Abstract
OBJECTIVE
To estimate prevalence of childhood-onset Duchenne and Becker muscular dystrophies (DBMD) in 6 sites in the United States by race/ethnicity and phenotype (Duchenne muscular dystrophy [DMD] or Becker muscular dystrophy [BMD]).
METHODS
In 2002, the Centers for Disease Control and Prevention established the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) to conduct longitudinal, population-based surveillance and research of DBMD in the United States. Six sites conducted active, multiple-source case finding and record abstraction to identify MD STARnet cases born January 1982 to December 2011. We used cross-sectional analyses to estimate prevalence of DBMD per 10 000 boys, ages 5 to 9 years, for 4 quinquennia (1991–1995, 1996–2000, 2001–2005, and 2006–2010) and prevalence per 10 000 male individuals, ages 5 to 24 years, in 2010. Prevalence was also estimated by race/ethnicity and phenotype.
RESULTS
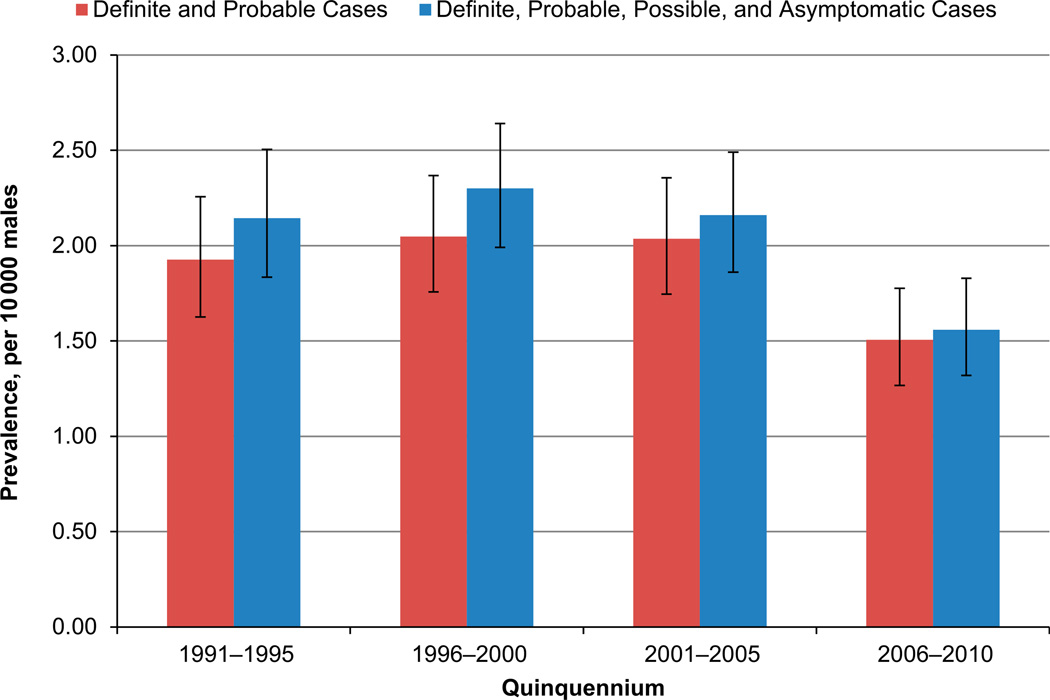
Overall, 649 cases resided in an MD STARnet site during $1 quinquennia. Prevalence estimates per 10 000 boys, ages 5 to 9 years, were 1.93, 2.05, 2.04, and 1.51, respectively, for 1991–1995, 1996–2000, 2001–2005, and 2006–2010. Prevalence tended to be higher for Hispanic individuals than non-Hispanic white or black individuals, and higher for DMD than BMD. In 2010, prevalence of DBMD was 1.38 per 10 000 male individuals, ages 5 to 24 years.
CONCLUSIONS
We present population-based prevalence estimates for DBMD in 6 US sites. Prevalence differed by race/ethnicity, suggesting potential cultural and socioeconomic influences in the diagnosis of DBMD. Prevalence also was higher for DMD than BMD. Continued longitudinal surveillance will permit us to examine racial/ethnic and socioeconomic differences in treatment and outcomes for MD STARnet cases.
Duchenne and Becker muscular dystrophies (DBMD) are allelic X-linked neuromuscular disorders. Worldwide, prevalence of DBMD has ranged from 0.1 to 1.8 per 10 000 male individuals.1–7 Studies of Duchenne muscular dystrophy (DMD) prevalence (per 10 000 male individuals) suggest estimates of 0.1 in South Africa,1 0.5 to 1.0 in Asian countries,2,3,8,9 0.7 to 1.0 in North America,5,10 and 0.2 to 2.8 in European countries.4,6,7,11–19 Some of these studies also examined Becker muscular dystrophy (BMD) prevalence, suggesting corresponding estimates of 0.01,1 0.1 to 0.2,2,3 0.2,5 and 0.1 to 0.7,4,6,7 respectively. A recent meta-analysis of worldwide prevalence of muscular dystrophies suggests prevalences of DMD and BMD as 0.5 and 0.1 per 10 000 male individuals, respectively.20 Differences in prevalence may reflect changes in diagnostic methods, along with racial/ethnic variations and age ranges of the populations studied.
Current clinical management, such as steroid use,21 ventilatory assistance,22–24 and scoliosis surgery,25 has improved survival for patients with DBMD. This improved survival is accompanied by new multisystem complications and the need for long-term care.26 Appropriate management is predicted to improve quality of life for patients and their families. For example, families with an affected child require more support/social services to help cope with caring for the child and the concomitant increase in health care costs.27,28 Improved understanding of overall and age-specific prevalence of DBMD is critical to help plan for medical and social services, particularly as children transition from pediatric to adult services, and to evaluate the impact of life-prolonging therapies.
In 2002, the Centers for Disease Control and Prevention established the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) to implement population-based surveillance and research for DBMD. In 2004, the MD STARnet began active, population-based surveillance. Previously, we reported preliminary point prevalence for 2007 by using data from 4 MD STARnet sites29; before our report, the most recent US prevalence estimates were published in 1974.30 In our current report, we present data from all MD STARnet sites through 2010 and estimate population-based prevalence for DBMD by age, race/ethnicity, and phenotype.
METHODS
The MD STARnet is a US, multisite population-based cohort for surveillance and research of DBMD.31 Sites comprise Arizona, Colorado, Georgia, Hawaii, Iowa, and the western 12 counties of New York State (henceforth termed New York). Public health authority for birth defects surveillance in Colorado, Georgia, Iowa, and New York was expanded to permit active case finding and record abstraction for DBMD; in Arizona and Hawaii, institutional review board approval was obtained for these activities from the University of Arizona and the Hawaii Department of Health, respectively, and as needed, health care facilities where data collection occurred.
Surveillance Data Collection
An eligible MD STARnet case had a birthdate on or after January 1, 1982, and on or before December 31, 2011, resided in an MD STARnet site during any part of that time period, and was diagnosed with childhood-onset DBMD. As described elsewhere,31 case finding was conducted in medical records (eg, neuromuscular clinics, hospitals) by using the International Classification of Diseases, Ninth Revision, Clinical Modification code 359.1 (hereditary progressive muscular dystrophy) and in death certificates by using International Classification of Diseases, 10th Revision, Clinical Modification code G71.0 (muscular dystrophy). For each case, demographic and clinical data were systematically abstracted from available medical records by using the MD STARnet surveillance instrument.
Selected, de-identified clinical data for each case were reviewed by a neuromuscular physician at each site and assigned an MD STARnet case definition (definite, probable, possible, asymptomatic, female) (Supplemental Table 4).32 If the individual reviews were concordant, the case was assigned to the consensus definition; if discordant, they were discussed by all physicians to generate a consensus definition.
Surveillance data collection in Arizona, Colorado, Iowa, and New York began in 2004; data collection in Georgia and Hawaii began in 2006 and 2010, respectively. For eligible cases, abstractors conducted retrospective longitudinal abstraction of medical records from the diagnosis date of DBMD until death, migration out of the site, or the start of prospective data collection (2004, 2006, or 2010) in a site. Cases who continued to reside in a site after the start of data collection were abstracted annually thereafter (eg, 2005–2012 for Arizona, Colorado, Iowa, and New York) until death or out-migration to obtain updated residence, diagnostic, comorbidity, and treatment data. Additional data obtained for a probable, possible, or asymptomatic case were re-reviewed to determine if the case definition needed revision. Newly diagnosed cases were enumerated prospectively and followed annually until death or out-migration.
Study Sample
MD STARnet surveillance data comprise 1054 cases. After excluding all elective terminations (n = 4), female (n = 9) and male individuals born before January 1, 1986, or after December 31, 2005 (n = 196), our sample included 845 (707 definite, 58 probable, 75 possible, 5 asymptomatic) male cases (Table 1). This birth period permitted follow-up to at least age 5 years (average age of diagnosis)33 and, because DBMD is rare, estimation of prevalence by quinquennia (ie, 5-year intervals: 1991–1995, 1996–2000, 2001–2005, 2006–2010) that corresponded to available census and midcensus data.
TABLE 1.
Selected Characteristics of Male Cases Identified by MD STARnet, 1991–2010
| All | Arizona | Colorado | Georgia | Hawaii | Iowa | New Yorka | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Case status | 845 | 182 | 176 | 261 | 23 | 101 | 102 | |||||||
| Definite | 707 | 83.7 | 165 | 90.7 | 158 | 89.8 | 195 | 74.7 | 12 | 52.2 | 95 | 94.1 | 82 | 80.4 |
| Probable | 58 | 6.9 | 6 | 3.3 | 4 | 2.3 | 25 | 9.6 | 6 | 26.1 | 3 | 3.0 | 14 | 13.7 |
| Possible | 75 | 8.9 | 10 | 5.5 | 11 | 6.3 | 41 | 15.7 | 5 | 21.7 | 3 | 3.0 | 5 | 4.9 |
| Asymptomatic | 5 | 0.6 | 1 | 0.5 | 3 | 1.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| Definite and probable cases | 765 | 171 | 162 | 220 | 18 | 98 | 96 | |||||||
| Race/ethnicity | ||||||||||||||
| Non-Hispanic white | 441 | 57.6 | 72 | 42.1 | 89 | 54.9 | 140 | 63.6 | 1 | 5.6 | 70 | 71.4 | 69 | 71.9 |
| Non-Hispanic black | 57 | 7.5 | 2 | 1.2 | 5 | 3.1 | 41 | 18.6 | 0 | 0.0 | 4 | 4.1 | 5 | 5.2 |
| Non-Hispanic AIAN | 3 | 0.4 | 3 | 1.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Non-Hispanic API | 20 | 2.6 | 0 | 0.0 | 2 | 1.2 | 6 | 2.7 | 7 | 38.9 | 4 | 4.1 | 1 | 1.0 |
| Non-Hispanic Other | 27 | 3.5 | 13 | 7.6 | 5 | 3.1 | 4 | 1.8 | 3 | 16.7 | 1 | 1.0 | 1 | 1.0 |
| Hispanic | 157 | 20.5 | 63 | 36.8 | 50 | 30.9 | 29 | 13.2 | 1 | 5.6 | 9 | 9.2 | 5 | 5.2 |
| Unknown | 60 | 7.8 | 18 | 10.5 | 11 | 6.8 | 0 | 0.0 | 6 | 33.3 | 10 | 10.2 | 15 | 15.6 |
| Phenotype | ||||||||||||||
| DMD | 543 | 71.0 | 124 | 72.5 | 108 | 66.7 | 149 | 67.7 | 13 | 72.2 | 65 | 66.3 | 84 | 87.5 |
| BMD | 219 | 28.6 | 46 | 26.9 | 54 | 33.3 | 70 | 31.8 | 5 | 27.8 | 32 | 32.7 | 12 | 12.5 |
| Unclassified | 3 | 0.4 | 1 | 0.6 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 |
| No. of cases/family | ||||||||||||||
| 1 | 601 | 78.6 | 130 | 76.0 | 119 | 73.5 | 181 | 82.3 | 16 | 88.9 | 77 | 78.6 | 78 | 81.3 |
| ≥2 | 164 | 21.4 | 41 | 24.0 | 43 | 26.5 | 39 | 17.7 | 2 | 11.1 | 21 | 21.4 | 18 | 18.8 |
AIAN, American Indian or Native American; API, Asian or Pacific Islander.
Includes 12 counties in western New York State.
Case race/ethnicity was assigned as recorded in medical record data, or if missing, birth parent data from birth certificates or medical records. If recorded maternal and paternal race/ethnicity matched (eg, both listed as Hispanic), the case was imputed by that value; if it differed (eg, one listed as Hispanic, the other as non-Hispanic white), the case was assigned as multiple race/ethnicity. If only maternal race/ethnicity was available, the case was assigned that value; missing maternal data generated missing case race/ethnicity.
Residence at diagnosis and at each subsequent identified clinic visit was used to systematically assign case annual residence from birth year through December 31, 2010. Cases who remained in an MD STARnet site from the previous calendar year or who moved into the site, died, or migrated out during a calendar year were assigned residence in that site for that year. Cases that relocated from one site to another were assigned residence in the most recent site the year after migration to maintain independent observations per year. Cases with 2 or more years between identified clinic visits in a site were assigned residence in that site for those years; those without an identified clinic visit for 3 or more years after their most recent identified clinic visit were censored in the year of their most recent identified visit.
Phenotype was assigned based on age of first symptoms. If the earliest MD symptoms reportedly occurred before the fifth birthday, the case was assigned as DMD. If symptoms occurred on or after the fifth birthday, the case was assigned as BMD.34
Survey Data Collection
Beginning in 2007, primary caregivers (in priority order: birth mother, birth father, legal guardian) of definite and probable cases were invited to participate in a telephone survey to supplement data (eg, socioeconomic factors, social support) collected by medical record abstraction.35 Data collection was conducted in 2 cycles. The first spanned April 2007 to May 2008 and included caregivers from Arizona, Colorado, Iowa, and New York. The second spanned April 2009 to March 2012 and included additional caregivers from these sites, as well as those from Georgia and Hawaii. For cases born 1986 to 2005, interviews were conducted with 298 (53% of eligible) caregivers (birth mothers = 258, 86.6%), which represented 298 cases and 38 affected male siblings. Survey data collection was approved by the institutional review board at each site.
Statistical Analysis
We calculated case frequency distributions by MD STARnet site (Arizona, Colorado, Georgia, Hawaii, Iowa, New York), case definition (definite, probable, possible, asymptomatic), race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic American Indian or Native American, non-Hispanic Asian or Pacific Islander, non-Hispanic other, Hispanic, unknown), phenotype (DMD, BMD), and cases per household (1, 2 or more); frequency distributions also were calculated for total male residents by site and race/ethnicity. For definite and probable cases combined, we explored changes in prevalence over time by calculating estimates for several quinquennia as follows:
We defined a case as a resident during a quinquennium if he was identified as a resident for at least part of the quinquennium. Census data for a particular quinquennium were those published 5 years after the end of the quinquennium (eg, 2005 estimates were used for 1996–2000). Population estimates for midcensus (1995 and 2005) and census (2000 and 2010) data were those estimated for July 1 of the respective year. We also estimated prevalence of DBMD in 2010 defined as follows:
We used the 2010 census estimates for male individuals, ages 5 to 9, 10 to 14, 15 to 19, and 20 to 24 years to estimate prevalence for each age group. Additionally, we estimated prevalence by the 3 largest racial/ethnic groups (non-Hispanic white, non-Hispanic black, and Hispanic), phenotype, and site; Hawaii was excluded from subgroup analyses because of having identified only 2 cases from these racial/ethnic groups. The Poisson approximation to the binomial distribution was used to calculate 95% confidence intervals.
We conducted 3 sensitivity analyses. We compared residence histories generated from surveillance data with self-reports from the telephone surveys to evaluate the use of available medical records to generate residence histories. We also estimated prevalence including possible and asymptomatic cases to examine potential bias introduced by variability in the quality and availability of clinical data among sites. Last, we estimated prevalence restricting the sample to the oldest case in each family to discern the impact that families with more than 1 case contributed to prevalence.
RESULTS
Overall, 707 (83.7%) of the 845 pooled cases (ie, all sites combined) were classified as definite (Table 1); case status for 82.0% of definite cases was based on DNA analysis demonstrating a dystrophin mutation (data not shown). Among the 765 definite and probable cases, most were non-Hispanic white (57.6%), diagnosed with DMD (71.0%), and the only case in the family (78.6%); this pattern was observed in each site except for race/ethnicity in Hawaii.
Among boys, ages 5 to 9 years, pooled prevalence for DBMD was 1.93 in 1991–1995, 2.05 in 1996–2000, 2.04 in 2001–2005, and 1.51 in 2006–2010 (Table 2). Racial/ethnic-specific prevalence was highest for Hispanic individuals in each quinquennium, except for 2006–2010, and lowest for non-Hispanic black individuals. Prevalence for DMD exceeded that of BMD in each quinquennium. Analysis of site-specific prevalence by race/ethnicity tended to parallel that for pooled prevalence, although meaningful comparisons were limited for non-Hispanic black and Hispanic cases due to single or zero counts; site-specific estimates by phenotype also showed similar patterns to those for all sites combined, but cell sizes limited meaningful comparisons for BMD (data not shown).
TABLE 2.
Prevalence of Definite and Probable Male Cases (n = 649) per 10 000 Boys, Ages 5 to 9 Years, Identified by MD STARnet, 1991–2010
| Quinquennium | Cases | Population | Prevalence | 95% CI | |
|---|---|---|---|---|---|
| Definite and probable cases | 1991–1995 | 150 | 778 716 | 1.93 | (1.63–2.26) |
| 1996–2000 | 178 | 869 321 | 2.05 | (1.76–2.37) | |
| 2001–2005 | 179 | 879 300 | 2.04 | (1.75–2.36) | |
| 2006–2010 | 142 | 942 791 | 1.51 | (1.27–1.78) | |
| Race/ethnicity | |||||
| Non-Hispanic white | 1991–1995 | 98 | 538 981 | 1.82 | (1.48–2.22) |
| 1996–2000 | 108 | 537 239 | 2.01 | (1.65–2.43) | |
| 2001–2005 | 105 | 512 206 | 2.05 | (1.68–2.48) | |
| 2006–2010 | 88 | 504 201 | 1.75 | (1.40–2.15) | |
| Non-Hispanic black | 1991–1995 | 9 | 121 965 | 0.74 | (0.34–1.40) |
| 1996–2000 | 16 | 139 199 | 1.15 | (0.66–1.87) | |
| 2001–2005 | 13 | 135 329 | 0.96 | (0.51–1.64) | |
| 2006–2010 | 10 | 144 476 | 0.69 | (0.33–1.27) | |
| Hispanic | 1991–1995 | 28 | 88 570 | 3.16 | (2.10–4.57) |
| 1996–2000 | 38 | 141 042 | 2.69 | (1.91–3.70) | |
| 2001–2005 | 38 | 176 768 | 2.15 | (1.52–2.95) | |
| 2006–2010 | 27 | 220 040 | 1.23 | (0.81–1.79) | |
| Phenotype | |||||
| DMD | 1991–1995 | 117 | 778 716 | 1.43 | (1.17–1.72) |
| 1996–2000 | 127 | 869 321 | 1.46 | (1.22–1.74) | |
| 2001–2005 | 141 | 879 300 | 1.60 | (1.35–1.89) | |
| 2006–2010 | 111 | 942 791 | 1.18 | (0.97–1.42) | |
| BMD | 1991–1995 | 39 | 778 716 | 0.50 | (0.36–0.68) |
| 1996–2000 | 50 | 869 321 | 0.58 | (0.43–0.76) | |
| 2001–2005 | 37 | 879 300 | 0.42 | (0.30–0.58) | |
| 2006–2010 | 30 | 942 791 | 0.32 | (0.21–0.45) |
CI, confidence interval.
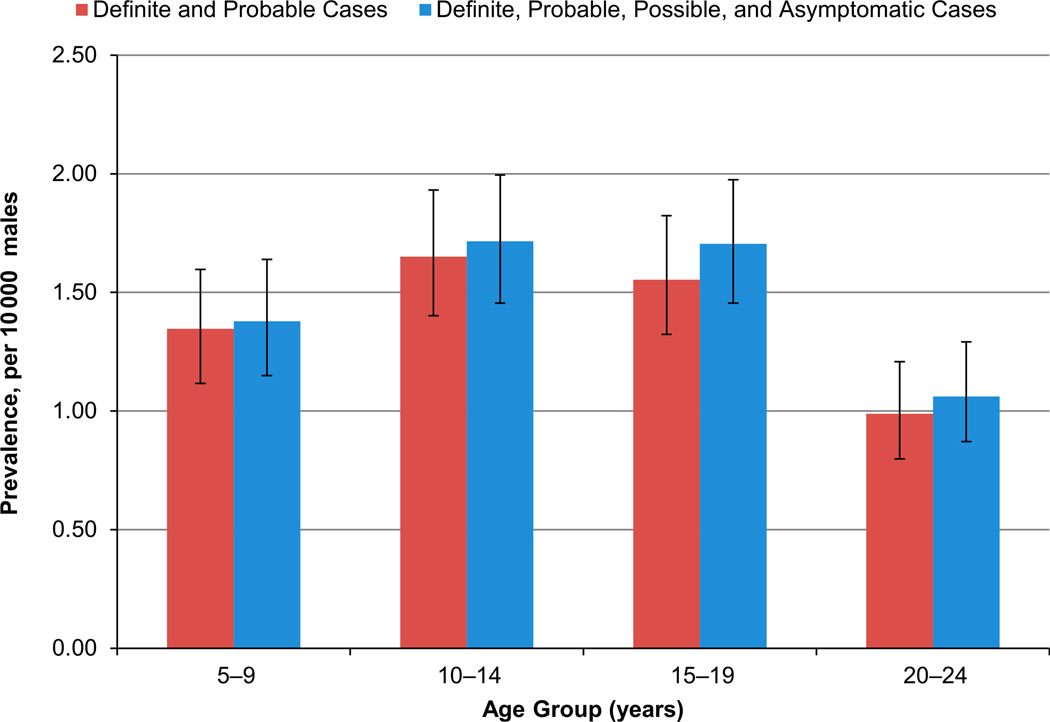
In 2010, pooled prevalence for the 530 definite and probable cases was 1.38 per 10 000; estimates ranged from 0.99 among cases ages 20 to 24 years to 1.65 among those ages 10 to 14 years (Table 3). Racial/ethnic-specific prevalence for all age groups combined was 0.63 for non-Hispanic black individuals, 1.45 for non-Hispanic white individuals, and 1.50 for Hispanic individuals. Estimates for Hispanic individuals exceeded those for non-Hispanic white individuals for all but the youngest age group; those for non-Hispanic black individuals were consistently the lowest for each age group. Prevalence for all age groups combined was 1.02 for DMD and 0.36 for BMD; estimates were lowest for DMD among the oldest age group, whereas the reverse was observed for BMD.
TABLE 3.
Prevalence in 2010 of Definite and Probable Male Cases (n = 530) per 10 000 Male Individuals, Ages 5 to 24 Years, Identified by MD STARnet, 1991–2010
| Age Group, y | Cases | Population | Prevalence | 95% CI | |
|---|---|---|---|---|---|
| Definite and probable cases | |||||
| All | 530 | 3 827 532 | 1.38 | (1.27–1.51) | |
| 20–24 | 95 | 960 866 | 0.99 | (0.80–1.21) | |
| 15–19 | 153 | 985 263 | 1.55 | (1.32–1.82) | |
| 10–14 | 155 | 938 612 | 1.65 | (1.40–1.93) | |
| 5–9 | 127 | 942 791 | 1.35 | (1.12–1.60) | |
| Race/ethnicity | |||||
| Non-Hispanic white | All | 312 | 2 147 039 | 1.45 | (1.30–1.62) |
| 20–24 | 61 | 564 779 | 1.08 | (0.83–1.39) | |
| 15–19 | 90 | 558 178 | 1.61 | (1.30–1.98) | |
| 10–14 | 85 | 519 881 | 1.63 | (1.31–2.02) | |
| 5–9 | 76 | 504 201 | 1.51 | (1.19–1.89) | |
| Non-Hispanic black | All | 38 | 605 232 | 0.63 | (0.44–0.86) |
| 20–24 | 4 | 143 512 | 0.28 | (0.08–0.71) | |
| 15–19 | 14 | 166 175 | 0.84 | (0.46–1.41) | |
| 10–14 | 10 | 151 069 | 0.66 | (0.32–1.22) | |
| 5–9 | 10 | 144 476 | 0.69 | (0.33–1.27) | |
| Hispanic | All | 120 | 797 913 | 1.50 | (1.25–1.80) |
| 20–24 | 20 | 185 830 | 1.08 | (0.66–1.66) | |
| 15–19 | 36 | 192 816 | 1.87 | (1.31–2.58) | |
| 10–14 | 37 | 199 227 | 1.86 | (1.31–2.56) | |
| 5–9 | 27 | 220 040 | 1.23 | (0.81–1.79) | |
| Phenotype | |||||
| DMD | All | 389 | 3 827 532 | 1.02 | (0.92–1.12) |
| 20–24 | 64 | 960 866 | 0.67 | (0.51–0.85) | |
| 15–19 | 106 | 985 263 | 1.08 | (0.88–1.30) | |
| 10–14 | 121 | 938 612 | 1.29 | (1.07–1.54) | |
| 5–9 | 98 | 942 791 | 1.04 | (0.84–1.27) | |
| BMD | All | 138 | 3 827 532 | 0.36 | (0.30–0.43) |
| 20–24 | 31 | 960 866 | 0.32 | (0.22–0.46) | |
| 15–19 | 46 | 985 263 | 0.47 | (0.34–0.62) | |
| 10–14 | 33 | 938 612 | 0.35 | (0.24–0.49) | |
| 5–9 | 28 | 942 791 | 0.30 | (0.20–0.43) |
CI, confidence interval.
Among the 296 caregivers (except Hawaii, n = 2) who completed the telephone survey, 261 (88.2%) had complete concordance by year for residence history constructed from surveillance data and that reported in the survey; 288 (97.3%) caregivers had concordant residence histories for each quinquennium analyzed (data not shown). Inclusion of possible and asymptomatic cases produced higher, but similar, prevalence patterns for each quinquennium or age group (Figs 1 and 2; Supplemental Tables 5 and 6); the larger increase in the earlier quinquennia reflected the higher number of possible and asymptomatic cases in those years. Also, racial/ethnic-specific estimates that included possible and asymptomatic cases were slightly increased compared with those for definite and probable cases (Supplemental Tables 5 and 6). Restriction of cases to the oldest case in each family attenuated the estimates, but preserved patterns identified for pooled prevalence (data not shown).
FIGURE 1.
Prevalence estimates per 10 000 male individuals by quinquennium with and without possible and asymptomatic cases, identified by MD STARnet, 1991–2010.
FIGURE 2.
Prevalence estimates for 2010 per 10 000 male individuals by age group, with and without possible and asymptomatic cases, identified by MD STARnet, 1991–2010.
DISCUSSION
We present population-based estimates of the prevalence of childhood-onset DBMD in 6 US sites. Prevalence among 5- to 9-year-olds was approximately 2 per 10 000 boys, in all quinquennia except 2006–2010. Prevalence among non-Hispanic white individuals paralleled that for all racial/ethnic groups combined, whereas prevalence among Hispanic individuals decreased across quinquennia, but tended to exceed that for other racial/ethnic groups examined; prevalence was lowest among non-Hispanic black individuals. Also, DMD was 3 times more prevalent than BMD. In 2010, prevalence of DBMD among 5- to 24-year-olds was 1.38 per 10 000 male individuals.
The lower prevalence in 2006–2010 may reflect delayed diagnosis of DBMD among MD STARnet cases.33 It also may reflect a change in parental reproductive choice; however, a separate analysis observed that reproductive patterns of MD STARnet mothers after knowledge of family history of DBMD tended to be similar to those without such family history.36 The similar estimates for all cases and non-Hispanic white cases reflect the sizable proportion of non-Hispanic white individuals in the MD STARnet; future efforts will include expanding surveillance among minority populations. It also may reflect racial/ethnic differences in time to diagnosis and access to care, random fluctuations, or true differences in prevalence of DBMD across these racial/ethnic groups. Conversely, the increased prevalence among Hispanic individuals may reflect suspected underreporting in census estimates for Hispanic children, ages 5 to 9 years.37 Additionally, our definitions for DMD and BMD, determined from age of onset of signs and symptoms, may have also biased the true prevalence of each phenotype.
Previous data are unavailable to compare prevalence of DBMD for consecutive cohorts of boys, ages 5 to 9 years. Our prevalence of 1.38 per 10 000 male individuals, ages 5 to 24 years was lower than the 1.8 per 10 000 boys younger than 16 years in western Sweden.7 It also was less comparable to other studies of original data on prevalence of DBMD among all male individuals in the population. By using 2010 US census data, which showed that 29.0% of all male individuals in the MD STARnet sites were ages 5 to 24 years,38 we can extrapolate our prevalence estimate (1.38 per 10 000) to all male individuals, regardless of age. Our extrapolated estimate (0.4 per 10 000) becomes more comparable to the combined estimates for DMD and BMD (per 10 000 male individuals) from Slovenia (0.4)4 and South Africa (0.1)1, but less comparable to the combined estimates from northern England (1.6)6; Alberta, Canada (1.1)5; Hong Kong (1.1)2; and southern Japan (0.9).3
In our subgroup analyses, prevalence patterns observed for non-Hispanic white and black individuals were similar to those reported in South Africa, being higher in white and lower in black individuals.1 Our prevalence of 1.12 for DMD among male individuals, ages 5 to 24 years, in 2010 was lower than the prevalence (2.8) for male individuals, ages 20 years or younger, in Bologna, Italy,18 and that (2.4) for boys, ages 5 to 16 years, in Birmingham, United Kingdom.19 Again, extrapolating our DMD prevalence estimate to all male individuals (0.3 per 10 000) was comparable to DMD prevalence in several other countries (0.2–0.8).8–17 Similarly, extrapolating our BMD prevalence estimate to all male individuals (0.1 per 10 000) was comparable to several other studies (0.1–0.2),2–5,7 although it was higher than the prevalence (0.01) in South Africa1 and lower than that (0.7) in northern England.6 Additional DMD or BMD studies were less comparable to ours, as they provided estimates per total residents rather than those for male individuals only, or reported estimates among live male births.39–47
Prevalence differences identified between our study and previous studies may reflect the changes in diagnostic methods over the past quarter century; many studies5,8–10,12,16,19 predated current diagnostic methods, potentially leading to misclassification of muscular dystrophy type. Prevalence differences identified may also be due, in part, to the racial/ethnic composition of our sample; specifically, our cases were mostly non-Hispanic white and had insufficient numbers of cases to examine estimates for some racial/ethnic groups (eg, Asian and Pacific Islander). Last, they may be due to differences in the age range of the comparison populations used1–19; given the life expectancy for DBMD, inclusion of all male individuals in the comparison population reduced estimates, as evidenced by our extrapolated estimates.
The strengths of our retrospective and prospective longitudinal surveillance approach included timely identification and follow-up of a sizable cohort. Our detailed case definitions required laboratory or family pedigree data for certainty of classification comparable to what would be obtained from ascertaining cases from specialized neuromuscular centers. Use of multiple-source population-based case finding improved our potential for comprehensive ascertainment across each MD STARnet site. It also permitted calculation of population parameters, which is difficult when ascertaining cases from specialized neuromuscular centers. Additionally, collection of longitudinal, population-based surveillance data permitted calculation of prevalence estimates for several quinquennia and an improved opportunity to generalize these estimates.
Our longitudinal surveillance approach was limited by its reliance on available medical record data. Only these data were available to generate residence history for each case; however, comparison of residence histories constructed from surveillance data with self-reports from a subgroup of caregivers showed a high concordance, suggesting we captured the years of residence for cases. Our previous comparison48 of selected characteristics showed little difference between caregiver participants and nonparticipants, suggesting that our comparison of residence histories may be extended to all caregivers. Also, available medical records may have contained insufficient information for diagnostic milestones and disease progression, reflected in part, by differing percentages of possible cases across sites in the earlier quinquennia. Estimation of prevalence, including possible and asymptomatic cases, increased the magnitude of prevalence estimates, as expected, but did not influence patterns identified. Additionally, reliance on medical records may have biased identification toward families with more than 1 affected case, as these families may have been more likely to have sufficiently detailed records for case definition. Although estimates using only the oldest case per family were attenuated, as expected, they showed similar patterns to those families with multiple affected cases.
CONCLUSIONS
Approximately 2 per 10 000 boys in 6 US sites, ages 5 to 9 years, were affected with DBMD. Prevalence differences were observed among selected racial/ethnic groups; DMD prevalence was threefold higher than BMD prevalence. In 2010, approximately 1.4 per 10 000 male individuals, ages 5 to 24 years, were affected. Our findings are of particular importance to health care providers and policy makers for planning needed services for cases as their disease progresses. Continued longitudinal surveillance will permit ongoing examination of racial/ethnic and socioeconomic differences in treatment and outcomes for MD STARnet cases.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT
Worldwide prevalence estimates of Duchenne and Becker muscular dystrophies (DBMD) vary, likely due to differences in diagnostic criteria, ascertainment, and survival. To date, no population-based prevalence data for DBMD by race/ethnicity have been published in the United States.
WHAT THIS STUDY ADDS
Approximately 2 per 10 000 boys, ages 5 to 9 years, in 6 sites in the United States have DBMD; prevalence remained rather constant across 4 birth cohorts that spanned 2 decades. Prevalence differed among selected racial/ethnic groups across the time period examined.
ACKNOWLEDGMENTS
We are grateful to the families who participated in the MD STARnet. We also acknowledge the contributions made to the MD STARnet and this manuscript by Dr Lisa Miller, Dr Shree Pandya, and Ms Sarah Scollon, as well as the many staff members at each MD STARnet site. Without their efforts, this project would not have been feasible.
Dr Mathews has received institutional support for clinical trials from Serepta Therapeutics, Prosensa, Eli Lilly, Viropharma, and PTC.
FUNDING: Supported by grants (U01DD000189 and U01DD000831) from the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Dr Romitti directed the surveillance and survey data collection in Iowa, led the study design, directed the statistical analyses, drafted the initial manuscript, and revised manuscript drafts; Dr Zhu performed statistical analysis, contributed to the initial draft, and reviewed and revised manuscript drafts; Mr Puzhankara assisted with the study design, performed initial statistical analyses, and reviewed and revised manuscript drafts; Dr James performed statistical analysis, and reviewed and revised manuscript drafts; Dr Nabukera assisted with data collection in Iowa, and reviewed and revised manuscript drafts; Dr Zamba assisted with the study design, assisted with the statistical analyses and prevalence estimation, and reviewed and revised manuscript drafts; Dr Ciafaloni contributed to the development of case definitions, led the development of the Duchenne and Becker phenotype classification, conducted clinical review of cases, and reviewed manuscript drafts; Drs Cunniff and Meaney codirected the surveillance and survey data collection in Arizona, assisted in conceptualization and design of the study, and reviewed and revised manuscript drafts; Dr Druschel directed the surveillance and survey data collection in New York, provided input and feedback on analyses, and reviewed and revised manuscript drafts; Dr Mathews led the development of case definitions, conducted clinical review of cases, and reviewed and revised manuscript drafts; Dr Matthews contributed to the development of case definitions, led the multisite clinical review committee and assignment of case definitions, conducted clinical review of cases, and reviewed manuscript drafts; Ms Andrews coordinated and supervised data acquisition in Arizona, and reviewed and revised manuscript drafts; Dr Caspers Conway assisted with oversight of data acquisition in Iowa, provided input and feedback on analyses, and reviewed and revised manuscript drafts; Ms Fox assisted with oversight of data collection in New York, provided input and feedback on analyses, and reviewed and revised manuscript drafts; Ms Street coordinated and supervised data acquisition in Georgia, and reviewed and revised manuscript drafts; Dr Adams reviewed and revised manuscript drafts; Dr Bolen coordinated the Muscular Dystrophy Surveillance, Tracking, and Research Network for the Centers for Disease Control and Prevention, and reviewed and revised manuscript drafts; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The other authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The other authors have indicated they have no potential conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Ballo R, Viljoen D, Beighton P. Duchenne and Becker muscular dystrophy prevalence in South Africa and molecular findings in 128 persons affected. S Afr Med J. 1994;84(8 pt 1):494–497. [PubMed] [Google Scholar]
- 2.Chung B, Wong V, Ip P. Prevalence of neuromuscular diseases in Chinese children: a study in southern China. J Child Neurol. 2003;18(3):217–219. doi: 10.1177/08830738030180030201. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa M, Nakahara K, Yoshidome H, et al. Epidemiology of progressive muscular dystrophy in Okinawa, Japan. Classification with molecular biological techniques. Neuroepidemiology. 1991;10(4):185–191. doi: 10.1159/000110268. [DOI] [PubMed] [Google Scholar]
- 4.Peterlin B, Zidar J, Meznaric-Petrusa M, Zupancic N. Genetic epidemiology of Duchenne and Becker muscular dystrophy in Slovenia. Clin Genet. 1997;51(2):94–97. doi: 10.1111/j.1399-0004.1997.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 5.Monckton G, Hoskin V, Warren S. Prevalence and incidence of muscular dystrophy in Alberta, Canada. Clin Genet. 1982;21(1):19–24. doi: 10.1111/j.1399-0004.1982.tb02074.x. [DOI] [PubMed] [Google Scholar]
- 6.Norwood FLM, Harling C, Chinnery PF, et al. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132(pt 11):3175–3186. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darin N, Tulinius M. Neuromuscular disorders in childhood: a descriptive epidemiological study from western Sweden. Neuromuscul Disord. 2000;10(1):1–9. doi: 10.1016/s0960-8966(99)00055-3. [DOI] [PubMed] [Google Scholar]
- 8.Kanamori M, Morton NE, Fujiki K, Kondo K. Genetic epidemiology of Duchenne muscular dystrophy in Japan: classical segregation analysis. Genet Epidemiol. 1987;4(6):425–432. doi: 10.1002/gepi.1370040604. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda N, Kondô K. No sex difference in mutations rates of Duchenne muscular dystrophy. J Med Genet. 1980;17(2):106–111. doi: 10.1136/jmg.17.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton NE, Chung CS. Formal genetics of muscular dystrophy. Am J Hum Genet. 1959;11(4):360–379. [PMC free article] [PubMed] [Google Scholar]
- 11.Hauser E, Toifl K, Mad A, Bittner R. The incidence of Duchenne muscular dystrophy in eastern Austria. The controversy regarding CK screening. Wien Klin Wochenschr. 1993;105(15):433–436. [PubMed] [Google Scholar]
- 12.Leth A, Wulff K, Corfitsen M, Elmgreen J. Progressive muscular dystrophy in Denmark. Natural history, prevalence and incidence. Acta Paediatr Scand. 1985;74(6):881–885. doi: 10.1111/j.1651-2227.1985.tb10052.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen J, Green A, Steffensen BF, Rahbek J. The Duchenne muscular dystrophy population in Denmark, 1977–2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscul Disord. 2003;13(10):804–812. doi: 10.1016/s0960-8966(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 14.Talkop UA, Kahre T, Napa A, et al. A descriptive epidemiological study of Duchenne muscular dystrophy in childhood in Estonia. Eur J Paediatr Neurol. 2003;7(5):221–226. doi: 10.1016/s1090-3798(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 15.van Essen AJ, Busch HFM, te Meerman GJ, ten Kate LP. Birth and population prevalence of Duchenne muscular dystrophy in The Netherlands. Hum Genet. 1992;88(3):258–266. doi: 10.1007/BF00197256. [DOI] [PubMed] [Google Scholar]
- 16.Prot J. Genetic-epidemiological studies in progressive muscular dystrophy. J Med Genet. 1971;8(1):90–95. doi: 10.1136/jmg.8.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes MI, Hicks EM, Nevin NC, Patterson VH. The prevalence of inherited neuromuscular disease in Northern Ireland. Neuromuscul Disord. 1996;6(1):69–73. doi: 10.1016/0960-8966(94)00017-4. [DOI] [PubMed] [Google Scholar]
- 18.Merlini L, Stagni SB, Marri E, Granata C. Epidemiology of neuromuscular disorders in the under-20 population in Bologna Province, Italy. Neuromuscul Disord. 1992;2(3):197–200. doi: 10.1016/0960-8966(92)90006-r. [DOI] [PubMed] [Google Scholar]
- 19.Bundey S. A genetic study of Duchenne muscular dystrophy in West Midlands. J Med Genet. 1981;18(1):1–7. doi: 10.1136/jmg.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mah JK, Kornqut L, Dykeman J, et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24(6):482–491. doi: 10.1016/j.nmd.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 23.Bach JR. A historical perspective on the use of noninvasive ventilatory support alternatives. Respir Care Clin N Am. 1996;2(2):161–181. [PubMed] [Google Scholar]
- 24.Yasuma F, Konagaya M, Sakai M, et al. A new lease on life for patients with Duchenne muscular dystrophy in Japan. Am J Med. 2004;117(5):363. doi: 10.1016/j.amjmed.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy—the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17(6):470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Birnkrant DJ, Noritz GH. Is there a role for palliative care in progressive pediatric neuromuscular diseases? The answer is “Yes!” J Palliat Care. 2008;24(4):265–269. [PubMed] [Google Scholar]
- 27.Bothwell JE, Dooley JM, Gordon KE, et al. Duchenne muscular dystrophy—parental perceptions. Clin Pediatr (Phila) 2002;41(2):105–109. doi: 10.1177/000992280204100206. [DOI] [PubMed] [Google Scholar]
- 28.Chen JY, Clark MJ. Family function in families of children with Duchenne muscular dystrophy. Fam Community Health. 2007;30(4):296–304. doi: 10.1097/01.FCH.0000290542.10458.f8. [DOI] [PubMed] [Google Scholar]
- 29.Romitti P, Puzhankara S, Mathews K, et al. Centers for Disease Control and Prevention (CDC). Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years—four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58(40):1119–1122. [PubMed] [Google Scholar]
- 30.Lubs ML. Hemophilia A and Duchenne muscular dystrophy in Colorado. Am J Hum Genet. 1974;26(6):56A. [Google Scholar]
- 31.Miller LA, Romitti PA, Cunniff C, et al. The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol. 2006;76(11):793–797. doi: 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews KD, Cunniff C, Kantamneni JR, et al. Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. J Child Neurol. 2010;25(9):1098–1102. doi: 10.1177/0883073810371001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) J Pediatr. 2009;155(3):380–385. doi: 10.1016/j.jpeds.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaha FJ, Quinlan JG. Dystrophinopathies: clarification and complication. J Child Neurol. 1996;11(1):13–20. doi: 10.1177/088307389601100103. [DOI] [PubMed] [Google Scholar]
- 35.Nabukera SK, Romitti PA, Campbell KA, et al. MD STARnet. Use of complementary and alternative medicine by males with Duchenne or Becker muscular dystrophy. J Child Neurol. 2012;27(6):734–740. doi: 10.1177/0883073811426501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabukera SK, Romitti PA, Caspers KM, et al. MD STARnet. Reproductive patterns among mothers of males diagnosed with Duchenne or Becker muscular dystrophy. Am J Med Genet A. 2013;161A(1):70–75. doi: 10.1002/ajmg.a.35682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Hare WP. Assessing net coverage error for young children in the 2010 US decennial census. [Accessed October 21, 2014];US Census Bureau Center for Survey Measurement Study Series. Available at: www.census.gov/srd/papers/pdf/ssm2014-02.pdf.
- 38.United States Census 2010. [Accessed February 3, 2014]; Available at: www.census.gov/2010census/.
- 39.Ahlström G, Gunnarsson LG, Leissner P, Sjödén PO. Epidemiology of neuromuscular diseases, including the postpolio sequelae, in a Swedish county. Neuroepidemiology. 1993;12(5):262–269. doi: 10.1159/000110327. [DOI] [PubMed] [Google Scholar]
- 40.Kvasnicová M, Styková J, Hudec P. Incidence of spinal muscular atrophy and Duchenne’s muscular dystrophy in the juvenile population of central Slovakia [in Slovak] Bratisl Lek Listy (Tlacene Vyd) 1994;95(2):78–82. [PubMed] [Google Scholar]
- 41.Dooley J, Gordon KE, Dodds L, MacSween J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin Pediatr (Phila) 2010;49(2):177–179. doi: 10.1177/0009922809347777. [DOI] [PubMed] [Google Scholar]
- 42.Bushby KMD, Thambyayah M, Gardner-Medwin D. Prevalence and incidence of Becker muscular dystrophy. Lancet. 1991;337(8748):1022–1024. doi: 10.1016/0140-6736(91)92671-n. [DOI] [PubMed] [Google Scholar]
- 43.El-Tallawy HN, Khedr EM, Qayed MH, et al. Epidemiological study of muscular disorders in Assiut, Egypt. Neuroepidemiology. 2005;25(4):205–211. doi: 10.1159/000088674. [DOI] [PubMed] [Google Scholar]
- 44.Mostacciuolo ML, Miorin M, Pegoraro E, et al. Reappraisal of the incidence rate of Duchenne and Becker muscular dystrophies on the basis of molecular diagnosis. Neuroepidemiology. 1993;12(6):326–330. doi: 10.1159/000110334. [DOI] [PubMed] [Google Scholar]
- 45.Siciliano G, Tessa A, Renna M, et al. Epidemiology of dystrophinopathies in North-West Tuscany: a molecular genetics-based revisitation. Clin Genet. 1999;56(1):51–58. doi: 10.1034/j.1399-0004.1999.560107.x. [DOI] [PubMed] [Google Scholar]
- 46.Holmgren J, Reyes J, Colombo M, Blanco MA. Duchenne and Becker muscular dystrophy in Chile [in Spanish] Rev Med Chil. 1992;120(3):288–292. [PubMed] [Google Scholar]
- 47.Helderman-van den Enden ATJM, Madan K, Breuning MH, et al. An urgent need for a change in policy revealed by a study on prenatal testing for Duchenne muscular dystrophy. Eur J Hum Genet. 2013;21(1):21–26. doi: 10.1038/ejhg.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Romitti PA, Conway KM, et al. Complementary and alternative medicine for Duchenne and Becker muscular dystrophies: characteristics of users and caregivers. Pediatr Neurol. 2014;51(1):71–77. doi: 10.1016/j.pediatrneurol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.