Abstract
Spectrins represent a family of membrane-associated proteins responsible for membrane flexibility and cell shape in erythrocytes, and probably in most nonerythroid cells. Spectrin functions as a tetramer consisting of two heterodimers each containing two subunits termed α and β. In humans, αI and αII spectrins but not β spectrins are characterized by the presence of an Src homology 3 (SH3) domain. As a tool to investigate the function of spectrin SH3 domains we derived several monoclonal antibodies (mAb) to the recombinant human αI or αII spectrin SH3 domain. Immunostaining using these monoclonal antibodies indicated expression of αI spectrin in cell bodies and αII spectrin in neurites of granule neurons in mouse primary cerebellar cultures. Monoclonal antibodies reactive to αI spectrin SH3 domain indicated expression of a protein(s) containing an αI-like SH3 domain in cytoplasmic vesicular-like structures in GFAP-positive cells in these cultures. In NIH 3T3 fibroblasts, these antibodies label macropinocytic vesicles. Together, these data and Western blotting results suggest expression of at least three spectrin-SH3 domain antibody-reactive proteins.
Keywords: Spectrin, Src homology 3 domain, Endocytosis, Macropinosome
It is known that αI (or erythroid) spectrin plays a vital role in the shape and stability of erythrocyte membranes. A similar function has been ascribed to αII spectrin (also called fodrin or nonerythroid spectrin) in neurons, which is an isoform of spectrin that is expressed in most tissues (reviewed in Refs. [1,7]). In the last decade, spectrin isoforms associated with intracellular organelles have been identified suggesting that spectrins play a universal structural role in intracellular membranes (reviewed in Refs. [4,5,9]).
Spectrin consists of two polypeptide chains, α and β, which associate as heterodimers. These heterodimers, in turn, associate head-to-head to form spectrin tetramers, which are considered a functional unit of spectrin (reviewed in Ref. [26,27]). Currently, two α-spectrin genes are known, encoding αI- and αII-spectrin, respectively [13,18], and five genes encode β-spectrins [10,13–16,21,22,30]. Considering the αβ heterodimer as a functional unit, the apparent imbalance in the number of α- vs. β-spectrins may be explained by hybrid heterodimer formation, e.g. αI with βII or βIII, or αII with βI or βIV, as previously suggested [2,3]; or by the existence of additional undetected genes encoding other α-like spectrins that form functional heterodimers with β spectrins.
Apart from several 106-amino acid repeat units common to both α- and β-spectrins [20]; mammalian α-spectrins are uniquely identified by the presence of calcium binding sites (EF hands) and an Src homology 3 (SH3) domain. In both αI- and αII-spectrin, the SH3 domain is located in the mid-region of the molecule between repeat units 9 and 11 [13,18,31]. Although most binding properties of spectrin to other proteins have been localized in β spectrins (reviewed in Refs. [5,9]) the spectrin SH3 domain may function through interactions with cytoplasmic ligands, and we recently identified a candidate αI SH3 domain binding protein [33]. This protein, designated, Hsshb3p1, belongs to a family of tyrosine kinase-binding proteins [19,28,34]. The spectrin SH3 domain binding site is highly conserved in these proteins suggesting that spectrin may provide a scaffold for intracellular signaling proteins [33]. As a tool to investigate spectrin function, we produced and characterized antibodies that detect SH3 domains from different isoforms of α-spectrin. Immunostaining and Western blotting analysis using these antibodies suggest expression of a protein(s) containing an αI-spectrin-like SH3 domain which associates with endocytic compartments in many nonerythroid cells, including GFAP-positive cells in mouse primary cerebellar cultures.
Purified GST fusion proteins containing the human αI- SH3 domain (GST-E-SH3) or the human αII-SH3 domain (GST-F-SH3) [33] were used for immunization of mice. Monoclonal antibodies were derived at the Institute for Basic Research in Developmental Disabilities Antibody Facility using standard techniques. Reactivities of antibodies to the recombinant spectrin SH3 domains were evaluated by ELISA and Western blotting. All antibodies reactive with GST and not to either of the spectrin SH3 domains were omitted from further analysis. Western blotting was performed using a PVDF membrane as described [11]. Polypeptides were separated on 7% SDS–Tricine polyacrylamide gels (GST fusion proteins), or on low-bis 6% SDS–Tris polyacrylamide gels [6] (NIH 3T3 cell lysates).
Cerebellar cell cultures were prepared as described [25,26]. Briefly, whole brains were removed from postnatal day-7 (P7) mouse pups (C57BL/6) and cell dissociation from cerebella was accomplished by trituration with a series of fire-polished Pasteur pipettes. After centrifugation cell pellets were resuspended in serum supplemented culture medium (10% horse serum; 5% FCS; 0.25% glucose (w/v); penicillin, 50 U/ml; streptomycin, 50 μg/ml, in MEM). Cells were seeded onto poly-D-lysine-coated (100 μg/ml) coverslips at a density of 1.875×106 cells/ml, and incubated at 37°C, in 5% CO2. After 24 h, the culture medium was changed to serum free medium (0.25% glucose (w/v); penicillin, 50 U/ml; streptomycin (50 μg/ml); 0.1% N2 supplement (Life Technologies, Rockville, MD)), in MEM. Cells were incubated an additional 48 h prior to processing for immunofluorescence. Cells were fixed in 100% methanol for 10 min at room temperature (cerebellar cultures) or with ice cold methanol (cultured cell lines). NIH 3T3, MDCK, HeLa, PTK1, and NRK cells were obtained from and grown according to ATCC (Rockville, MD) instructions. Tissue culture media were from Life Technologies or from Fisher Scientific (Pittsburgh, PA).
Uptake of the fluorescent markers wheat germ agglutinin–texas red conjugate (WGA-TR) (at 10 μg/ml) and Alexa 594 (1 mg/ml) (Molecular Probes, Eugene, OR) by NIH 3T3 cells was monitored at several time points (from 5 min up to 2 h after addition of the marker to medium) following fixation of cells. MAbs to spectrin SH3 domain were used as primary antibodies in Western blotting and in indirect immunofluorescence. Secondary antibodies were from Jackson ImmunoResearch, Inc. (West Grove, PA) and from Molecular Probes. Coverslips were rinsed extensively with PBS and mounted on ethanol-cleaned glass slides with mounting media as described [33]. High-resolution images (1024×1024 pixels) were collected using a 60× NA 1.4 objective and a Nikon PCM 2000 Confocal Imaging System.
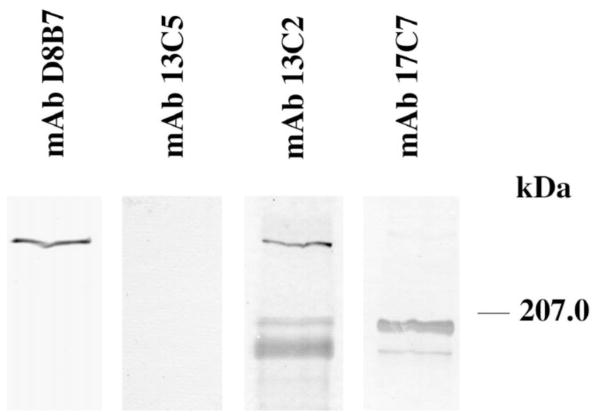
Out of 17 mAb clones, reactive with spectrin SH3 domain but not with GST, three groups of clones were defined based on their immunoreactivity in Western blotting (Fig. 1). One group of clones, represented by mAb 13C2, reacted with both αI and αII spectrin SH3 domains. While mAb 15A5 is also in this group, it showed less reactivity with the αII SH3 domain than did mAb 13C2. The second group of clones, represented by mAb 13C5 and mAb 17C7, reacted specifically with the αI SH3 domain. The third group of clones, represented by mAb D8B7, reacted specifically with the αII SH3 domain. Immunoreactivity of these mAbs in ELISA (not shown) was very similar to that in Western blot analysis. All antibodies except mAb D8B7 reacted with αI spectrin (240-kDa band) extracted from human ghosts (Table 1). MAbs 13C2 and 15A5 reacted with the 240-kDa species in lysates of NIH 3T3 cells. The same reactivity was observed with mAb D8B7 in NIH 3T3 cells (Fig. 2). MAb 17C7 reacted with αI spectrin in mouse ghosts and with a 200-kDa protein in NIH 3T3 cells and in mouse cerebellum [35]. MAb 17C7 showed weak reactivity with a 180-kDa band in NIH 3T3 cells, while mAb 13C2 showed reactivity with a 200-kDa band and a 180-kDa band in these cells (Fig. 2).
Fig. 1.
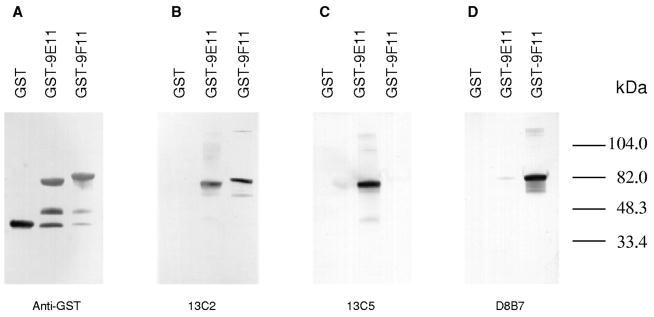
Specificity of monoclonal antibodies to recombinant spectrin Src homology 3 domain. Equal amounts of IPTG-induced bacterial lysates of the GST-SH3 domain fusion polypeptides of αI spectrin (GST-9E11, containing spectrin repeat units 9 through 11, as defined in Ref. [11]) and αII spectrin (GST-9F11, containing αII spectrin repeat units 9 through 11; it includes two sequence insertions not present in αI spectrin and accounting for an apparent difference in migration between GST-9E11 and GST-9F11) were solubilized in Laemmli buffer and separated on 7% SDS–Tricine polyacrylamide gels. Identical blotted transfers of gels were incubated with the following antibodies: anti-GST, panel A; mAb 13C2, panel B; mAb 13C5, panel C, and mAb D8B7, panel D. mAb 17C7 showed reactivity identical to mAb 13C5; mAb 15A5 was similar to mAb 13C2 but was less immunoreactive to GST-9F11. All monoclonal antibodies were raised to recombinant GST fusion polypeptides as indicated in Table 1.
Table 1.
Characterization of monoclonal antibodies to αI and αII spectrin SH3 domains
| MAb clone | Antigena | Immunoreactivity in Western blotting
|
Immunofluorescence in cultured cells
|
|||||
|---|---|---|---|---|---|---|---|---|
| E-SH3 | F-SH3 | Human ghostsb | NIH 3T3 cellsc | NIH 3T3 cells | Primary cerebellar culture
|
|||
| Neurons | Glial cellsd | |||||||
| D8B7 | F-SH3 | − | +++ | − | 240 kDa | Cell periphery | Cell extensions and periphery | Cell periphery (weak) |
| 13C2 | E-SH3 | ++ | ++ | 240 kDa | 240 kDa 200 kDae 180 kDa |
|||
| 13C5 | E-SH3 | +++ | − | 240 kDa | – | Punctate cytoplasmic | Cell bodies | Punctate cytoplasmic |
| 15A5 | E-SH3 | +++ | +/− | 240 kDa | 240 kDa | |||
| 17C7f | E-SH3 | +++ | − | 240 kDa | 200-kDa 180 kDae |
– | ||
Recombinant GST-SH3 polypeptides containing αI spectrin (E-SH3) and αII spectrin (F-SH3) SH3 domains [33] were used as antigens. ‘−’, indicates no reactivity; ‘+/−’ weak reactivity; ‘++’ moderate reactivity; ‘+++’ strong reactivity.
αI spectrin was extracted from human ghosts as described in Ref. [3].
Total cell lysate of NIH 3T3 cells was used. Apparent molecular size of immunoreactive bands is indicated (see also Fig. 2).
GFAP-positive cells.
Weak reactivity.
MAb 17C7 was previously reported in Ref. [35]. Weak reactivity to the 180-kDa is observed.
Fig. 2.
Reactivity of antibodies in NIH 3T3 cells. Samples containing 20 μg of total cell lysate were separated on 6% low-bis SDS–Tris poly-acrylamide gels. Identical blotted transfers of gels were incubated with the antibodies indicated above panels. MAb 15A5 reactivity (not shown) was similar to mAb D8B7.
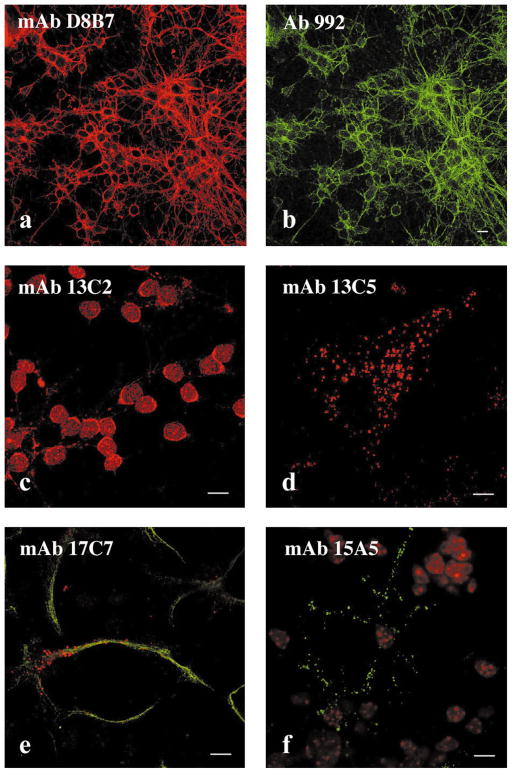
Immunoreactivity of spectrin SH3 domain antibodies was evaluated in primary mouse cerebellar cultures. MAb D8B7 labeled neurites and the periphery of cell bodies in granule neurons (Fig. 3a). In cerebellar neurons mAb D8B7 staining colocalized with that of polyclonal antibody 992 raised to axonal spectrin (Fig. 3b) [17]. MAbs 15A5, 13C5, and 13C2 showed immunoreactivity with granule neuron cell bodies (Fig. 3c). MAb 17C7 as well as the other antibodies reactive with the αI SH3 domain (mAbs 13C2, 13C5, and 15A5) showed a cytoplasmic punctate immunostaining pattern in GFAP-positive cells (Fig. 3e and f) and in fibroblastic-like cells present in primary cerebellar cultures. Furthermore, these antibodies stained cytoplasmic vesicular-like structures in mouse NIH 3T3 fibroblasts (Fig. 3d) and in HeLa, MDCK and NRK, and PTK1 cells (not shown). MAb 13C2 (Fig. 4) as well as other mAbs to the αI spectrin SH3 domain colocalized with vesicles containing fluorescent WGA-TR endocytosed by cells within 5 min. The functional properties of these vesicles are consistent with macropinocytic vesicles [35]. Thus, mAbs to the αI SH3 domain serve as markers of macropinosomes.
Fig. 3.
Immunostaining pattern of monoclonal antibodies to spectrin SH3 domains. Mouse cerebellar cultures (a–c and e and f) and NIH 3T3 cells (d) were stained with monoclonal antibodies to spectrin SH3 domain. A culture enriched for granule neurons costained with mAb D8B7 to αII SH3 domain (a, red) and with the polyclonal antibody 992 to axonal spectrin [17] (Chemicon, Temecula, CA) (b, green), or stained with mAb 13C2 (c) (red). A culture of cerebellar cells enriched for astrocytic cells costained with mAb 17C7 (e, red) and with the polyclonal antibody to GFAP (Sigma, St. Louis, MO) (f, green). An NIH 3T3 fibroblast stained with mAb 15A5 (d) (green), nuclear autofluorescence (red). Antibodies 17C7, 13C2, and 15A5 produce very similar pattern in NIH 3T3 cells to that observed with mAb 13C5. Note coincident pattern in (a) and (b), indicating colocalization of antibody staining. Bar, 10 μm.
Fig. 4.
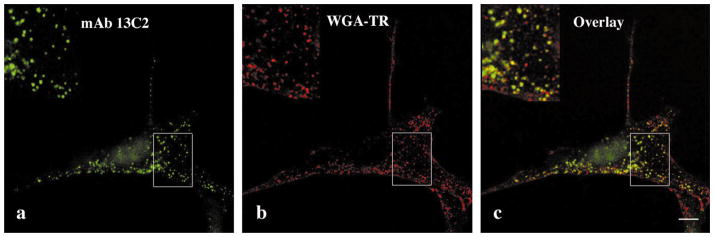
MAb 13C2 to αI spectrin SH3 domain labels vesicles participating in endocytosis of fluorescent wheat germ agglutinin in NIH 3T3 cells. (a–c) Cells were allowed to endocytose WGA-TR (red) for 5 min and stained with mAb 15A5 (green); (c) is overlay of (a) and (b) images. Similar result was observed with all monoclonal antibodies reactive to the αI SH3 domain. Bar, 10 μm.
The SH3 domain is highly conserved in spectrins. Nonerythroid spectrins from Drosophila, mouse, chicken, rat and human all contain an SH3 domain [24]. Sequences of the SH3 domain in human αII-spectrin and chicken α-spectrin are 100% identical [13,31]. αI- and αII-spectrins are the most homologous in the SH3 domain region, with 65% identity compared to an overall identity between these spectrins of 54%. The specificity of mAb D8B7 for the αII spectrin SH3 domain was consistent with expression of αII spectrin in neurites of granule neurons and the plasma membrane of nonerythroid cells [12,17]. MAbs reactive with the αI SH3 domain indicated expression of αI spectrin in cell bodies of granule neurons consistent with observation by Clark et al. [2]. Interestingly, all mAbs reactive with the αI SH3 domain associated with macropinocytic vesicles in NIH 3T3 cells as defined by Xu et al. [35]. The macropinocytic compartment was originally characterized in macrophages and in tumor cell lines (reviewed in Ref. [23]). Macropinocytic vesicles are resistant to brefeldin A treatment, do not contain a clathrin coat, are heterogeneous in size, and are larger than clathrin-coated vesicles [8,32]. The similar staining pattern of GFAP-positive cells in cerebellar primary cultures and in many nonerythroid cultured cell lines suggest the presence of macropinocytic vesicles in astrocytes and in many cells originating from different tissues. Our antibodies provide useful markers in further defining the function of this non-clathrin pinocytic compartment.
The staining of macropinocytic vesicles with anti-αI SH3 domain antibodies suggests expression of proteins containing an SH3 domain similar to the αI SH3 domain. A 200-kDa protein that reacts with mAb 17C7 and is a Hsshb3p1 interacting protein [35] is also detected by mAb 13C2, which detects an additional 180-kDa band. The lack of mAb 13C5 reactivity with these bands, and the labeling of macropinocytic vesicles by this antibody suggest the presence of a conformational epitope shared by the αI spectrin SH3 domain, the 200-kDa protein and the 180-kDa band. Our mAbs will help to determine whether these bands represent novel α-spectrins.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke grant R29 NS32874 (L.K.) and in part by funds provided by the New York State Office of Mental Retardation and Developmental Disabilities. We thank Drs. K.S. Kim and Richard Kascsak (NYS IBR, Staten Island, NY) and the technical staff of the IBR Monoclonal Antibody Facility, Mr. James Chen, Ms. Heni Hong, and Mr. Victor Sapienza, for help in developing monoclonal antibodies to spectrin SH3 domain. We also thank Dr. Ekkhart Trenkner (NYS IBR, Staten Island, NY) for help in mouse primary cerebellar cultures, and Joanne Stocker for help in preparation of the manuscript.
Abbreviations
- ATCC
American Tissue Culture Collection
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- GFAP
glial fibrillary acidic protein
- GST
glutathione S-transferase
- MEM
minimal essential medium
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PVDF
polyvinylidene difluoride
- SH3
Src homology 3
- Tricine
N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
Footnotes
Nomenclature for spectrin isoforms used in this paper is according to Winkelmann and Forget [29].
References
- 1.Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- 2.Clark MB, Ma YP, Bloom ML, Barker JE, Zagon LS, Zimmer WE, Goodman SR. Brain alpha-erythroid spectrin identification, compartmentalization, and beta-spectrin associations. Brain Res. 1994;663:223–236. doi: 10.1016/0006-8993(94)91267-x. [DOI] [PubMed] [Google Scholar]
- 3.Davis J, Bennett V. Brain spectrin. Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem. 1983;258:7757–7766. [PubMed] [Google Scholar]
- 4.De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol. 1998;10:542–549. doi: 10.1016/s0955-0674(98)80071-9. [DOI] [PubMed] [Google Scholar]
- 5.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JD, Swartz DR, Greaser ML. Factors affecting poly-acrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Ann Biochem. 1989;180:205–210. doi: 10.1016/0003-2697(89)90116-4. [DOI] [PubMed] [Google Scholar]
- 7.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 8.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holleran EA, Holzbaur ELF. Speculating about spectrin: new insights into the Golgi-associated cytoskeleton. Trends Cell Biol. 1998;8:26–29. doi: 10.1016/s0962-8924(97)01195-1. Review. [DOI] [PubMed] [Google Scholar]
- 10.Hu RJ, Watanabe M, Bennett V. Characterization of human brain cDNA-encoding the general isoform of beta-spectrin. J Biol Chem. 1992;267:18715–18722. [PubMed] [Google Scholar]
- 11.Kotula L, DeSilva T, Speicher D, Curtis PJ. Functional characterization of recombinant human red cell α-spectrin polypeptides containing the tetramer binding site. J Biol Chem. 1993;268:14788–14793. [PubMed] [Google Scholar]
- 12.Levin J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–643. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon AP, Giebelhaus DH, Champion JE, Bailes JA, Lacey S, Carritt B, Henchman SK, Moon RT. cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin. Differentiation. 1987;34:68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 14.Mishra L, Cai T, Levine A, Weng D, Mezey E, Mishra B, Gearhart J. Identification of elf1, a beta-spectrin, in early mouse liver development. Int J Dev Biol. 1998;42:221–224. [PubMed] [Google Scholar]
- 15.Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–364. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- 16.Ohara O, Ohara R, Yamakawa H, Nakajima D, Nakayama M. Characterization of a new beta-spectrin gene which is predominantly expressed in brain. Mol Brain Res. 1998;57:181–192. doi: 10.1016/s0169-328x(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 17.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin (240.235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol. 1986;102:2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, Leto TL, Linnenbach AJ, Winkelmann JC, Speicher DW, Marchesi VT, Curtis PJ, Forget BG. The complete cDNA and polypeptide sequences of human erythroid α-spectrin. J Biol Chem. 1990;265:4434–4443. [PubMed] [Google Scholar]
- 19.Shi Y, Alin K, Goff S. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- 20.Speicher DW, Marchesi VT. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984;311:177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- 21.Stabach PR, Morrow JS. Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila Beta H Spectrin. J Biol Chem. 2000;275:21385–21395. doi: 10.1074/jbc.C000159200. [DOI] [PubMed] [Google Scholar]
- 22.Stankewich MC, Tse WT, Peters LL, Ch’ng Y, John KM, Stabach PR, Devarajan P, Morrow JS, Lux SE. A widely expressed beta III spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–427. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 24.Thomas GH, Newbern EC, Korte CC, Bales MA, Muse SV, Clark AG, Kiehart DP. Intragenic duplication and divergence in the spectrin superfamily of proteins. Mol Biol Evol. 1997;14:1285–1295. doi: 10.1093/oxfordjournals.molbev.a025738. [DOI] [PubMed] [Google Scholar]
- 25.Trenkner E. Cerebellar cells in culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT; Cambridge, MA: 1991. pp. 283–307. [Google Scholar]
- 26.Trenkner E, Sidman RL. Histogenesis of mouse cerebellum in microwell cultures. Cell reaggregation and migration, fiber and synapse formation. J Cell Biol. 1977;75:915–940. doi: 10.1083/jcb.75.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viel A, Branton D. Spectrin: on the path from structure to function. Curr Opin Cell Biol. 1996;8:49–55. doi: 10.1016/s0955-0674(96)80048-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Mysliwiec T, Krainc D, Jensen RA, Sonoda G, Testa JR, Golemis EA, Kruh GD. Identification of ArgBP1, an Arg protein tyrosine kinase binding protein that is the human homologue of CNS-specific Xenopus gene. Oncogene. 1996;12:1921–1929. [PubMed] [Google Scholar]
- 29.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81:3173–3185. Review. [PubMed] [Google Scholar]
- 30.Winkelmann JC, Leto TL, Watkins PC, Eddy R, Shows TB, Linnenbach AJ, Sahr KE, Kathuria N, Marchesi VT, Forget BG. Molecular cloning of the cDNA for human erythrocyte beta-spectrin. Blood. 1988;72:328–334. [PubMed] [Google Scholar]
- 31.Wasenius VM, Saraste M, Salven P, Eramaa M, Holm L, Lehto VP. Primary structure of the brain alpha-spectrin. J Cell Biol. 1989;108:79–93. doi: 10.1083/jcb.108.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West MA, Bretscher MS, Watts C. Distinct endocytic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L. Identification of a candidate spectrin SH3 binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem. 1998;273:13681–13692. doi: 10.1074/jbc.273.22.13681. [DOI] [PubMed] [Google Scholar]
- 34.Zonghan D, Pendegrast M. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Ziemnicka D, Merz GS, Kotula L. Human spectrin SH3 domain binding protein 1 regulates macropinocytosis in NIH 3T3 cells. J Cell Sci. 2000;113:3805–3814. doi: 10.1242/jcs.113.21.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]