Abstract
Advances in genetics research have greatly expanded our ability to accurately diagnose gliomas and provide more useful prognostic information. Herein specific examples are used to show how highyield targets such as EGFR, 1p/19q, IDH1/2, MGMT, and BRAF can expand the power of the surgical neuropathologist. To avoid errors, however, the significance and controversies associated with each test must be thoroughly understood.
Keywords: Glioma, EGFR, 1p/19q, BRAF, IDH1/2, MGMT
As is the case in other pathology subspecialties, molecular diagnostics is now a prominent component of surgical neuropathology. Not long ago, an H&E–stained section and perhaps a few immunostains were considered adequate, but this is no longer true. As our knowledge of the molecular events underpinning gliomagenesis and progression have exploded, so too has the demand for greater diagnostic and prognostic accuracy from neuro-oncologists and neurosurgeons. However, the “hyper-subspecialized” nature of molecular neuro-oncology means that, even in centers offering molecular testing of gliomas, it is difficult to keep abreast of new insights on when to conduct specific tests, how to interpret test results, and how to integrate new biomarkers such as isocitrate dehydrogenase 1 and 2 (IDH1/2) and BRAF into a workup.
Herein a real-life, case-based approach is used to illustrate the power of key glioma molecular biomarkers, including 1p/19q codeletion, EGFR amplification, IDH1/2 mutations, MGMT promoter methylation, BRAF fusion, and BRAF V600E Table 1. But as these cases demonstrate, molecular tests cannot only improve diagnostic classification and prognostic accuracy in challenging biopsies, they can also create confusion and errors if misapplied or misinterpreted.
Table 1.
Key Genetic Alterations and Their Use in Glioma Diagnosticsa
| Genetic Alteration | Tumor | Diagnostic Value | Prognostic Value | Predictive Value | Most Common Methods |
|---|---|---|---|---|---|
| 1p/19q codeletion | ∼80% of grade II and III oligodendro gliomas |
Differentiates most oligos from astrocytic gliomas and oligo mimickers (eg, neurocytoma, clear cell ependymoma, small cell GBM) |
Longer survival | Associated with better response to PCV therapy |
FISH, PCR-based LOH |
| EGFR amplification | ∼40% of GBMs | Often detects scattered tumor cells in under- sampled GBMs; differentiates small cell GBM from AO |
Controversial | None; anti-EGFR therapies have failed thus far |
FISH |
| IDH1/2 mutation | ∼80% of grades II-III astrocytomas and oligodendrogliomas; >90% of secondary GBMs |
Presence strongly suggests an infiltrative glioma; negative in non- neoplastic glioma mimickers and non- infiltrative gliomas |
Longer survival, may even trump WHO grade |
No specific therapy to date but associated with better response to adjuvant therapy, including radiation |
PCR and sequencing, IHC |
|
MGMT promoter methylation |
GBM | None | None in the absence of adjuvant therapy |
Predicts better response to temozolomide |
Methylation- sensitive PCR |
| BRAF fusion | >75% of PAs (mostly fusions), ∼50% PMA |
Fusion suggests a PA or PMA |
Fusion may be a favorable marker |
No specific therapy to date; anti-MEK clinical trials ongoing |
PCR breakpoint analysis, FISH |
|
BRAF V600E mutation |
80% PXA; 25% GG | Mutation suggests PXA or GG (although not a perfect discriminator from PAs or diffusely infiltrative gliomas) |
V600E may be unfavorable |
No specific therapy to date; anti-BRAF V600E clinical trials ongoing |
PCR and sequencing, IHC |
AO, grade III anaplastic oligodendroglioma; FISH, fluorescence in situ hybridization; GBM, glioblastoma; GG, ganglioglioma; IHC, immunohistochemistry. LOH, loss of heterozygosity; PA, pilocytic astrocytoma; PCR, polymerase chain reaction; PCV, procarbazine/CCNU/vincristine; PMA, pilomyxoid astrocytoma; PXA, pleomorphic xanthoastrocytoma; WHO, World Health Organization.
1p/19q codeletion, EGFR amplification, IDH1/2 mutations, and BRAF fusion or V600E mutation are all useful in resolving diagnostic dilemmas and/or refining patient prognosis in gliomas. With the exception of BRAF and MGMT, none of these tests are useful in preadolescent patients.
Case 1: Is It Glioblastoma, Glioblastoma With Oligodendroglial Component, Anaplastic Oligoastrocytoma, or Anaplastic Oligodendroglioma?
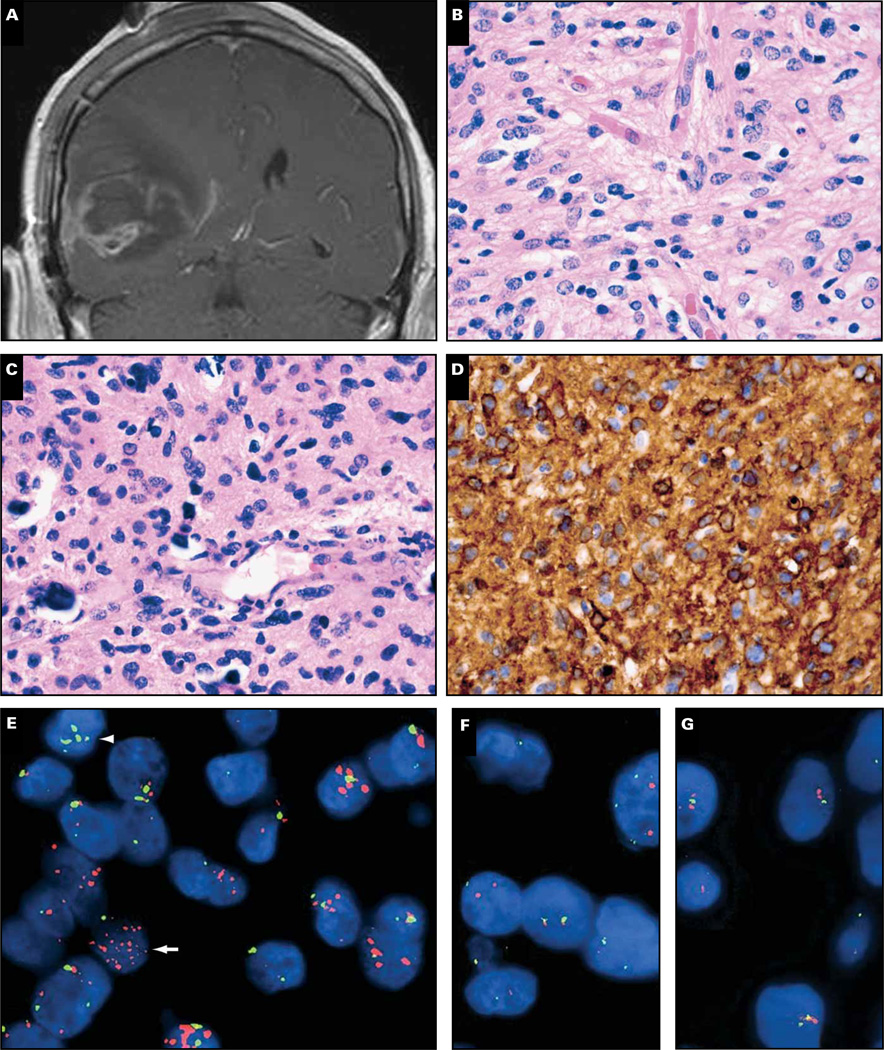
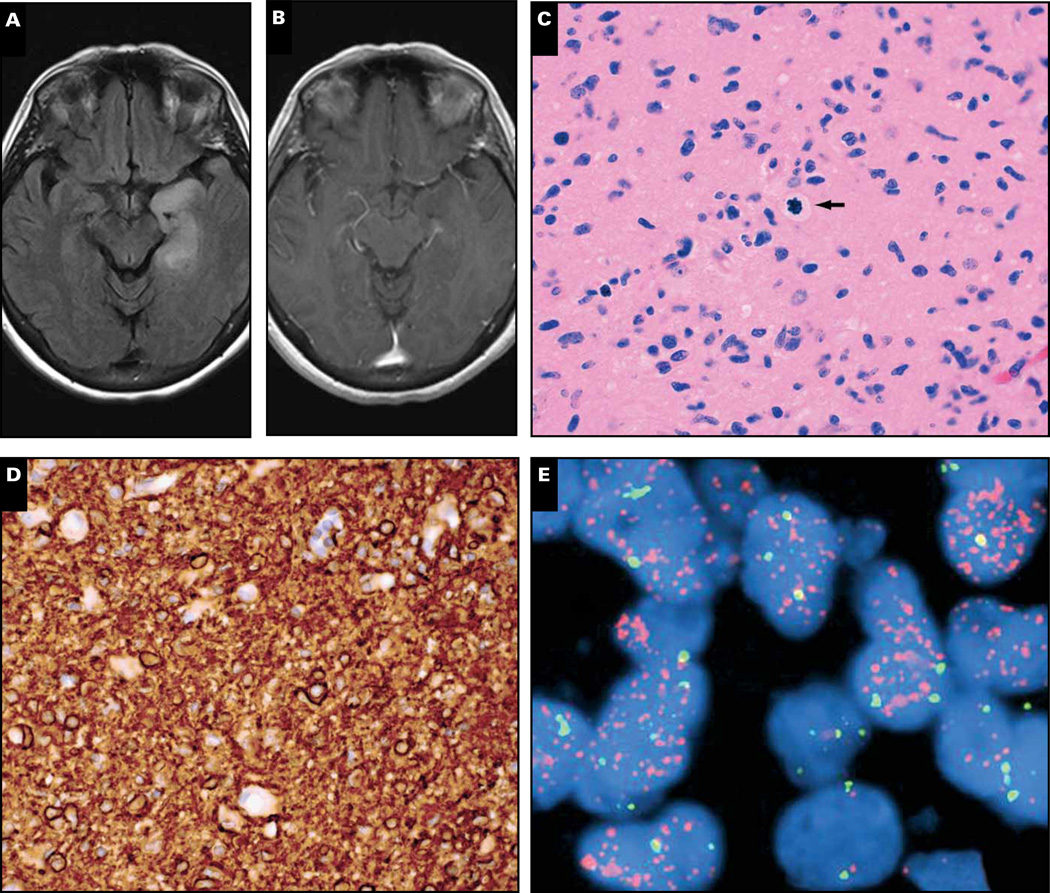
Magnetic resonance imaging in a 71-year-old man revealed a contrast-enhancing mass of the right temporal lobe with surrounding edema Image 1A. Histologically, the resected lesion was an obvious glioma but had mixed nuclear morphology, demonstrating round and irregular angulated nuclei, “chickenwire” branching capillaries, and scattered microcalcifications Image 1B and Image 1C. The tumor also had numerous mitoses and large areas of necrosis (not shown).
Image 1.
Case 1. A 71-year-old man had a right temporal enhancing mass (A) that showed frequent mitoses and large areas of necrosis (not shown), “chickenwire” branching capillaries and microcalcifications (B, C), and both rounded and angulated nuclei. Epidermal growth factor receptor (EGFR) immunostain was strongly positive (D), and the tumor showed scattered cells with EGFR amplification (E, arrow) but not 1p/19q codeletion (F, G). Of note, some tumor cells had polysomy 7 but not EGFR amplification (E, arrowhead). R1232H IDH1 immunostain was negative, as was IDH1/2 mutation sequencing (not shown). Despite treatment with radiation and temozolomide, the man died 4 months later. Orange signals in E, EGFR; green signals, chromosome 7 centromeric enumeration probe. In F and G, orange signals, 1p36 and 19q13; green signals, 1q25 and 19p13.
At this point the differential diagnosis includes glioblastoma (GBM), glioblastoma with an oligodendroglial component (GBM-O), anaplastic oligoastrocytoma (AOA), and an anaplastic oligodendroglioma (AO).1 In large outcome-based studies the entities appear to behave differently, with AO having the best prognosis, followed (in order of decreasing survival) by AOA, GBM-O, and GBM.2 Not surprisingly, even among board-certified neuropathologists, the interobserver variability on a case like this is high.3 The glioma is obviously lethal, but without accurate, consistent classification it is difficult to estimate exactly how long the patient has to live, and whether he might be a candidate for a particular clinical trial.
Testing key oncogenes can help resolve such cases. Epidermal growth factor receptor (EGFR) is a powerful receptor tyrosine kinase (RTK) that activates the mitogen-activated protein kinase (MAPK)/ERK and PI3K/Akt pathways, both of which promote cellular proliferation, migration, and resistance to apoptosis. The EGFR gene is amplified in about 40% of GBMs, is specifically associated with primary (ie, de novo) GBMs, and is more likely in GBMs of elderly patients.4 EGFR immunohistochemistry (IHC) is a good predictor of amplification, insofar as amplification is virtually never seen when protein expression is weak. In contrast, high-grade gliomas with strong EGFR staining as seen in this case Image 1D also show amplification more than 50% of the time,5 as was shown here via EGFR fluorescent in situ hybridization Image 1E. When some parts of the high-grade glioma have an oligodendroglial-like component but EGFR is amplified, the best diagnosis is “small cell GBM.”6 Such tumors might look like AOs or AOAs but never have the 1p/19q codeletion that is characteristic of most oligodendroglial tumors. Indeed, this tumor was negative for codeletion Image 1F and Image 1G and R132H IDH1 (not shown), both of which are strongly inversely related to EGFR amplification.7–10 The other option, GBM-O, also is not likely because GBM-O often has IDH1/2 mutations, mucin-filled microcystic spaces, and/or minigemistocytes, none of which were present in this case (Arie Perry, MD, personal communication, December 18, 2012).11
After surgery the patient underwent treatment with radiotherapy and adjuvant temozolomide but unfortunately died 4 months later. This outcome is much more consistent with a GBM than an AO or AOA, but whether EGFR amplification is an adverse independent prognostic marker in GBMs is unclear.12–18 Adding to the controversy is our data suggesting that GBMs with high levels of EGFR amplification via fluorescence in situ hybridization (FISH) (EGFR:CEP7 > 20) have longer survivals than those with low to moderate levels of amplification (EGFR:CEP7 = 2–20).19 Consistent with this finding, the EGFR:CEP7 ratio in the current case was 14.
Another interesting facet to this case is that not all tumor cells had EGFR amplification even though they had polysomy 7 (Image 1E, arrowhead). Recent work has shown that many GBMs contain heterogeneous mosaic amplification of RTKs, including EGFR, PDGFRA, and MET.20 It is therefore possible that the nonamplified cells in this tumor actually did have amplification, but of other RTKs. This could help explain the rather disappointing response to anti-EGFR therapies such as erlotinib.21,22
Cases 2 and 3: Be Careful When Interpreting 1p/19q Results!
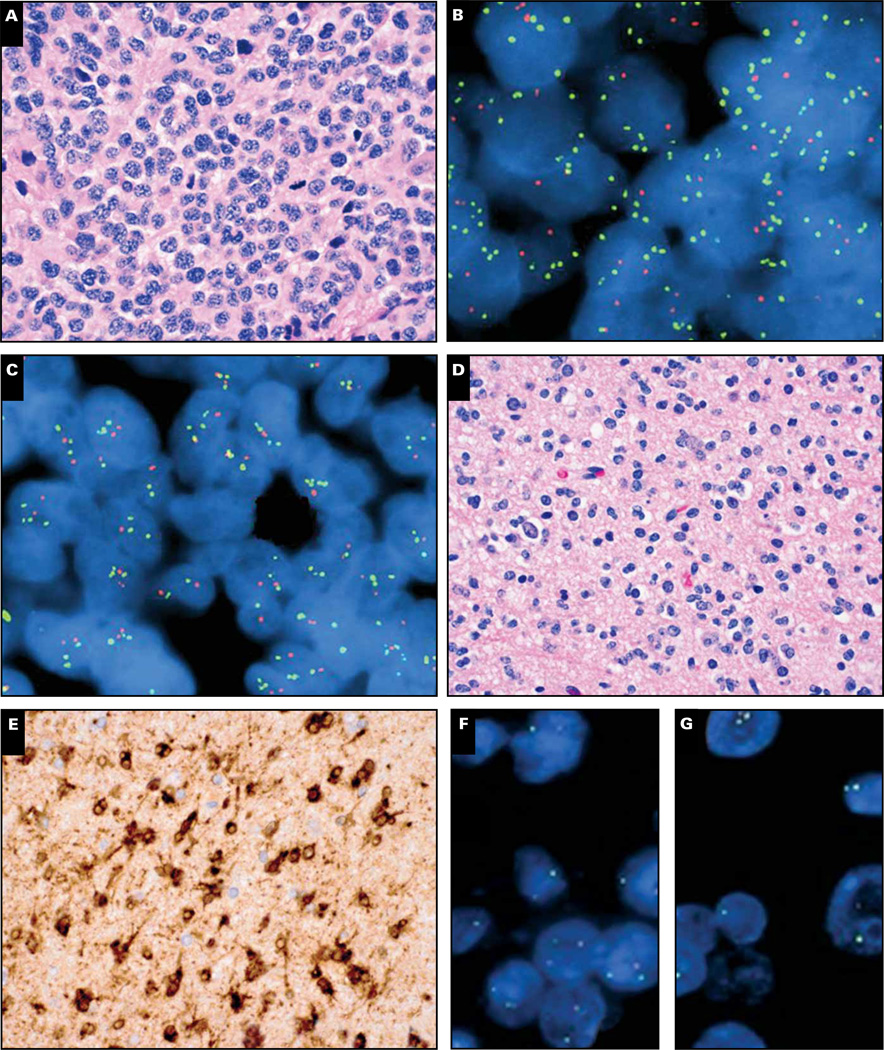
A 62-year-old man had a left frontal/sphenoid enhancing tumor that was suggestive of a grade III AO or AOA Image 2A. The tumor was highly cellular with scattered mitoses, very focal necrosis, and predominantly round cell morphology. On FISH the tumor showed relative codeletion of 1p36 (ratio of 1p36/1q25 = 0.42 with 93% of the cells showing loss of 1p36) and 19q13 (ratio of 19q13/19p13 = 0.48 with 92% of the cells showing loss of 19q13) Image 2B and Image 2C. The tumor also had 3 or more 1q25 and 19p13 signals in more than 70% of the nuclei. The tumor was negative for EGFR amplification and IDH1/2 mutations (not shown). Despite the codeletion result, the patient’s clinical course was extremely aggressive, with death occurring only 3 months after initial tumor resection.
Image 2.
Cases 2 (A–C) and 3 (D–G). Left frontal/sphenoid high-grade glial tumor with predominantly round cell morphology and brisk mitotic activity (A). Fluorescence in situ hybridization (FISH) analysis of the lesion showed relative codeletion of 1p36 and 19q13 with polysomy of 1q25 and 19p13 (B, C). The tumor was negative for EGFR amplification with FISH and negative for IDH1/2 mutations with gene sequencing (not shown). Another glial tumor with round cell morphology and no mitotic activity, suggestive of World Health Organization grade II oligodendroglioma (D), showed strong immunoreactivity for R132H IDH1 (E) as well as classic 1p/19q codeletion by FISH (F, G). Orange signals, 1p36 and 19q13; green signals, 1q25 and 19p13.
Codeletion of 1p and 19q has long been known to be a hallmark of oligodendroglial tumors, specifically those that will respond better to adjuvant therapy. Recent clinical trials have suggested that codeletion can now be regarded as a bona fide predictive (rather than just prognostic) marker, associated with better response to procarbazine, lomustine, and vincristine chemotherapy.23,24 However, it is critical to remember that a true, clinically relevant codeletion is the product of an unbalanced translocation between chromosomes 1 and 19, with loss of the derivative chromosome resulting in whole-arm 1p and 19q deletions.25
FISH is the most popular way to test for 1p/19q codeletion because morphologic subregions can readily be targeted, it requires only 2 additional unstained slides, and most laboratories have the necessary reagents and equipment for other FISH tests (eg, HER2 in breast cancer). The most widely used probes are commercially available, targeting 1p36 and 19q13. These regions were initially chosen because they are minimally deleted in gliomas,26 but subsequent work has shown that although this approach is very sensitive for detecting whole-arm codeletions, its specificity is lower compared with other assays such as polymerase chain reaction (PCR)– based loss of heterozygosity (LOH) analysis.27 This occurs because some higher-grade gliomas have random interstitial deletions on multiple chromosomes, including 1p36 and 19q13, which can mimic codeletion on FISH.
Commercially available 1p/19q FISH probes are still useful but need to be applied judiciously. For example, testing should only be undertaken in cases that are plausibly oligodendroglial—there is no reason for up-front 1p/19q testing of all gliomas. In our experience, fewer than 3% of histologically unequivocal GBMs will show apparent codeletion on FISH; such cases show very poor correlation with PCR-based LOH and do not behave differently than other GBMs (unpublished data). Furthermore, other molecular markers can help confirm or contradict a 1p/19q codeletion result. EGFR amplification and/or 10q loss are practically mutually exclusive with wholearm 1p/19q codeletion.9,10 On the other hand, virtually all whole-arm codeleted gliomas should have an accompanying mutation in either IDH1 or IDH2.28,29 Thus if a glioma does not plausibly look oligodendroglial, has EGFR amplification or 10q deletion, and/or is wild-type for IDH1/2, 1p/19q testing can safely be withheld.
Empiric analysis has shown that maximal sensitivity and specificity, as well as prognostic stratification power, are obtained with ratio cutoffs lower than 0.75 per probe pair or at least 40% of tumor nuclei showing relative deletion of both 1p36 and 19q13.27,30 Case 2 met both criteria for codeletion and did not have EGFR amplification. Yet it also lacked IDH1/2 mutations, strongly suggesting that 1p/19q FISH results were actually false positive. In contrast, a grade II tumor with oligodendroglial morphology Image 2D showed the R132H IDH1 mutation on IHC Image 2E and 1p/19q codeletion on FISH Image 2F and Image 2G. In case 3, the 1p/19q result was trustworthy, and the patient has had no recurrences in more than a year.
Finally, case 2 illustrates the newer issue of polysomy. About 40% of codeleted AOs will have more than two 1q25 and 19p13 FISH signals in at least 30% of glioma nuclei; such tumors have outcomes intermediate between codeleted AOs without polysomy and those without codeletion at all.31 This finding has been verified in a separate published study32 as well as our own unpublished data (not shown). It is not yet clear whether this finding occurs because those tumors have a higher rate of false-positive codeletion, as this case probably did. However, in our cohort of codeleted AOs, the polysomy and nonpolysomy cases have comparable rates of concordance with PCR-based LOH analyses of 1p and 19q (not shown). Thus, the presence of polysomy itself does not appear to invalidate a codeletion result, but it should be added to the report.
Cases 4–6: IDH1/2 Mutation Screening Improves Diagnostic Accuracy, but the R132H IDH1 Antibody Is Not Infallible
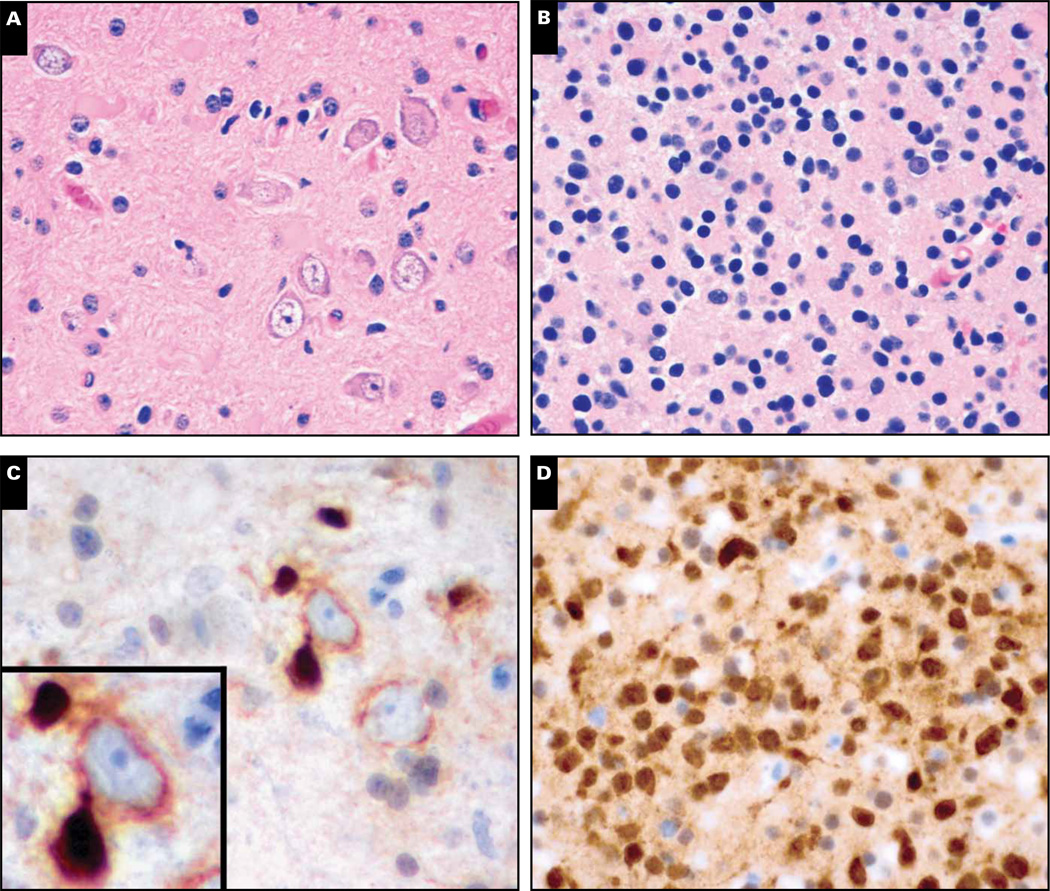
Case 4 was a 36-year-old man who developed seizures and headaches and was found to have a 2 × 2-cm nonenhancing mass in the left frontal lobe (not shown). Histology from the initial resection showed mildly pleomorphic neoplastic glial cells admixed with disordered neuronal/ganglion cells suggestive of a low-grade glioneuronal tumor such as dysembryoplastic neuroepithelial tumor (DNET) or ganglioglioma Image 3A. No further treatment other than radiologic follow-up was done. Four years later the tumor recurred, this time as an unequivocal grade II oligodendroglioma expressing R132H IDH1 Image 3B and Image 3D. Retrospective analysis of the original tumor, which predated IDH1/2 testing, showed scattered R132H IDH1–positive cells Image 3C. Many of those cells were wrapping around immunonegative neurons (Image 3C, inset).
Image 3.
Case 4. A 36-year-old man had a 2 × 2-cm nonenhancing mass in the left frontal lobe (radiology not shown). Histologic features of the initial biopsy (A) suggested a low-grade glioneuronal tumor with disordered ganglion cells, such as a dysembryoplastic neuroepithelial tumor or ganglioglioma. Four years later the tumor recurred, this time showing unequivocal grade II oligodendroglioma round cell morphology (B). Immunohistochemical staining for R132H IDH1 mutation was strongly positive in the tumor cells (D). Retrospective analysis of the original tumor also showed immunopositive cells (C), many of which were wrapping around and physically distorting immunonegative neurons (C, inset).
Several years ago, high-resolution sequencing of GBMs identified point mutations in codons 132 and 172 of IDH1 and IDH2, respectively.33,34 Both of these codons normally encode arginine amino acid residues, which help bind isocitrate during its oxidation into α-ketoglutarate. IDH1 and IDH2 are single-gene enzymes, with IDH1 localizing to the cytosol and peroxisomes and IDH2 residing in the mitochondria.35 (Interestingly, mitochondrial IDH2 does not appear to contribute to the Krebs cycle; that task is left to the multigene IDH3 enzyme complex, which is not mutated in gliomas.) The point mutations confer neoenzymatic properties onto IDH1 and IDH2, as both mutant enzymes convert α-ketoglutarate into D-2-hydroxyglutarate.36
Details of the biochemistry and effects of D-2-hydroxyglutarate are beyond the scope of this discussion; however, from a surgical neuropathology perspective, the key point is that IDH1/2 mutations are only seen in diffusely infiltrative gliomas. These mutations are present in 70% to 80% of grades II and III astrocytomas and oligodendrogliomas, as well as in approximately 10% of grade IV GBMs that arise from lower-grade gliomas (so-called “secondary” GBMs).34 Noninfiltrative, potentially curable grade I gliomas such as pilocytic astrocytomas, gangliogliomas, and DNETs do not contain IDH1/2 mutations. The mutations are also not present in conditions mimicking gliomas such as demyelination and viral encephalitides.37–39
In case 4, the original resection was misinterpreted as a grade I glioneuronal tumor because the infiltrating glioma cells were physically warping nonneoplastic cortical neurons, making them look like part of the tumor (Image 3A and Image 3C). Had IDH1/2 testing been available at that time, such a mistake could have been prevented. In fact, a larger multi-institutional cohort of tumors originally diagnosed as gangliogliomas showed that, in tumors with IDH1/2 mutations, outcomes were far more consistent with diffuse gliomas.40
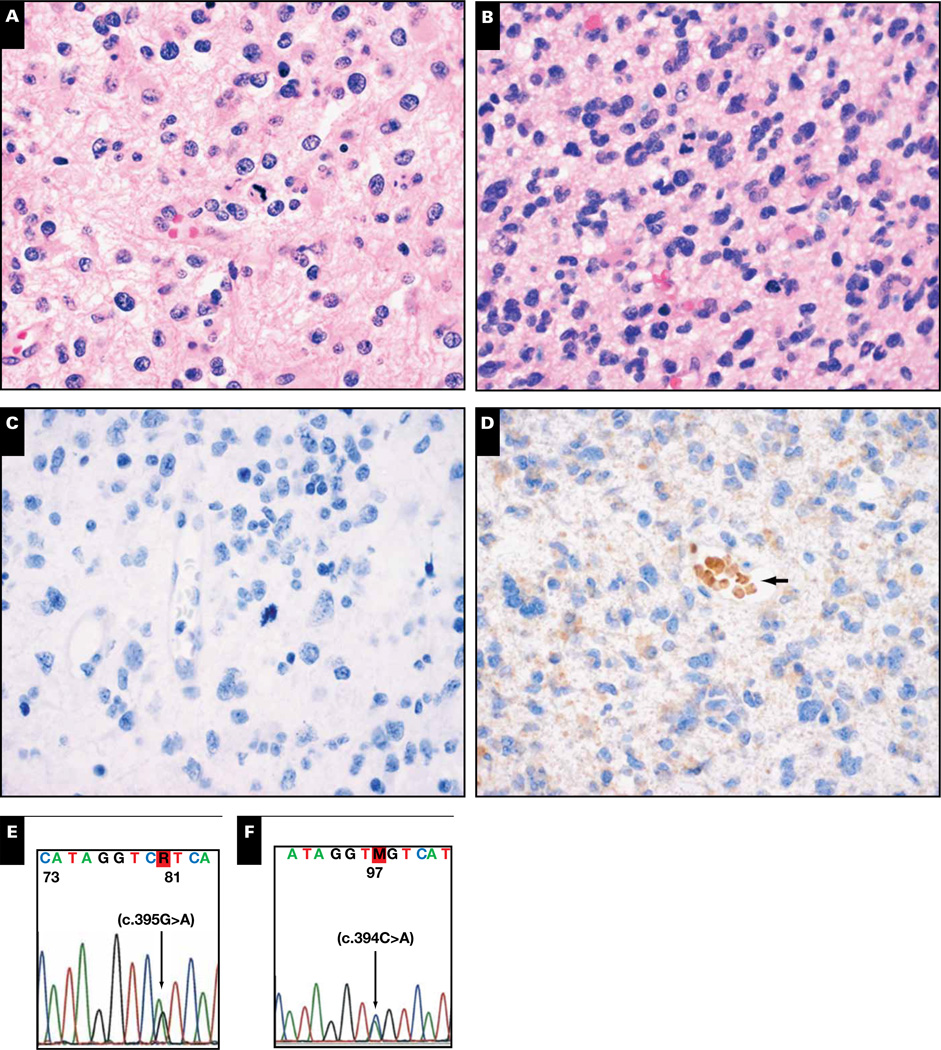
Because about 80% to 90% of IDH-mutant gliomas contain the arginine-to-histidine R132H IDH1 variant, it was practical to generate a mutation-specific antibody as a rapid, sensitive immunohistochemical screen on formalin-fixed, paraffin-embedded tissue specimens.41–43 In our experience, roughly 10% to 15% of R132H IDH1 immunonegative gliomas will be positive for less common IDH1/2 mutations, or are false negative for R132H IDH1 on IHC, on follow-up sequencing Image 4. Indeed, although the R132H IDH1 antibody is virtually 100% specific, it will miss about 1 in 20 R132H IDH1-mutant gliomas (case 5, Image 4A, Image 4C, and Image 4E).42 Various molecular methods can be used to detect less common IDH1/2 mutations and can improve sensitivity beyond the 20% mutant allele limit of traditional PCR and Sanger sequencing.44–46 As case 6 illustrates, follow-up testing of immunonegative cases is definitely worthwhile in grades II-III gliomas and known secondary GBMs, as well as in patients between 20 and 60 years of age, in patients with tumors that manifest with seizures, and in those with gliomas with low to absent levels of necrosis (Image 4B, Image 4D, and Image 4F).8,47,48
Image 4.
Cases 5 (A, C, E) and 6 (B, D, F). A World Health Organization (WHO) grade III anaplastic oligoastrocytoma of the right frontotemporal lobe (A) was immunonegative for R132H IDH1 (C), even though it showed an R132H IDH1 mutation on sequencing (E). In another case, a 36-year-old man had a right temporal WHO grade III anaplastic astrocytoma (B) that, aside from false-positive staining in red blood cells (D, arrow), was negative for R132H IDH1 on immunohistochemistry. Sequencing revealed an uncommon R132S IDH1 mutation (F).
R132H IDH1 shows strong cytoplasmic localization, extending out into the tumor cell processes (Image 3C). Sometimes even tumor nuclei will show staining; this is thought to be the result of antigen diffusion during tissue processing.41,42,49
Case 7: IDH1/2 Mutation Screening Also Improves Prognostic Accuracy
A 61-year-old woman with seizures had a left temporal lesion with increased T2 signal but no significant contrast enhancement Image 5A and Image 5B. The tumor was extensively resected, revealing an astrocytoma with mitoses but no necrosis or microvascular proliferation Image 5C. Thus, using a strict application of the World Health Organization criteria, this tumor would be called a grade III anaplastic astrocytoma. However, not only was the tumor strong for EGFR Image 5D but it also was negative for IDH1/2 mutations on both IHC and PCR (not shown). As expected, based on the EGFR IHC (see also case 1), FISH analysis showed EGFR amplification Image 5E.
Image 5.
Case 7. A 61-year-old woman with left-sided seizures was found to have a left temporal lesion that showed a T2 signal but no significant contrast enhancement on T1 magnetic resonance imaging (MRI) (A, B). Histologic examination of the resection specimen showed an infiltrating glial tumor with grade III histology, including angulated atypical nuclei and readily identified mitoses (C, arrow); no microvascular proliferation or necrosis was seen. The tumor showed strong, diffuse immunohistochemical expression of EGFR (D), and EGFR was amplified (E). The tumor was negative for IDH1/2 mutations via both immunohistochemistry and polymerase chain reaction (not shown). Orange signals (E), EGFR; green signals, chromosome 7 centromeric enumeration probe.
Case 7 was a recent one, and therefore the follow-up period has been insufficient for more definitive recurrence and survival data. However, a recent series showed that non-enhancing grade III gliomas with wild-type IDH1/2 generally progress to classic ring-enhancing grade IV GBM lesions within a few months. In contrast, similar-appearing lesions with IDH1/2 mutations progress far more slowly.50
This case underscores a key feature of IDH1/2 mutations—diffuse gliomas with the mutation tend to do a lot better than their grade-matched wild-type counterparts. This survival difference is so stark that grade III anaplastic astrocytomas without the mutation fare just as badly as wild-type grade IV GBMs.34,51 In fact, a major reason why advanced age is an adverse prognostic indicator in GBMs is because elderly patients are less likely to have IDH1/2-mutant tumors.34,51 Whether this favorable effect extends to grade II gliomas is highly debatable; some have suggested a better prognosis52,53 while others found no difference.54–58
In case 7, not only was the “anaplastic astrocytoma” IDH1/2 of wild type, but it also had EGFR amplification, which as previously discussed is far more commonly associated with GBMs than with grade II-III tumors.59 Therefore, even though this patient’s tumor was well-sampled and showed no grade IV histologic features, the molecular profile (and her age) were far more consistent with a not yet fully developed primary GBM. The molecular testing did not alter her treatment because she would have been given temozolomide and radiation either way. However, it did make her survival estimate more realistic and also prevented her from being misassigned to a clinical trial aimed at patients with grade III tumors.
Case 8: Which Is More Important: MGMT Promoter Methylation or IDH1/2 Mutations?
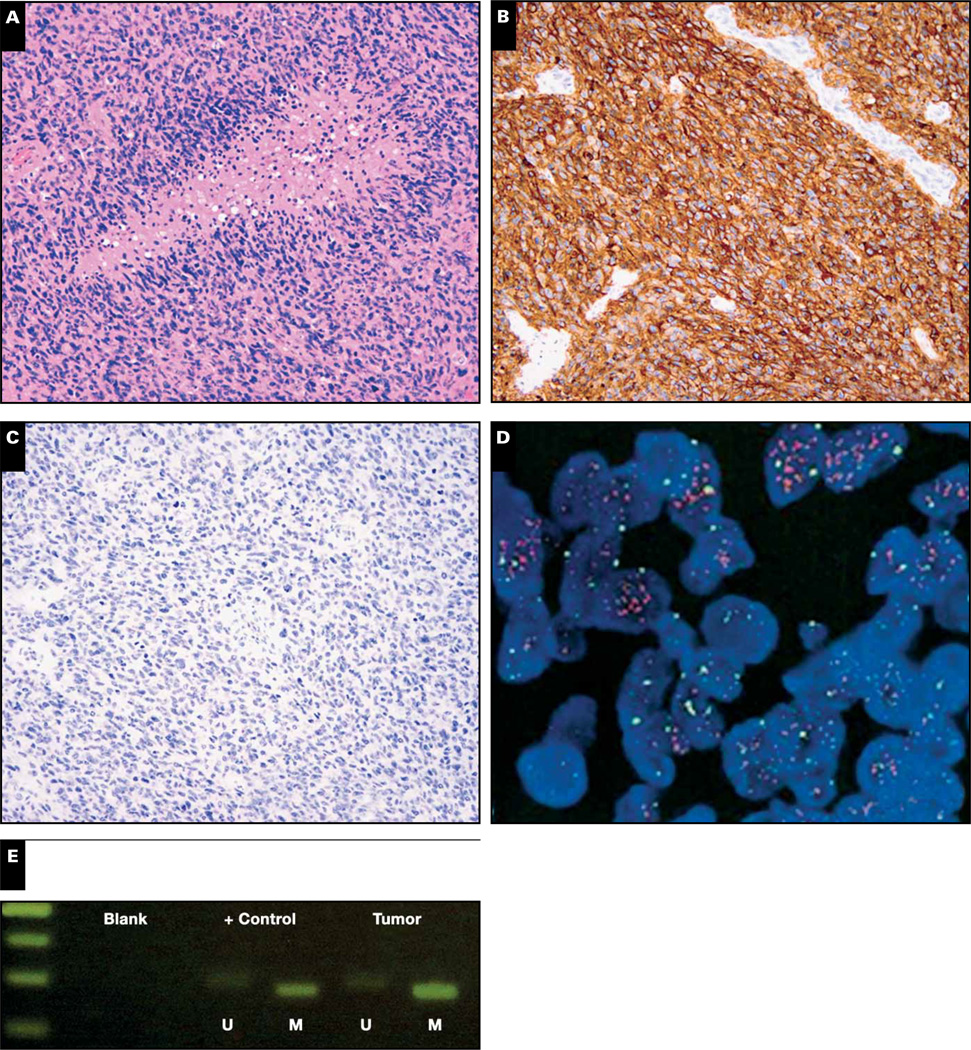
A 56-year-old man had a right frontal GBM featuring abundant necrosis with pseudopalisading Image 6A. The tumor was strongly positive for EGFR via IHC Image 6B, negative for R132H IDH1 Image 6C, and also negative for less common IDH1/2 mutations on sequencing (not shown). As predicted with the strong and diffuse EGFR reaction on IHC, it was positive for EGFR amplification on FISH Image 6D but had MGMT promoter methylation Image 6E. The patient received radiation and temozolomide and was still working full-time more than a year after his initial surgery.
Image 6.
Case 8. A glioblastoma had abundant pseudopalisading necrosis (A). The tumor was strongly and diffusely positive for EGFR via immunohistochemistry (B) and was immunonegative for R132H IDH1 (C) as well as less common IDH1/2 mutations on sequencing (not shown). The tumor was, however, positive for EGFR amplification (D) and MGMT promoter methylation (E). U, unmethylated; M, methylated; orange signals in D, EGFR; green signals, chromosome 7 centromeric enumeration probe.
O6-Methylguanine-DNA methyltransferase (MGMT) is a DNA repair protein that specifically removes alkyl groups from the O6 position of guanine in DNA, making cells resistant to chemotherapeutic alkylating agents.60 When the gene promoter is methylated, MGMT expression decreases and temozolomide sensitivity increases. Ever since the landmark 2005 GBM study by Hegi,61 testing for MGMT promoter methylation has become standard of care in the workup of GBMs. Curiously, the 2005 Hegi study also showed that GBMs with methylation responded better to a radiation-only regimen, which is inconsistent with the known mechanism of MGMT. In 2005, the existence of IDH1/2 mutations was unknown, as was the ability of those mutations to promote global hypermethylation, including methylation of the MGMT promoter.62–67
IDH1/2 mutations may promote sensitivity to radiation,24,53,68 but this is also controversial.69,70 If this theory proves to be true, it begs the question as to whether the favorable prognostic effect of MGMT promoter methylation is really due to MGMT itself or is merely a byproduct of the fact that methylated tumors are simply more likely to harbor IDH1/2 mutations. The reverse may be true, and the effect of IDH1/2 mutations occurs mostly because of methylation of the MGMT promoter. This question is not easy to directly address because, although tumors like that in case 8 are not uncommon, it is difficult to accumulate enough gliomas with IDH1/2 mutations but without MGMT promoter methylation. Some multivariate analyses have given greater prognostic importance to IDH1/2 status than MGMT promoter methylation.71,72 However, a recent study of elderly patients (in whom IDH1/2 mutations are uncommon) showed that MGMT promoter methylation was associated with better response to temozolomide-containing regimens but not to radiotherapy alone.73 Furthermore, it is not clear whether IDH1/2 mutations have any direct effect on response to temozolomide.74 Thus, it is likely that MGMT promoter methylation is still favorable, independent of IDH1/2, but only in regimens containing temozolomide. IDH1/2 mutations, on the other hand, may be relevant to a broader spectrum of adjuvant therapies. Thus, having both molecular alterations is likely even better than just MGMT methylation.
Case 9: BRAF in Pediatric Low-Grade Gliomas
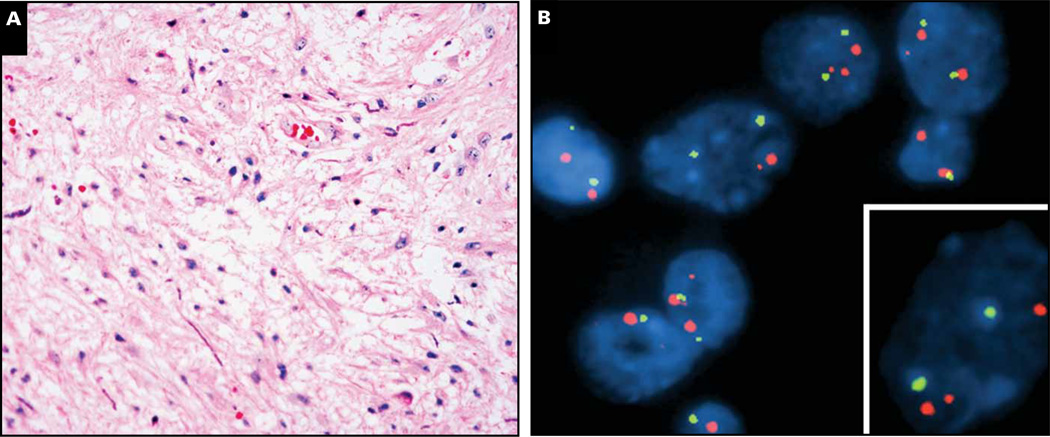
Case 9 is a 14-year-old boy with a thalamic tumor featuring low cellularity and loose organization Image 7A. No high-grade features were seen, but neither were biphasic morphology, Rosenthal fibers, or eosinophilic granular bodies, so it was called “low-grade glioma, not otherwise specified.” Despite its location, the patient has been completely recurrence-free for the past 26 years, never requiring any adjuvant therapy or re-resection. Recent BRAF FISH analysis performed on archived paraffin blocks of the tumor revealed an abnormal signal pattern in nearly 50% of the cells, consisting of a small orange signal near one of the larger orange BRAF signals Image 7B (lower right inset).
Image 7.
Case 9. A 14-year-old boy had a thalamic tumor (radiology not available) that was clearly a low-grade glioma but did not show conclusive diagnostic features of a pilocytic astrocytoma (A). A BRAF rearrangement pattern was apparent on fluorescence in situ hybridization (B), with an extra smaller signal near one of the larger BRAF signals (B, inset). Orange signals, BRAF; green signals, CEP7.
Unlike adult gliomas, those arising in the pediatric population are usually low grade, such as pilocytic astrocytomas or gangliogliomas. Until recently, the molecular underpinnings of these tumors were a mystery because older-generation, whole-genome arrays failed to detect any consistent abnormalities. Now we know that the BRAF oncogene is frequently altered in a large proportion of these tumors, usually via a tandem duplication and fusion event on 7q34 (reviewed by Horbinski75). B-Raf is an intracellular serine/threonine kinase component of the MAPK pathway. The BRAF portion of the fusion gene contains only its kinase domain, ie, it does not require Ras binding for activation. KIAA1549-BRAF is by far the most common fusion, but FAM131B-BRAF and SRGAP3-RAF1 also rarely occur. On histologic examination, the fusions are mostly in pilocytic and pilomyxoid astrocytomas; by location, infratentorial and optic nerve tumors are more likely to have BRAF fusions than gliomas of the supratentorium.
The best method for detecting BRAF fusion has not yet been established, but case 9 used a 3-probe FISH cocktail that spans the entire length of the BRAF gene.76 A tumor nucleus that harbors a BRAF fusion will show 2 large signals representing 2 sets of the 3 contiguous probes as well as a smaller signal near 1 of the larger signals that represents the BRAF kinase domain of a fusion gene (Image 7B, inset). Although this can detect the duplicated portion of BRAF irrespective of its fusion partner, limitations include resolution, truncation artifact, and difficulty interpreting tumors that have high overall chromosome 7 polysomy. Other methods such as breakpoint PCR might be useful, but whether one approach is superior to another has yet to be proven.
The BRAF V600E point mutation that has been seen in other cancers such as melanomas also occurs in some pediatric low-grade gliomas, in particular gangliogliomas and pleomorphic xanthoastrocytomas.75 Grade II diffusely infiltrative gliomas can also harbor the mutation. When the point mutation occurs, the supratentorium and optic nerve are the most frequent sites. PCR and sequencing are currently in widespread use, but a V600E-specific antibody for paraffin-embedded tissues is forthcoming.77
From diagnostic and prognostic perspectives, the presence of a BRAF fusion suggests either a pilocytic or pilomyxoid astrocytoma, whereas the differential for a V600E–mutant tumor is broader. In case 9, histologic examination could not resolve the diagnosis beyond “low-grade glioma, not otherwise specified,” but the BRAF fusion signal is consistent with the indolent nature of the tumor. Indeed, the fusion tends to be a favorable prognostic marker, whereas V600E may be slightly unfavorable.78,79 Current thinking is that detecting a fusion in an otherwise equivocal biopsy should tilt the diagnosis in favor of a pilocytic or pilomyxoid astrocytoma. Ongoing clinical trials with B-Raf and MEK inhibitors will determine whether these markers also have any predictive relevance.
In conclusion, the cases presented herein illustrate how far our understanding of glioma genetics has come in recent decades and how much that understanding has translated into better diagnoses and prognostic stratification. As molecular techniques advance even further into array-based platforms, as integrated pathway analyses further substratify tumors, and as tailored antiglioma therapies are developed, it will be interesting to see whether molecular diagnostics supersedes traditional light microscopic examination as the mainstay of glioma workup.
Upon completion of this activity you will be able to:
list the main glioma molecular biomarkers used in surgical neuropathology.
outline the proper use of each molecular biomarker, including when to test specific markers and how to interpret the results.
explain the limitations of each biomarker regarding prognostic and predictive information, including how patient management might be altered.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 396. Exam is located at www.ascp.org/ajcpcme.ascp.org/ajcpcme.
Acknowledgments
This study was supported in part by grant 1-K08 CA155764-01A1 from the National Cancer Institute (Dr Horbinski), grant 2P20 RR020171 from the National Institute of General Medical Sciences (Dr Horbinski), and the University of Kentucky College of Medicine Physician Scientist Program (Dr Horbinski).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al., editors. WHO Classification of Tumors of the Central Nervous System. 4th ed. Lyon: IARC; 2007. [Google Scholar]
- 2.Miller CR, Dunham CP, Scheithauer BW, et al. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol. 2006;24:5419–5426. doi: 10.1200/JCO.2006.08.1497. [DOI] [PubMed] [Google Scholar]
- 3.Castillo MS, Davis FG, Surawicz T, et al. Consistency of primary brain tumor diagnoses and codes in cancer surveillance systems. Neuroepidemiology. 2004;23:85–93. doi: 10.1159/000073980. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 5.Horbinski C, Hobbs J, Cieply K, et al. EGFR expression stratifies oligodendroglioma behavior. Am J Pathol. 2011;179:1638–1644. doi: 10.1016/j.ajpath.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry A, Aldape KD, George DH, et al. Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer. 2004;101:2318–2326. doi: 10.1002/cncr.20625. [DOI] [PubMed] [Google Scholar]
- 7.Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62:1118–1128. doi: 10.1093/jnen/62.11.1118. [DOI] [PubMed] [Google Scholar]
- 8.Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 9.Idbaih A, Criniere E, Marie Y, et al. Gene amplification is a poor prognostic factor in anaplastic oligodendrogliomas. Neuro Oncol. 2008;10:540–547. doi: 10.1215/15228517-2008-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idbaih A, Marie Y, Lucchesi C, et al. BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer. 2008;122:1778–1786. doi: 10.1002/ijc.23270. [DOI] [PubMed] [Google Scholar]
- 11.Joseph NM, Phillips J, Dahiya S, et al. Diagnostic implications of IDH1-R132H and OLIG2 expression patterns in rare and challenging glioblastoma variants [published online ahead of print October 5] Mod Pathol. 2012 doi: 10.1038/modpathol.2012.173. [DOI] [PubMed] [Google Scholar]
- 12.Benito R, Gil-Benso R, Quilis V, et al. Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathology. 2009;30:392–400. doi: 10.1111/j.1440-1789.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 13.Houillier C, Lejeune J, Benouaich-Amiel A, et al. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer. 2006;106:2218–2223. doi: 10.1002/cncr.21819. [DOI] [PubMed] [Google Scholar]
- 14.Korshunov A, Sycheva R, Golanov A. The prognostic relevance of molecular alterations in glioblastomas for patients age < 50 years. Cancer. 2005;104:825–832. doi: 10.1002/cncr.21221. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Backlund LM, Nilsson BR, et al. Clinical significance of EGFR amplification and the aberrant EGFRvIII transcript in conventionally treated astrocytic gliomas. J Mol Med. 2005;83:917–926. doi: 10.1007/s00109-005-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 17.Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 pt 1):228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 18.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs J, Nikiforova MN, Fardo DW, et al. Paradoxical relationship between the degree of EGFR amplification and outcome in glioblastomas. Am J Surg Pathol. 2012;36:1186–1193. doi: 10.1097/PAS.0b013e3182518e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Nicholas MK, Lukas RV, Chmura S, et al. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38:243–253. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Yung WK, Vredenburgh JJ, Cloughesy TF, et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 2010;12:1061–1070. doi: 10.1093/neuonc/noq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 24.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951 [published online ahead of print October 15] J Clin Oncol. 2012 doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins RB. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 26.Barbashina V, Salazar P, Holland EC, et al. Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene. Clin Cancer Res. 2005;11:1119–1128. [PubMed] [Google Scholar]
- 27.Horbinski C, Nikiforova MN, Hobbs J, et al. The importance of 10q status in an outcomes-based comparison between 1p/19q fluorescence in situ hybridization and polymerase chain reaction-based microsatellite loss of heterozygosity analysis of oligodendrogliomas. J Neuropathol Exp Neurol. 2012;71:73–82. doi: 10.1097/NEN.0b013e318240fa65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2 . Neurology. 2010;74:1886–1890. doi: 10.1212/WNL.0b013e3181e1cf3a. [DOI] [PubMed] [Google Scholar]
- 29.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woehrer A, Sander P, Haberler C, et al. FISH-based detection of 1p 19q codeletion in oligodendroglial tumors: procedures and protocols for neuropathological practice—a publication under the auspices of the Research Committee of the European Confederation of Neuropathological Societies. Clin Neuropathol. 2011;30:47–55. doi: 10.5414/npp30047. [DOI] [PubMed] [Google Scholar]
- 31.Snuderl M. Polysomy for chromosomes 1 and 19 predicts earlier recurrence in anaplastic oligodendrogliomas with concurrent 1p/19q loss. Clin Cancer Res. 2009;15:6430–6437. doi: 10.1158/1078-0432.CCR-09-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiens AL, Cheng L, Bertsch EC, et al. Polysomy of chromosomes 1 and/or 19 is common and associated with less favorable clinical outcome in oligodendrogliomas: fluorescent in situ hybridization analysis of 84 consecutive cases. J Neuropathol Exp Neurol. 2012;71:618–624. doi: 10.1097/NEN.0b013e31825b5f7a. [DOI] [PubMed] [Google Scholar]
- 33.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CB. Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med. 2009;360:813–815. doi: 10.1056/NEJMe0810213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horbinski C, Kofler J, Kelly LM, et al. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68:1319–1325. doi: 10.1097/NEN.0b013e3181c391be. [DOI] [PubMed] [Google Scholar]
- 38.Camelo-Piragua S, Jansen M, Ganguly A, et al. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119:509–511. doi: 10.1007/s00401-009-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 40.Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21:564–574. doi: 10.1111/j.1750-3639.2011.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capper D, Reuss D, Schittenhelm J, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121:241–252. doi: 10.1007/s00401-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 42.Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2009;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capper D, Zentgraf H, Balss J, et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 44.Meyer J, Pusch S, Balss J, et al. PCR- and restriction endonuclease-based detection of IDH1 mutations. Brain Pathol. 2009;20:298–300. doi: 10.1111/j.1750-3639.2009.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horbinski C, Kelly L, Nikiforov YE, et al. Detection of IDH1 and IDH2 mutations by fluorescence melting curve analysis as a diagnostic tool for brain biopsies. J Mol Diagn. 2010;12:487–492. doi: 10.2353/jmoldx.2010.090228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felsberg J, Wolter M, Seul H, et al. Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol. 2010;119:501–507. doi: 10.1007/s00401-010-0647-4. [DOI] [PubMed] [Google Scholar]
- 47.Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21:194–197. doi: 10.1016/j.seizure.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2012;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preusser M, Wöhrer A, Stary S, et al. Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens. J Neuropathol Exp Neurol. 2011;70:715–723. doi: 10.1097/NEN.0b013e31822713f0. [DOI] [PubMed] [Google Scholar]
- 50.Olar A, Raghunathan A, Albarracin CT, et al. Absence of IDH1-R132H mutation predicts rapid progression of nonenhancing diffuse glioma in older adults. Ann Diagn Pathol. 2012;16:161–170. doi: 10.1016/j.anndiagpath.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 52.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120:719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 53.Okita Y, Narita Y, Miyakita Y, et al. IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy [published online ahead of print July 20] Int J Oncol. 2012 doi: 10.3892/ijo.2012.1564. [DOI] [PubMed] [Google Scholar]
- 54.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177:2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takano S, Kato Y, Yamamoto T, et al. Immunohistochemical detection of IDH1 mutation, p53, and internexin as prognostic factors of glial tumors. J Neurooncol. 2012;108:361–373. doi: 10.1007/s11060-012-0837-0. [DOI] [PubMed] [Google Scholar]
- 56.Ahmadi R, Stockhammer F, Becker N, et al. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol. 2012;109:15–22. doi: 10.1007/s11060-012-0863-y. [DOI] [PubMed] [Google Scholar]
- 57.Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18:2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 58.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 59.Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27:105–113. doi: 10.1053/j.semdp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Tabatabai G, Hegi M, Stupp R, et al. Clinical implications of molecular neuropathology and biomarkers for malignant glioma. Curr Neurol Neurosci Rep. 2012;12:302–307. doi: 10.1007/s11910-012-0263-x. [DOI] [PubMed] [Google Scholar]
- 61.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 62.Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 64.Toedt G, Barbus S, Wolter M, et al. Molecular signatures classify astrocytic gliomas by IDH1 mutation status. Int J Cancer. 2011;128:1095–1103. doi: 10.1002/ijc.25448. [DOI] [PubMed] [Google Scholar]
- 65.Glas M, Bahr O, Felsberg J, et al. NOA-05 phase 2 trial of procarbazine and lomustine therapy in gliomatosis cerebri. Ann Neurol. 2011;70:445–453. doi: 10.1002/ana.22478. [DOI] [PubMed] [Google Scholar]
- 66.Mulholland S, Pearson DM, Hamoudi RA, et al. MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer. 2012;131:1104–1113. doi: 10.1002/ijc.26499. [DOI] [PubMed] [Google Scholar]
- 67.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S, Chou AP, Chen W, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2012;15:57–68. doi: 10.1093/neuonc/nos261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi ST, Yu L, Lu YT, et al. IDH mutations occur frequently in Chinese glioma patients and predict longer survival but not response to concomitant chemoradiotherapy in anaplastic gliomas. Oncol Rep. 2011;26:1479–1485. doi: 10.3892/or.2011.1428. [DOI] [PubMed] [Google Scholar]
- 70.Ducray F, El Hallani S, Idbaih A. Diagnostic and prognostic markers in gliomas. Curr Opin Oncol. 2009;21:537–542. doi: 10.1097/CCO.0b013e32833065a7. [DOI] [PubMed] [Google Scholar]
- 71.Juratli TA, Kirsch M, Geiger K, et al. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol. 2012;110:325–333. doi: 10.1007/s11060-012-0977-2. [DOI] [PubMed] [Google Scholar]
- 72.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 73.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 74.Taal W, Dubbink HJ, Zonnenberg CB, et al. First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol. 2011;13:235–241. doi: 10.1093/neuonc/noq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horbinski C. To BRAF or not to BRAF: is that even a question anymore? J Neuropathol Exp Neurol. 2012;72:2–7. doi: 10.1097/NEN.0b013e318279f3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11–19. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 78.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17:4790–4788. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 79.Horbinski C, Nikiforova MN, Hagenkord JM, et al. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol. 2012;14:777–789. doi: 10.1093/neuonc/nos077. [DOI] [PMC free article] [PubMed] [Google Scholar]