Abstract
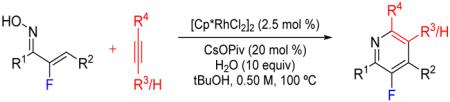
A Rh(III)-catalyzed C–H functionalization approach was developed for the preparation of multi-substituted 3-fluoropyridines from α-fluoro-α,β-unsaturated oximes and alkynes. Oximes substituted with aryl, heteroaryl and alkyl β-substituents were effective coupling partners, as were symmetrical and unsymmetrical alkynes with aryl and alkyl substituents. The first examples of coupling α,β-unsaturated oximes with terminal alkynes was also demonstrated and proceeded with uniformly high regioselectivity to provide single 3-fluoropyridine regioisomers. Reactions were also conveniently set up in air on the bench top.
Nitrogen heterocycles and their fluorinated analogues are ubiquitous and highly desirable motifs in pharmaceutical compounds.1-3 While facile new syntheses of fluorinated pyri-dines have emerged in recent years,4 current methods of constructing pyridines with fluorine substitution at the 3-position require either functional group transformations upon preinstalled functionality at this site on the pyridine ring5–9 or rely on heavily functionalized building blocks.10–13 Herein we describe a new Rh(III)-catalyzed C–H functionalization approach to prepare 3-fluoropyridines bearing multiple substituents from α-fluoro-α,β-unsaturated oximes and alkynes.
Chiba14 and Rovis15 have established the utility of [Cp*RhCl2]2/metal acetate salt catalyst systems for the synthesis of multi-substituted pyridines from α,β-unsaturated oximes and internal alkynes.16-17 However, we found that the nucleophilic alcoholic solvents utilized in their protocols, MeOH or 2,2,2-trifluoroethanol (TFE), posed a problem for the construction of fluorinated analogues due to alcohol displacement of the fluorine under the basic reaction conditions (Table 1, entries 1–2). To avoid fluoride displacement we examined a range of nonhydroxylic solvents, and while most proved to be ineffective (see Table S1 in the SI), ethyl acetate resulted in complete conversion to fluoropyridine 3a with minimal byproduct formation as determined by 19F NMR (entry 3). Unfortunately, very low conversion to fluoropyridine 3b was observed when diphenylacetylene (2b) was used as the alkyne partner, both with CsOPiv (entry 4) and the more soluble Bu4NOAc as the acetate base (entry 5) even at a higher reaction temperature (entry 6). The sterically hindered alcohol solvents i-PrOH (entry 7) and t-BuOH (entry 8) were explored with the goal of improving reaction rate while minimizing fluoride displacement. t-BuOH proved to be the most effective in providing complete conversion with minimal byproduct formation (entry 8). Additionally, the loading of CsOPiv was evaluated, and 20 mol % was determined to be optimal (see Table S2 in the SI).
Table 1.
Solvent screen for Rh(III)-catalyzed fluoro-pyridine formationa

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | R | solvent | temp (°C) |
oxime 1ab |
pyridine 3b |
byprodb |
|
| ||||||
| 1 | Et | MeOH | 60 | 0% | 48% | 52%b |
| 2 | Ph | TFE | 60 | 36% | 29% | 35%b |
| 3 | Et | EtOAc | 60 | 0% | 88% | 12% |
| 4 | Ph | EtOAc | 60 | 95% | 2% | 3% |
| 5 | Ph | EtOAcc | 60 | 91% | 4% | 5% |
| 6 | Ph | EtOAcc | 80 | 59% | 22% | 19% |
| 7 | Ph | i-PrOH | 80 | 18% | 57% | 25% |
| 8 | Ph | t-BuOH | 80 | 0% | 89% | 11% |
All reaction were set up in the glove box and run under nitrogen.
Percentages determined by 19F NMR with pen-tafluorobenzaldehyde as an external standard. Byproduct isolated by column chromatography and determined to be the product of fluoride displacement by the methoxy group.
Run with 20 mol % of Bu4NOAc instead of CsOPiv.
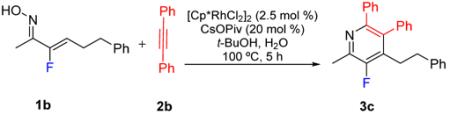
CsOPiv is highly hygroscopic as are the other carboxylate salts that have been used with Rh(III) catalysts in pyridine synthesis. For bench-top reactions we therefore envisaged that it would be important to determine the tolerance of the reaction to moisture. This was investigated by evaluating the effect of increasing amounts of water upon the reaction of oxime 1b and alkyne 2b, which are two of the more challenging coupling partners (Table 2). Significantly, up to stoichiometric amounts of water had minimal effect on either the yield of 3c or the formation of byproducts (entries 1-5). Furthermore, at 10 or more equivalents of added water, the reaction conversion was actually higher and was accompanied by only a small increase in byproduct formation (entries 6 and 7). Finally, increasing the reaction concentration from 0.1 M to 0.5 M, which is desirable for preparative reactions, resulted in a modest increase in conversion and yield (entry 8).
Table 2.
Concentration and added water screen for Rh(III)-catalyzed fluoropyridine formationa
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | [1a] | H2O | oxime 1bb |
pyridine 3cb |
byprodb |
|
| |||||
| 1 | 0.10 M | none | 50% | 27% | 23% |
| 2 | 0.10 M | 10% | 43% | 32% | 25% |
| 3 | 0.10 M | 20% | 43% | 33% | 26% |
| 4 | 0.10 M | 40% | 43% | 32% | 25% |
| 5 | 0.10 M | 100% | 33% | 42% | 25% |
| 6 | 0.10 M | 1000% | 7% | 62% | 31% |
| 7 | 0.10 M | 2000% | 9% | 61% | 28% |
| 8 | 0.50 M | 1000% | 3% | 68% | 29% |
All reactions were set up in a glove box and run under nitrogen.
Percentages were determined by 19F NMR with pen-tafluorobenzaldehyde as an external standard.
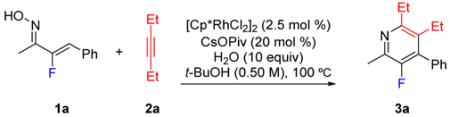
Because the synthesis protocol uses water and a high oxidation state catalyst, we also investigated the feasibility of pyridine synthesis with the reaction set up on the benchtop in air (Table 3). For the coupling of oxime 1a to alkyne 2a, no detrimental effect on the reaction rate or selectivity was observed when the reaction was set up in air (see entry 1 vs 2).
Table 3.
Comparison of Rh(III)-catalyzed fluoropyridine formations run under nitrogen and aira
| ||||
|---|---|---|---|---|
|
| ||||
| entry | time | oxime 1ac |
pyridine 3ac |
byprodc |
|
| ||||
| 1a | 15 min | 28% | 63% | 9% |
| 30 min | 9% | 81% | 10% | |
| 2b | 15 min | 18% | 74% | 8% |
| 30 min | 1% | 91% | 8% | |
Set up in a glove box and run under nitrogen.
Set up on bench-top in air.
Percentages were determined by 19F NMR with pentafluorobenzaldehyde as an external standard.
With optimized bench-top conditions established, we next explored the scope and generality of fluoropyridine synthesis (Scheme 1). Oximes 1 substituted with phenyl (3a, 3b, 3d-3g), alkyl (3c, 3h, 3i) and the electron-rich furyl (3j-3l) at the β-position each provided 3-fluoropyridines in moderate to excellent yields (Scheme 1). Symmetrical dialkyl and diaryl alkynes coupled in comparable yields for the different oxime coupling partners, as exemplified by 3-fluoropyridine 3a versus 3b, 3h versus 3c, and 3j versus 3k. Unsymmetrical internal alkynes also provided 3-fluoropyridines 3f, 3g, 3i and 3lin good yields, but with variable regioselectivities as has been previously reported for the preparation of non-fluorinated pyridines.14-15 Attempts to incorporate internal alkynes with bulky t-butyl or TMS substituents were not successful.
Scheme 1.
Scope of Rh(III)-catalyzed fluoropyridine formation from oximes and internal alkynesa
a All reactions were set up in air on bench-top. Yields are based upon the mass balance of pure material after column chromatography.
To the best of our knowledge, terminal alkynes have not previously been demonstrated to be viable partners for Rh(III)-catalyzed pyridine formation.18 Moreover, while we had reported conditions for the Rh(I)-catalyzed synthesis of pyridines from α,β-unsaturated oximes and terminal alkynes, the regioselectivities were generally modest.19 In the current study, a range of terminal alkynes 2 coupled efficiently with oximes 1 to give single regioisomers of the 3-fluoropyridines 3(Scheme 2). Straight-chain alkyl (3m, 3r-3s) and branched alkyl (3n, 3p-3q) terminal alkynes, and even neohexyne (3o), were effective coupling partners. The complete selectivity for the formation of the 5-substituted 3-fluoropyridines 3 is consistent with the regioselectivity observed by Fagnou et al. for terminal alkyne insertion in their Rh(III)-catalyzed synthesis of isoquinolones.20 Coupling of phenylacetylene with oxime 1a was also attempted, but did not yield any of the desired 3-fluoropyridine.
Scheme 2.
Scope of Rh(III)-catalyzed fluoropyridine formation from oximes and terminal alkynesa
a All reactions were set up in air on the bench-top. Yields are based upon the mass balance of pure material after column chromatography.
In conclusion, we have developed a one-step method for the preparation of 3-fluoropyridines from α-fluoro-α,β-unsaturated oximes and alkynes by Rh(III)-catalyzed C–H functionalization. The method is straightforward with reaction set up on the bench-top. α-Fluoro-α,β-unsaturated oximes and alkynes with a variety of alkyl, aryl and heteroaryl substituents are effective coupling partners. Moreover, the first examples of coupling terminal alkynes with α,β-unsaturated oximes with uniformly high selectivity provides an efficient approach to obtain single isomers of the 3-fluoropyridine products with predictable regioselectivity.
Supplementary Material
ACKNOWLEDGMENT
Support has been provided by the National Institutes of Health (R01-GM069559).
Footnotes
Supporting Information
Full experimental procedures, 1H, 13C and 19F NMR spectra of 3-fluoropyridines and intermediates. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- 1.Erian AW. J. Heterocycl. Chem. 2001;38:793. [Google Scholar]
- 2.Kirsch P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]
- 3.Begue J–P, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 4.Fier PS, Hartwig JF. Science. 2013;342:956. doi: 10.1126/science.1243759. [DOI] [PubMed] [Google Scholar]
- 5.(a) Shestopalov AM, Shestopalov AA, Rodinovskaya LA, Gromova AV. Synthesis of Ring-Fluorinated Pyridines. In: Petrov VA, editor. Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications Part I. Vol. 1. Wiley; Hoboken: 2009. pp. 243–272. For recent reviews on fluorination of prefunctionalized pyridines, see: [Google Scholar]; (b) Shestopalov AM, Rodinovskaya LA, Mortikov VY, Fedorov AE. Synthesis of Fluorinated Pyridines. In: Nenajdenko V, editor. Fluorine in Heterocyclic Chemistry: 6-Membered Heterocycles. Vol. 2. Springer International Publishing; Switzerland: 2006. pp. 1–58. [Google Scholar]
- 6.Beaty RD, Musgrave WKR. J. Chem. Soc. 1952:875. [Google Scholar]
- 7.Fukuhara T, Yoneda N, Suzuki A. J. Fluorine Chem. 1988;38:435. [Google Scholar]
- 8.Boudakian MM. J. Fluorine Chem. 1981;18:497. [Google Scholar]
- 9.Ryan SJ, Schimler SD, Bland DC, Sanford MS. Org. Lett. 2015;17:1866. doi: 10.1021/acs.orglett.5b00538. [DOI] [PubMed] [Google Scholar]
- 10.(a) Chen Z, Zhu J, Xie H, Li S, Wu Y, Gong Y. Org. Lett. 2010;12:4376. doi: 10.1021/ol101859p. [DOI] [PubMed] [Google Scholar]; (b) Chen Z, Zhu J, Xie H, Li S, Wu Y, Gong Y. Org. Biomol. Chem. 2011;9:5682. doi: 10.1039/c1ob05371j. [DOI] [PubMed] [Google Scholar]
- 11.Dolbier WR, Jr., Xu Y–L. J. Fluorine Chem. 2003;123:71. [Google Scholar]
- 12.Pews RG, Lysenko Z. J. Org. Chem. 1985;50:5115. doi: 10.1021/jo961675g. [DOI] [PubMed] [Google Scholar]
- 13.Jones G. Pyridines and their Benzo Derivatives: (v) Synthesis. In: Katritzky AR, Rees CW, editors. Comprehensive Heterocyclic Chemistry. Vol. 2. Pergamon; Oxford: 1984. pp. 395–510. [Google Scholar]
- 14.Too PC, Noji T, Lim YJ, Li X, Chiba S. Synlett. 2011:2789. See Scheme 4 and the associated discussion for a proposed mechanism for the Rh(III)-catalyzed synthesis of pyridines from α,β-unsaturated oximes and internal alkynes. [Google Scholar]
- 15.Hyster TK, Rovis T. Chem. Commun. 2011;47:11846. doi: 10.1039/c1cc15248c. “See Scheme 2 and the associated discussion for an analogous mechanism to that described in reference 14 for the Rh(III)-catalyzed synthesis of pyridines from α,β-unsaturated oximes and internal alkynes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Satoh T, Miura M. Chem. –Eur. J. 2010;16:11212. doi: 10.1002/chem.201001363. For recent reviews on Rh(III)-catalyzed C-H functionalization, see: [DOI] [PubMed] [Google Scholar]; b) Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]; c) Song G, Wang F, Li X. Chem. Soc. Rev. 2012;41:3651. doi: 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; d) Patureau FW, Wencel-Delord J, Glorius F. Aldrichimica Acta. 2012;45:39. [Google Scholar]
- 17.(a) Neely JM, Rovis T. J. Am. Chem. Soc. 2013;135:66. doi: 10.1021/ja3104389. For additional examples of pyridine synthesis catalyzed by Rh(III), see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Neely JM, Rovis T. J. Am. Chem. Soc. 2014;136:2735. doi: 10.1021/ja412444d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Luo C–Z, Jayakumar J, Gandeepan P, Wu Y–C, Cheng C–H. Org. Lett. 2015;17:924. doi: 10.1021/acs.orglett.5b00028. [DOI] [PubMed] [Google Scholar]; (d) Shi Z, Koester DC, Boultadakis-Arapinis M, Glorius F. J. Am. Chem. Soc. 2013;135:12204. doi: 10.1021/ja406338r. For additional examples of Rh-catalyzed isoquinoline synthesis from N-oximes, see: [DOI] [PubMed] [Google Scholar]; (e) Zhang X, Chen D, Zhao M, Zhao J, Jia A, Li X. Adv. Synth. Catal. 2011;353:719. [Google Scholar]; (f) Too PC, Wang Y-F, Chiba S. Org. Lett. 2010;12:5688. doi: 10.1021/ol102504b. [DOI] [PubMed] [Google Scholar]; (g) Parthasarathy K, Cheng C-H. J. Org. Chem. 2009;74:9359. doi: 10.1021/jo902084j. [DOI] [PubMed] [Google Scholar]
- 18. For regioselective syntheses of pyridines from α,β-unsaturated oxime esters with functionalized alkenes, see 17a and 17b.
- 19.(a) Martin RM, Bergman RG, Ellman JA. J. Org. Chem. 2012;77:2501. doi: 10.1021/jo202280e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2008;10:325. doi: 10.1021/ol7028367. For the first example of Rh(I)-catalyzed synthesis of pyridines from α,β-unsaturated oximes and internal alkynes, see: [DOI] [PubMed] [Google Scholar]
- 20.Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




