Abstract
An appetite for CaCl2 and NaCl occurs in young rats after they are fed a diet lacking Ca or Na, respectively. Bilateral lesions of the parabrachial nuclei (PBN) disrupt normal taste aversion learning and essentially eliminate the expression of sodium appetite. Here we tested whether similar lesions of the PBN would disrupt the calcium-deprivation-induced appetite for CaCl2 or NaCl. Controls and rats with PBN lesions failed to exhibit a calcium-deprivation-induced appetite for CaCl2. Nevertheless, both groups did exhibit a significant calcium-deprivation-induced appetite for 0.5 M NaCl. Thus, while damage to the second central gustatory relay in the PBN disrupts the appetite for 0.5 M NaCl induced by furosemide, deoxycorticosterone acetate, and polyethylene glycol, the sodium appetite induced by dietary CaCl2 depletion remains intact.
Keywords: Mineral appetite, Parabrachial, Calcium depletion, Sodium depletion
1. Introduction
The gustatory system helps regulate the nutrient and mineral selection required for homeostasis [1, 2]. The central gustatory system plays an important role in this adaptive process [3]. In rodents, the second central gustatory relay in the pontine parabrachial nuclei (PBN) is critical for the acquisition of a conditioned taste aversion (CTA) and for the expression of a sodium appetite [4–6]. Specifically, when assessed using a 0.5 M concentration of NaCl, rats with bilateral lesions of the PBN failed to demonstrate a sodium appetite following treatment with the diuretic, furosemide, with the mineralocorticoid, deoxycorticosterone acetate (DOCA), or with 30% polyethylene glycol (PEG). These same rats also failed to exhibit a LiCl-induced conditioned taste aversion, now the behavioral index of well-placed bilateral PBN lesions [7].
We sought to further examine the role of this region with regard to another phenomenon, the ingestion of calcium and sodium salts after dietary calcium deprivation. Rats on a calcium deficient diet [8–11], or that are parathyroidectomized, will ingest both calcium [12–14] and sodium salts [10, 11, 13] at concentrations they normally avoid. Calcium deprivation also raises both CaCl2 and NaCl intake during sham drinking [15], and increases the palatability of calcium salts [16]. One suggestion for why rats that are calcium deprived also ingest NaCl is that short-term ingestion of NaCl may raise ionized calcium and, therefore, may confer relief of the deficit [17]. The question addressed here is whether rats with lesions of the PBN also will be able to express either or both of these appetites.
Calcium deprivation changes gustatory afferent neural activity. There are known differences in calcium solution preferences and the activity of the chorda tympani in calcium-deprived rats [18, 19] and in the gustatory region of the nucleus of the solitary tract [20]. In addition, large lesions of the medial region of the thalamus that include the gustatory relay, compromise calcium intake after parathyroidectomy [21]. The pontine parabrachial nuclei receive gustatory projections from the nucleus of the solitary tract and, in turn, project to the gustatory thalamus [22, 23] (for a review see [3]). Thus, in the following experiments we compared PBN–intact controls with rats that had taste-guided bilateral PBN lesions (PBNx) for differences in the following three phenomena: 1) Expression of a calcium and sodium appetite following calcium deprivation, 2) Expression of a furosemide-induced sodium appetite, and 3) the ability to learn a LiCl-induced conditioned taste aversion. The latter two tests serve as functional indexes for PBN lesions. Thus, if the lesions are well placed, both the furosemide-induced sodium appetite and the LiCl-induced conditioned taste aversion should be disrupted.
2. Methods
2.1 Subjects
Sprague-Dawley rats (n = 36, Charles River Laboratories, Wilmington, MA) weighed between 302 – 456 g at the time of surgery. Between surgery and the current tests these rats were used in another (unpublished) experiment that involved testing their ability to learn a LiCl-induced conditioned taste aversion with corn oil as the conditioned stimulus (CS). The rats were housed in individual, hanging, wire mesh cages. The room was temperature controlled, with an automatic light:dark cycle (12 hr, lights on at 7AM). All experimental manipulations were performed during the lights on period. Rats were maintained on ad libitum food and water except where noted below.
2.2. Surgery
Seven rats were non-surgical controls. The remaining 29 animals consisted of 7 full surgical controls that were subjected to all procedures except that their intracerebral infusions were saline rather than ibotenic acid. The remaining 22 rats had bilateral ibotenic acid lesions centered on electrophysiologically-identified gustatory responses in the pontine parabrachial nuclei (PBN; 0.2 µl per side, 20 µg/µl in phosphate buffered saline, pH = 7.4). The procedures are essentially identical to those used previously and will be only summarized here [see 24, 25]. All procedures conformed to NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine.
The rats were pretreated with atropine sulfate (0.1 mg, ip) and then anesthetized with pentobarbital sodium (50 mg/kg, ip). Anesthesia was supplemented every 45 – 60 min as needed (5 – 8 mg, ip). The rats were mounted in a Kopf stereotaxic apparatus with the skull flat between β and λ, the skull bared, and holes burred over the intended recording targets. First, gustatory responses were located bilaterally in the dorsal pons using standard recording techniques and glass insulated tungsten electrodes (Z = 1.0 – 2.5 MΩ at 1kH). The initial penetration ranged between –11.0 and –12.0 mm posterior to β and 1.8 – 2.0 mm lateral to the midline with the electrode inclined caudally 20° off vertical. Testing began ~5.5 mm deep to the dura and consisted of noting neural activity on an audio monitor and an oscilloscope while washing the anterior tongue with distilled water and 0.1 M NaCl, both at room temperature. When a multiunit taste response was encountered, typically after 1–5 penetrations, the electrode was removed and the process repeated on the contralateral side. With the taste responses located, the recording electrode was replaced with a glass micropipette that had been glued to the shaft of a Hamilton 1.0 µl syringe loaded with ibotenic acid. The pipette was positioned using the coordinates established previously. Typically, a gustatory response also was recorded through the pipette. The ibotenic acid (0.2 µl) was then infused over a period of 10 min. The pipette remained in place for an additional 10 min before being withdrawn. After both infusions, the wound was closed with clips and the rat given gentamicin prophylactically (0.15 ml, im).
2.3 Test Conditions
Three experiments were performed on these rats. In Experiment 1, the ingestion of water, calcium salt (0.5 M CaCl2), and sodium salt (0.5 M NaCl) was measured under baseline conditions and then after Ca deprivation. The 0.5 M CaCl2 was chosen to match the concentration of the 0.5 M NaCl solution, a concentration that is consistently rejected by intact, Na+ replete controls. In Experiment 2, ingestion of water, CaCl2, and NaCl was measured after furosemide-induced sodium depletion. For each of these experiments, control and PBNx rats were separated into three groups -- one was given a choice between water and 0.5 M CaCl2 (group WC), another had water and 0.5 M NaCl (group WN), and the third, all three fluids (group WCN). Experiment 3 examined the ability of the rats to learn a LiCl-induced conditioned taste-aversion to a sweet CS. Neither CaCl2 nor NaCl was provided.
2.3.1 Experiment 1: Calcium Deficient Diet
For 10 days all rats were placed on a calcium deficient diet (AIN 76A, Dyets Inc, Bethlehem, PA) with the calcium added back. As mentioned above, in addition to distilled water (dH2O), one group had access to a 0.5 M CaCl2 solution, the second to 0.5 M NaCl, and the third to both 0.5 M NaCl and 0.5 M CaCl2. Water and the tastants were presented in graduated cylinders affixed to the front of the cages with springs and intake was recorded daily. For the two groups with only 2 tubes, the left/right positions were switched daily. For the group with 3 choices, the tubes were repositioned randomly among the 6 possible orders. Following this 10 day baseline period, the salt solutions were removed, and the rats were fed only the calcium deficient diet for 30 days. Subsequently, the respective salts were replaced on the front of the cage for each group and 24 h intake was measured daily for 5 days.
2.3.2 Experiment 2: Sodium Depletion Test Conditions
At the end of the Ca-appetite test period, the diet was changed to the base diet (AIN 76, ICN Biochemicals #902903, Irvine, CA), which had adequate Ca++ included, but was without added NaCl. During this baseline period (11 days), the appropriate amount of NaCl was added back to this diet. The rats were maintained in the same groups and offered the same fluid choices described above. Intake stabilized over the last 7 days of this period. On day 8, the rats received an injection of furosemide (Furo, 10.0 mg in two equal doses 2 hours apart, sc; see [26]). Furosemide promotes sodium excretion and a subsequent sodium appetite. Between the injection and the test the following morning, the rats had access only to water and the sodium deficient diet without added NaCl. The fluids were then returned to the cages and intake of the fluids was measured at 15, 30, 60, and 120 minutes, and again at 24 hours. Normal chow (with NaCl) was returned 120 min into the test period. After a week on the normal chow, the entire regimen was replicated but, rather than Furo, the rats were injected with an equivalent volume of saline (sc).
2.3.3 Experiment 3: Conditioned Taste Aversion (CTA)
All rats were water deprived and adapted to a regimen in which they had access to a water bottle at the front of the cage every morning for 15 minutes and every afternoon for 1 hour. Water intake stabilized over 10 ten days. On the morning of day 11, all rats were presented with 0.15% Na-saccharin for 15 min instead of the water. Five minutes later, the rats were injected with lithium chloride (0.15 M; 1.5 mEq/kg body weight, ip). In the afternoon they were again offered water for 1 hour. This was repeated twice with 2 water days (15 min a.m., 1 h p.m.) intervening between each cycle. On the fourth trial the rats were offered saccharin again but without the subsequent lithium injection.
2.4 Statistical analysis
2.4.1 Effect of calcium deprivation
Data are expressed as means and standard deviations. Mean baseline intake for each fluid for each rat was calculated across the 10-day baseline period. The baseline intake for each group (WC, WN, WCN) was subjected to a 3-way mixed factorial analysis of variance (ANOVA) varying solution (water vs. CaCl2 and/or NaCl), lesion (Control vs. PBNx) and Trial (Days 1–10). When indicated, the data for the PBNx rats were analyzed alone varying solution and trials. Post hoc tests were conducted, where appropriate, using Newman-Keuls tests with alpha set at 0.05. Calcium appetite data were analyzed similarly varying solution (CaCl2 and/or NaCl), lesion (Control vs. PBNx), and trial, including terminal baseline (intake averaged across baseline trials 9 + 10) and post Ca deprivation test trials 1 – 5.
2.4.2 Furosemide-induced sodium appetite
Intake for group WC, WN, and WCN was analyzed using separate mixed factorial ANOVAs varying solution (CaCl2 and/or NaCl), lesion (Control vs. PBNx), and time (15min, 30min, 60 min, 120 min, 24 h) following the injection of furosemide and, again, in a separate iteration, following the injection of saline. Post hoc Newman-Keuls tests were conducted as described. For groups WCN and WN, mixed factorial ANOVAs were conducted varying lesion (Control vs. PBNx) and Drug (Saline vs. Furosemide) at the 24 h time point. Post hoc Newman-Keuls tests were conducted when indicated as described.
2.4.3 CTA analysis
Intake of 0.15% saccharin was examined using a mixed factorial ANOVA varying lesion (Control vs. PBNx) and trials (1 – 4). Newman-Keuls tests were employed for post hoc analysis.
2.5 Histology
When the experiments were completed, all rats with PBN lesions and a subsample of the full surgical controls were sacrificed with an overdose of pentobarbital sodium (Nembutal, 100 mg/kg, ip) and, once totally unresponsive, perfused through the heart with saline, then 10% Formalin. The brains were extracted and relevant blocks sectioned at 50 µm on a freezing microtome. Alternate series of sections were stained with cresyl violet and the Weil procedures. The lesions were assessed by an evaluator (RN) who had no knowledge of the data from individual rats.
3.Results
3.1 Histology
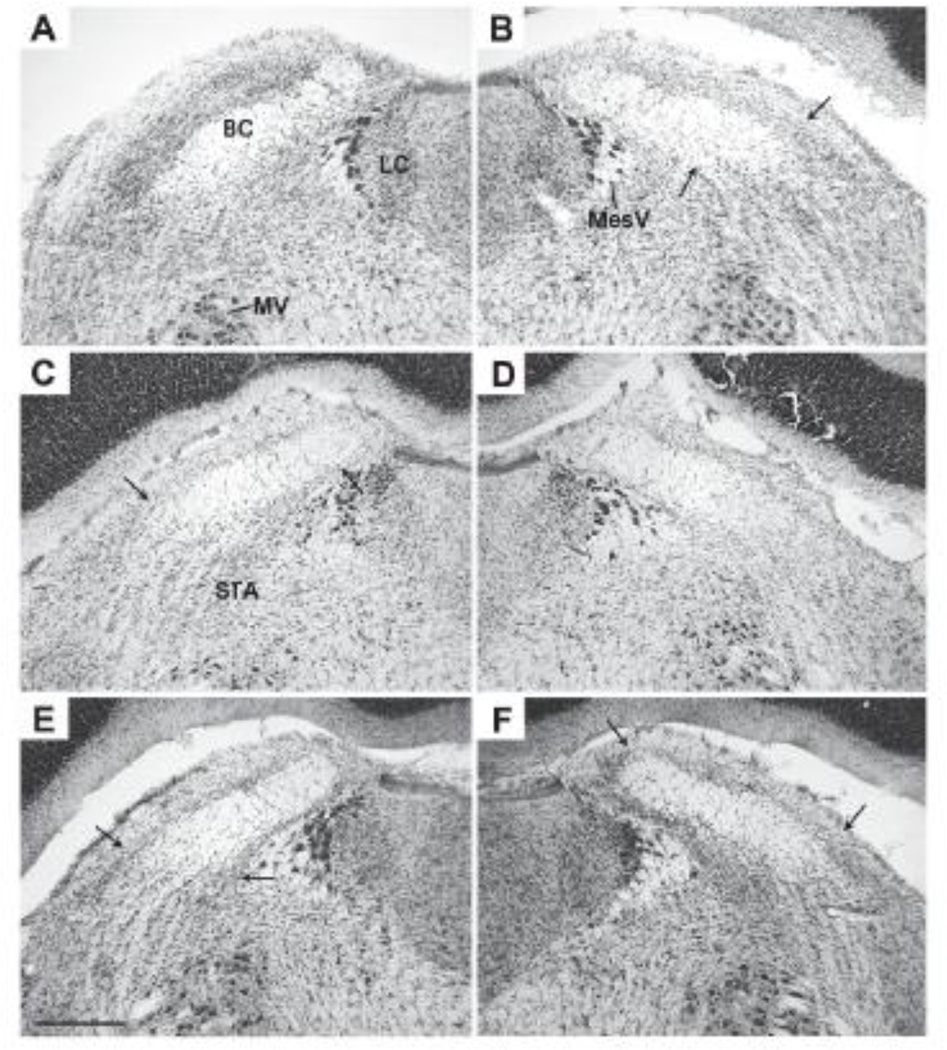
All save one of the 22 rats with lesions had histologically confirmed bilateral damage to the PBN. Based on the smallest unilateral lesion, the damage to the gustatory area of the PBN was judged to be complete (C = 5), partial complete (PC = 12), partial (P = 4), or none (ND = 1). In the coronal plane, the area assessed extended from the point at which the inferior colliculus separates from the pons caudally to the point at which the brachium conjunctivum (BC) enters the cerebellum, a distance of approximately 1.0 mm [see 27, Fig. 108–116]. This includes the caudal two thirds of the PBN and all of the area in which taste responsive neurons are located (Fig 1A [28]). Complete lesions encircled the BC except at the rostral extreme where the gliosis often was confined dorsal to the fiber tract (Fig. 1D). Partial-complete damage also surrounded the BC except for varying amounts of cell sparing extending from ventrolateral to the tract and around the lateral tip (Fig. 1B, C, & E). Partial damage was confined to neurons dorsal to and within the BC (Fig. 1F). Some of the complete lesions extended beyond the PBN into the supratrigeminal area, the locus coeruleus, or Barrington’s nucleus, usually unilaterally.
Figure 1.
Photomicrographs of 50 µm coronal sections from the dorsolateral pons from 3 rats in these experiments (Cresyl violet stain). Each section is at approximately the same rostrocaudal level, midway through the gustatory area of the parabrachial nucleus (PBN). Medial is to the right in panels A, C, and E, and to the left in panels B, D, and F. Dorsal is up. In Panels B, C, & E, the arrows indicate the lateral boundaries of the lesion gliosis dorsal and ventral to the BC. In Panel E, the arrows are placed at the medial and lateral edges of the gliosis dorsal to BC. In brain #491, the left PBN exhibited little if any damage (Panel A) but on the right almost all the parabrachial neurons were destroyed (Panel B, partial-complete). This animal exhibited a modest Na-appetite (5.0 ml at 2 h) but failed to acquire a CTA after 3 CS-US pairings). In animal #479, the left PBN was intact only in the ventrolateral quadrant below the BC (Panel C, partial-complete) but on the right the neuronal damage ringed the BC (Panel D, complete). This rat failed to demonstrate a Na-appetite or a CTA. In rat #478, the left-hand lesion was also partialcomplete (Panel E) but on the right damage was confined dorsal to the BC (Panel F, partial). This animal failed to exhibit a Na-appetite (2 ml at 2 h) and failed to reduce CS intake until trials 3 & 4 of the CTA test (5.0 & 0.0 ml, respectively). Except for Panel A, few if any neurons were evident within the BC. The horizontal line in panel E represents approximately 500 µm. Abbreviations: BC – brachium conjunctivum, LC – locus coeruleus, MV – trigeminal motor nucleus, MesV – mesencephalic trigeminal nucleus, STA – supratrigeminal area.
The criterion for including rats with PBN damage (PBNx) in the analysis was an inability to form a CTA, to express a Na-appetite, or both. Rats with complete or partial complete lesions failed on one or both of these tests. The four animals with partial PBN lesions (Fig. 1F) and the one with no obvious damage unilaterally (Fig. 1A) were impaired on at least one of the screening tests. Two rats with partial damage had been assigned to group WC (water and CaCl2) and thus were not exposed to NaCl in the Na-appetite tests. In the CTA test, however, neither rat reduced intake of the CS after three pairings with LiCl injections, suggesting that these lesions, too, were adequate. Thus, based on these screening tests, the data from all 22 PBNx rats were included in the statistics.
3.2 Behavioral Tests
3.2.1 Experiment 1: Calcium Appetite
3.2.1.1 Baseline Intake
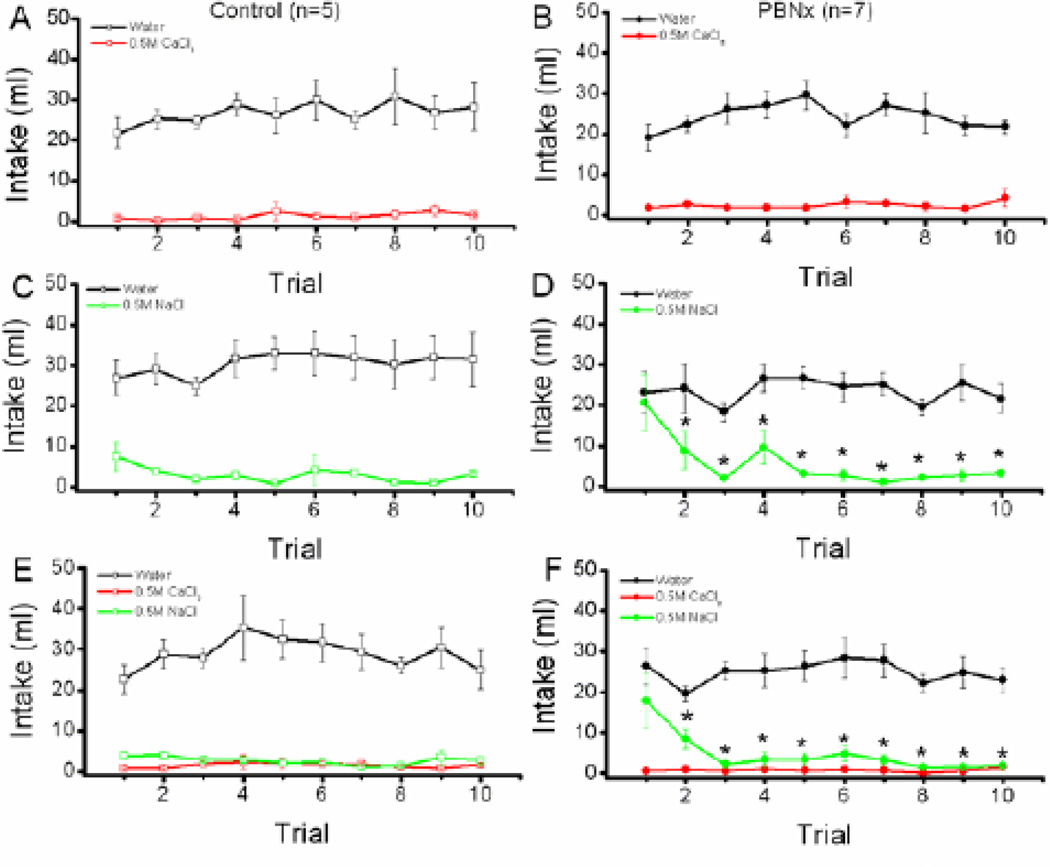
As shown in Figure 2, baseline intake of fluids (water, CaCl2, and NaCl) was evaluated within each of the three treatment groups as a function of lesion condition (Control vs. PBNx) and trials (1–10). Group WC. The main effect of solution (water vs. CaCl2) was significant, F (1,10) = 164.2, p < 0.0001, indicating that the control and PBNx groups combined consumed more water than CaCl2 during the 10 day baseline period (see Figure 2, panels A & B). The main effect of trial also was significant, F (9,90) = 2.03, p < 0.04, demonstrating that intake of the fluids (water and CaCl2) changed over trials overall. Neither the main effect of lesion, F < 1, nor the lesion × solution × trial interaction, F (9,90) = 1.51, p < 0.15, however, attained statistical significance.
Figure 2.
Baseline CaCl2 intake. Top panels: Intake (ml/24h) of water and 0.5 M CaCl2 by Control (A) and PBNx (B) rats in the Water-CaCl2 (WC) condition across 10 days of baseline. Middle panels: Intake (ml/24h) of water and 0.5 M NaCl by Control (C) and PBNx (D) rats in the water-NaCl (WN) condition across 10 days of baseline. Bottom panels: Intake (ml/24h) of water, 0.5 M CaCl2, and 0.5 M NaCl by Control (E) and PBNx (F) rats in the water-CaCl2-NaCl (WCN) condition across 10 days of baseline. * indicates that NaCl intake on Trial 1 differs significantly from intake of NaCl on Trials 2 – 10, ps < 0.05.
Group WN. A fairly similar pattern was evident in group WN (see Figure 2, panels C & D). Thus, the main effect of solution was significant, F (1,10) = 103.1, p <0 .0001, demonstrating that all rats consumed more water than 0.5 M NaCl overall. The solution × trial interaction was significant, F(9,90) = 7.3, p <0 .0001. Even so, post hoc Newman-Keuls tests showed that water intake was greater than NaCl intake on all trials. The main effect of lesion was not significant, F < 1, nor did it interact significantly with any other factors, ps > 0.05. That said, visual inspection of the data suggests that the initial high intake of 0.5 M NaCl was carried primarily by the PBNx rats. When the data for the PBNx rats were analyzed separately for group WN, a significant solution × trials interaction was found, F (9,63) = 8.09, p < 0.001. Post hoc tests revealed that, for PBNx rats, intake of NaCl on trial 1 was greater than intake of NaCl on trials 2 – 10, ps < 0.05. Further, while PBNx rats generally consumed more water than NaCl over trials, ps < 0.05, upon first exposure, post hoc tests confirmed that PBNx rats in the WN condition consumed as much 0.5 M NaCl as water, p > 0.05. In short, rats with PBN lesions initially over-consumed 0.5 M NaCl and then reduced intake to levels equivalent to the Control subjects.
Group WCN. The pattern of baseline intake for group WCN was in keeping with that of group WC and WN (see Figure 2, panels E & F). Thus, the main effect of solution was significant, F (2,20) = 141.2, p < 0.0001, and post hoc tests confirmed that all rats consumed more water than CaCl2, p < 0.05, and NaCl, p < 0.05, and that intake of these two solutions did not differ one from the other overall, p > 0.05. The solution × trial interaction was significant, F (18,180) = 3.1, p < 0.0001. The results of the post hoc tests, however, revealed little meaningful about the data. Neither the main effect of lesion, nor the lesion × solution × trial interaction attained statistical significance, ps > 0.05. Again, as with the WN group, the PBNx rats in group WCN appeared to consume more 0.5 M NaCl on the first exposure. When the PBNx trial 1 data were analyzed alone, the results revealed a significant main effect of solution, F (2,12) = 13.7, p < 0.0008. Post hoc tests confirmed that trial 1 intake of NaCl by the PBNx rats did not differ from trial 1 intake of water, p > 0.05, but was greater than trial 1 intake of CaCl2, p < 0.05.
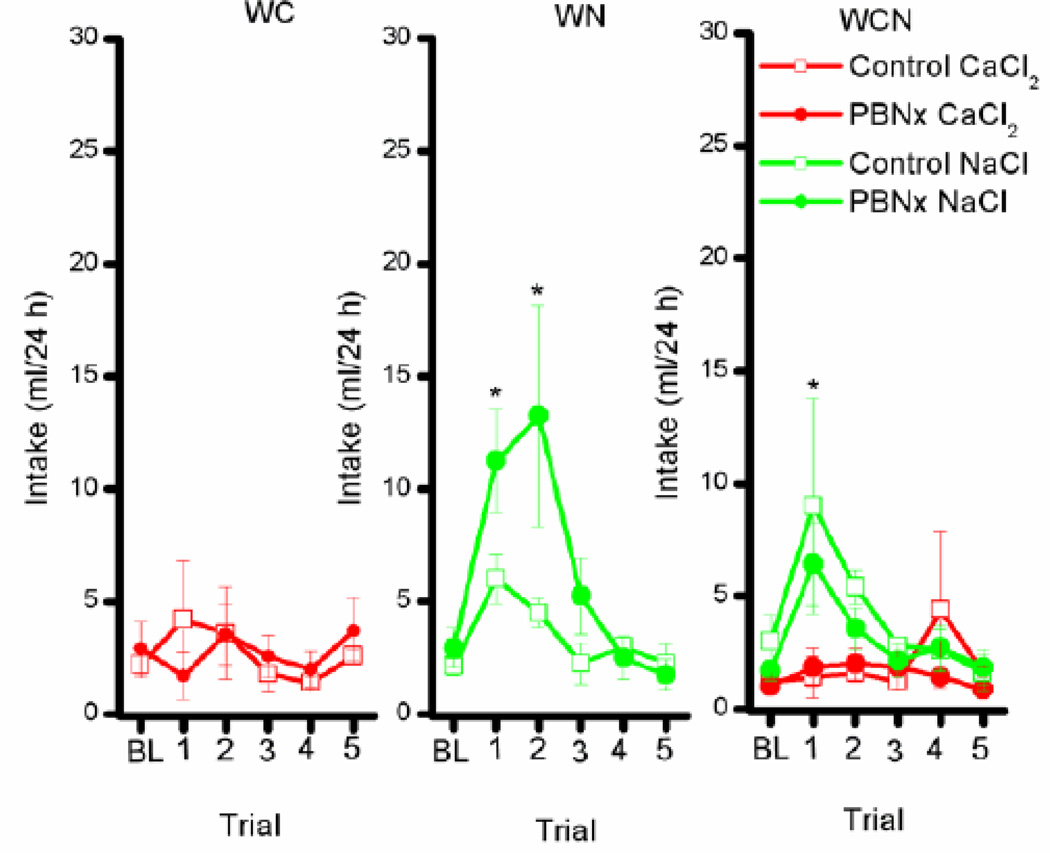
3.2.1.2 Calcium Appetite: Deprivation Test
During the Ca appetite test, all rats consumed about 25 ml of water/day regardless of lesion or solution condition. As such, these data will not be considered further here. Baseline (BL) intake of CaCl2 and NaCl for the three solution conditions (WC, WN, WCN) was averaged across trials 9 and 10 prior to dietary Ca deprivation. After 30 days on the Ca-free diet, the control and PBNx rats were given five 24h test trials. Group WC. The lesion × trial interaction revealed that the main effect of lesion, trial, and the lesion × trial interaction was not significant, Fs < 1 (see Figure 3, left panel). In this study, thirty days of calcium deprivation failed to affect calcium intake in the Control or the PBNx rats in group WC. Group WN. Overall these rats increased intake of NaCl from terminal baseline (BL) to trial 1 and 2 (Figure 3, middle panel). This conclusion was supported by post hoc analysis of a significant main effect of trial, F(5,50) = 4.02, p < 0.004. The PBNx rats tended to consume more NaCl than Controls in group WN, but neither the main effect of lesion, F(1,10) = 1.91, p < 0.20, nor the lesion × trial interaction, F(5,50) = 1.36, p < 0.25, was significant. Group WCN. Once again, NaCl intake was augmented by 30 days on a calcium deficient diet for both the Control and the PBNx rats (Figure 3, right panel). In accordance, the main effect of solution was significant, F(1,10) = 9.6, p < 0.01, indicating that all rats, Control and PBNx alike, consumed more NaCl than CaCl2 overall. Further, post hoc tests of a significant solution × trial interaction, F(5,50) = 3.62, p < 0.007, found that, on trial 1, all rats consumed more NaCl than CaCl2, p < 0.05. Lesion condition, however, had no impact on the data as evidenced by a non-significant main effect of lesion, F(1,00) = 1.64, p > 0.05, or any interaction, Fs < 1.0
Figure 3.
Calcium and sodium intake after 30 days of calcium deprivation. Left panel: Intake (ml/24h) of 0.5 M CaCl2 by Control (open symbols) and PBNx rats (closed symbols in the water-CaCl2 (WC) condition. Middle panel: Intake (ml/24h) of 0.5 M NaCl by Control (open symbols) and PBNx (closed symbols) rats in the water-NaCl (WN) condition. Right panel: Intake (ml/24h) of 0.5 M CaCl2 and 0.5 M NaCl by Control (open symbols) and PBNx (closed symbols) rats in the water-CaCl2-NaCl (WCN) condition. * indicate significant differences in NaCl intake compared with baseline for both Controls and PBNx groups overall, p < 0.05. For the WCN group (Right Panel), on Trial 1 NaCl intake also differed significantly from CaCl2 consumption.
3.2.2 Experiment 2: Sodium appetite
3.2.2.1 Furosemide
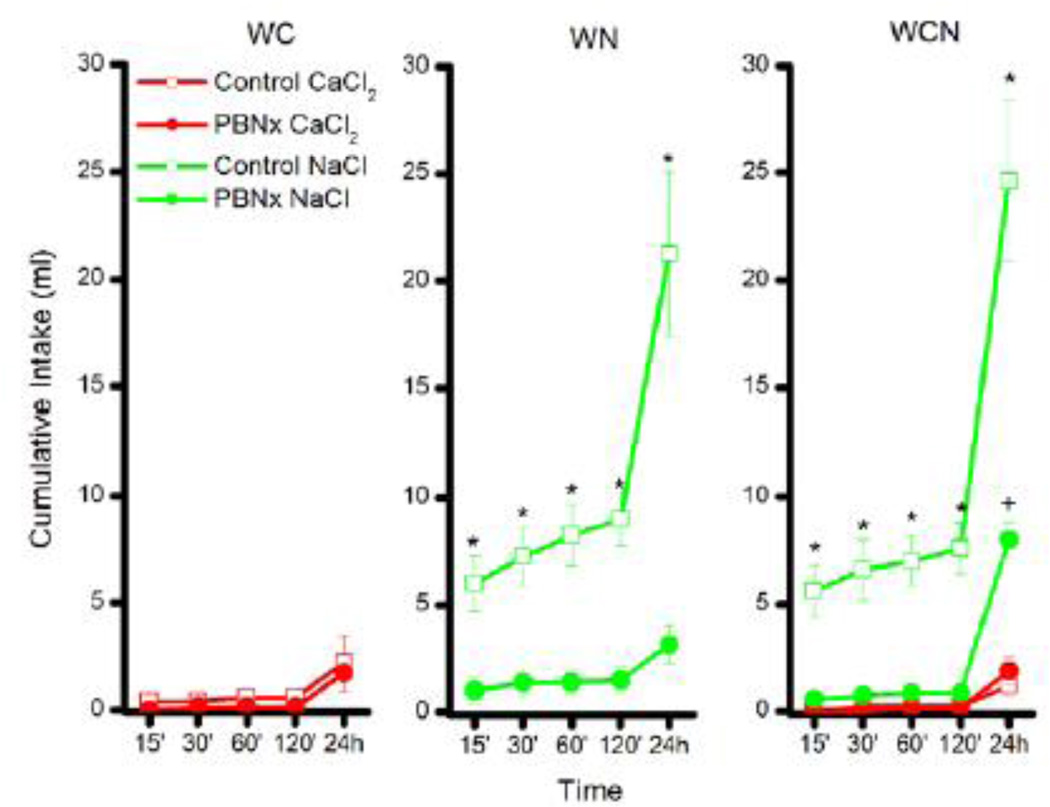
The rats were given access to water in all tests, but water intake differed little as a function of lesion or solution condition. Consequently, water intake data will not be addressed further. Body weight, analyzed at the start of the furosemide study, revealed a significant main effect of lesion, F(1,30) = 8.5, p < 0.007, with the PBNx rats (mean = 618.8 +/− 12.9) weighing less than the controls (mean = 679.3 +/− 16.2). There was no effect of stimulus group on body weight, F < 1, and no significant group × lesion interaction, F < 1. Intake of NaCl and CaCl2 across the 24 h test period following the Furo injection is shown in Figure 4 for the Control and PBNx rats in group WC, WN, and WCN.
Figure 4.
The effects of furosemide injections on intake of 0.5 M CaCl2 and 0.5 M NaCl for control and PBNX rats. Left panel: Intake (ml) of CaCl2 by Control (open symbols) and PBNx (closed symbols) rats in the water-CaCl2 (WC) condition. Middle panel: Intake (ml) of NaCl by Control (open symbols) and PBNx (closed symbols) rats in the water-NaCl (WN) condition. Right panel: Intake (ml) of CaCl2 and NaCl by Control (open symbols) and PBNx (closed symbols) rats in the water-CaCl2-NaCl (WCN) condition. * indicate significant differences in intake between Control and PBNx rats. + indicates a significant increase in NaCl intake for the PBNx rats in group WCN at the 24 h time point between the furosemide and the saline trial < 0.05.
Group WC. The furosemide-induced sodium appetite failed to increase intake of CaCl2 in either the Control or the PBNx rats over 24 h (Fig 4, left panel). This observation was confirmed by a non-significant lesion × time interaction, F < 1.0. There was, however, an increase in cumulative intake of CaCl2 from 15 min to 24 h, overall, as indicated by post hoc tests of a significant main effect of time, F (4,40) = 8.03, p < 0.0001. Even so, CaCl2 intake was negligible following the injection of furosemide (Control: 2.2 ml/24h; PBNx: 1.7 ml/24h). Likewise, intake of CaCl2 also was low for group WC when injected with saline (Control: 1.8 ml/24h; PBNx: 1.28 ml/24h).
Group WN. Analysis of the data for group WN (Fig 4, middle panel) revealed a significant main effect of lesion, F (1,10) = 32.82, p < 0.0002, and lesion × time interaction, F (4,40) = 37.0, p < 0.0001. Post hoc tests on the two-way interaction showed that Control rats consumed more NaCl than did PBNx rats at all time points tested, ps < 0.05. Further, while cumulative intake of NaCl increased from 15 min to 24 h for the Control rats, p < 0.05, it failed to significantly increase across the same time period for the PBNx subjects, p > 0.05. Finally, intake of NaCl by the PBNx subjects did not differ at the 24 h time point, whether injected with furosemide or saline (saline data not shown). This conclusion was confirmed by post hoc tests of a significant lesion × drug (saline vs. furosemide) interaction, F (1,10) = 41.9, p < 0.0001. Thus, while PBNx rats in the WN group increased consumption of NaCl when calcium deprived in Experiment 1, they failed to do so when sodium depleted in Experiment 2.
Group WCN. Results showed that the lesion × solution × time interaction was significant, F(4,40) = 12.5, p < 0.0001. Post hoc tests of this 3-way interaction revealed that Control rats treated with furosemide drank more NaCl than PBNx rats treated with furosemide beginning with the 15 min test and at all time points tested thereafter, ps < 0.05. At 24 h, intake of NaCl was increased in the furosemide-treated PBNx rats compared to intake at all earlier time points, but 24 h intake was significantly lower in PBNx rats (8 ml) compared to Controls (about 25 ml), p < 0.05. Interestingly, while 24 h NaCl intake by furosemide-treated PBNx rats was markedly reduced compared to furosemide-treated Control subjects, post hoc tests of a significant lesion × drug interaction, F (1,10) = 25.9, p < 0.0005, revealed that 24 h NaCl intake by PBNx rats in group WCN was significantly greater following the injection of furosemide (again about 8 ml) than following the subsequent injection of saline (about 1 ml), p < .05. Thus, PBNx rats in the WCN group exhibited a statistically significant, albeit greatly reduced, Na-appetite on the furosemide trial, that was not evident in the WN cohort. This may be due to slight differences in lesion placement between the cohorts or to the presence of the CaCl2 solution. No other effects of the saline injection were of note. Relative to NaCl, intake of CaCl2 was negligible for both the Control and the PBNx rats. Controls ingested only 1.2 ml/24h, p < 0.05, and the PBNx rats 1.9 ml/24h, p > 0.05. The furosemide-induced sodium appetite, then, was greatly and significantly impaired in the PBNx rats. This finding suggests that the increase in NaCl intake by these same subjects following calcium deprivation in Experiment 1 was not likely due to misplaced lesions.
3.2.3 Experiment 3: Conditioned Taste Aversion
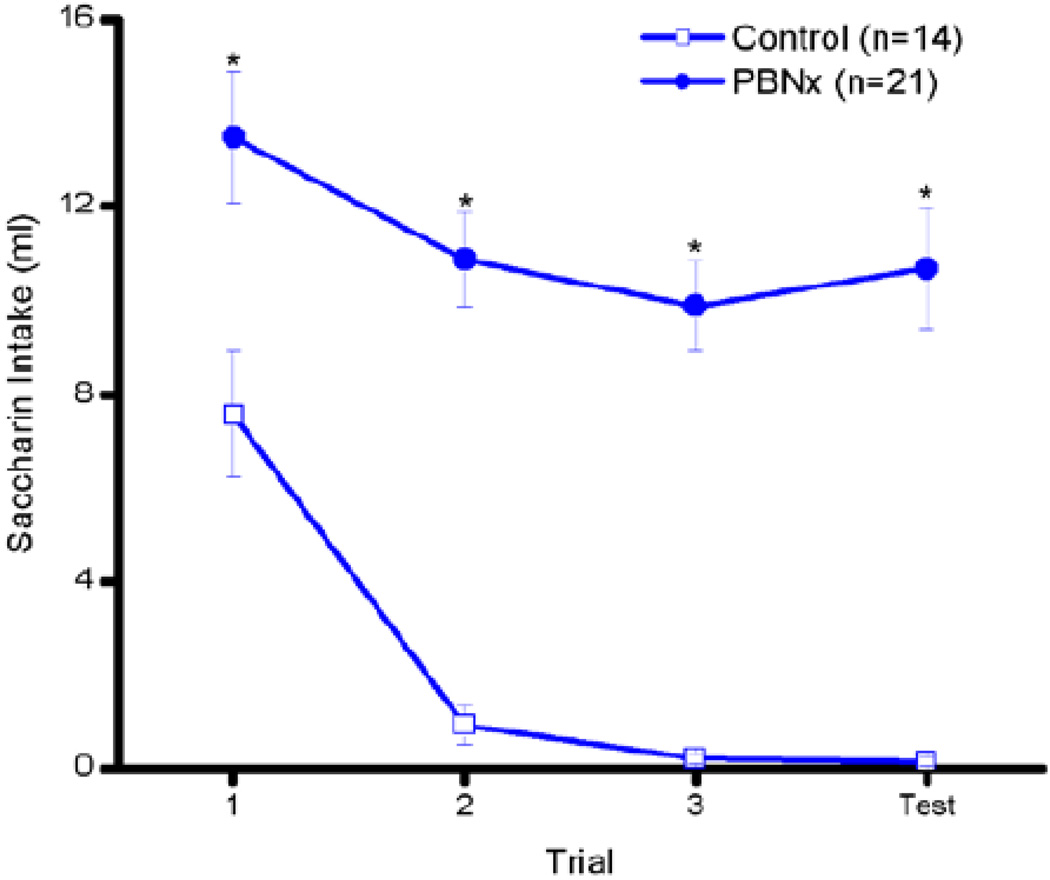
As discussed, disruption of a LiCl-induced conditioned taste aversion stands as a second highly reliable test of accurate PBN lesion placement. In the present case, despite 3 taste-LiCl pairings, the PBNx rats had significantly higher intakes of the saccharin cue overall (see Figure 5). In accordance, the main effect of lesion was statistically significant, F (1,33) = 71.9, p < 0.0001, confirming that the PBNx rats drank more of the LiCl-paired saccharin cue than the Control rats overall. The main effect of trial also was statistically significant, F (3,99) = 14.83, p < 0.0001. The lesion × trial interaction, however, only approached statistical significance, F (3,99) = 2.48, p < 0.065. Post hoc tests on this nonsignificant interaction revealed that, while the Control rats significantly reduced intake beginning with trial 2, ps < 0.05, the PBNx rats exhibited a reduction in saccharin intake only on trial 3 (compared with trial 1), p< 0.05, and the PBNx rats consumed more of the LiCl-paired saccharin cue than Controls on all trials, ps < 0.05. Interestingly, while a small furosemide-induced sodium appetite was evidenced in the PBNx rats in the WCN condition, this may have had more to do with the simultaneous availability of the CaCl2 solution than with slight differences in lesion placement, as the CTA was equally disrupted amongst the PBNx rats previously assigned to the WC, WN, or WCN conditions, all Fs < 1. Bilateral lesions of the PBN, then, greatly disrupted not only a furosemide-induced sodium appetite, as shown in Experiment 2, but also a LiCl-induced conditioned taste aversion.
Figure 5.
LiCl-induced conditioned taste aversion. Intake of 0.15% Na-saccharin by Control (open symbols) and PBNx (closed symbols) rats across three saccharin-LiCl pairings and one saccharin only test. * indicate trials in which there was a significant difference between PBNx intake and that of Controls, p < 0.05.
4. Discussion
Control and PBNx rats fed Ca-deficient diet for 30 days increased intake of 0.5 M NaCl but not 0.5 M CaCl2. In the Controls, the rise in NaCl intake was not unexpected, as previous reports demonstrated this effect following calcium deprivation in normal rats [10, 11, 13, 29, 30]. The finding was unexpected in the rats with PBN lesions. Rats with PBN lesions consistently fail to exhibit an appetite for 0.5 M NaCl when challenged with furosemide, DOCA, and even PEG [7]. Rats with PBN lesions also fail to exhibit an appetite for 0.3 M NaCl when made deficient with 10 days on a Na-deficient diet [31]. This failure of the PBN lesion to disrupt the Ca-deprivation-induced sodium appetite cannot be attributed to misplaced lesions because the same rats failed to exhibit a furosemide-induced appetite for 0.5 M NaCl, a LiCl-induced conditioned taste aversion, or both. These latter two deficits are characteristic of accurate PBN lesion placement [see 4, 5, 6, 24, 25].
Rats placed on a calcium deficient diet [8–11] or parathyroidectomized ingest calcium [12–14]. In the present experiment, however, none of the animals evinced a Ca appetite. This finding may have been due, at least in part, to age. Potent calcium appetite (e.g., intake up to 60 ml/h of 0.3 M CaCl2 when sham feeding) was observed when the 3 – 6 week period of Ca deprivation began at about 3 – 4 weeks of age [15, 16]. The rats in this earlier study were typically about 200g or so when depletion began. In contrast, our rats were comparatively quite old. They weighed between 302 to 456 g at the time of surgery and they participated in an oil-LiCl conditioned aversion study thereafter. These data suggest there may be a critical period during which calcium deficiency can support the development of a calcium appetite. Alternatively, the failure to evidence a Ca appetite may have been due to the use of a high concentration of CaCl2. The 0.5 M CaCl2 solution is completely avoided by rats. Further, in another study rats exhibited strongly aversive taste reactivity behavior following the intraoral infusion of 0.3 M CaCl2 [16]. Thus, our failure to obtain a calcium appetite in the current experiments may have reflected the age of the rats, the use of a strong 0.5 M CaCl2 solution, or both.
In contrast, the Ca-deprivation-induced sodium appetite persisted, despite the use of an otherwise aversive 0.5 M NaCl solution. Moreover, this sodium appetite was evident in both the Control and the PBNx rats. In the earlier study that used younger animals, the intake of sodium was slightly higher and more sustained than in the present report [15]. This difference may be due to a number of factors including adolescent exposure to Ca deficiency or to their use of a 0.3 M rather than a 0.5 M NaCl solution (7). Regardless, in the present experiments both the Ca-deprived Control and the PBNx rats exhibited a substantial sodium appetite. This finding is of interest because, as mentioned, rats with PBN lesions fail to exhibit a sodium appetite following furosemide induced body Na+ depletion [32], following direct [33, 34] and indirect [35] stimulation with DOCA treatment, and following PEG induced plasma volume deficits [4, 6, 7]. Thus, as suggested [17], if NaCl intake serves to raise ionized calcium in calcium-deficient rats, rats with PBN lesions apparently remain sensitive to this effect.
Our data also demonstrate that, after Ca deprivation, the ingestion of sodium salts by normal rats is not due to their inability to distinguish Ca from Na. Intake of 0.5 M Ca is selectively negligible and published data show that rats with PBN lesions can taste NaCl across a range of concentrations [36]. Under a number of conditions, both salts are ingested by calcium deprived rats (e.g. [10]). The ingestion is motivated because calcium deprived rats will bar press for sodium as well as calcium salts [37]. When Ca deprived, it would be interesting to know how rats respond to the two salts on progressive ratio schedules. Our experiments do not speak to this issue or, more generally, to the neural mechanisms by which Ca deprivation induces motivated behavior.
Two mechanisms have been suggested for why normally aversive concentrations of sodium are ingested after Ca deprivation. One is physiological; raising serum Na produces a short term increase in serum Ca [15]. The other is evolutionary; sources of sodium are more common in the environment but they frequently contain other salts including Ca [38]. The two possibilities are not mutually exclusive. One produces a short-term increase in circulating Ca, the other an actual increase in body Ca. In both cases ingesting the Na salt would relieve the symptoms of Ca deficiency and thus result in a learned preference for that stimulus. This mechanism was first described by Rozin for thiamine deficiency [39]. The sodium appetite that results from Na depletion, on the other hand, appears to be innate [30, 38, 40]. If true, this distinction could provide one avenue for exploring why Ca-deprived PBNx rats demonstrate a Na appetite but when Na depleted, they do not.
Baseline intake of 0.5 M NaCl revealed yet another deficit and competency. Whether in group WN (water and NaCl offered) or WCN (water, CaCl2, and NaCl offered), the rats with lesions of the PBN initially over-consumed 0.5 M NaCl compared with controls. Indeed, the PBNx rats initially consumed as much salt as water. This finding is consistent with other reports in which rats with PBN lesions over-consume various solutions including capsaicin [41], 0.3 M sucrose, and 100% corn oil [42]. Of course, in the absence of salt need, 0.5 M NaCl is not only aversive, but potentially lethal (unpublished observations in PBNx rats). Here, the PBNx rats were able to detect the solution, distinguish it from water, and begin to avoid it during the second 24 h access period. Thus, while the PBNx rats initially over-consumed half molar salt, they began to avoid it perhaps because of its osmotic effects in the gastrointestinal system.
Summary
Neither the Control nor the PBNx rats exhibited a calcium appetite following 30 d on a calcium-deplete diet. This null effect may be due to the age at which the subjects were calcium deprived, to the concentration of the CaCl2 solution employed, or both. Nevertheless, all rats, Control and PBNx alike, exhibited a reliable sodium appetite following exposure to the calciumdeplete diet. This observation replicates earlier findings in non-lesioned subjects, but was unexpected in rats with PBN lesions, as these rats fail to exhibit an appetite for 0.5 M NaCl following treatment with furosemide or DOCA. Finally, when Na-replete, the PBN lesioned rats over-consumed half molar salt upon the first exposure, but reduced intake subsequently.
Highlights.
Controls and rats with parabrachial lesions (PBNx) failed to ingest 0.5 M CaCl2 after extended dietary calcium depletion.
Both groups did ingest significantly more 0.5 M NaCl after the same calcium depletion.
Following sodium depletion with a diuretic, the PBNx rats ingested only one-third as much 0.5 M NaCl as the Controls.
Damage to the second central gustatory relay in the parabrachial nuclei disrupts an appetite for 0.5 M NaCl in sodium but not calcium depleted rats.
Acknowledgements
Han Li performed the surgery, Nelli Horvath collected the data, Kathy Matyas and Nelli Horvath did the histology, and Erin Handly assisted with Fig. 1. They each have our thanks. We also thank Sarah Ballard for her careful editorial assistance. Supported by NIH DC05435, DC00240, DA012473 and DA009815.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
P.S. Grigson, Email: psg6@psu.edu.
E.M. Colechio, Email: emc231@psu.edu.
M.L. Power, Email: mpower@acog.org.
J. Schulkin, Email: Jschulkin@acog.org.
R. Norgren, Email: rxn5@psu.edu.
References
- 1.Pfaffmann D. The sense of taste. In: Code CF, editor. Handbook of Physiology. Washington, DC: American Physiologic Society; 1959. pp. 507–533. [Google Scholar]
- 2.Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lectrues. 1942;38:63–102. [Google Scholar]
- 3.Lundy RF, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. 3rd ed. San Diego, CA: Elsevier Academic Press; 2004. pp. 891–921. [Google Scholar]
- 4.Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- 5.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci. 1992;106:147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci. 1995;109:997–1008. [PubMed] [Google Scholar]
- 7.Stricker EM, Grigson PS, Norgren R. Variable effects of parabrachial nucleus lesions on salt appetite in rats depending upon experimental paradigm and saline concentration. Behav Neurosci. 2013;127:275–284. doi: 10.1037/a0031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers WL. Specificity of specific hungers. J Comp Physiol Psychol. 1967;64:49–58. doi: 10.1037/h0024802. [DOI] [PubMed] [Google Scholar]
- 9.Scott EM, Verney EL, Morissey PD. Self selection of diet; appetites for sodium, chloride and sodium chloride. J Nutr. 1950;41:173–201. doi: 10.1093/jn/41.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Tordoff MG, Ulrich PM, Schulkin J. Calcium deprivation increases salt intake. Am J Physiol. 1990;259:R411–R419. doi: 10.1152/ajpregu.1990.259.3.R411. [DOI] [PubMed] [Google Scholar]
- 11.Schulkin J. The appetite for salts in mineral and vitamin deficient rats. Federation Proceedings. 1981;40:292. (abstract). [Google Scholar]
- 12.Richter CP, Eckert JF. Mineral appetite of parathyroidectomized rats. N AM J Med Sci. 1938;198:9–16. [Google Scholar]
- 13.Lewis M. Behavior Resulting from Calcium Deprivation in Parathyroidectomized Rats. J Comp Physiol Psychol. 1964;57:348–352. doi: 10.1037/h0044487. [DOI] [PubMed] [Google Scholar]
- 14.Leshem M, Del Canho S, Schulkin J. Calcium hunger in the parathyroidectomized rat is specific. Physiol Behav. 1999;67:555–559. doi: 10.1016/s0031-9384(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 15.McCaughey SA, Tordoff MG. Calcium-deprived rats sham-drink CaCl2 and NaCl. Appetite. 2000;34:305–311. doi: 10.1006/appe.1999.0317. [DOI] [PubMed] [Google Scholar]
- 16.McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solutions in rats. Physiol Behav. 2005;84:335–342. doi: 10.1016/j.physbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Tordoff MG. NaCl ingestion ameliorates plasma indexes of calcium deficiency. Am J Physiol. 1997;273:R423–R432. doi: 10.1152/ajpregu.1997.273.1.R423. [DOI] [PubMed] [Google Scholar]
- 18.Cherukuri CM, McCaughey SA, Tordoff MG. Comparison of differences between PWD/PhJ and C57BL/6J mice in calcium solution preferences and chorda tympani nerve responses. Physiol Behav. 2011;102:496–502. doi: 10.1016/j.physbeh.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Tordoff MG. Calcium deficiency alters chorda tympani nerve responses to oral calcium chloride. Physiol Behav. 1998;63:297–303. doi: 10.1016/s0031-9384(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 20.McCaughey SA, Tordoff MG. Calcium deprivation alters gustatory-evoked activity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2001;281:R971–R978. doi: 10.1152/ajpregu.2001.281.3.R971. [DOI] [PubMed] [Google Scholar]
- 21.Emmers R, Nocenti MR. Role of thalamic gustatory nucleus in diet selection by normal and parathyroidectomized rats. Proc Soc Exp Biol Med. 1967;125:1264–1270. doi: 10.3181/00379727-125-32331. [DOI] [PubMed] [Google Scholar]
- 22.Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173:1136–1139. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- 23.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol. 1973;150:217–237. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 24.Grigson PS, Reilly S, Scalera G, Norgren R. The parabrachial nucleus is essential for acquisition of a conditioned odor aversion in rats. Behav Neurosci. 1998;112:1104–1113. [PubMed] [Google Scholar]
- 25.Sclafani A, Azzara AV, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci. 2001;115:920–933. doi: 10.1037/0735-7044.115.4.920. [DOI] [PubMed] [Google Scholar]
- 26.Wolf G. Refined salt appetite methodology for rats demonstrated by assessing sex differences. J Comp Physiol Psychol. 1982;96:1016–1021. [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 5th ed. New York: Elsevier Academic Press; 2005. [Google Scholar]
- 28.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- 29.Tordoff MG. Calcium: taste, intake, and appetite. Physiol Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- 30.Schulkin J. Calcium Hunger. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 31.Scalera G, Norgren R. Taste functions and Na+-appetite after excitotoxic lesions of the parabrachial nuclei in rat. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and Tast XI. Tokyo: Springer Verlag; 1994. p. 533. [Google Scholar]
- 32.Jalowiec JE. Sodium appetite elicited by furosemide: effects of differential dietary maintenance. Behav Biol. 1974;10:313–327. doi: 10.1016/s0091-6773(74)91914-2. [DOI] [PubMed] [Google Scholar]
- 33.Geerling JC, Loewy AD. Aldosterone-sensitive NTS neurons are inhibited by saline ingestion during chronic mineralocorticoid treatment. Brain Res. 2006;1115:54–564. doi: 10.1016/j.brainres.2006.07.091. [DOI] [PubMed] [Google Scholar]
- 34.Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol. 1986;250:R267–R275. doi: 10.1152/ajpregu.1986.250.2.R267. [DOI] [PubMed] [Google Scholar]
- 36.Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci. 1995;109:939–954. [PubMed] [Google Scholar]
- 37.Lewis M. Discrimination between drives for sodium chloride and calcium. J Comp Physiol Psychol. 1968;65:208–212. doi: 10.1037/h0025551. [DOI] [PubMed] [Google Scholar]
- 38.Schulkin J. Sodium Hunger: The Search for a Salty Taste. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 39.Rozin P. Specific hunger for thiamine: Recovery from deficiency and thiamine preference. J Comp Physiol Psychol. 1965;59:98–101. doi: 10.1037/h0021612. [DOI] [PubMed] [Google Scholar]
- 40.Wolf G. Innate mechanisms for regulation of sodium appetite. In: Pfaffmann C, editor. Olfaction and Taste: Proceedings of the Third International Symposium. New York: The Rockafeller University Press; 1969. pp. 548–553. [Google Scholar]
- 41.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- 42.Liang NC, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: II. Sucrose and corn oil conditioned aversions. Physiol Behav. 2012;105:589–594. doi: 10.1016/j.physbeh.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]