Abstract
Docosahexaenoic acid (DHA, 22:6 n-3) from fish oil, and butyrate, a fiber fermentation product, work coordinately to protect against colon tumorigenesis in part by inducing apoptosis. We have recently demonstrated that dietary DHA is incorporated into mitochondrial membrane phospholipids, thereby enhancing oxidative stress induced by butyrate metabolism. In order to elucidate the subcellular origin of oxidation induced by DHA and butyrate, immortalized young adult mouse colonocytes were treated with 0–200 μM DHA or linoleic acid (LA, 18:2 n-6; control) for 72 h with or without 5 mM butyrate for the final 24 h. Cytosolic reactive oxygen species, membrane lipid oxidation, and mitochondrial membrane potential (MP), were measured by live-cell fluorescence microscopy. After 24 h of butyrate treatment, DHA primed cells exhibited a 151% increase in lipid oxidation (P < 0.01), compared with no butyrate treatment, which could be blocked by a mitochondria-specific antioxidant, 10-(6′-ubiquinoyl) decyltriphenylphosphonium bromide (MitoQ) (P < 0.05). Butyrate treatment of LA pretreated cells did not show any significant effect. In the absence of butyrate, DHA treatment, compared with LA, increased resting MP by 120% (P < 0.01). In addition, butyrate-induced mitochondrial membrane potential (MP), dissipation was 21% greater in DHA primed cells as compared with LA at 6 h. This effect was reversed by preincubation with inhibitors of the mitochondrial permeability transition pore, cyclosporin A or bongkrekic acid (1 μM). The functional importance of these events is supported by the demonstration that DHA and butyrate-induced apoptosis is blocked by MitoQ. These data indicate that DHA and butyrate potentiate mitochondrial lipid oxidation and the dissipation of MP which contribute to the induction of apoptosis.
Introduction
Colon cancer is one of the leading causes of cancer death in essentially all economically developed countries (1). It is estimated that 70% of colorectal cancers are preventable by moderate changes in diet and lifestyle (2). Epidemiological studies indicate that populations ingesting higher amounts of fish are at a lower risk for colon cancer and a lower mortality rate of colorectal cancer (3–5), compared with those ingesting diets high in saturated fat or other polyunsaturated fatty acids (PUFA). In addition, other dietary components also appear to favorably impact colonic epithelial cell biology. For example, the daily consumption of dietary fiber, which enhances butyrate luminal production, can decrease the incidence of cancers of the digestive tract (6). Interestingly, a number of animal model studies conducted in our laboratory have consistently demonstrated that the combination of fermentable fiber, e.g. pectin and fish oil have an enhanced protective effect against colon tumorigenesis (7,8). These studies suggest that the combination of dietary fish oil and fermentable fiber, may provide the basis for a novel chemotherapeutic strategy for the colon. However, the molecular mechanism(s) by which dietary fish oil and fermentable fibers synergistically reduce malignant transformation in the colon remain to be elucidated.
Fish oil is highly enriched in two long chain n-3 PUFA, eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3). These lipids are incorporated into cell membrane phospholipids and have effects on membrane composition and function (8), reactive oxygen species (ROS) production (8,9), membrane lipid oxidation (10), transcriptional or translational regulation (11,12), eicosanoid biosynthesis (13) and intracellular signal transduction (14). These pleiotropic effects are believed to mediate the n-3 PUFA-induced suppression of colon cancer (15). Cumulative evidence shows that n-3 PUFA, in contrast to n-6 PUFA, uniquely affect cell cytokinetics by altering the cellular microenvironment in part through changes to membrane composition, thereby promoting apoptosis (7,15–17). In animal studies, highly purified n-3 PUFA ethyl esters were incorporated into colonocyte mitochondrial membrane phospholipids (18,19), which coincided with the enhancement of apoptosis in the colon (8,19). A similar trend was observed utilizing colonocyte cell lines, in which the incorporation of DHA into cardiolipin, a mitochondrial inner membrane phospholipid, was associated with the induction of oxidative stress and apoptotic signaling (8,17,19). We have also shown that an increase in ROS production following fish oil supplementation in the diet is correlated with the induction of apoptosis in colonic crypts (20,21). Furthermore, lipophilic antioxidants, which specifically target membrane lipid oxidation, partially reverse the effect of DHA-induced apoptosis in human colon carcinoma cells (17,22). This evidence in its entirety suggests that oxidative stress, in particular membrane lipid oxidation, may favorably modulate apoptosis in cells enriched with n-3 PUFA.
The fermentation of dietary fiber provides colonic epithelial cells with a direct energy source, and creates an environment for butyrate to modulate colon cancer development through epigenetic and genetic means (23,24). It is noteworthy that the efficacy of butyrate uptake is dependent on factors such as co-existing substrates in the lumen (23) and the pathogenesis phase of the mucosa (24). Hence, the luminal effects of butyrate may change over time and could be subtle in nature. Existing evidence shows that butyrate can induce cellular oxidative stress and modulate changes in cell cycle arrest, maturation, differentiation, as well as apoptosis (25–28). These apparent paradoxical effects of butyrate may be determined by the state of mutational activation of the cells, the timing and the amount of butyrate administered, the source of butyrate, and the interaction with dietary fat (15,29).
With respect to why a diet containing highly fermentable fiber is only protective when fish oil is the lipid source (30), we recently demonstrated that the ingestion of long-chain n-3 PUFA may prime colonocytes for butyrate-induced apoptosis by enhancing the unsaturation of mitochondrial phospholipids, especially cardiolipin, resulting in an increase in ROS (8,20). In the present study, to further elucidate the subcellular origin of oxidation induced by n-3 PUFA and butyrate combination, mouse colonocytes were treated with DHA or linoleic acid (LA) (an n-6 PUFA control) with and without butyrate. Cellular membrane lipid oxidation and mitochondrial permeability transition (MPT) were measured by live-cell fluorescence microscopy. In addition, we have addressed the specific role of mitochondrial oxidative stress in butyrate and DHA induced apoptosis using a targeted derivative of ubiquinol [10-(6′-ubiquinoyl) decyltriphenylphosphonium bromide (MitoQ)].
Materials and methods
Materials
RPMI 1640 and Hanks’ balanced salt solution (HBSS) were from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Insulin, transferrin, selenium without linoleic acid were obtained from Collaborative Biomedical Products (Bedford, MA). Leibovitz buffer, Glutamax™ and recombinant mouse interferon-γ (γ-IFN) were from GIBCO BRL (Grand Island, NY). Fatty acids were purchased from NuChek (Elysian, MN). Cell Death Detection ELISA and Cytotoxicity-Detection (LDH) kits were from Roche Applied Science (Indianapolis, IN). Fluorescent probes, diphenyl-1-pyrenylphosphine (DPPP) and Rhodamine 123 (Rhd 123), 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CMH2-DCFDA) were from Molecular Probes (Eugene, OR). Cyclosporin A (CsA) was from Calbiochem (San Diego, CA). MitoQ was a gift from Dr Michael Murphy (Medical Research Council Dunn Human Nutrition Unit, Cambridge, UK). All other reagents were obtained from Sigma (St Louis, MO).
Cell culture
YAMC cells were obtained from R.H. Whitehead, Ludwig Cancer Institute, Melbourne, Australia (31). For all fluorescence assays, prior to fatty acid pretreatment, 4–5 × 103 cells (passages 17–23) were seeded onto borosilicate chambered cover glasses (Nalge Nunc, No. 155380) to achieve 50–70% confluence. Under a permissive temperature, 33°C, mycoplasma free cultures were grown in RPMI 1640 complete media supplemented with 5000 U/l γ-IFN, 5% fetal calf serum (FCS), and 1% insulin/transferrin/selenium. For the Cell Death Detection assay, cells were incubated in 35 mm cell culture dishes at 35 000 cells per dish.
Fatty acids were added to cultures complexed to bovine serum albumin (BSA) (32). LA (18:2 n-6) which is considered chemopromotive, served as the control in all experiments (13,16,19). Cultures were pretreated with or without DHA- or LA-BSA complex (0–200 μM) for 72 h. Co-incubation with 0–10 mM sodium butyrate in complete RPMI 1640 media was initiated during the final 6, 12 or 24 h of fatty acid pre-treatment.
Oxidative damage assays
Following fatty acid treatment, cells were washed twice with phosphate buffer saline solution (PBS) and loaded with 5 μM DPPP to measure membrane lipid hydroperoxide formation or 5 μM CMH2-DCFDA to determine the levels of cytosolic ROS. Probes were added to cells 10–15 min in the dark at room temperature prior to image acquisition. Control experiments were conducted under basal or oxidative stress conditions, i.e. with 25–100 μM cumene hydroperoxide (CumOOH) or 10 μM hydrogen peroxide (H2O2). Fluorescence was measured using an Ultima confocal microscope (Meridian Instruments, Okemos, MI). The relative levels of DPPP-oxide fluorescence intensities were monitored using 351 (excitation) and 460 nm (emission), and CMH2-DCFDA was monitored at 530 nm (emission) following excitation at 488 nm. Treatments from a minimum of 10 cells per area, 15 areas per well, and 4 wells per experiment were performed on different days. Due to the hydrophobicity of the standard compounds in the membrane phospholipid bilayers, CumOOH (positive control) initiated phospholipid hydroperoxide formation, while H2O2 (negative control) generated peroxides primarily in the cytosol without affecting membrane lipid oxidation (33). To determine the association between PUFA treatment and mitochondrial membrane lipid oxidation, select cultures were treated with the mitochondrial targeted antioxidant, MitoQ, at 1–50 μM (34). MitoQ was loaded 1 or 24 h prior to butyrate exposure and fluorophore loading. In complementary experiments, non-selective lipid soluble antioxidants, vitamin E-succinate (α-tocopherolsuccinate) and vitamin E (α-tocopherol, α–TOC) were utilized at 0–60 μM.
Measurement of mitochondrial membrane potential (MP)
Following fatty acid pretreatment, cells were rinsed with PBS, incubated with 656 nM Rhd123 for 15 min at 33°C in the dark, washed twice with PBS and replenished with phenol-free Leibovitz media. The relative levels of Rhd123 fluorescence intensities were monitored at 488 nm (excitation) and 530 nm (emission) using confocal microscopy (Meridian Ultima), as described previously (8). Groups of cells (>5 cells) from at least 14 separate fields for each sample were captured. Average fluorescence intensity was quantified for each treatment. In select experiments, cells were incubated with MPT pore inhibitors, Bongkrekic acid or Cyclosporin A (1 μM), 30 min prior to the addition of butyrate, and subsequently co-incubated with butyrate with and without fatty acid for an additional 24 h.
Apoptosis analyses
The level of apoptosis was measured by cellular fragmentation enzyme linked immunosorbent assay (ELISA) (Roche) and caspase 3 activity (Molecular Probes) assays. Floating and adherent cells were harvested and supernatants were utilized, and values normalized to the number of adherent cells per dish as described previously (32).
Measurement of cell cytotoxicity
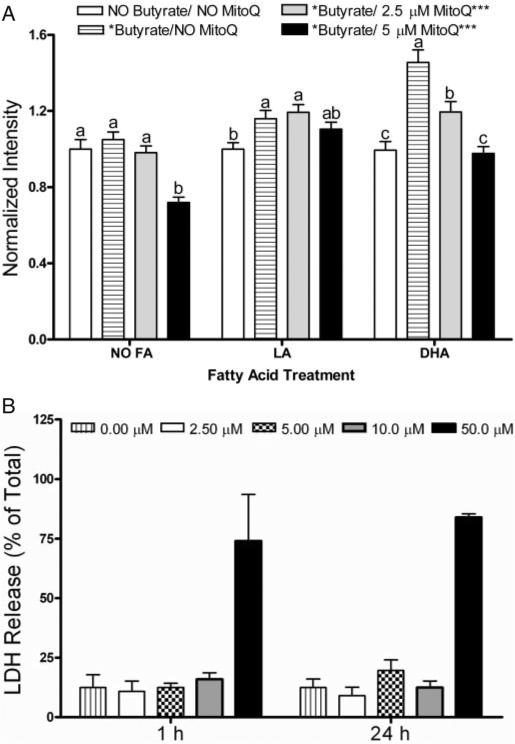
YAMC cells (1.4 × 104) per well were seeded into 96-well tissue culture dishes and incubated for 24 h with RPMI 1640 medium supplemented with γ-interferon (γ-IFN). Cells were incubated with MitoQ at 0–50 μM for 0–72 h. Supernatants were harvested, and the level of LDH release was assayed by ELISA. LDH release was compared with untreated wells, which were lysed with 1% Triton-X 100, i.e. total releasable LDH present in untreated cells, according to the manufacturer's instructions.
Statistical analysis
For all lipid oxidation and cytosolic ROS experiments, data were analyzed using linear mixed models (35). Fixed effects were constructed to indicate the treatments under which the data points were generated. Sample level random effects were used to account for the dependency of cell culture conditions within the same dish. When one set of experiments was conducted over a prolonged time frame, a factor accounting for the day to day variation was also added to the model. For all other studies, the effects of independent variables (main treatment effects) were assessed using SuperAnova. Differences among means were determined using t/F type tests of contrast. A <5% P-value in a test was considered as being statistically significant.
Results
DHA and butyrate synergistically enhance lipid oxidation
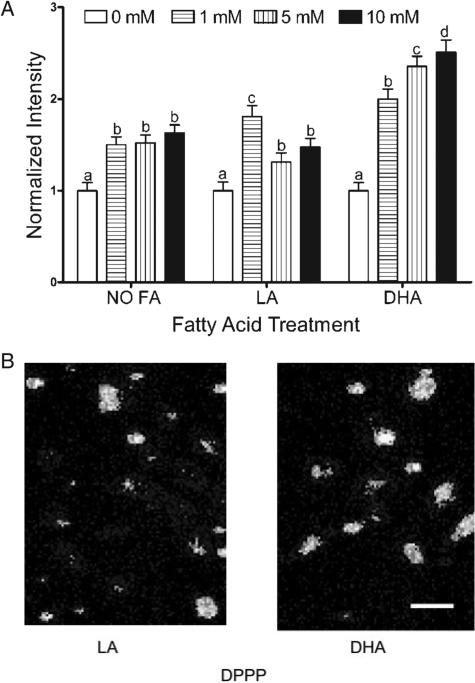
Butyrate treatment (0–10 mM) over a 24 h period increased (P < 0.05) lipid oxidation in DHA (50 μM) primed cells in a dose-dependent manner (Figure 1A). In contrast, a variable response was observed upon treatment with LA, a control PUFA. The levels of butyrate and fatty acid are considered physiologically relevant because they lie well within the range found within the colonic lumen and blood, respectively (36). Neither DHA nor LA treatment increased lipid oxidation in the absence of butyrate co-incubation. DHA primed cells co-incubated with 5 mM butyrate for 24 h exhibited a >2-fold increase in lipid oxidation (P < 0.001) relative to no butyrate treatment. The increase in oxidation was detected as early as 12 h following butyrate treatment with DHA (data not shown). Representative photomicrographs demonstrating oxidation of DPPP in YAMC cells are shown in Figure 1B. Similar to previous studies, butyrate increased (by 22%) the level of cytosolic ROS (Figure 2) (8,9). In comparison, butyrate and DHA co-incubation modestly increased (P < 0.05) ROS production by 13% compared with no butyrate control.
Fig. 1.
Effect of butyrate on membrane lipid oxidation in the presence of fatty acids. Select YAMC cultures were incubated with BSA-complexed DHA or LA (50 μM) for 72 h and butyrate (0–10 mM) for the final 24 h. (A) Butyrate dose dependently increased lipid oxidation in DHA primed cells. DHA primed cells consistently exhibited a greater increase in lipid oxidation when compared with no butyrate treatment, whereas LA or no fatty acid treatment did not show an effect. Lipid oxidation was measured by quantifying the fluorescence intensity of oxidized DPPP incorporated into cellular membranes. Data are expressed as the means ± SE of changes in average pixel intensities of all cells in each treatment group. All data were divided by the average fluorescence intensity of the control. The number of cells analyzed (n) ranged from 181 to 280, collected from five independent experiments. Values not sharing the same letters are significantly different (P < 0.05). (B) Representative photomicrographs (200×) of DPPP loaded YAMC cells treated with DHA and LA, respectively, for 72 h and with 5 mM butyrate for the final 24 h. Scale bar, 60 μm in all images. See online Supplementary material for a color version of this figure.
Fig. 2.
Effect of butyrate on ROS production in YAMC cells in the presence or absence of fatty acids. Select YAMC cultures were incubated with BSA-complexed DHA or LA (50 μM) for 72 h and butyrate (0 or 5 mM) for the final 24 h. Cytosolic ROS were measured by quantifying the fluorescence intensity of CMH2-DCFDA. The number of cells analyzed (n) ranged from 434 to 735, collected from two independent experiments. All data were divided by the average fluorescence intensity of the control. Values not sharing the same letters are significantly different (P < 0.05).
Mitochondrial-targeted antioxidant protects cells from oxidative stress
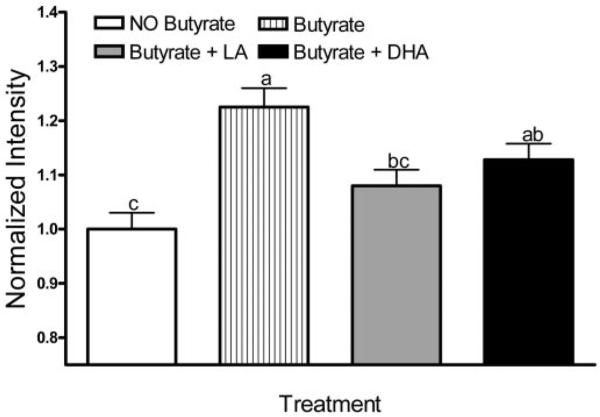
To investigate the role of mitochondria-derived oxidative stress in cell signaling, we utilized MitoQ, a mitochondria-targeted antioxidant that is rapidly taken up into living cells where it protects against oxidative damage (37). MitoQ dose-dependently inhibited butyrate-induced lipid oxidation in DHA primed cells when added during the final 1 h of butyrate co-treatment (data not shown) or following 24 h of co-incubation with butyrate (Figure 3A). In comparison, in LA treated cultures, MitoQ did not affect lipid oxidation. In order to rule out any non-specific effects on cell function, MitoQ toxicity was evaluated by determining the percentage of lactate dehydrogenase (LDH) release in YAMC cultures over a 12–24 h incubation period using 0–50 μM MitoQ. MitoQ concentrations up to 10 μM did not perturb cell viability (Figure 3B). In complementary experiments, the accumulation of lipid hydroperoxides induced by DHA and butyrate co-treatment at 24 h, was partially suppressed (P < 0.05) by treatment with vitamin E succinate, a non-selective lipid antioxidant (data not shown). These results suggest that the subcellular origin of oxidation induced by DHA and butyrate was localized to the mitochondrial membrane lipid bilayers.
Fig. 3.
MitoQ reduces lipid oxidation induced by butyrate in the presence of fatty acids. Select YAMC cultures treated with DHA (50 μM) exhibited an increase in lipid oxidation following 24 h butyrate (5 mM) co-treatment. (A) Lipid oxidation was reversed by MitoQ incubation in a dose-dependent manner when MitoQ was loaded for the final 24 h of butyrate co-treatment. The number of cells analyzed (n) ranged from 283 to 655, collected from five independent experiments. All data were divided by the average fluorescence intensity of the control. Values not sharing the same letters are significantly different (P < 0.05). (B) The level of LDH release induced by MitoQ incubation indicates a lack of cytotoxicity. YAMC cells were incubated with complete medium containing MitoQ at 0–50 μM, for 1 or 24 h. Supernatants were harvested, and the level of LDH release was assayed. Values were compared with an untreated well and expressed as percentage of LDH present in cultures lysed with 1% Triton-X 100 (total releasable LDH). Data represent n = 3 wells.
DHA enhances mitochondrial membrane potential
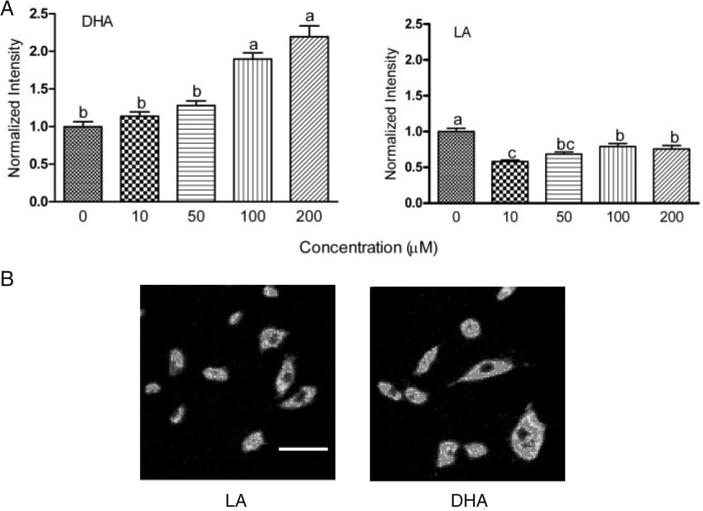
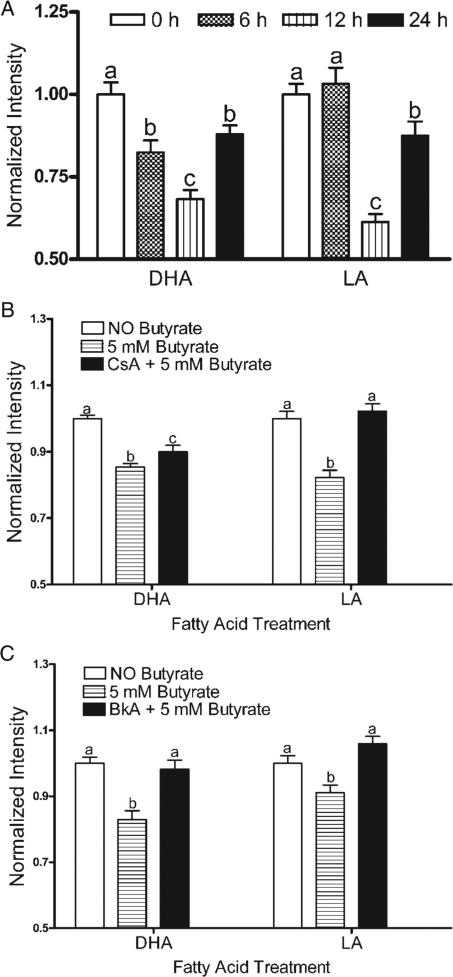
DHA incubation increased the MP in a dose-dependent manner in the absence of butyrate, whereas the opposite effect was observed in LA treated cells (Figure 4A). Compared with LA, 72 h DHA treatment increased resting MP by 120% (P < 0.01), consistent with the reported capacity of this compound to hyperpolarize mitochondria (8,9). Representative photomicrographs of cells incubated with 50 μM DHA or LA are shown in Figure 4B. In the presence of butyrate, DHA co-incubation reduced the fluorescence intensity, indicating a decrease in MP (Figure 5A). Following 6 h co-incubation with butyrate, the decrease in MP was 21% greater in DHA compared with the LA treated cells. Pretreatment with the MPT pore inhibitors CsA partially reversed, and BkA completely blocked butyrate-induced loss of MP in DHA-supplemented cells (Figure 5B and C). Comparable observations were made upon co-incubation with CsA or BkA with butyrate for the final 6 and 12 h (data not shown).
Fig. 4.
Fatty acids selectively alter mitochondria membrane potential. (A) YAMC cultures were treated with DHA or LA at 0, 10, 50, 100 and 200 μM for 72 h. To measure the changes in the basal level of MP following fatty acid treatment, cells (post-treatment) were loaded with Rhodamine 123. Each treatment group contained from 107 to 221 cells from two independent experiments. All data were divided by the average fluorescence intensity of the control. Values not sharing the same letters are significantly different (P < 0.05). (B) Representative photomicrographs (400×) of cells incubated with 50 μM DHA or LA for 72 h. DHA enriched cells demonstrated a higher fluorescence intensity as compared with LA. Scale bar, 60 μm in all images. See online Supplementary material for a color version of this figure.
Fig. 5.
Effect of butyrate on MP in the presence of fatty acids. (A) MP was dissipated by butyrate (5 mM) co-incubation, for the final 6, 12 and 24 h, following 50 μM DHA or LA treatment for 72 h, in a time dependant manner. After 6 h butyrate co-incubation, the dissipation of MP was 20% greater in DHA compared with LA treated cells. Mean ± SE fluorescence values were collected from four wells from two independent experiments. The number of cells analyzed (n) ranged from 106 to 362. (B) CsA at 1 μM, loaded 30 min prior to butyrate incubation and replenished 12 h after the initial dose, blocked the dissipation of MP following 50 μM DHA or LA treatment (72 h) and butyrate (5 mM) co-incubation. (C) BkA at 1 μM inhibited MP dissipation induced by butyrate (5 mM) co-incubation for 24 h. Similar observations were made upon co-incubation with CsA or BkA with butyrate for the final 6 and 12 h (data not shown). All data were divided by the average fluorescence intensity of the control. Values not sharing the same letters are significantly different (P < 0.05).
Mitochondrial-targeted antioxidant suppresses butyrate-induced apoptosis
We have previously demonstrated that the YAMC cell line is a good model system to examine the molecular mechanisms by which chemoprotective agents modulate colonocyte apoptosis (38). As shown in Table I, supplementation of YAMC cells with DHA and LA treatments (72 h) differently affected the extent of apoptosis induced following exposure to butyrate. Cells preincubated with DHA exhibited a significantly (P < 0.05) higher (by 66%) level of apoptosis in the presence of butyrate compared with cells preincubated with LA. Similar results were obtained upon examination of caspase 3 activity (data not shown). Since exogenous fatty acids are readily incorporated into colonocyte membranes (8), these results suggest that fatty acid-induced changes in membrane lipid composition alter butyrate-induced apoptosis. To examine the involvement of mitochondrial oxidative stress in butyrate-induced apoptosis in YAMC cells, we evaluated the effects of mitochondrial-specific (MitoQ) and non-specific (α-TOC) membrane-targeted antioxidants. MitoQ significantly (P < 0.01) inhibited apoptosis in all treatment groups (Table II). In contrast, α-TOC modestly suppressed (P < 0.05) apoptosis only in DHA-butyrate treated cells across a range of concentrations (20–60 μM) (Table III). BkA significantly (P < 0.05) reduced butyrate-induced apoptosis across all treatments (Table II) under conditions which also inhibited dissipation of MP (Figure 5C).
Table I.
Induction of apoptosis in fatty acid and butyrate-treated colonocytes
| Untreated | 0.29 ± 0.02a,b |
| LA | 0.14 ± 0.06a |
| DHA | 0.22 ± 0.04a |
| Butyrate | 0.62 ± 0.04c |
| Butyrate + LA | 0.44 ± 0.06b |
| Butyrate + DHA | 0.74 ± 0.08c |
Cultures were incubated with 5 mM butyrate and 50 μM DHA, LA or no fatty acid treatment as described in the Materials and methods. Apoptosis was measured by DNA fragmentation ELISA. Data represent mean absorbance at 405 nm ± SE divided by the total number of adherent cells per dish, n = 4-6 separate wells from 1 of 4 representative experiments. Values not sharing the same letters are significantly different (P < 0.05).
Table II.
Effect of anti-oxidants and mitochondrial permeability transition inhibitor on butyrate-induced apoptosisa
| Control | α-TOCb | MitoQc | BKAd | |
|---|---|---|---|---|
| Butyrate | 1.00 ± 0.05 | 1.02 ± 0.04 | 0.34 ± 0.01* | 0.81 ± 0.05* |
| LA + butyrate | 1.00 ± 0.06 | 1.09 ± 0.08 | 0.30 ± 0.02* | 0.77 ± 0.03* |
| DHA + butyrate | 1.00 ± 0.05 | 0.89 ± 0.05* | 0.33 ± 0.03* | 0.78 ± 0.05* |
Significantly different from control (P < 0.05).
Caspase 3 activity data are expressed as fold over control (without anti-oxidant or mitochondrial permeability transition inhibitor added) within each fatty acid treatment group, n = 4-6, mean ± SE). Cells were incubated with or without 50 μM LA or DHA for 72 h and 5 mM butyrate was added during the last 24 h of incubation.
α-TOC (20 μM) was co-incubated with fatty acid for the entire 72 h.
MitoQ (2.5 μM) was co-incubated with butyrate ± fatty acid for an additional 24 h.
BKA (1 μM) was added 30 min prior to addition of butyrate, and subsequently co-incubated with butyrate ± fatty acid for an additional 24 h.
Table III.
Dose-response of apoptosis in α-TOC-treated colonocytes
| α-TOC | 0 (μM) | 20 (μM) | 40 (μM) | 60 (μM) |
|---|---|---|---|---|
| LA + But | 1.00 ± 0.06 | 0.99 ± 0.06 | 0.98 ± 0.04 | 0.92 ± 0.04 |
| DHA + But | 1.00 ± 0.06 | 0.84 ± 0.03* | 0.88 ± 0.04 | 0.84 ± 0.05* |
Refer to Table II for experimental details, n = 4-5.
Significantly different from control (no α-TOC added), P < 0.05.
Discussion
We have previously demonstrated that dietary fish oil and soluble fiber, e.g. pectin, coordinately modulate the colonic epithelial prooxidant-antioxidant balance (21). This is noteworthy because these dietary constituents work in concert to create an environment permissive for apoptosis, thereby reducing colon cancer risk (7,13,21) With respect to a molecular mechanism of action, it appears as though the incorporation of n-3 PUFA into mitochondrial membrane phospholipids increases colonocyte susceptibility to butyrate-induced oxidative stress (19). These data suggest that oxidative stress may mediate the pro-apoptotic effect of butyrate and dietary fish oil in colonic crypts (7,8,19). The current experiments support this hypothesis, because DHA, a major fatty acid found in fish oil, enhanced membrane lipid oxidation induced by co-incubation with physiological concentrations (0–10 mM) of butyrate (Figure 1). These results add to the growing evidence that mitochondrial phospholipids are an important target for lipid oxidation induced by butyrate and DHA. The capacity of the mitochondrial antioxidant, MitoQ, to block the effect of butyrate (Figure 3A) also supports a mitochondrial-based mechanism for the effects of DHA and butyrate on promotion of apoptosis in colonocytes.
The opening of the MPT pore is mechanistically linked to cytochrome c release in some models of apoptosis (39). However, little is known regarding the ability of membrane fatty acids to alter the dynamics of the MPT pore. We noted that in the absence of butyrate, DHA-pretreated cells exhibited a higher resting MP compared with LA-pretreated cells. Because DHA incorporation may favor MPT pore opening (40), it is likely that DHA treatment primes cells for butyrate-induced apoptosis via a mechanism involving the permeability transition pore. Thus, we propose that DHA be added to the list of physiological regulators of the MPT pore, which already includes Ca2+ (41). Interestingly, Malis et al. (18) have demonstrated that the incorporation of PUFA into mitochondrial membrane phospholipids sensitizes cells towards the activation of apoptosis by Ca2+. Overloading of Ca2+ has been shown to activate the MPT pore by increasing mitochondrial ROS (42–45). In addition, Ca2+ accumulation within the mitochondrial compartment can trigger the release of PUFA enriched at the sn-2 position of phospholipids in mitochondrial membranes (18). Therefore, when cells are co-incubated with butyrate and DHA, these short and long chain fatty acids may induce Ca2+ overloading and subsequent induction of the MPT pore. Current experiments are exploring the role of calcium release from intracellular stores in DHA and butyrate-induced apoptosis in colonocytes.
This study also demonstrates that the combination of DHA and butyrate potentiates mitochondrial membrane lipid oxidation and the dissipation of membrane potential. These observations extend our previous finding that a functional relationship exists among butyrate, n-3 PUFA, and cytosolic ROS production in colonocytes (8,20). Since oxidative damage of mitochondrial membrane proteins and phospholipids can directly trigger MPT (45), these observations implicate a pivotal cross-talk between membrane lipid oxidation and the dynamics of the mitochondrial membrane pore.
Our findings demonstrate that cells preincubated with DHA exhibit a higher level of apoptosis relative to LA in the presence of butyrate. However, compared with the unsupplemented control, butyrate treatment alone induced a comparable level of apoptosis. This result is somewhat difficult to interpret because, in vivo, there is no comparable fiber-free control diet. Collectively, these observations are consistent with previous in vivo data from our laboratory demonstrating that ROS generation by dietary fish oil (containing DHA) and pectin (which generates butyrate in the lumen of the colon) appears to protect the colon by promoting apoptosis (12,21,22).
To explore the connection between membrane lipid oxidation and apoptosis, we examined the effects of two different membrane antioxidants: MitoQ, whose antioxidant activity is confined to the mitochondria (34,37) and α-TOC, which has a lesser capacity to scavenge free radicals in the mitochondrion. MitoQ effectively reduced apoptosis in all treatments, whereas α-TOC modestly reduced apoptosis but only in the DHA and butyrate treatment (Table II). These outcomes provide clear evidence that an increase in mitochondrial lipid oxidation and the resultant change in MP contribute directly to the induction of apoptosis by DHA and butyrate co-treatment. With respect to how mitochondria translate/interpret oxidant signals that ultimately trigger apoptosis, it has been shown that the incorporation of DHA into cardiolipin following dietary fish oil supplementation favors oxidative stress accumulation and apoptosis induction (21,22). Recent studies have demonstrated that ROS production is correlated to the unsaturation index of cardiolipin acyl chains (8,9). In addition, the oxidation of the cardiolipin acyl chains is associated with the release of cytochrome-C from mitochondria triggering caspase activation (46), an event inhibited by α-TOC, resveratrol, and Bcl-xl protein expression (46,47). It is possible therefore, that cardiolipin, a unique mitochondrial phospholipid localized with the inner membrane, may act as an essential signal for the execution of DHA-butyrate apoptotic program.
Why does an enhanced level of n-3 PUFA in mitochondrial membrane phospholipids promote lipid peroxidation in the colon? DHA with 22 carbons and 6 double bonds is the longest and most unsaturated fatty acid commonly found in membranes (48). Under certain biological conditions the formation of oxidative insult is proportional to the number of double bonds of PUFA (49). Therefore, it is understandable why incorporation of DHA but not LA (containing only two double bonds) into mitochondrial membranes provides the substrate for lipid oxidation following exposure to butyrate (50). Additional experiments are needed in order to determine whether other long chain PUFA, including arachidonic acid (20:4 n-6) and eicosapentaenoic acid (20:5 n-3), promote lipid oxidation. n-3 PUFA can also promote lipid oxidation by suppressing the level of expression of antioxidant enzymes (51). In addition, n-3 PUFA and butyrate have been shown to decrease (antiapoptotic) Bcl-2 expression in colon cancer cell lines (52) and in rat colonic crypts (53), respectively. Since Bcl-2 possesses antioxidant properties and is capable of suppressing membrane lipid oxidation which occurs with apoptotic cell death (54,55), it is conceivable that a reduction in Bcl-2 explains, at least in part, the ability of DHA and butyrate to promote apoptosis in the colon.
Our observations are consistent with previous in vivo dietary studies indicating that DHA exposure increases oxidative stress and primes colonocytes for apoptosis induction by butyrate (8,19,21). This raises an interesting question regarding the safety of long-term antioxidant administration as part of an anti-aging chemotherapeutic regimen. Indeed, it is possible that antioxidant supplements might not be beneficial for cancer prevention, and may on the contrary, increase overall mortality. The concept that antioxidant administration may block apoptosis and enhance colonic tumorigenesis was recently addressed in a systematic review and meta-analysis (56).
In conclusion, our results add to growing evidence that DHA alters colonocyte mitochondrial membrane composition and function, thereby creating a permissive environment for apoptosis induced by lumenal metabolites, such as butyrate. Further, our results indicate that the effects of individual chemoprotective nutrients may not be as important as the combination of foods consumed in the diet.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Michael P.Murphy for generously providing the MitoQ and Mary E.Murphy for statistical assistance. This study was supported by grants from NIH CA59034, CA82907 and P30ES09106.
Abbreviations
- α-TOC
alpha-tocopherol
- BKA
bongkrekic acid
- CSA
cyclosporine A
- CumOOH
cumene hydroperoxide
- DHA
docosahexaenoic acid
- DPPP
diphenyl-1-pyrenylphosphine
- EPA
eicosapentaenoic acid
- ELISA
enzyme-linked immunosorbent assay
- LA
linoleic acid
- LDH
lactate dehydrogenase
- MitoQ
10-(6′-ubiquinoyl) decyltriphenylphosphonium bromide
- MP
mitochondrial membrane potential
- MPT
mitochondrial permeability transition
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- YAMC
young adult mouse colonocyte
Footnotes
Supplementary material
Supplementary material can be found at: http://carcin.oxfordjournals.org/
Conflict of Interest Statement: None declared.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J. Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Willett WC. Dietary factors and risk of colon cancer. Ann. Med. 1994;26:443–52. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez E, Chatenoud L, LaVecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am. J. Clin. Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Schloss I, Kidd MS, Tichelaar HY, Young GO, O'Keefe SJ. Dietary factors associated with a low risk of colon cancer in coloured west coast fishermen. S. Afr. Med. J. 1997;87:152–158. [PubMed] [Google Scholar]
- 5.Caygill CP, Hill MJ. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur. J. Cancer Prev. 1995;4:329–332. doi: 10.1097/00008469-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European prospective investigation into cancer and nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 7.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 8.Hong MY, Chapkin RS, Barhoumi R, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 9.Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J. Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- 10.Hawkins RA, Sangster K, Arends MJ. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J. Pathol. 1998;185:61–70. doi: 10.1002/(SICI)1096-9896(199805)185:1<61::AID-PATH49>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan BA, Narayanan NK, Reddy BS. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int. J. Oncol. 2001;19:1255–1262. doi: 10.3892/ijo.19.6.1255. [DOI] [PubMed] [Google Scholar]
- 12.Davidson LA, Nyuyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Lupton JR, Carroll RJ, Chapkin RS. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptido leukotriene synthesis. J. Lipid Res. 1991;32:1205–1213. [PubMed] [Google Scholar]
- 14.Ma D, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 15.Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J. Nutr. 2004;134:479–482. doi: 10.1093/jn/134.2.479. [DOI] [PubMed] [Google Scholar]
- 16.Turner ND, Zhang J, Davidson LA, Lupton JR, Chapkin RS. Oncogenic ras alters sensitivity of mouse colonocytes to butyrate and fatty acid mediated growth arrest and apoptosis. Cancer Lett. 2002;186:29–35. doi: 10.1016/s0304-3835(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZY, Istfan NW. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:301–308. doi: 10.1054/plef.2000.0218. [DOI] [PubMed] [Google Scholar]
- 18.Malis CD, Weber PC, Leaf A, Bonventre JV. Incorporation of marine lipids into mitochondrial membranes increases susceptibility to damage by calcium and reactive oxygen species: evidence for enhanced activation of phospholipase A2 in mitochondria enriched with n-3 fatty acids. Proc. Natl Acad. Sci. USA. 1990;87:8845–8849. doi: 10.1073/pnas.87.22.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 20.Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol. Biomarkers Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 21.Sanders LM, Henderson CE, Hong MY, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J. Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 22.Latham P, Lund EK, Brown JC, Johnson IT. Effects of cellular redox balance on induction of apoptosis by eicosapentaenoic acid in HT29 colorectal adenocarcinoma cells and rat colon in vivo. Gut. 2001;49:97–105. doi: 10.1136/gut.49.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G197–G203. doi: 10.1152/ajpgi.00144.2003. [DOI] [PubMed] [Google Scholar]
- 24.Moreau NM, Champ MM, Goupry SM, Le Bizec BJ, Krempf M, Nguyen PG, Dumon HJ, Martin LJ. Resistant starch modulates in vivo colonic butyrate uptake and its oxidation in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2004;134:493–500. doi: 10.1093/jn/134.3.493. [DOI] [PubMed] [Google Scholar]
- 25.Giardina C, Boulares H, Inan MS. NSAIDs and butyrate sensitize a human colorectal cancer cell line to TNF-alpha and Fas ligation: the role of reactive oxygen species. Biochim. Biophys. Acta. 1999;1448:425–438. doi: 10.1016/s0167-4889(98)00156-6. [DOI] [PubMed] [Google Scholar]
- 26.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic. Biol. Med. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Shimoyama T, Suzuki K, Umeda T, Nakaji S, Sugawara K. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand J. Gastroenterol. 2001;36:744–750. doi: 10.1080/003655201300192012. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 29.Gibson PR, Rosella O, Wilson AJ, Mariadason JM, Rickard K, Byron K, Barkla DH. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis. 1999;20:539–544. doi: 10.1093/carcin/20.4.539. [DOI] [PubMed] [Google Scholar]
- 30.Lee I, Bender E, Arnold S, Kadenbach B. New control of mitochondrial membrane potential and ROS formation—a hypothesis. Biol. Chem. 2001;382:1629–1636. doi: 10.1515/BC.2001.198. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc. Natl Acad. Sci. USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner ND, Lupton JR, Chapkin RS. Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells. Am. J. Physiol. 1999;277:C310–C319. doi: 10.1152/ajpcell.1999.277.2.C310. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi M, Shibata M, Niki E. Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radic. Biol. Med. 2001;31:164–174. doi: 10.1016/s0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 34.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 35.McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. Wiley; New York: 2000. [Google Scholar]
- 36.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J. Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 37.Smith RA, Kelso GF, James AM, Murphy MP. Targeting coenzyme Q derivatives to mitochondria. Methods Enzymol. 2004;382:45–67. doi: 10.1016/S0076-6879(04)82003-2. [DOI] [PubMed] [Google Scholar]
- 38.Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner ND, Lupton JR, Chapkin RS. Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells. Am. J. Physiol. 1999;277:C310–C319. doi: 10.1152/ajpcell.1999.277.2.C310. [DOI] [PubMed] [Google Scholar]
- 39.Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broekemeier KM, Pfeiffer DR. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry. 1995;34:16440–16449. doi: 10.1021/bi00050a027. [DOI] [PubMed] [Google Scholar]
- 41.Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca(2+)-induced membrane permeability transition in brain mitochondria. J. Neurochem. 2001;79:1237–1245. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- 42.van de Water B, Zoeteweij JP, de Bont HJ, Mulder GJ, Nagelkerke JF. Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential. Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine. J. Biol. Chem. 1994;269:14546–14552. [PubMed] [Google Scholar]
- 43.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 44.Gunter TE, Gunter KK. Uptake of calcium by mitochondria: transport and possible function. IUBMB Life. 2001;52:197–204. doi: 10.1080/15216540152846000. [DOI] [PubMed] [Google Scholar]
- 45.Takeyama N, Miki S, Hirakawa A, Tanaka T. Role of the mitochondrial permeability transition and cytochrome c release in hydrogen peroxide-induced apoptosis. Exp. Cell Res. 2002;274:16–24. doi: 10.1006/excr.2001.5447. [DOI] [PubMed] [Google Scholar]
- 46.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 47.Priault M, Bessoule JJ, Grelaud-Coq A, Camougrand N, Manon S. Bax-induced cell death in yeast depends on mitochondrial lipid oxidation. Eur. J. Biochem. 2002;269:5440–5450. doi: 10.1046/j.1432-1033.2002.03234.x. [DOI] [PubMed] [Google Scholar]
- 48.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 49.Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem. Res. Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 50.Udilova N, Jurek D, Marian B, Gille L, Schulte-Hermann R, Nohl H. Induction of lipid peroxidation in biomembranes by dietary oil components. Food Chem. Toxicol. 2003;41:1481–1489. doi: 10.1016/s0278-6915(03)00164-9. [DOI] [PubMed] [Google Scholar]
- 51.Ding WQ, Vaught JL, Yamauchi H, Lind SE. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: the potential importance of down-regulation of superoxide dismutase 1 expression. Mol. Cancer Ther. 2004;3:1109–1117. [PubMed] [Google Scholar]
- 52.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Different molecular events account for butyrate-induced apoptosis in two human colon cancer cell lines. J. Nutr. 2002;132:1812–1818. doi: 10.1093/jn/132.7.1812. [DOI] [PubMed] [Google Scholar]
- 53.Hong MY, Chapkin RS, Davidson LA, Turner ND, Morris JS, Carroll RJ, Lupton JR. Fish oil enhances targeted apoptosis during colon tumor initiation in part by downregulating Bcl-2. Nutr. Cancer. 2003;46:44–51. doi: 10.1207/S15327914NC4601_06. [DOI] [PubMed] [Google Scholar]
- 54.Hockenberry DM, Oltavai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 function in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 55.Albrecht H, Tschopp J, Jongeneel CV. Bcl-2 protects from oxidative damage and apoptotic cell death without interferring with activation of NF-kB by TNF. FEBS Lett. 1994;351:45–48. doi: 10.1016/0014-5793(94)00817-5. [DOI] [PubMed] [Google Scholar]
- 56.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.