Abstract
Background
The Herpesviridae encode a family of protein homologs that function as the “port of entry” for insertion of the viral DNA into preformed capsids during encapsidation.
Methods
Transmission electron microscopy of recombinant Varicella-zoster virus pORF54.
Results
Results suggest that pORF54 forms higher order structures with itself. Enriched fractions analyzed by TEM revealed non-axial oriented portals with defined central channels and distinguishable crown and tail regions.
Conclusion
These morphological features are consistent with those previously reported for other herpesvirus and bacteriophage portal proteins.
Herpesviruses are enveloped, double stranded DNA viruses that infect both vertebrate and invertebrate animals. Currently eight different herpesviruses are known to cause disease in humans. All herpesviruses establish latent infections for the lifetime of the host. Human herpesviruses (HHVs) have a distinct tropism for nervous and lymphoid cells, where the body’s immune surveillance is limited and the virus can remain undetected. This typically results in cycles of active viral replication (reactivation) and latent periods of infection.
Vaccination has proven valuable for VZV and will likely prove useful for the other HHVs in the future. Despite intense efforts by the biomedical research community, there is no effective cure for any established herpesvirus infection. HHVs, including our model organism, Varicella-zoster virus, can maintain a latent presence in human populations. As a consequence of latency, therapies which inhibit viral replication cannot effectively eliminate infection. Until a means of resolving latent infection is found, replication inhibitors (i.e. acyclovir) will remain the treatment of choice for suppressing and controlling symptoms of recurrent disease.
Most of the available inhibitors share the same mechanism of action, targeting viral DNA polymerase and interfering with DNA synthesis. These drugs include acyclovir, ganciclovir, penciclovir, brivudin, cidofovir, and foscarnet. They can be categorized into two chemical classes: nucleoside analogues and pyrophosphate analogues [1]. Following phosphorylation of their respective prodrug form, these compounds act as a substrate for viral DNA polymerase and once incorporated into the nascent DNA chain, block strand elongation. Some of these drugs have severe negative side effects, limited viral specificity, and poor bioavailability and/or toxicity profiles. Additionally, since all of these drugs share the same target, development of resistance is possible. Resistant strains of HHVs have been identified for all currently approved drugs [1].
Although therapeutic options are available for certain herpesviruses, shortcomings due to specificity, bioavailability, host toxicity and drug resistance warrant continued research aimed at identifying and developing novel therapies. Proteins that play a role in herpesviral DNA encapsidation have become promising novel chemotherapeutic targets. Two series of related non-nucleoside compounds, N-α-methylbenzyl-N’-aryl thiourea analogs, that inhibit either HSV-1 [1, 2] or VZV [1, 3] DNA encapsidation, have been described. In the presence of thiourea inhibitors, only B-capsids were observed in the nuclei of HSV or VZV infected cells. Electron microscopy revealed a lack of DNA-filled capsids in the nucleus for HSV-1 or VZV infected cells treated with their respective thiourea inhibitor [2, 3]. HSV and VZV mutant viruses resistant to thiourea compounds were found to contain mutations in their putative portal proteins, pUL6 and pORF54 respectively [2, 3]. In a separate study, the HSV-1 portal protein homolog, pUL6, was shown to be the likely target of the HSV-1 specific thiourea compounds [4]. Previously, pUL6 was shown to localize to a single vertex of the viral capsid and is the likely site of entry for viral genomic DNA during the encapsidation process [5, 6]. Additionally, HSV-1 UL6 deletion mutants are defective in both DNA cleavage and packaging, which results in large numbers of B-capsids in the nuclei of mutant-infected cells. The effect of inhibiting pUL6 or pORF54 function via the thiourea compounds is consistent with the genetic evidence provided by studies with HSV-1 deletion mutants [7, 8]. Thus, a thorough understanding of the interactions between herpesvirus portal proteins and thiourea compounds is of significant interest in the context of developing novel drug treatments for any of the herpesviruses.
Herpesviruses and dsDNA bacteriophages both utilize a common process to package their viral genomes into empty procapsids during replication. The specific chain of events following assembly of the procapsid and preceding egress of the viral particle from the nucleus (for herpesviruses [9]) or cells (in bacteriophage [10]) is known as DNA encapsidation. The packaging of viral DNA into procapsids is a critical process involving the coordinated interactions of several viral proteins. These include the portal protein, which is located at a single 5-fold vertex of the procapsid, and a complex of several other proteins known as the terminases. The terminase complex binds and cleaves viral DNA into single genome lengths while interacting directly with the portal protein to translocate viral DNA into the procapsid in an ATP dependent manner.
The eight portal homologs in human herpesviruses range in molecular mass from 68.0 kDa in HHV-8 to 86.8 kDa in VZV (Table 1A). With the exception of VZV, viruses of the same subfamily tend to have portal proteins of similar mass, most notably the gamma herpesviruses EBV (68.4 kDa) and HHV-8 (68.0 kDa). All of the herpesviruses contain a conserved central core that is in part responsible for the amino acid homology observed between viruses of all subfamilies (Table 1B, Figs. S1 and S2). For example, VZV pORF54 amino acid identity ranges from a low of 42% with HCMV to a high of 64% with HSV-1.
Table 1.
Portal protein homologs of human herpesviruses.
| A. Characteristics | ||||||
|---|---|---|---|---|---|---|
| Virus | Subfamily | Portal | Monomer (kDa) | Length (AA) | Portal Complex Mass (Mda) | Accession |
| HSV-1 | α | pUL6 | 74.1 | 676 | 0.889 | ADM22788 |
| HSV-2 | α | pUL6 | 74.9 | 678 | 0.899 | AEV91344 |
| VZV | α | pORF54 | 86.8 | 769 | 1.042 | ABJ98891 |
| HCMV | β | pUL104 | 78.5 | 697 | 0.942 | F5HBR4 |
| HHV-6a | β | pU76 | 77.2 | 662 | 0.926 | P52453 |
| HHV-7 | β | pU76 | 74.7 | 640 | 0.896 | P52455 |
| EBV | γ | pBBRF1 | 68.4 | 613 | 0.821 | AFY97948 |
| HHV-8 | γ | pORF43 | 68 | 605 | 0.816 | AAC57125 |
| B. Sequence homology* | |||||||
|---|---|---|---|---|---|---|---|
| Virus | HSV-1 | HSV-2 | VZV | HCMV | HHV-6a | HHV-7 | EBV |
| HSV-1 | |||||||
| HSV-2 | 85 / 88 | ||||||
| VZV | 44 / 64 | 43 / 63 | |||||
| HCMV | 24 / 39 | 25 / 40 | 23 / 42 | ||||
| HHV-6a | 23 / 42 | 24 / 43 | 24 / 44 | 36 / 57 | |||
| HHV-7 | 22 / 42 | 23 / 41 | 22 / 43 | 37 / 60 | 59 / 77 | ||
| EBV | 23 / 41 | 24 / 41 | 25 / 43 | 24 / 41 | 24 /46 | 27 / 47 | |
| HHV-8 | 23 / 41 | 23 / 42 | 25 / 45 | 22 / 41 | 27 / 49 | 26 / 48 | 51 / 70 |
Amino acid identity / similarity. The amount of shading indicates level of homology between pairs of viruses.
It is reasonable to speculate that pORF54 performs a functional role similar to that of pUL6 and other HHV portal proteins as pORF54 shows 44% amino acid identity with its HSV-1 homolog (Table 1B) [1]. These data, in addition to the similar results observed via electron microscopy for inhibitor treated, infected cells [2, 3] are predictive of conserved functions for HSV-1 pUL6, VZV pORF54, and the other HHV portals. The location and arrangement of multimeric pUL6 has been resolved within a unique 5-fold vertex of HSV-1 B capsids. More extensive studies of bacteriophage portals have produced X-ray crystal structures of dodecameric portal proteins for bacteriophage SPP1 [11, 12]. Based on previous studies for the HSV-1 and HCMV portal proteins [13, 14] it is reasonable to assume pORF54 can self-assemble into larger, multimeric structures. Assuming that the VZV portal behaves similarly to other herpesvirus portals, a dodecameric structure would yield a complex of greater than 1.04 MDa – the largest of the HHV portal proteins (Table 1A).
Previously we performed TEM on purified pORF54 expressed in a recombinant baculovirus system. It was the first report of the Varicella-zoster virus portal protein [15]. However, we were not able to obtain any non-axial orientations of the portal complex. In this study, samples of the previously isolated pORF54 were diluted in 1.0 M arginine buffer at room temperature and gently sonicated with a probe sonicator. TEM revealed a more even distribution of portals, i.e. less aggregation and elimination of portal “balls”, and showed structural features of the VZV portal not observed previously.
Transmission electron microscopy of purified, diluted, and sonicated VZV pORF54 stained with 1% PTA on glow discharged grids is shown in Fig. 1.
Fig. 1.
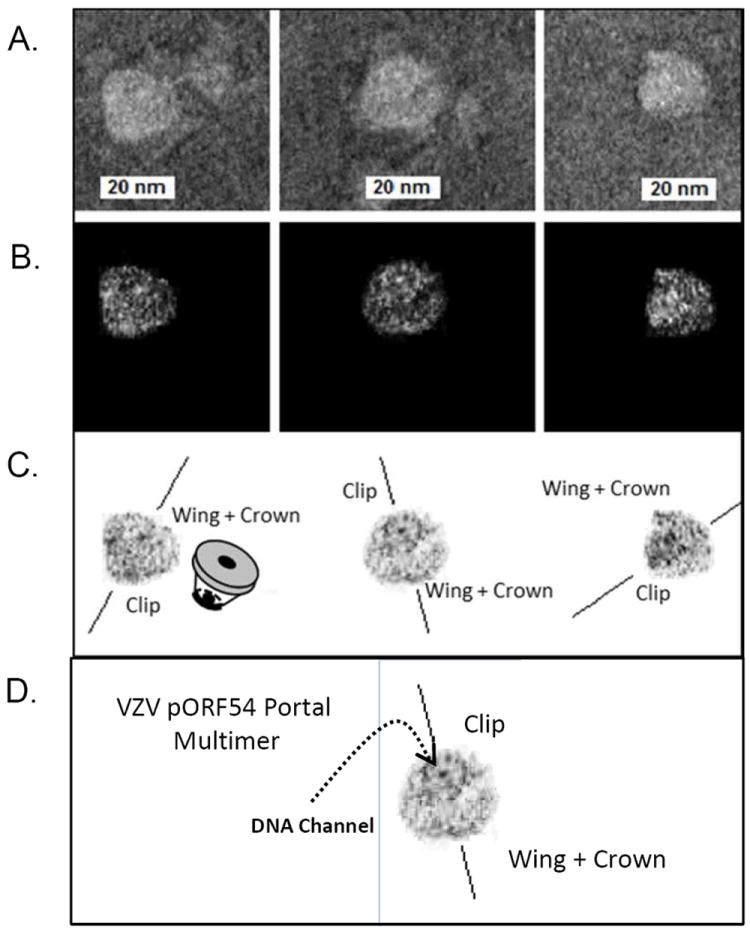
TEM of purified VZV pORF54 stained with 1% PTA, glow-discharged and imaged at 100kV using a Philips CM-100 TEM. Individual portals with non-axial perspectives. (A) Untouched images. The central channel is visible in two of the three portals (middle and left) portals, while the last presents a more lateral view (right). (B) Contrast enhanced view, with back-ground noise removed. (C) Contrast enhanced negative images with background noise removed. The crown and stem faces are indicated. (D) DNA channel is visible in the center of the portal multimer.
Individual portal complexes with non-axial perspectives were observed. Three examples of typical portal proteins, with sizes ~25 nm in diameter are shown (Fig. 1A-C). The central channel is visible in two of the three portals (left and center panels), while the last presents a more lateral view (right). The crown and stem faces are indicated. In Fig. 1D, a very distinct central channel, presumably the entry point for viral DNA, was observed. The VZV portal is predicted to be larger than any other portals studied to date. This may explain why certain structural features such as the crown/wing and stem are readily visible in the three examples.
In other TEM analysis (Fig. 2), samples were stained with 1% PTA but not glow discharged. Particles were observed with a mushroom like appearance consistent with the predicted multimeric structure of phage portal proteins for which crystallographic data exists (Fig. 2A). Fig. 2B shows contrast enhanced images of non-glow discharged portals. The central channel can be observed in all three cases. The similarity of these images to those observed for the HCMV portal by Holzenburg et al. [13] is striking. These TEM studies represent the most revealing examples to date of intact VZV portal proteins.
Fig. 2.
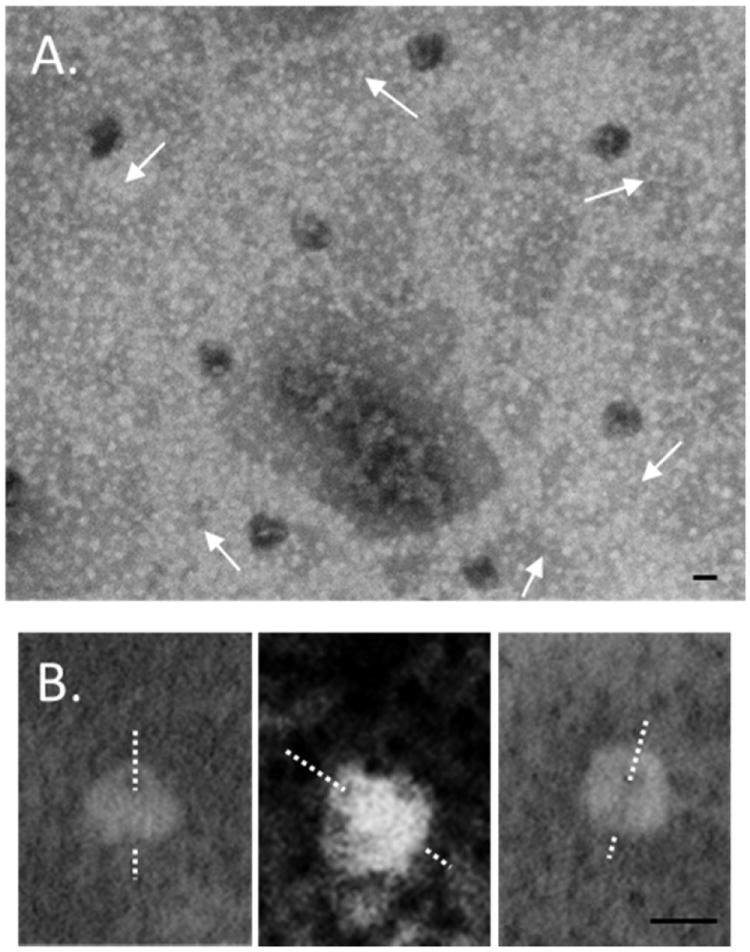
TEM of VZV portal images stained with 1% PTA without glow-discharge. (A) Portals were observed in multiple non-axial orientations. The crown/wing versus the clip can be distinguished in most cases. Arrows indicate similar orientation of the portals. (B) Contrast-enhanced, reverse-images of non-glow-discharged portal proteins. The dashed lines indicate the approximate channel path. Bars = 25 nm.
A number of unique non-nucleoside alphaherpesvirus inhibitors have been identified. Novel small molecules that target VZV capsid formation [16] and the HSV helicase-primase [17-19] are under study. Further investigation of herpesvirus portals and their associated terminase proteins will likely yield new targets for antiviral drug development. The identification of encapsidation specific antiviral inhibitors for HSV-1, HCMV, and VZV suggests that the encapsidation process is a valid antiviral target for herpesviral chemotherapy [1-3, 20, 21]. It will be interesting to examine portal formation in the presence of inhibitors in order to identify the precise mechanism of action of the thiourea inhibitor series. Future studies will focus on the effects of inhibitor treatment on the functional and structural characteristics of VZV portal protein.
Supplementary Material
Fig. S1 describes the highly conserved portal core region found in ds DNA viruses. Fig. S2 compares the conserved core of Herpesvirus family portal proteins via Raptor X.
Acknowledgments
The manuscript was written by RJV and AJH. We would like to thank M.A. Visalli for editorial assistance and D.M. Sherman of the Life Science Microscopy Facility at Purdue University. These studies were supported by National Institutes of Health grant 1 R15 AI062713-02.
References
- 1.Visalli RJ, van Zeijl M. DNA encapsidation as a target for anti-herpesvirus drug therapy. Antiviral research. 2003;59(2):73–87. doi: 10.1016/s0166-3542(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 2.van Zeijl M, Fairhurst J, Jones TR, Vernon SK, Morin J, LaRocque J, Feld B, O’Hara B, Bloom JD, Johann SV. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. Journal of virology. 2000;74(19):9054–9061. doi: 10.1128/jvi.74.19.9054-9061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visalli RJ, Fairhurst J, Srinivas S, Hu W, Feld B, DiGrandi M, Curran K, Ross A, Bloom JD, van Zeijl M, et al. Identification of small molecule compounds that selectively inhibit varicella-zoster virus replication. Journal of virology. 2003;77(4):2349–2358. doi: 10.1128/JVI.77.4.2349-2358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcomb WW, Brown JC. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. Journal of virology. 2002;76(19):10084–10088. doi: 10.1128/JVI.76.19.10084-10088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC. Visualization of the herpes simplex virus portal in situ by cryoelectron tomography. Virology. 2007;361(2):426–434. doi: 10.1016/j.virol.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. Journal of virology. 2001;75(22):10923–10932. doi: 10.1128/JVI.75.22.10923-10932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamberti C, Weller SK. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226(2):403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- 8.Patel AH, Rixon FJ, Cunningham C, Davison AJ. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217(1):111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 9.Brown JC, Newcomb WW. Herpesvirus capsid assembly: insights from structural analysis. Curr Opin Virol. 2011;1(2):142–149. doi: 10.1016/j.coviro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- 11.Lebedev AA, Krause MH, Isidro AL, Vagin AA, Orlova EV, Turner J, Dodson EJ, Tavares P, Antson AA. Structural framework for DNA translocation via the viral portal protein. EMBO J. 2007;26(7):1984–1994. doi: 10.1038/sj.emboj.7601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olia AS, Prevelige PE, Jr, Johnson JE, Cingolani G. Three-dimensional structure of a viral genome-delivery portal vertex. Nat Struct Mol Biol. 2011;18(5):597–603. doi: 10.1038/nsmb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzenburg A, Dittmer A, Bogner E. Assembly of monomeric human cytomegalovirus pUL104 into portal structures. The Journal of general virology. 2009;90(Pt 10):2381–2385. doi: 10.1099/vir.0.013292-0. [DOI] [PubMed] [Google Scholar]
- 14.Rochat RH, Liu X, Murata K, Nagayama K, Rixon FJ, Chiu W. Seeing the portal in herpes simplex virus type 1 B capsids. Journal of virology. 2011;85(4):1871–1874. doi: 10.1128/JVI.01663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard AJ, Sherman DM, Visalli MA, Burnside DM, Visalli RJ. The Varicella-zoster virus ORF54 gene product encodes the capsid portal protein, pORF54. Virus Res. 2012;167(1):102–105. doi: 10.1016/j.virusres.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue N, Matsushita M, Fukui Y, Yamada S, Tsuda M, Higashi C, Kaneko K, Hasegawa H, Yamaguchi T. Identification of a varicella-zoster virus replication inhibitor that blocks capsid assembly by interacting with the floor domain of the major capsid protein. Journal of virology. 2012;86(22):12198–12207. doi: 10.1128/JVI.01280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vere Hodge RA, Field HJ. Antiviral agents for herpes simplex virus. Advances in pharmacology. 2013;67:1–38. doi: 10.1016/B978-0-12-405880-4.00001-9. [DOI] [PubMed] [Google Scholar]
- 18.Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, Handke G, Eckenberg P, Hewlett G, Pevzner V, et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nature medicine. 2002;8(4):392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 19.Chono K, Katsumata K, Kontani T, Kobayashi M, Sudo K, Yokota T, Konno K, Shimizu Y, Suzuki H. ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. The Journal of antimicrobial chemotherapy. 2010;65(8):1733–1741. doi: 10.1093/jac/dkq198. [DOI] [PubMed] [Google Scholar]
- 20.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral research. 2006;71(2-3):154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12(2):115–127. doi: 10.1002/rmv.344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 describes the highly conserved portal core region found in ds DNA viruses. Fig. S2 compares the conserved core of Herpesvirus family portal proteins via Raptor X.


