Summary
Background
As part of a pilot U.S. inhibitor surveillance project initiated at the Centers for Disease Control and Prevention (CDC) in 2006, centralized inhibitor measurement was instituted.
Objective
To validate a modified method for inhibitor measurement suitable for surveillance of treated and untreated patients.
Methods/Results
710 subjects with hemophilia A were enrolled; 122 had history of inhibitor (HI). Nijmegen-Bethesda assay (NBA) results on 50 split specimens shipped on cold packs and frozen were equivalent (r=0.998). Because 55% of 228 initial specimens had factor VIII (FVIII) activity (VIII:C) present, a heat treatment step was added. Heating specimens to 56°C for 30 minutes and centrifuging removed FVIII, as demonstrated by reduction of VIII:C and FVIII antigen to <1 U/dL in recently treated patients. Among specimens inhibitor-negative before heating, 1 of 159 with negative HI and 5 of 30 with positive HI rose to ≥0.5 Nijmegen Bethesda units (NBU) after heating. Correlation of heated and unheated inhibitor-positive specimens was 0.94 (P=0.0001). The modified method had a CV for a 1 NBU positive control of 10.3% and for the negative control of 9.8%. Based on results on 710 enrollment specimens, a positive CDC inhibitor was defined as ≥ 0.5 NBU. Results were similar when 643 post-enrollment specimens were included. Of 160 enrolled hemophilia B patients, 2 had HI. All others had NBU ≤ 0.2 at enrollment.
Conclusion
The CDC experience demonstrates that this modified NBA can be standardized to be within acceptable limits for clinical tests and can be used for national surveillance.
Keywords: factor VIII, factor IX, inhibitor
Introduction
Measurement of factor VIII (FVIII) inhibitors in the U.S. was standardized in 1975 at a meeting in Bethesda, Maryland, which produced a method bearing the name of the conference site [1]. The Bethesda assay (BA) calls for a two-hour incubation of a mixture of patient plasma with normal pool plasma (NPP) and comparison of the FVIII activity remaining with a similarly treated control mixture containing buffer and NPP. The percent residual activity in the patient mix is converted to Bethesda units (BU). One BU is defined as the amount of inhibitor producing a residual activity of 50%. This method has persisted virtually unchanged in the majority of laboratories in the U.S. despite the introduction of modifications to the method in Europe in 1995 [2]. The Nijmegen modification [3], or Nijmegen-Bethesda assay (NBA), calls for two changes in the standard BA: 1) buffering of the NPP used in patient and control mixtures for incubation, and 2) use of FVIII-deficient plasma (VIIIDP) instead of buffer in the control mixture and for dilution. A subsequent modification allowed substitution of 4% bovine serum albumin for the FVIIIDP [4], thereby reducing cost. While studies have documented that these modifications have improved the performance of the BA for measurement of FVIII inhibitors [5-6], both the BA and the NBA have shown poor inter-laboratory correlation in four different proficiency testing programs [2, 7-9]. For the U.S. inhibitor surveillance pilot project conducted at the Centers for Disease Control and Prevention (CDC) in 2006-2009 [10], centralized inhibitor measurement was instituted using a method modified to facilitate national inhibitor surveillance by allowing simplified sample handling and valid testing of treated patients. This paper presents the details of the adopted method and its validation.
Materials and Methods
Subjects
People with hemophilia with FVIII or FIX levels of <50 International Units per deciliter (IU/dL) were enrolled at 12 U.S. Hemophilia Treatment Centers. Using standardized data collection tools, HTC staff reported demographic data as well as information about any previous history of inhibitor such as the peak inhibitor titer, a recently obtained inhibitor titer, and whether or not immune tolerance induction (ITI) had been performed. A positive history of inhibitor was defined as evidence of a current or previous clinically relevant inhibitor as determined by the enrolling site. Detailed treatment product exposure records were collected prospectively from the time of enrollment. Inhibitor measurements were performed at CDC at study entry, annually, prior to any planned product switch, or for clinical indication of an inhibitor. The protocol was approved by the investigational review boards of CDC and each participating site, and all participants or parents of minor children gave informed consent.
Specimen Collection
Blood was collected into evacuated siliconized glass tubes (Becton Dickinson, Franklin Lakes, NJ) containing 3.2% sodium citrate in a ratio of 1:9 with blood and centrifuged at 1,600 × g for 20 minutes at 4°C, followed by repeat centrifugation of the separated plasma at 1,600 × g for 20 minutes at 4°C in polypropylene tubes. Blood processing was completed within two hours. Separated plasma was shipped to CDC overnight on cold packs. At CDC, the plasma samples were aliquoted and stored in polypropylene tubes at −70°C. For comparison purposes, some specimens were split, with a portion of the plasma shipped frozen on dry ice and a portion shipped as above.
Measurement Methods
FVIII coagulant activity (VIII:C) and FIX coagulant activity (IX:C) in IU/dL were measured by one-stage clot-based assay using PTT-A reagent (Diagnostica Stago, Parsippany, N.J., U.S.A.), 0.1M imidazole buffer, pH 7.4 (Siemens, Marburg, Germany) as diluent, and FVIII deficient plasma (VIIIDP) or FIX deficient plasma (IXDP) from hemophilic subjects (George King Biomedical, Overland Park KS) as substrate on a STAR or Evolution coagulation analyzer (Diagnostica Stago). Calibration curves were prepared using Normal Reference Plasma (CCNRP, Precision Biologic, Dartmouth, Nova Scotia, Canada) diluted in 0.1M imidazole buffer, pH 7.4 (Siemens). Positive controls for FVIII inhibitors were individual patient plasmas of known inhibitor titer (George King Biomedical) diluted in VIIIDP to approximately 1 Nijmegen-Bethesda unit/mL (NBU). Normal pool plasma buffered with 0.1M imidazole to pH 7.4 (BNPP) was prepared in house by addition of solid imidazole (Acros Organics, Geel, Belgium) or commercially by Precision Biologic. Bovine serum albumin buffer (BSA) was prepared of 4% bovine serum albumin (Sigma, St. Louis, MO) in imidazole buffer (Siemens).
Specimens were thawed at 37°C, mixed, and heated to 56°C for 30 minutes. After centrifugation at 5,000 rpm for 5 minutes at room temperature in a table-top centrifuge, the supernatant was removed for testing. Mixtures of 1 part patient plasma to 1 part BNPP and a negative control of 1 part VIIIDP to 1 part BNPP were covered and incubated for 2 hours at 37°C. VIII:C in each mixture was measured. The VIII:C remaining in the patient mixture was divided by the VIII:C remaining in the control mixture and expressed as % residual activity (%RA). %RA was converted to Nijmegen-Bethesda units/mL (NBU) using the formula:
which was calculated by linear regression of a curve containing 1 NBU=50%RA and 0 NBU=100%RA. Each specimen was initially screened undiluted. If %RA was ≥100, the NBU was reported as 0. If the %RA was >75, the NBU of that dilution was reported. If the first dilution was less than 75%RA, the assay was repeated at dilutions of patient plasma of 1:1, 1:2, 1:4, and 1:8 with VIIIDP for quantitation and assessment of parallelism. If no dilution had greater than 25 % RA, additional dilutions were added until %RA between 25 and 75 was reached. NBU was calculated by the equation and then multiplied by the dilution factor. Results were rounded to one decimal place for reporting. FIX inhibitors were measured similarly with omission of the 2-hour incubation.
FVIII antigen (VIII:Ag) was measured by ELISA (Asserochrom VIII:Ag, Diagnostica Stago). Dilute Russell's Viper Venom Time (DRVVT) was measured using DVVtest and DVVconfirm reagents (American Diagnostica, Stamford, CT).
Coefficient of variation (CV) was calculated for the VIII:C or IX:C of the negative control (BNPP plus VIII DP or IXDP; BNPP plus BSA) after incubation and for the NBU of the inhibitor positive control, using GraphPad Prism, Version 5 (GraphPad Software Inc., San Diego, CA). Spearman correlation coefficient and comparisons by Fisher's exact test also were calculated using GraphPad Prism, with a significance level set at P < 0.05.
Results
Results on 1353 specimens from 710 patients with hemophilia A (HA) and 289 specimens from 160 patients with hemophilia B (HB) were examined. Characteristics of enrolled patients are shown in Table 1. As determined by the enrolling sites, 122 HA patients and 2 HB patients had a history of a clinically significant inhibitor (HI) at the time of study enrollment. One HB patient and 63 of 122 HA patients were reported to have previously had an inhibitor titer >5 BU.
Table 1.
Characteristics of study subjects
| Hemophilia A | Hemophilia B | |||
|---|---|---|---|---|
| History of inhibitor | Negative | Positive | Negative | Positive |
| Number of patients | 588 | 122 | 158 | 2 |
| Severity: | ||||
| Severe (<1 U/dL) | 324 (55.1) | 99 (81.1) | 49 (31.0) | 2 (100) |
| Moderate (1-5 U/dL) | 117 (19.9) | 16 (13.1) | 71 (44.9) | 0 |
| Mild (>5 U/dL) | 147 (25.0) | 7 (5.7) | 38 (24.1) | 0 |
| Race: | ||||
| White Non-Hispanic | 493 (83.8) | 84 (68.8) | 138 (87.3) | 1 (50) |
| Black Non-Hispanic | 38 (6.5) | 16 (13.1) | 11 (7.0) | 0 |
| Hispanic | 29 (4.9) | 18 (14.8 | 5 (3.2) | 0 |
| Asian | 9 (1.5) | 2 (1.6) | 0 | 0 |
| Native American | 1 (0.2) | 1 (0.8) | 0 | 0 |
| Other or mixed | 18 (3.1) | 1 (0.8) | 4 (2.5) | 1 (50) |
Modifications of the published NBA were validated during the implementation of the project. Two shipping methods were compared on split samples. Fifty specimens with inhibitor titers ranging from 0 to 900 were split and shipped to CDC both on cold packs and frozen on dry ice; their NBUs showed excellent correlation (r=0.998). The cold pack method was chosen to simplify specimen handling.
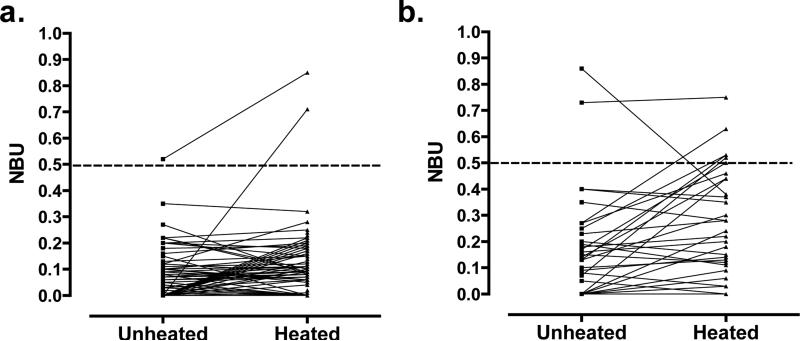
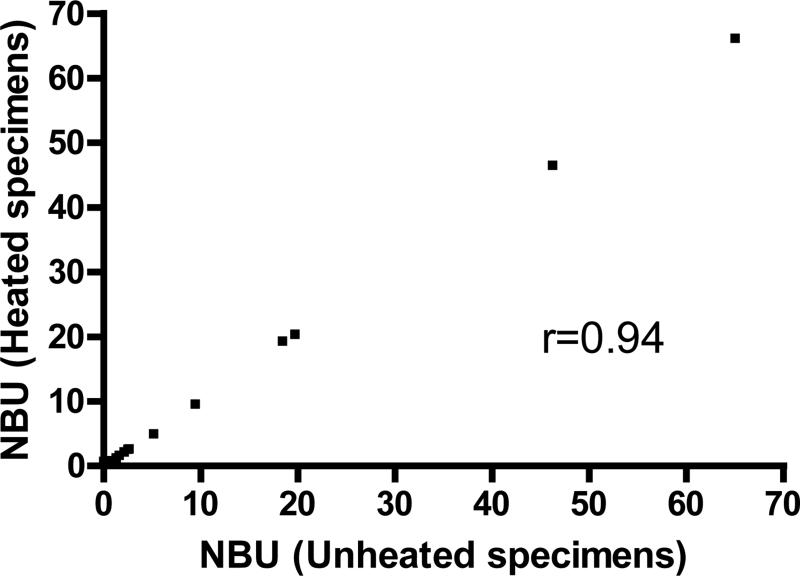
Initial tests of 228 frozen specimens from severe HA patients showed that126 (55%) had measurable VIII:C. All were from patients reported to have been treated with FVIII-containing products within 72 hours of blood collection. In the unmodified NBA, these specimens showed a residual activity of ≥100% and an inhibitor titer of 0. In order to eliminate the VIII:C while preserving the inhibitory antibodies, plasmas were heated to 56°C for 30 minutes and centrifuged. Inhibitor results on 202 patient specimens were compared with and without heat treatment (Table 2). Of 159 specimens with negative HI, 120 had unheated NBU of 0. After heating, 45 (37.5%) of these remained 0, 74 (61.7%) increased from 0 to 0.1-0.2 NBU, and one increased from 0 to 0.7 NBU (Figure 1a). Among 37 specimens with unheated NBU greater than 0 but below 0.5, 31 (83.8%) remained constant, 6 (16.2%) decreased below 0.1, and none increased. Of 30 specimens with positive HI but with inhibitor results below 0.5 NBU at enrollment, 5 (16.7%) rose to ≥0.5 NBU after heating, significantly more than the 1 of 159 (0.6%) with negative HI (P=0.0004); one positive specimen decreased from 0.9 to 0.4 NBU (Figure 1b). In a series of 21 specimens with results ≥0.5 NBU (Figure 2), the correlation of the inhibitor titers in heated and unheated specimens was 0.94 (P<0.0001). Plasma from 30 individuals without hemophilia was also heat-treated and tested for inhibitor. Of those, 20 had 0 NBU, 9 had 0.1 NBU, and 1 had 0.2 NBU (Table 2).
Table 2.
Comparison of factor VIII inhibitor results in Nijmegen-Bethesda units (NBU) on heated and unheated specimens
| NBU Pre Heating | NBU Post Heating n (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1-0.4 | 0.5-0.9 | ≥1.0 | All | ||
| Negative History of Inhibitor n=159 | 0 | 45 (37.5) | 74 (61.7) | 1 (0.8) | 0 | 120 |
| 0.1-0.4 | 6 (16.2) | 31 (83.8) | 0 | 0 | 37 | |
| 0.5-0.9 | 0 | 0 | 1 (100) | 0 | 1 | |
| ≥1.0 | 0 | 0 | 0 | 1(100) | 1 | |
| Positive History of Inhibitor n=43 | 0 | 4 (44.4) | 5 (55.6) | 0 | 0 | 9 |
| 0.1-0.4 | 2 (9.5) | 14 (66.7) | 5 (23.8) | 0 | 21 | |
| 0.5-0.9 | 0 | 0 | 2 (100) | 0 | 2 | |
| ≥1.0 | 0 | 0 | 0 | 11 (100) | 11 | |
| Normals n=30 | NA | 20 (66.7) | 10 (33.3) | 0 | 0 | 30 |
Figure 1.
Change in factor VIII inhibitor titer with heat treatment. Results are shown prior to rounding to one decimal place. a. Patients with negative history of inhibitor. b. Patients with positive history of inhibitor.
Figure 2.
Correlation of factor VIII inhibitors in heated and unheated specimens with positive results.
VIII:C and VIII:Ag were measured in heated and unheated specimens from patients who had been infused with FVIII products within 24 hours of blood draw. Among 15 severe patients, all had measurable VIII:C and VIII:CAg before heating and <1 IU/dL after heating. In 7 patients with moderate HA, VIII:C and VIII:Ag also decreased to <1 IU/dL after heating.
The steps of the method adopted for the CDC surveillance are shown in Table 3. Coefficient of variation (CV) was 9.8% for VIII:C of the negative control (n=117) and 10.3% for NBU of a 1 NBU positive control (n=114). Within-runs variation of the negative control was 5.3% (n=10) and of the positive control 4.8% (n=10). Substitution of 4% bovine serum albumin buffer (BSA) for the VIIIDP in the incubation mixture was also tested but was not adopted as part of the standard procedure. In 71 specimens, the correlation of BSA and VIIIDP was 0.990. There were no differences in results if BSA was used for the incubation mixture and for dilution of specimens with inhibitor titers ranging from 0 to 56.5. CV for the negative control was 10.6%.
Table 3.
Diagram of the inhibitor method adopted for surveillance by the Centers for Disease Control and Prevention (CDC)
| CDC Modification of the Nijmegen-Bethesda Method | |
|---|---|
| Heat patient and control plasmas to 56°C and centrifuge. | |
| Dilute patient plasma in VIIIDP* if an inhibitor is expected. | |
| Patient Mix | Control Mix |
| 1 part patient or dilution: 1 part BNPP** | 1 part VIIIDP:1 part BNPP |
| ↘ | ↙ |
| Incubate for 2 hours at 37°C. | |
| Measure Factor VIII activity. | |
| Patient mix/control mix X 100 = % residual activity (RA) | |
| Convert RA to NBU by formula. | |
| Adjust for dilution, if necessary. | |
| Nijmegen modifications: BNPP used in mixes, VIIIDP used to dilute plasma and in control mix; CDC modification: heating of plasma to destroy infused and endogenous FVIII | |
VIIIDP=factor VIII-deficient plasma
BNPP=imidazole-buffered normal pooled plasma
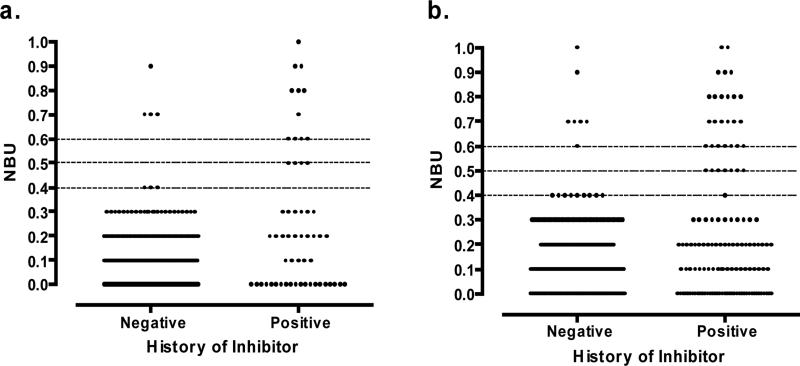
In order to establish a reference range for the assay, results at enrollment were compared for the 588 patients with negative HI and 86 of the 122 patients with positive HI. Thirty-six patients who had undergone successful ITI were excluded. Figure 3a shows those with NBU below 1.0 with lines plotted at NBU of 0.4, 0.5, and 0.6. No specimen from the 588 negative HI patients (left) had NBU between 0.4 and 0.7. Among 56 specimens from patients with positive HI (right), none showed NBU of 0.4. A cut-off for a positive inhibitor of ≥0.5 misclassified 4 fewer specimens with positive HI than a cut-off of ≥0.6, showing a higher sensitivity. A positive CDC inhibitor was defined as ≥0.5. The results in this range for all inhibitor tests completed on these subjects (Figure 3b) were similar to those at enrollment, supporting the cut-off assignment.
Figure 3.
Factor VIII inhibitor titers below 1.0 NBU. a. in enrollment specimens and b. in all specimens.
Table 4 shows all of the results at enrollment. Of 588 patients with negative HI reported by the sites, 581 (98.8%) had NBU <0.5. Six positive specimens ranged from 0.5 to 1.1, and one was 10.2. All were negative for DRVVT and were drawn peripherally. Three, including the high titer inhibitor, were confirmed on repeat specimens; one was not confirmed in a specimen drawn 3 months later but reappeared after 2 years of surveillance; three have not been retested. Of 122 patients with a positive HI, 51 (41.8%) had a positive CDC inhibitor at enrollment, including 18 (14.8%) with high titer antibodies (>5.0 NBU). Thirty-six (29.5%) had undergone successful ITI, and 35 others (28.7%) were negative at the time of enrollment. A maximum inhibitor titer >5 BU was reported for 65 of 122 patients, and 3 more had a high titer when tested at CDC, for a total of 68 (55.7%) with high titer inhibitors.
Table 4.
Factor VIII inhibitor tests at enrollment on patients with and without a previous history of an inhibitor, using the method defined in Table 3
| NBU | Negative History (n=588) | Positive History (n=122) | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| 0 | 304 | 51.7 | 32 | 26.2 |
| 0.1-0.4 | 277 | 47.1 | 39 | 32.0 |
| 0.5-0.9 | 5 | 0.9 | 20 | 16.4 |
| 1.0-4.9 | 1 | 0.2 | 13 | 10.7 |
| ≥5 | 1 | 0.2 | 18 | 14.8 |
Some characteristics of the adopted assay method allow evaluation of its limitations. If a true negative specimen would be expected to have a result within 2 standard deviations of the mean of the normal control (30.55±2.98), the lowest %RA expected would be 67, producing an inhibitor titer of 0.6 NBU in a specimen that is actually negative. Additionally, using the regression equation for the NBU curve, the change in the VIII:C of the incubation mix with change in NBU was calculated to be 2.9 IU/dL per 0.1 NBU. A change from a titer of 0 to one of 0.5 would result from a change in the incubation mix of only 14.5 IU/dL.
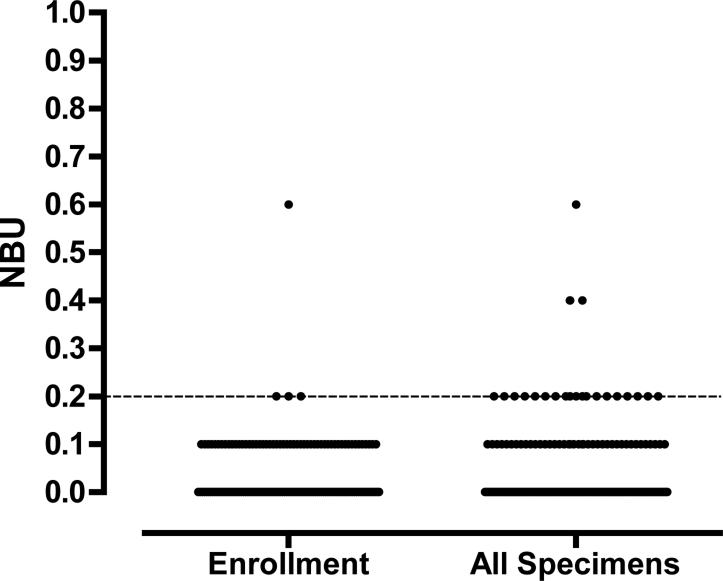
Factor IX inhibitor assays were performed with and without heat treatment on 17 specimens. All had NBU of 0 pre heating. One specimen from a patient with positive HI rose from 0 to 0.6 NBU after heating. Of 160 HB patients enrolled, 2 were reported to have a positive HI. Both were positive at enrollment at 0.6 and 5.6 NBU using the modified method. All other patients (98.9%) had NBU ≤0.2 at enrollment, including 115 (61.5%) with 0 NBU (Figure 4a). Results for all 289 specimens tested are shown in Figure 4b. CV was 6.8 % for the negative control. Within runs CV was 5.8% for the negative control (n=10). No positive control was used.
Figure 4.
Factor IX inhibitor titers in a. Enrollment specimens and b. All specimens.
Discussion
Because of the increased use of prophylaxis and ITI, sampling a hemophilia patient in the baseline state, without circulating FVIII, has become difficult; however, the presence of even a minimum amount of patient FVIII violates an assay principle of the BA, that the patient and control mixtures be equivalent before incubation. While it is possible to arithmetically account for the additional FVIII in the mix, the kinetics of the antigen-antibody reaction may be altered. There may be competition between the infused FVIII and that provided in the assay, which are likely to be different, or the antibody may be bound to the infused factor, leading to a false-negative result. If the additional FVIII is not accounted for, a residual activity of 100% or higher will be measured leading to an NBU result of 0.
To avoid this problem, we added a heat treatment step to eliminate FVIII from the sample prior to testing. As antibodies, inhibitors are more stable than many coagulation proteins, a characteristic that allows conditions under which the inhibitor remains but the FVIII, both activity and antigen, is eliminated. Heat treatment of plasmas has been reported by others [11, 12], but few data on the efficacy of this procedure have been provided. Verbruggen and colleagues [12] reported no change with heat treatment in inhibitor positive specimens. We, too, found little change in inhibitor positive plasmas, however, heating did appear to cause increase in some specimens that were inhibitor negative before heating. Five of 30 specimens from patients with positive HI which were <0.5 NBU rose into the positive range after heating. Only one of 159 patients with negative HI and none of the normal subjects increased to that range. This suggests that the modified method increases sensitivity to low titer inhibitors.
In the clinical setting, this modified assay allows testing of patients receiving replacement therapy when an urgent inhibitor titer is needed during emergency treatment or hospitalization, as well as inhibitor screening at routine clinic visits in patients receiving prophylaxis, ITI, or episodic care, when washout is not feasible or might place the patient at risk of breakthrough bleeding. It cannot, however, detect all inhibitors; some are not detected in clot-based assays, and some may be cleared in vivo [13]. The use of appropriate washout and pharmacokinetic studies is warranted when there is clinical suspicion of an inhibitor and a negative test. Alternative measurement methods, such as ELISA and fluorescence assays, may also prove useful.
Our data support the use of ≥0.5 NBU to define a positive inhibitor when the CDC method is used. A similarly large study of the unaltered Nijmegen method also provides data using that cutoff [5]. Assigning significance to inhibitor titers in the 0.1-0.4 range stretches the capability of the clot-based methodology. We and others [14] have demonstrated that some normal subjects show such low titers with both the BA and NBA. These assays assume that the curve of log %RA versus dilution is linear between 25 and 75 %RA, allowing quantitation of inhibitors in that range, which covers 0.4-2.0 BU. It is generally assumed in practice that the curve is also linear between 75 and 100 %RA, i.e., between 0 and 0.4 BU, however, it has been suggested that the sensitivity of the inhibitor assay does not extend below 0.4 BU (75 %RA), the limit of the range used for calculation in the original Bethesda assay, and that any inhibitor titer <0.4 BU should be considered negative [2, 11]. Our data are consistent with that concept. More than 98% of our patients without clinical history fall into that range. From a practical standpoint, there is sufficient variation in the VIII:C assay to make low titer inhibitor readings difficult to interpret. We have demonstrated mathematically that an inhibitor titer as high as 0.6 NBU is possible in a specimen that is actually negative. Using the regression equation for the NBU curve, it can be calculated that the difference in the VIII:C of the incubated mix varies by only 2.9 IU/dL per 0.1 NBU. The difference between a titer of 0 and one of 0.5 is only 14.5 IU/dL. Maintaining sufficient control of the test to achieve reproducible results in this range is challenging in many clinical laboratories. Other methods, such as chromogenic, ELISA, or fluorescence assays, may prove to be more sensitive and specific.
Evaluation of inhibitor test methods is difficult, because there is no gold standard against which to compare them. In practice, both laboratory and clinical evidence is used to determine whether a patient has an inhibitor. The inhibitor-negative population is defined largely by test, although non-neutralizing antibodies that are not detected in clot-based assays are suspected in some patients [13]. A significant portion of patients with past inhibitors test negative because they have been successfully treated, either by ITI or use of alternatives to factor replacement. A limitation of this study is the difficulty in defining the inhibitor and non-inhibitor populations, for which we have relied on clinical judgment. Another drawback is the possibility that heating and slow centrifugation of the patient specimens might have introduced some inhibitory substance causing prolonged clotting times in the patient mix but not in the control mix, which was not heated, leading to higher inhibitor titers. The data suggest that that is not the case. Only one patient with negative HI and negative titer and no normal subject developed a positive titer after heating, as compared to 16.7% of patients with positive HI (p=0.0004) in the direct comparison study, and only 7 positive titers (1.2%) occurred among a total of 588 tests on patients with negative HI at enrollment when the described method was used.
Our experience with inhibitor measurement in large numbers of specimens by a central laboratory demonstrates that the CDC modification of the NBA can be standardized to be within acceptable limits for clinical tests and is suitable for large-scale surveillance without regard for treatment status. Harmonization of regional or local laboratories for the same purpose could conceivably be accomplished by adoption of a detailed standard method with controls and uniform reagents.
Acknowledgements
The work was supported by the CDC Foundation through a grant from Pfizer Inc. The authors wish to thank the patients who participated and the study coordinators and administrators at the sites.
Footnotes
Addendum
The Hemophilia Inhibitor Research Study Investigators include authors from the following study sites: Thomas C. Abshire, Amy L. Dunn, and Christine L. Kempton, Emory University, Atlanta GA; Paula L. Bockenstedt, University of Michigan Hemophilia and Coagulation Disorders, Ann Arbor, MI; Doreen B. Brettler, New England Hemophilia Center, Worcester, MA; Jorge A. Di Paola, Mohamed Radhi, and Steven R. Lentz, University of Iowa Carver College of Medicine, Iowa City, IA; Gita Massey and John C. Barrett, Virginia Commonwealth University, Richmond, VA; Anne T. Neff, Vanderbilt University Medical Center, Nashville, TN; Amy D. Shapiro, Indiana Hemophilia and Thrombosis Center, Indianapolis, IN; Michael Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; Brian M. Wicklund, Kansas City Regional Hemophilia Center, Kansas City, MO; Marilyn J. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, University of Colorado and The Children's Hospital, Aurora, CO; Christine Knoll, Phoenix Children's Hospital Hemophilia Center, Phoenix, AZ.
J.M.Soucie and C.H.Miller designed the research project. C.H. Miller analyzed the data and wrote the paper. S.J. Platt and Anne S. Rice performed testing and analyzed data. T.C. Abshire, P.L. Bockenstedt, D.B. Brettler, J.A. DiPaola, G. Massey, A.T. Neff, A.D. Shapiro, M. Tarantino, B.M. Wicklund, M.J. Manco-Johnson, A.L. Dunn, C. Knoll, J.C. Barrett, M. Radhi, S.R. Lentz, and C.L. Kempton provided data and patient specimens and revised the manuscript. F. Kelley coordinated the research project and collected data. All authors edited and approved the final paper.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Kasper CK, Aledort LM, Counts RB, Edson JR, Frantantoni J, Green D, Hampton JW, Hilgartner MW, Lazerson J, Levine PH, McMillan CW, Pool JG, Shapiro SS, Schulman NR, van Eys J. A more uniform measurement of factor VIII inhibitors. Thrombos Diathes Haemorrh. 1975;34:869–72. [PubMed] [Google Scholar]
- 2.Peerschke EIB, Castellone DD, Ledford-Kraemer M, Van Cott EM, Meijer P, NASCOLA Proficiency Testing Committee Laboratory assessment of factor VIII inhibitor titer. Am J Clin Pathol. 2009;131:552–8. doi: 10.1309/AJCPMKP94CODILWS. [DOI] [PubMed] [Google Scholar]
- 3.Verbruggen B, Novakova I, Wessels H, Boezeman, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:c inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- 4.Verbruggen B, van Heerde W, Novakova I, Lillicrap D, Giles A. A 4% solution of bovine serum albumin may be used in place of factor VIII:C deficient plasma in the control sample in the Nijmegen modification of the Bethesda factor VIII:C inhibitor assay. Thromb Haemost. 2002;88:362–4. [PubMed] [Google Scholar]
- 5.Giles AR, Verbruggen B, Rivard GE, Teitel J, Walker I, Association of Hemophilia Centre Directors of Canada A detailed comparison of the performance of the standard versus the Nijmegen modification of the Bethesda assay in the haemophilia A population of Canada. Thromb Haemost. 1998;79:872–5. [PubMed] [Google Scholar]
- 6.Reber G, Aurousseau MH, Dreyfus M, Delahousse B, Caron C, Trzeciack MC, Aillaud MF, Horellou MH, Laurian Y, Sie P. Inter-laboratory variability of the measurement of low titer factor VIII:C inhibitor in haemophiliacs: improvement by the Nijmegen modification of the Bethesda assay and the use of common lyophilized plasmas. Haemophilia. 1999;5:292–3. doi: 10.1046/j.1365-2516.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- 7.Kitchen S, Jennings I, Preston FE, Kitchen DP, Woods TAL, Walker ID. Interlaboratory variation in factor VIII:C inhibitor assay results is sufficient to influence patient management: data from the UK National Quality External Assessment Scheme for Blood Coagulation. Semin Thromb Hemost. 2009;35:778–85. doi: 10.1055/s-0029-1245110. [DOI] [PubMed] [Google Scholar]
- 8.Meijer P, Verbruggen B. The between-laboratory variation of factor VIII inhibitor testing: the experience of the External Quality Assessment Program of the ECAT Foundation. Semin Thromb Hemost. 2009;35:786–93. doi: 10.1055/s-0029-1245111. [DOI] [PubMed] [Google Scholar]
- 9.Favaloro EJ, Bonar R, Kershaw G, Duncan E, Sioufi J, Marsden K. Investigations from external quality assurance programs reveal a high degree of variation in the laboratory identification of coagulation factor inhibitors. Semin Thromb Hemost. 2009;35:794–805. doi: 10.1055/s-0029-1245112. [DOI] [PubMed] [Google Scholar]
- 10.Miller CH, Benson J, Ellingsen D, Driggers J, Payne A, Kelly FM, Soucie JM, Hooper WC, the Hemophilia Inhibitor Research Study Investigators F8 and F9 mutations in U.S. hemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2011 doi: 10.1111/j.1365-2516.2011.02700.x. DOI: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw G, Jayakodi D, Dunkley S. Laboratory identification of factor inhibitors: the perspective of a large tertiary hemophilia center. Semin Thromb Hemost. 2009;35:760–8. doi: 10.1055/s-0029-1245108. [DOI] [PubMed] [Google Scholar]
- 12.Verbruggen B, van Heerde WL, Laros-Gorkom BAP. Improvements in factor VIII inhibitor detection: from Bethesda to Nijmegen. Semin Thromb Hemost. 2009;35:752–9. doi: 10.1055/s-0029-1245107. [DOI] [PubMed] [Google Scholar]
- 13.Gilles JGG, Amout J, Vermylen J, Saint-Remy JMR. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993;82:2452–61. [PubMed] [Google Scholar]
- 14.Aligman M, Dietrich G, Nydegger UE, Boieldieu D, Sultan Y, Kazatchkine MD. Natural antibodies to factor VIII (antihemophilic factor) in healthy individuals. Proc Natl Acad Sci USA. 1992;89:3795–9. doi: 10.1073/pnas.89.9.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]