several limitations to the data presented herein. First, study sample selection was based on self-reported tobacco use and is not necessarily a representative sample of all lung, breast, or prostate cancer in newly diagnosed cancer population. Another limitation is that the DBBR questionnaire asked about past exposures to secondhand smoke, ever use of other tobacco products, and ever use of NRT. There was no information regarding current tobacco exposure or use in these categories. As a result, though data suggest that cotinine levels exceed exposure from secondhand smoke or NRT, it is not possible to directly compare real-time self-reported secondhand smoke exposure or NRT in this cohort. As a part of the DBBR cohort, samples were collected over a protracted period of time between 2004 and 2010, thereby subjecting the accuracy of self-report to potential changes in social norms associated with tobacco use during this period of time. Also, blood was not obtained on the same day as self-reported tobacco. Thus, it is possible that the cotinine values observed differ from self-report because of a true change in smoking status; however, length of time between completion of questionnaire and blood specimen collection was not associated with a significant difference in rate of discordance (Table 2). Finally, there was no further information on recent quit status beyond information knowing that patients quit within the past 12 months. As a result, there were no data that could be used to assess whether misreporting was associated with a shorter recent quit status (such as within the past month) as compared with a longer recent quit status (such as 10–11 months). Additional studies will need to be performed to assess whether accuracy is related to a more stringent recent quit status definition.
In conclusion, there are three important implications of this research. First, former smokers are at risk for misrepresenting true tobacco use, and patients who quit within the past year are at highest risk for misrepresentation. Second, patients with tobacco-related or non-tobacco-related cancers are at risk for misrepresentation, but patients with a tobacco-related cancer (lung cancer) may be at a higher risk of misrepresentation as compared with patients having a non-tobacco-related cancer. Third, combining a tobacco-related disease site with a 10+ pack-year history and unemployed work status may be useful in the optimal identification of patients who would most benefit from the addition of biochemical confirmation to ascertain true tobacco use status. Biochemical confirmation of all cancer patients may not be practical, but these factors can help facilitate implementation of a useful strategy to more accurately identify tobacco use in cancer patients.
Acknowledgments
The authors wish to thank Cheryl Rivard and Jennifer Graf for their assistance with data analysis and comments on earlier drafts of this paper. This work was supported in part by Roswell Park Cancer Institute, National Cancer Institute (NCI) grant #P30 CA016056, an American Cancer Society Mentored Research Scholar Grant (MRSG-11-031-01-CCE), and an American Cancer Society Institutional Research Grant.
Footnotes
Conflict of interest Dr Cummings provides expert testimony against the tobacco industry.
Contributor Information
Nelson A. Morales, Department of Natural Sciences, Roswell Park Cancer Institute, Buffalo, NY, USA
Michelle A. Romano, Department of Radiation Oncology, Medical University of South Carolina, 169 Ashley Ave. Rm 168, Charleston, SC 29425, USA
K. Michael Cummings, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
James R. Marshall, Department of Cancer Prevention, Roswell Park Cancer Institute, Buffalo, NY, USA
Andrew J. Hyland, Department of Health Behavior, Roswell Park Cancer Institute, Buffalo, NY, USA
Alan Hutson, Department of Biostatistics, Roswell Park Cancer Institute, Buffalo, NY, USA.
Graham W. Warren, Email: warrengw@musc.edu, Department of Radiation Oncology, Medical University of South Carolina, 169 Ashley Ave. Rm 168, Charleston, SC 29425, USA. Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, Charleston, SC, USA.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- 2.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45:S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 3.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 4.Videtic GM, Stitt LW, Dar AR, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. 2003;21:1544–1549. doi: 10.1200/JCO.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 5.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst. 2011;103:835–838. doi: 10.1093/jnci/djr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisawa T, Iizasa T, Saitoh Y, et al. Smoking before surgery predicts poor long-term survival in patients with stage I non-small-cell lung carcinomas. J Clin Oncol. 1999;17:2086–2091. doi: 10.1200/JCO.1999.17.7.2086. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman EL, Jacobson JS, Hershman DL, et al. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol. 2008;26:392–398. doi: 10.1200/JCO.2007.13.3033. [DOI] [PubMed] [Google Scholar]
- 8.Boorjian S, Cowan JE, Konety BR, et al. Cancer of the prostate strategic urologic research endeavor investigators. Bladder cancer incidence and risk factors in men with prostate cancer: results from Cancer of the prostate strategic urologic research endeavor. J Urol. 2007;177:883–887. doi: 10.1016/j.juro.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 9.Waggoner SE, Darcy KM, Fuhrman B, et al. Gynecologic Oncology Group. Association between cigarette smoking and prognosis in locally advanced cervical carcinoma treated with chemoradiation: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:853–858. doi: 10.1016/j.ygyno.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Kenfield SA, Stampfer MJ, Chan JM, et al. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aveyard P, Adab P, Cheng KK, et al. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90:228–239. doi: 10.1046/j.1464-410x.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 12.Bross I. Misclassification in 2 × 2 tables. Biometrics. 1954;10:478–486. [Google Scholar]
- 13.Goldberg JD. The effects of misclassification on the bias in the difference between two proportions and the relative odds in the fourfold table. J Am Stat Assoc. 1975;70:561–567. [Google Scholar]
- 14.Marshall JR, Priore R, Graham S, et al. On the distortion of risk estimates in multiple exposure level case-control studies. Am J Epidemiol. 1981;113:464–473. doi: 10.1093/oxfordjournals.aje.a113114. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JR, Hastrup JL. Mismeasurement and the resonance of strong confounders: uncorrelated errors. Am J Epidemiol. 1996;143:1069–1070. doi: 10.1093/oxfordjournals.aje.a008671. [DOI] [PubMed] [Google Scholar]
- 16.Gorber SC, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Schultz KE, Haller CA, et al. Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170:885–891. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attebring M, Herlitz J, Berndt AK, et al. Are patients truthful about their smoking habits? A validation of self-report about smoking cessation with biochemical markers of smoking activity amongst patients with ischaemic heart disease. J Intern Med. 2001;249:145–151. doi: 10.1046/j.1365-2796.2001.00770.x. [DOI] [PubMed] [Google Scholar]
- 19.Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in health and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2:261–270. [PubMed] [Google Scholar]
- 20.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 21.Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status—a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2003;42:154–159. doi: 10.1080/02841860310005020. [DOI] [PubMed] [Google Scholar]
- 22.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assaf AR, Parker D, Lapane KL, et al. Are there gender differences in self-reported smoking practices? Correlation with thiocyanate and cotinine levels in smokers and nonsmokers from the Pawtucket Heart Health Program. J Womens Health (Larchmt) 2002;11:899–906. doi: 10.1089/154099902762203731. [DOI] [PubMed] [Google Scholar]
- 24.Dietz PM, Homa D, England LJ, et al. Estimates of non-disclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173:355–359. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- 25.Pell JP, Haw SJ, Cobbe SM, et al. Validity of self-reported smoking status: comparison of patients admitted to hospital with acute coronary syndrome and the general population. Nicotine Tob Res. 2008;10:861–866. doi: 10.1080/14622200802023858. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 2009;107:349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, et al. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaporiciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg. 2002;73:420–425. doi: 10.1016/s0003-4975(01)03443-9. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin SJ, McCarthy CM, Pusic AL, et al. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg. 2005;55:16–19. doi: 10.1097/01.sap.0000168282.81348.b3. [DOI] [PubMed] [Google Scholar]
- 31.Komenaka IK, Hsu CH, Martinez ME, et al. Preoperative chemotherapy for operable breast cancer is associated with better compliance with adjuvant therapy in matched stage II and IIIA patients. Oncologist. 2011;16:742–751. doi: 10.1634/theoncologist.2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Land SR, Cronin WM, Wickerham DL, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the national surgical adjuvant breast and bowel project p-1 breast cancer prevention trial. Cancer Prev Res (Phila) 2011;4:1393–1400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eifel PJ, Jhingran A, Bodurka DC, et al. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 34.Jang S, Prizment A, Haddad T, et al. Smoking and quality of life among female survivors of breast, colorectal and endometrial cancers in a prospective cohort study. J Cancer Surviv. 2011;5:115–122. doi: 10.1007/s11764-010-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 36.Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 37.Bittner N, Merrick GS, Galbreath RW, et al. Primary causes of death after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008;72:433–440. doi: 10.1016/j.ijrobp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med. 1993;119:383–390. doi: 10.7326/0003-4819-119-5-199309010-00006. [DOI] [PubMed] [Google Scholar]
- 40.Marin VP, Pytynia KB, Langstein HN, et al. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121:451–457. doi: 10.1097/01.prs.0000297833.53794.27. [DOI] [PubMed] [Google Scholar]
- 41.Cooley ME, Wang Q, Johnson BE, et al. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer. 2012;76:144–149. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khuri FR, Kim ES, Lee J, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]