Abstract
BK virus nephropathy is a common cause of graft loss in kidney transplant recipients. Cases of BK nephropathy following allogeneic hematopoietic cell transplantation (HCT) are underreported. An increased incidence of BK virus-associated nephropathy is being seen in the setting of more profound and prolonged immunosuppression following solid organ transplantation and HCT. We will review diagnostic and treatment modalities for BK-associated nephropathy following allogeneic HCT.
Keywords: BK virus nephropathy, Hematopoietic cell transplantation, Antiviral therapy
INTRODUCTION
The BK virus is a member of the Polyomavirus family and, although ubiquitous in the general population, is not pathologic in immunocompetent adults. The BK virus seroprevalence is 90% in adults [1], with the virus in latent dormant stage embedded in the urothelium. Intermittent reactivation with low level viruria (<106 virus copies/mL of urine) may be noted in approximately 5% of immunocompromised patients without consequence [2,3]. However, higher levels of BK shedding in the urine (>107 virus copies/mL of urine) has been noted in 20% to 60% of patients after solid organ transplantation, and is associated with the risk of developing hemorrhagic cystitis and Polyomavirus-associated nephropathy, including ureteral obstruction, hydronephrosis, and renal allograft loss [2,4-7]. The most reported cases of BK virus nephropathy or hemorrhagic cystitis occur after solid organ transplantation secondary to immunosuppression, but have also been reported in recipients of allogeneic hematopoietic cell transplants (HCT) [8-14]. BK viruria has been reported in 50% of patients after HCT, within 2 month of transplantation [8,9,15,16]. Hemorrhagic cystitis is commonly associated with BK infection, occurring in 10% to 25% of recipients [8,15], although a direct correlation for BK viruria and hemorrhagic cystitis has not been established. In fact, in a multivariate analysis for predictors of hemorrhagic cystitis in patients receiving HCT at M. D. Anderson, the presence of BK viruria was not found to be a significant factor [17].
Pathogenesis of BK-Associated Nephropathy
Development of BK viruria and associated nephropathies, including hemorrhagic cystitis, is dependent on several factors including type of conditioning regimen, use of T cell-depleted grafts, and use of related or unrelated donor grafts [17,18]. Leung et al. suggested 3 phases for the development of BK virus hemorrhagic cystitis [19]. First, the conditioning regimen damages the bladder mucosa, providing a hospitable environment for the BK virus to replicate. Second, viral replication becomes unchecked in the absence of functional immunity. Third, there is further damage to the bladder mucosa with immune reconstitution and the return of anti-BK virus immunity [19]. Review of the literature suggests a higher incidence of BK hemorrhagic cystitis with the use of T cell-depleted grafts [20]. The introduction of more powerful immunosuppressive agents, such as mycophenolate mofetil and tacrolimus, have also likely increased the reported incidence of BK virus-induced nephropathy and hemorrhagic cystitis [21].
Diagnosis of BK-Associated Nephropathy
The current diagnostic modalities most commonly used include screening for the BK viral load in the urine and serum, and microscopic examination of the urine to check for Polyomavirus sediments in patients presenting with hematuria and abnormal renal function. In 1 report, the group of patients who developed hemorrhagic cystitis showed evidence for BK viruria at a median of 23 days following HCT, with hemorrhagic cystitis subsequently developing on average 44 days following HCT [21]. A recent report suggested the importance of BK viremia as a prognostic marker for BK nephropathy in children undergoing HCT [22]. Patients with a high BK viral load in the serum (>10,000 copies/mL) had more severe BK-associated nephropathy requiring more aggressive treatment, including dialysis, with survival approximately 48% at 1 year. In contrast, the group of patients with low serum BK titers (<10,000 copies/mL) had less aggressive disease with an estimated survival of 89% at 1 year [22].
The definitive diagnosis for BK nephropathy is a renal biopsy with immunohistochemistry showing presence of the virus in the renal cells. When, and if, this test is performed depends on the severity of presentation and whether a definitive diagnosis will change the treatment plan. Figure 1 illustrates the positive SV-40T stain noted in the tubulointerstitium of the kidney, nuclear enlargement, and smudge chromatin, suggesting viral inclusions, as well as the presence of IgA tubular casts associated with viral infection, which were noted in 1 of our patients who developed BK-associated nephropathy 214 days following a double cord blood transplant for relapsed acute lymphoblastic leukemia (ALL). This patient’s clinical course, illustrated in Figure 2, and described in the following paragraphs, is representative of the challenges of treatment for BK-associated nephropathy.
Figure 1.
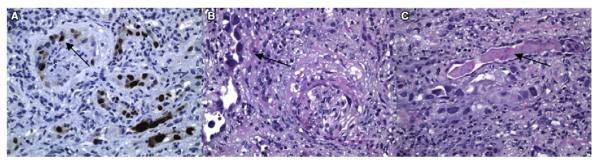
Immunohistochemistry images of renal biopsy. SV-40T stain was positive, suggesting BK virus involvement of the tubulointerstitium (A). Nuclear enlargement and smudge chromatin suggest viral inclusion (B). The presence of tubular casts for IgA is a nonspecific finding associated with tubulitis and interstitial lymphoplasmacytic infiltrates (C).
Figure 2.
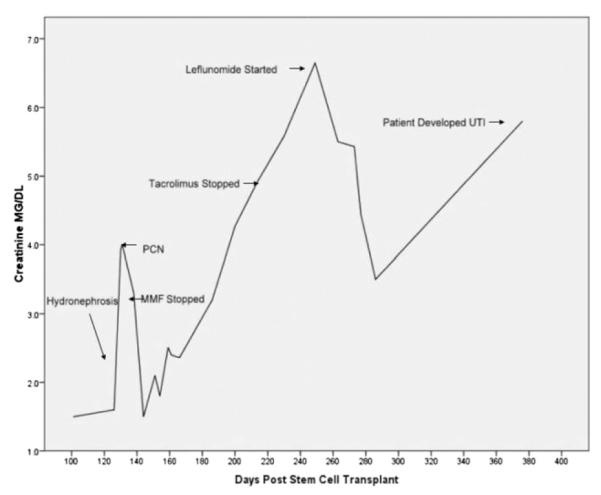
Serum creatinine measurements beginning 100 days following transplantation in patient diagnosed with BK-associated hemorrhagic cystitis and nephropathy following a double cord blood transplant. The patient developed BK viruria, with >5 × 108 virus copies/mL urine, associated with hemorrhagic cystitis, 39 days post-HCT. At 105 days post-HCT, there was a rise in creatinine up to 3.6 mg/dL that was noted to be because of bilateral hydronephrosis, diagnosed by renal ultrasound, in the setting of persistent BK viruria, with improvement in creatinine following placement of bilateral percutaneous nephropathy tubes. Immunosuppression was also discontinued at this time. The presence of renal failure prohibited the use of cidofovir, so leflunomide was initiated on day +263, and over the course of 3 weeks, the creatinine decreased from 6.7 mg/dL to 3.5 mg/dL, with a 75% reduction in BK viruria from >5 × 108 to 1.25 × 108 copies of virus/mL urine. At approximately 1 year posttransplantation, 6.86 × 105 copies of virus/mL of urine was noted after taking leflunomide for approximately 15 weeks. However, the patient subsequently developed another rise in her creatinine concurrent with the development of a pseudomonas urinary tract infection. The patient remains on leflunomide at 20 mg daily, with no liver or bone marrow toxicities noted.
Treatment of BK-Associated Nephropathy
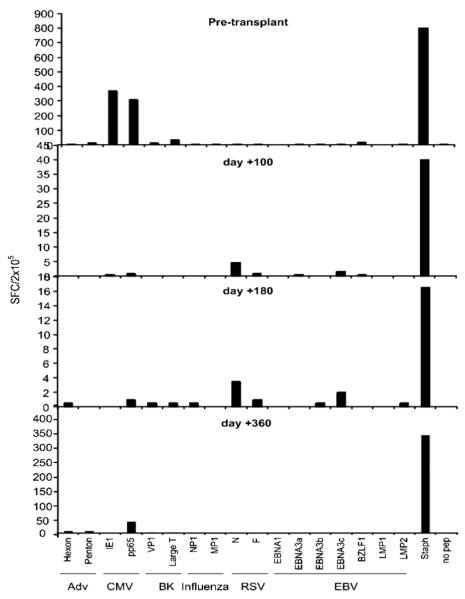
The heterogeneous and complex clinical situations of patients who develop BK viruria and nephropathy, as well as the lack of large clinical studies, make it challenging to develop standard guidelines for the treatment of BK virus-associated hemorrhagic cystitis and nephropathy. Studies suggest that BK virus nephropathy is caused by an imbalance between BK virus replication and BK virus cellular mediated immunity [23,24], although the degree of cellular immunity necessary to prevent reactivation of BK virus after HCT is not known. Thus, the standard treatment for BK nephropathy is to first reduce immunosuppression with the aim of improving the T cell-mediated immunity against the virus [23,25], followed by the use of antiviral agents. Figure 3 shows enzyme-linked immunosorbent spot (ELISPOT) responses to several common viruses peritransplantation, including BK virus, in our patient who developed BK nephropathy. In this patient, varying levels of immunity against BK virus was present before the cord blood transplant, but became absent at 100 days following transplantation with transient recovery of immunity at 180 days. Despite discontinuation of all immunosuppression at 210 days posttransplantation, BK-virus reactive T cells remained undetectable at 360 days posttransplantation (Figure 3).
Figure 3.
Summary of ELISPOT responses as measured by SFC/105 peripheral blood mononuclear cells (PBMC) against the BK virus antigens LT and VP1. Pretransplantation, the patient was noted to have 10 SFC/2 × 105 PBMC against the VP1 antigen and 40 SFC/2 × 105 PBMC against the LT antigen. BK-virus reactive T cells remain undetectable at 360 days posttransplantation. The previously described ELISPOT assay was used to determine the frequency of virus-specific IFN-γ-secreting T cells.38 Briefly, PBMCs were isolated by ficoll gradient and plated at 2 × 105 cells/well. We measured the viral-specific activity of responder cells after direct stimulation with pepmixes spanning Hexon and Penton (Adv), IE1 and pp65 (CMV), VP1 and Large T (BK), MP1 and NP1 (Influenza), N and F (RSV), and EBNA1, EBNA3a, EBNA3b, EBNA3c, LMP1, LMP2, and BZLF1 (EBV). All pepmixes, which are overlapping peptide libraries (15mers overlapping by 11 amino acids) were purchased from JPT Technologies (Berlin, Germany). Each culture condition was run in triplicate. After 20 hours of incubation, plates were developed as previously described [38], dried overnight at room temperature in the dark, then sent to Zellet Consulting (New York, New York) for quantification. The frequency of T cells specific to each antigen was expressed as specific SFC per input cell numbers.
A variety of antiviral agents have been tried, including cidofovir and leflunomide, but the duration of treatment is not standardized [26-30]. Cidofovir ([S]-1-[3-hydroxy-2-(phosphonomethoxy) propyl] cytosine [HPMPC]) is an acyclic phosphonate analogue of dCMP, which has shown antiviral activity against several DNA viruses, including BK virus, although its mechanism of action is still under investigation. New classes of nucleoside phosphonate derivatives are under investigation [31]. Leflunomide is an immunomodulatory agent that inhibits pyrimidine synthesis and results in antiproliferative and anti inflammatory effects. Leflunomide is converted to its active metabolite, A77 1726 (M1), which inhibits BK virus DNA replication in the renal tubular epithelium. Thus, the assembly and release of the virus is inhibited [32-34]. Leflunomide has been extensively studied in the setting of BK virus nephropathy developing after renal allografts, but has not been well studied in HCT. The recommended starting dose for leflunomide is 20 mg daily, with the effective drug level of leflunomide to achieve 50% and 90% reduction in BK virus replication reported to be 15 to 30 μg/mL and 35 to 100 μg/mL in the blood, respectively [29]. Associated toxicities include myelosuppression and liver toxicity [29,33]. The duration of therapy is not clear, but treatment is usually continued until there is resolution of viruria. Because of ongoing kidney failure and the difficulty of using cidofivir in this setting, we treated our patient with leflunomide, with a reduction noted in BK viruria but persistent kidney dysfunction as manifested by a persistently elevated creatinine (Figure 2). In a comparison study of cidofovir against leflunomide for the treatment of BK nephropathy in solid organ transplants, both drugs had similar rates of clearing BK viremia, but there was less graft loss seen with the use of leflunomide [35]. Of interest, in 1 study evaluating the inhibitory effects of cidofivir against leflunomide against BK virus replication in vitro, cidofivir was noted to be superior [31].
In addition to antiviral therapies, intravenous immunoglobulin and fluoroquinolones [36] have been evaluated for treatment, and were noted to be inferior to cidofivir and leflunomide [35,37]. Finally, in addition to drug therapy, the use of cellular-mediated therapies hold great promise in this setting, because the pathogenesis of BK-associated complications is based on poor cellular immunity against the virus. Based on promising work infusing cytotoxic T lymphocytes (CTLs) with tri-viral specificity for Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus (ADV) into patients with these persistent viral infections despite traditional drug therapy, Gerdemann and colleagues [38] are currently developing an approach to develop multivirus-reactive CTLs with broader antiviral activity, including activity against the BK virus.
SUMMARY
In conclusion, BK nephropathy developing after HCT is a pathologic entity that occurs in the setting of profound immunosuppression. The complexity of patients makes it difficult to currently standardize treatment recommendations. Aggressive diagnosis of patients at higher risk of developing BK-associated nephropathy (known preexisting kidney disease, persistent BK viruria, persistent hemorrhagic cystitis) with renal biopsy, followed by aggressive immunosuppresion withdrawal as feasible and earlier treatment initiation, may help in preventing kidney failure. The most effective intervention is the reduction in immunosuppression. The addition of antiviral agents such as cidofovir or leflunomide in the setting of persistent BK viremia/viruria is helpful. New nucleoside phosphonate analogues, currently in development, should be widely studied in the setting of HCT to provide more antiviral agent choices for treatment. The choice of antiviral agent at this time is limited but should be based on patient tolerability and side effect profile. Finally, the development of cellular-mediated therapies holds great promise.
Footnotes
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis. 2005;41:354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 3.Randhawa P, Uhrmacher J, Pasculle W, et al. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol. 2005;77:238–243. doi: 10.1002/jmv.20442. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 5.Nickeleit V, Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–1315. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 6.Ahsan N, Shah KV. Polyomaviruses and human diseases. Adv Exp Med Biol. 2006;577:1–18. doi: 10.1007/0-387-32957-9_1. [DOI] [PubMed] [Google Scholar]
- 7.Bohl DL, Brennan DC. BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007;2(Suppl 1):S36–S46. doi: 10.2215/CJN.00920207. [DOI] [PubMed] [Google Scholar]
- 8.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 9.Arthur RR, Shah KV, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 10.Apperley JF, Rice SJ, Bishop JA, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84:243–246. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto S, Azuma E, Hori H, et al. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatr Hematol Oncol. 2002;19:255–261. doi: 10.1080/08880010252899424. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro S, Robin M, Esperou H, et al. Polyomavirus nephropathy in the native kidneys of an unrelated cord blood transplant recipient followed by a disseminated polyomavirus infection. Transplantation. 2006;82:292–293. doi: 10.1097/01.tp.0000226172.68372.f9. [DOI] [PubMed] [Google Scholar]
- 14.Limaye AP, Smith KD, Cook L, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5:614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 16.Azzi A, Cesaro S, Laszlo D, et al. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J Clin Virol. 1999;14:79–86. doi: 10.1016/s1386-6532(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 17.Silva Lde P, Patah PA, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti S, Osman H, Collingham K, et al. Polyoma viruria following T-cell-depleted allogeneic transplants using Campath-1H: incidence and outcome in relation to graft manipulation, donor type and conditioning. Bone Marrow Transplant. 2003;31:379–386. doi: 10.1038/sj.bmt.1703847. [DOI] [PubMed] [Google Scholar]
- 19.Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant. 2005;36:929–937. doi: 10.1038/sj.bmt.1705139. [DOI] [PubMed] [Google Scholar]
- 20.Childs R, Sanchez C, Engler H, et al. High incidence of adenoand polyomavirus-induced hemorrhagic cystitis in bone marrow allotransplantation for hematological malignancy following T cell depletion and cyclosporine. Bone Marrow Transplant. 1998;22:889–893. doi: 10.1038/sj.bmt.1701440. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 22.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Binggeli S, Egli A, Schaub S, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131–1139. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 24.Egli A, Kohli S, Dickenmann M, Hirsch HH. Inhibition of polyomavirus BK-specific T-Cell responses by immunosuppressive drugs. Transplantation. 2009;88:1161–1168. doi: 10.1097/TP.0b013e3181bca422. [DOI] [PubMed] [Google Scholar]
- 25.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 26.Araya CE, Lew JF, Fennell RS, III, Neiberger RE, Dharnidharka VR. Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy. Pediatr Transplant. 2006;10:32–37. doi: 10.1111/j.1399-3046.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 27.Bjorang O, Tveitan H, Midtvedt K, Broch LU, Scott H, Andresen PA. Treatment of polyomavirus infection with cidofovir in a renal-transplant recipient. Nephrol Dial Transplant. 2002;17:2023–2025. doi: 10.1093/ndt/17.11.2023. [DOI] [PubMed] [Google Scholar]
- 28.Faguer S, Hirsch HH, Kamar N, et al. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transpl Int. 2007;20:962–969. doi: 10.1111/j.1432-2277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 29.Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 30.Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057–1070. doi: 10.1097/TP.0b013e3181d0e15e. [DOI] [PubMed] [Google Scholar]
- 31.Topalis D, Lebeau I, Krecmerova M, Andrei G, Snoeck R. Activity of different classes of acyclic nucleoside phosphonates against BK virus in primary human renal cells. Antimicrob Agents Chemother. 2011;55:1961–1967. doi: 10.1128/AAC.01809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldman WJ, Knight DA, Blinder L, et al. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42:412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 33.Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352:1157–1158. doi: 10.1056/NEJM200503173521125. [DOI] [PubMed] [Google Scholar]
- 34.Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84:2150–2156. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilton R, Tong CY. Antiviral therapy for polyomavirus-associated nephropathy after renal transplantation. J Antimicrob Chemother. 2008;62:855–859. doi: 10.1093/jac/dkn305. [DOI] [PubMed] [Google Scholar]
- 36.Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 37.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]