Abstract
Previous research has suggested autoimmune diseases are risk factors for developing venous thromboembolism (VTE). We assessed whether having diagnoses of selected autoimmune diseases associated with anti-phospholipid antibodies—autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE)—were associated with having a VTE diagnosis among US adult hospitalizations. A cross-sectional study was conducted using the 2010 Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. VTE and autoimmune diseases were identified using International Classification of Diseases, Ninth Revision, Clinical Modification coded diagnoses information. The percentages of hospitalizations with a VTE diagnosis among all non-maternal adult hospitalizations without any of the four autoimmune diseases of interest and among those with AIHA, ITP, RA, and SLE diagnoses were 2.28, 4.46, 3.35, 2.65 and 2.77 %, respectively. The adjusted odds ratios (OR) for having a diagnosis of VTE among non-maternal adult hospitalizations with diagnoses of AIHA, ITP, RA, and SLE were 1.25 [95 % confidence interval (CI) 1.05–1.49], 1.20 (95 % CI 1.07–1.34), 1.17 (95 % CI 1.13–1.21), and 1.23 (95 % CI 1.15–1.32), respectively, when compared to those without the corresponding conditions. The adjusted OR for a diagnosis of VTE associated with a diagnosis of any of the four autoimmune diseases was 1.20 (95 % CI 1.16–1.24). The presence of a diagnosis of AIHA, ITP, RA, and SLE was associated with an increased likelihood of having a VTE diagnosis among the group of all non-maternal adult hospitalizations.
Keywords: Autoimmune diseases, Autoimmune hemolytic anemia, Immune thrombocytopenic purpura, Rheumatoid arthritis, Systemic lupus erythematosus, Venous thromboembolism
Introduction
Venous thromboembolism (VTE), which consists of deep vein thrombosis (DVT) and pulmonary embolism (PE), is an important health concern [1–3]. Research in the past several years has increasingly suggested that autoimmune diseases may be risk factors for VTE occurrence [4]. These have included studies that have reported a higher risk of VTE associated with autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE), as well as several other autoimmune diseases [5–11]. For example, investigators in a case-control study among outpatients in a hospital in Italy found that SLE was one of the factors significantly associated with idiopathic DVT (OR 4.31, 95 % CI 3.14–5.48) [12]. A study among 30 patients with AIHA in the US found that 8 (27 %) of these patients suffered a VTE event, either during the initial hemolytic crisis, relapse, or (as in one case) a surgical procedure [8].
Hospitalized patients with autoimmune disorders may particularly be at risk of VTE. Hospitalization itself is a strong risk factor for VTE [13, 14]; reasons for this likely include the VTE risks associated with immobility, surgery, and co-morbid conditions. Hospitalized patients with autoimmune diseases therefore may be at risk of hospital associated VTE because of the effects of both factors. Increased epidemiologic understanding of the association between autoimmune diseases and VTE can help to better assess the risk for VTE among hospitalized patients and develop and implement appropriate prevention strategies. This study assesses the association between VTE, whether occurring during or before hospitalization, and selected autoimmune diseases in a large sample of hospitalizations in the US; we focus on the autoimmune diseases, SLE, RA, ITP, and AIHA. Our objectives were to estimate the percent of hospitalizations with recorded diagnoses of AIHA, ITP, RA, SLE, or any combination of these diseases that also had recorded diagnoses of VTE and to assess if those with these autoimmune diseases had an increased likelihood of having a VTE diagnosis compared to hospitalizations without these diagnoses.
Methods
This study used data from the National Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. In 2010, the Nationwide Inpatient Sample database included information on inpatient stays from approximately 1,000 hospitals (~20 % of community hospitals in the US) in 45 states (comprising 96 % of the US population). Information collected on each discharge included dates of hospitalization, patient’s sex, age, and race/ethnicity as well as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coded information on diagnoses and performed procedures. The dataset also includes information on whether the patient underwent major surgery during the hospitalization (derived from ICD-9-CM coded information available for the hospitalization). More detailed information about the Nationwide Inpatient Sample data is available at the following website: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
A cross-sectional approach was used with hospitalization as the unit of analysis. All hospitalizations for non-maternal causes (did not have a maternal diagnosis or procedure) where the patient’s age was ≥19 years were included in this study. Hospitalizations with recorded diagnoses of VTE were defined as those with ICD-9-CM codes indicating diagnoses of DVT, PE, or both, as any of the up to 25 listed diagnoses. The diagnosis of the four selected autoimmune diseases and the presence of selected medical conditions (cancer, congestive heart failure, stroke, long bone fracture, infection) that have been reported to be risk factors [3, 15] for VTE as well as venous catheterization [3] (also a reported risk factor for VTE) during hospitalization were also identified using ICD-9-CM coded diagnoses and procedure information. The ICD-9-CM codes used in this study to identify various diagnoses and venous catheterization are presented in Appendix.
Analysis
The association of a diagnosis of VTE with the recorded diagnosis of each or any of the 4 selected autoimmune diseases was assessed using logistic regression analysis. Unadjusted odds ratios (OR) for recorded diagnoses of VTE associated with hospitalizations with recorded diagnoses of AIHA, ITP, RA, or SLE (when compared to hospitalizations without a diagnosis of the respective autoimmune disease) were derived using a separate model for each disease where only the disease was included as the explanatory variable; adjusted OR were derived using a model where the explanatory variables were all four autoimmune diseases of interest, patient sex, age, race/ethnicity, length of hospital stay, selected medical conditions, venous catheterization, and major surgery. Similarly, in separate models, unadjusted and adjusted OR were also derived for hospitalizations with a recorded diagnosis of any (i.e. one or more) of the four autoimmune diseases (a composite variable). To assess if there were effect modifications by sex and age-group, we conducted logistic regression analysis where we included in the model two-way interaction terms between each of the autoimmune diseases and sex and between each of the autoimmune diseases and age-group, and three-way interaction terms between each of the autoimmune diseases, sex and age-group, as well as the main effects variables for these factors and the other adjustment variables.
SAS version 9.3 (SAS Institute, Inc.) was used to conduct the analysis. Complex survey data analysis procedures (proc surveyfreq and proc surveylogistic) were used to adjust for the Nationwide Inpatient Sample sampling design, accounting for weights, strata, and clusters. The analysis was weighted to represent all non-maternal hospitalizations in the US among adults ≥19 years of age occurring in the types of hospitals included in the Health-care Cost and Utilization Project, Nationwide Inpatient Sample data base. Observations with missing information on the patient’s sex (0.1 %) and length of hospital stay (0.01 %) were excluded from regression analyses. Observations with missing race/ethnicity information (11 %) were classified into a ‘missing information’ response category for the race/ethnicity variable.
Results
In the study sample, there were 5,675,929 observations of hospitalizations among adults ≥19 years of age that were not because of maternal causes (hereafter referred to simply as hospitalizations of both women and men) and of these, 136,120 hospitalizations had recorded diagnoses of one or more of the four autoimmune diseases of interest; 103,653 of these were among non-maternal hospitalizations of adult women (hereafter referred to simply as hospitalizations of women) and 32,445 were among hospitalizations of adult men (hereafter referred to simply as hospitalizations of men). The number of hospitalizations with a recorded diagnosis of AIHA, ITP, RA, and SLE were 2,866, 9,646, 94,585, and 34,112, respectively, among hospitalizations of both women and men, 1,650, 5,305, 71,032, and 30,202, respectively, among hospitalizations of women, and 1,216, 4,336, 23,538, and 3,908, respectively, among hospitalizations of men. There were 5,007 hospitalizations that had diagnoses of more than one of the four autoimmune diseases. The estimates presented here onwards in this paper are all weighted estimates. A diagnosis of VTE was recorded in 2.28 % (95 % CI 2.22–2.34 %) of hospitalizations of both women and men that did not have a diagnosis of any of the four autoimmune diseases of interest (Fig. 1). In comparison, among hospitalizations of both women and men with recorded diagnoses of AIHA, ITP, RA, and SLE, a diagnosis of VTE was recorded in 4.46 % (95 % CI 3.70–5.22 %), 3.35 % (95 % CI 2.98–3.72 %), 2.65 % (95 % CI 2.77–2.89 %), and 2.77 % (95 % CI 2.57–2.97 %) of hospitalizations, respectively. Among hospitalizations of both women and men with recorded diagnoses of any of the autoimmune diseases of interest, a diagnosis of VTE was recorded in 2.84 % (95 % CI 2.72–2.96 %). The estimated percent of hospitalizations with a diagnosis of VTE are also presented separately for women and men in Fig. 1. Among hospitalizations of women without any of the autoimmune diseases of interest and among those with a diagnosis of SLE, the percent with a VTE diagnosis were 2.23 % (95 % CI 2.18–2.28 %) and 2.45 % (95 % CI 2.65–2.85 %), respectively. The corresponding percents among hospitalizations of men were 2.33 % (95 % CI 2.27–2.39 %) and 3.64 % (95 % CI 3.11–4.17 %), respectively.
Fig. 1.
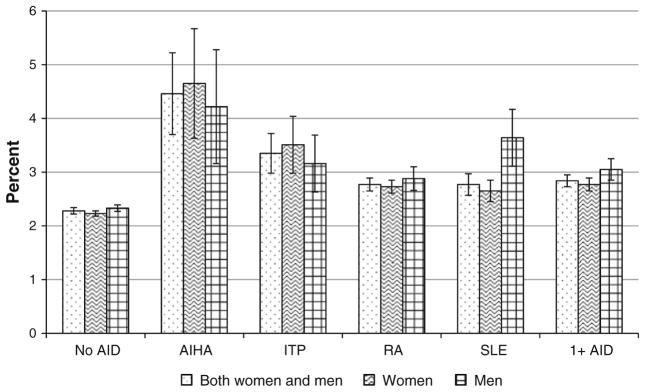
Rate of hospitalizations with a recorded diagnosis of venous thromboembolism among hospitalizations with and without autoimmune diseases. No AID no autoimmune disease, AIHA autoimmune hemolytic anemia, ITP immune thrombocytopenic purpura, RA rheumatoid arthritis, SLE systemic lupus erythematosus, 1+ AID one or more of the four autoimmune diseases. Hairlines indicate 95 % confidence limits of the respective estimates
The unadjusted and adjusted ORs for recorded diagnosis of VTE among hospitalizations with recorded diagnoses of autoimmune diseases are presented in Table 1. The adjusted OR for recorded diagnoses of VTE was highest among hospitalizations of both women and men with recorded diagnoses of AIHA, followed by SLE, ITP, and RA (when compared to hospitalization of men and women without the respective autoimmune disease in each case). The adjusted OR for a diagnosis of VTE among hospitalizations of both women and men with any of the four autoimmune disorders was 1.20 (95 % CI 1.16–1.24) compared to hospitalizations of both women and men without the four autoimmune diseases. Of the two-way interactions with sex, only those between sex and SLE and sex and ITP were statistically significant (p < 0.05); with regard to age-group, a two-way interaction was statistically significant (p < 0.05) only for SLE. A three-way interaction was statistically significant (p < 0.05) only for ITP. Table 2 presents ORs for VTE stratified by sex. The adjusted OR for VTE diagnosis among hospitalizations with a diagnosis of SLE (compared to hospitalizations without an SLE diagnosis) was higher among hospitalizations of men (OR 1.55, 95 % CI 1.33–1.80) than among hospitalizations of women (OR 1.19, 95 % CI 1.11–1.28). When compared to hospitalizations not having a diagnosis of ITP, the adjusted OR for VTE associated with a diagnosis of ITP was higher among hospitalizations of women (OR 1.29, 95 % CI 1.10–1.51) than among hospitalizations of men, among whom the OR was not statistically significant (OR 1.10, 95 % CI 0.94–1.29).
Table 1.
Unadjusted and adjusted odds ratios for recorded diagnoses of venous thromboembolism among adult non-maternal hospitalizations with selected autoimmune diseases, Nationwide Inpatient Sample, 2010
| Characteristic | Unweighted sample size | Unadjusted OR (95 % CI) for VTE | Adjusted OR (95 % CI) for VTE |
|---|---|---|---|
| Autoimmune hemolytic anemia | 2,866 | 2.00 (1.68–2.38) | 1.25 (1.05–1.49) |
| Immune thrombocytopenic purpura | 9,646 | 1.49 (1.33–1.66) | 1.20 (1.07–1.34) |
| Rheumatoid arthritis | 94,585 | 1.22 (1.17–1.27) | 1.17 (1.13–1.21) |
| Systemic lupus erythematosus | 34,112 | 1.22 (1.13–1.31) | 1.23 (1.15–1.32) |
| One or more of the above diseases | 136,120 | 1.25 (1.21–1.30) | 1.20 (1.16–1.24) |
The analysis was weighted to represent all non-maternal hospitalizations among adults ≥19 years age in non-rehabilitation community hospitals
The reference group for the odds ratios is all hospitalizations without the respective autoimmune disease
In adjusted analysis, the factors adjusted for are sex, race/ethnicity, age, selected medical risk factors (cancer, congestive heart failure, stroke, long bone fracture, and infection), venous catheterization, major surgery, and length of hospital stay. Since all four autoimmune diseases were included in a model, the results for each are adjusted with respect to the other three
OR odds ratio, CI confidence interval, VTE venous thromboembolism
Table 2.
Unadjusted and adjusted odds ratios for recorded diagnoses of venous thromboembolism among adult non-maternal hospitalizations with selected autoimmune diseases, by sex, Nationwide Inpatient Sample, 2010
| Characteristic | Women OR (95 % CI) for VTE | Men OR (95 % CI) for VTE |
|---|---|---|
| Unadjusted odds ratios | ||
| Autoimmune hemolytic anemia | 2.13 (1.70–2.66) | 1.84 (1.42–2.38) |
| Immune thrombocytopenic purpura | 1.59 (1.36–1.86) | 1.36 (1.16–1.60) |
| Rheumatoid arthritis | 1.23 (1.18–1.29) | 1.24 (1.15–1.34) |
| Systemic lupus erythematosus | 1.19 (1.11–1.28) | 1.57 (1.36–1.83) |
| One or more of the above diseases | 1.25 (1.21–1.30) | 1.32 (1.23–1.41) |
| Adjusted odds ratios | ||
| Autoimmune hemolytic anemia | 1.36 (1.08–1.71) | 1.14 (0.88–1.48) |
| Immune thrombocytopenic purpura | 1.29 (1.10–1.51) | 1.10 (0.94–1.29) |
| Rheumatoid arthritis | 1.16 (1.11–1.21) | 1.17 (1.08–1.26) |
| Systemic lupus erythematosus | 1.19 (1.11–1.28) | 1.55 (1.33–1.80) |
| One or more of the above diseases | 1.20 (1.15–1.24) | 1.21 (1.14–1.29) |
The analysis was weighted to represent all non-maternal hospitalizations among adults ≥19 years age in non-rehabilitation community hospitals
The reference group for the odds ratios is all hospitalizations without the respective autoimmune disease
In adjusted analysis, the factors adjusted for are race/ethnicity, age, selected medical risk factors (cancer, congestive heart failure, stroke, long bone fracture, and infection), venous catheterization, major surgery, and length of hospital stay. Since all four autoimmune diseases were included in a model, the results for each are adjusted with respect to the other three
OR odds ratio, CI confidence interval, VTE venous thromboembolism
Table 3 presents estimates of ORs for VTE stratified by age group. The adjusted ORs for VTE associated with a diagnosis of SLE (when compared to not having an SLE diagnosis) was higher among hospitalizations of both women and men who were 19–40 years of age (OR 1.82, 95 % CI 1.61–2.07) and 41–60 years of age (OR 1.14, 95 % CI 1.02–1.28) than those in the older age group, among which the OR did not reach statistical significance, (OR 0.94, 95 % CI 0.84–1.05). Results of analysis stratified by sex and age-group are presented in Table 4. The highest OR for VTE associated with a diagnosis of ITP (when compared to not having a diagnosis of ITP) was among hospitalizations of women 19–40 years of age (OR 1.66, 95 % CI 1.12–2.45). Though the three-way interaction was not statistically significant for SLE, the highest OR for VTE associated with a diagnosis of SLE (compared to not having a diagnosis of SLE) was among hospitalizations of men 19–40 years of age (OR 2.79, 95 % CI 2.16–3.61).
Table 3.
Unadjusted and adjusted odds ratios for recorded diagnoses of venous thromboembolism among adult non-maternal hospitalizations with selected autoimmune diseases, by age-group, Nationwide Inpatient Sample, 2010
| Characteristic | 19–40 Years of age OR (95 % CI) | 41–60 Years of age OR (95 % CI) | ≥61 Years of age OR (95 % CI) |
|---|---|---|---|
| Unadjusted odds ratios | |||
| Autoimmune hemolytic anemia | 2.03 (1.16–3.57) | 2.62 (1.91–3.58) | 1.76 (1.42–2.18) |
| Immune thrombocytopenic purpura | 1.72 (1.23–2.41) | 1.49 (1.19–1.86) | 1.43 (1.25–1.64) |
| Rheumatoid arthritis | 1.47 (1.22–1.78) | 1.12 (1.02–1.21) | 1.16 (1.11–1.21) |
| Systemic lupus erythematosus | 2.28 (2.02–2.57) | 1.15 (1.02–1.29) | 1.00 (0.90–1.11) |
| Overall (one or more of above) | 1.95 (1.75–2.17) | 1.17 (1.08–1.26) | 1.17 (1.13–1.22) |
| Adjusted odds ratios | |||
| Autoimmune hemolytic anemia | 1.14 (0.64–2.03) | 1.53 (1.10–2.13) | 1.19 (0.96–1.48) |
| Immune thrombocytopenic purpura | 1.40 (1.01–1.95) | 1.22 (0.97–1.53) | 1.17 (1.02–1.35) |
| Rheumatoid arthritis | 1.20 (0.99–1.45) | 1.17 (1.08–1.28) | 1.16 (1.11–1.21) |
| Systemic lupus erythematosus | 1.82 (1.61–2.07) | 1.14 (1.02–1.28) | 0.94 (0.84–1.05) |
| Overall (one or more of above) | 1.67 (1.48–1.85) | 1.19 (1.10–1.28) | 1.14 (1.10–1.18) |
The analysis was weighted to represent all non-maternal hospitalizations among adults ≥19 years age in non-rehabilitation community hospitals
The reference group for the odds ratios is all hospitalizations without the respective autoimmune disease
In adjusted analysis, the factors adjusted for are sex, race/ethnicity, selected medical risk factors (cancer, congestive heart failure, stroke, long bone fracture, and infection), venous catheterization, major surgery, and length of hospital stay. Since all four autoimmune diseases were included in a model, the results for each are adjusted with respect to the other three
OR odds ratio, CI confidence interval
Table 4.
Adjusted odds ratios for recorded diagnoses of venous thromboembolism among adult non-maternal hospitalizations with selected autoimmune diseases, by sex and age-group, Nationwide Inpatient Sample, 2010
| Characteristic | 19–40 Years of age OR (95 % CI) | 41–60 Years of age OR (95 % CI) | ≥61 years of age OR (95 % CI) |
|---|---|---|---|
| Among women | |||
| Autoimmune hemolytic anemia | 1.14 (0.58–2.23) | 1.67 (1.07–2.61) | 1.30 (0.97–1.75) |
| Immune thrombocytopenic purpura | 1.66 (1.12–2.45) | 1.48 (1.11–1.97) | 1.14 (0.94–1.38) |
| Rheumatoid arthritis | 1.15 (0.92–1.45) | 1.21 (1.10–1.33) | 1.15 (1.09–1.21) |
| Systemic lupus erythematosus | 1.68 (1.47–1.93) | 1.10 (0.98–1.24) | 0.92 (0.82–1.03) |
| One or more of the above | 1.58 (1.40–1.78) | 1.21 (1.11–1.31) | 1.13 (1.08–1.18) |
| Among men | |||
| Autoimmune hemolytic anemia | 1.23 (0.50–3.04) | 1.40 (0.85–2.30) | 1.06 (0.77–1.46) |
| Immune thrombocytopenic purpura | 0.94 (0.54–1.62) | 0.91 (0.63–1.30) | 1.21 (0.99–1.48) |
| Rheumatoid arthritis | 1.47 (0.96–2.24) | 1.12 (0.95–1.31) | 1.18 (1.09–1.29) |
| Systemic lupus erythematosus | 2.79 (2.16–3.61) | 1.31 (1.02–1.69) | 1.07 (0.81–1.42) |
| One or more of the above | 1.98 (1.59–2.46) | 1.14 (1.00–1.31) | 1.18 (1.10–1.27) |
The analysis was weighted to represent all non-maternal hospitalizations among adults ≥19 years age in non-rehabilitation community hospitals
The reference group for the odds ratios is all hospitalizations without the respective autoimmune disease
In adjusted analysis, the factors adjusted for are race/ethnicity, selected medical risk factors (cancer, congestive heart failure, stroke, long bone fracture, and infection), immune thrombocytopenic purpura, rheumatoid arthritis, venous catheterization, major surgery, and length of hospital stay. Since all four autoimmune diseases were included in a model, the results for each are adjusted with respect to the other three
OR odds ratio, CI confidence interval
Discussion
Our findings indicate that among non-maternal hospitalizations of adult patients in the United States, a recorded diagnosis of AIHA, ITP, RA, and SLE was significantly associated with an increased likelihood of having a recorded diagnosis of VTE. The percent of hospitalizations with a VTE diagnosis among those with a diagnosis of SLE was somewhat higher among hospitalizations of men compared to hospitalizations of women; the odds ratio for VTE diagnosis associated with an SLE diagnosis (compared to not having an SLE diagnosis) was also somewhat higher among hospitalizations of men than among hospitalizations of women. Age also appeared to be a significant effect modifier for the association with SLE where the OR for a VTE diagnosis associated with an SLE diagnosis (compared to not having an SLE diagnosis) was higher among hospitalizations of both women and men 19–40 years of age compared to hospitalizations of older women and men, with the OR being less than one and not statistically significant for hospitalizations of those ≥61 years of age. When stratified by both sex and age-group, the highest odds ratio for a VTE diagnosis associated with an ITP diagnosis (compared to not having an ITP diagnosis) was among hospitalizations of women 19–40 years of age. The findings of the current study add to the evidence suggesting that selected autoimmune diseases are associated with VTE occurrence.
There are several reasons why autoimmune disease may increase the risk of VTE. These reasons include that autoimmune diseases are commonly marked by inflammation, which increases the likelihood of thrombosis through several mechanisms including dysfunction of endothelium, increased tissue factor expression, increased levels of plasminogen activator inhibitor 1, and impairment of the protein C anticoagulant system [11, 16]. Also, higher levels of antiphospholipid antibodies (aPL) have been reported for SLE, RA, thyroid disease, and ITP, and other autoimmune diseases [11, 17]. Antiphospholipid antibodies may result in hypercoagulability and thrombosis by affecting the interaction between cell surface phospholipids and coagulation related proteins and possibly through other mechanisms as well [5, 11, 18, 19]. It is important to note there may be a higher risk of VTE regardless of the aPL status as other factors may influence thrombosis [5, 9, 11]. For example, it has been found that a substantial percent (~40 %) of SLE patients that suffer thrombosis do not have aPL [5]. It is also possible that an increased risk of VTE results, at least partially, from glucocorticoid therapy that may be associated with autoimmune diseases. A number of studies have indicated that glucocorticoids may increase the risk of VTE [20–24]. However, a review study reported that research findings showed that glucocorticoids had differential effects depending on the situation assessed [21]. Possible mechanisms of how glucocorticoids may increase VTE risk may include increased clotting factor and fibrinogen levels resulting from glucocorticoids [20, 21]. Another possible reason for the increased risk of VTE is that it is, at least in part, because of the increased risk of VTE that may be associated with chemotherapy, as chemotherapeutic agents may be used in the treatment of autoimmune diseases [25, 26]. More research is needed to better understand the reasons for the possible increased risk of VTE among patients with autoimmune diseases.
The increased OR for recorded diagnoses of VTE diagnosis among hospitalizations with diagnoses of the autoimmune diseases assessed in this study are consistent with a number of previous reports suggesting these disorders increase risk for developing VTE [5–10, 27, 28]. In a population-based cohort study conducted in Denmark that investigated the association between chronic ITP and VTE, it was found that the incidence risk ratio (IRR) for a primary diagnosis of VTE among patients with chronic ITP, compared to those in the reference group, was 2.65 (95 % CI 1.27–5.50); for provoked VTE (those with VTE related to cancer, fracture, trauma, surgery, or pregnancy/delivery) the IRR was 3.16 (95 % CI 1.11–8.98), and for unprovoked VTE, the IRR was 2.26 (95 % CI 0.81–6.30) [7]. Because of unavailable information, our study did not identify chronic ITP cases separately. A very serious possible complication of ITP is severe bleeding. Therefore, the possible increased risk of VTE among at least some ITP patients may cause additional difficulties in the clinical management of these patients; in addition, more investigation of this is needed.
A study using US hospital discharge data reported that among hospitalizations without joint surgery, the relative risk for VTE among hospitalizations of patients with RA, compared to those without the disorder, was 1.99 (95 % CI 1.98–2.00) [6]. In addition, in a study conducted in China, the standard incidence ratio (SIR) for VTE among SLE patients in two hospitals (as compared to the general regional population) was found to be 11.9 (95 % CI 7.3–19.6) [29]. In that study, the SIR was higher for SLE patients in younger age groups than for those in older age groups. The magnitude of the ORs associated with autoimmune diseases that have been reported by a number of other studies are substantially higher than found in our study [7, 9, 29]. Possible reasons for this may include differences in study designs and populations assessed. For instance, we used a cross-sectional approach to assess the risk of VTE diagnosis among hospitalizations with the four autoimmune diseases where the comparison group was all other no-maternal adult hospitalizations which themselves may have been at a higher risk of VTE diagnosis because hospitalized patients are more likely to have a VTE [1, 3] and because of other factors (e.g. other comorbidities that are risk factors for VTE). This could have resulted in the ORs found in our study being lower than they would have been if the comparison group was non-hospitalized persons.
Limitations of this study include that we used secondary data, and not data directly collected by the investigators through medical chart review. ICD-9-CM coded VTE diagnosis information recorded in discharge data are not always accurate. However, very good positive predictive values, 75–95 %, have been reported to be associated with many of the ICD-9-CM codes used to identify VTE diagnosis in this study [30]. The unit of study was the hospitalization and not the patient and it is likely some patients had more than one hospitalization in the study sample. Many of the VTE events among patients in this study may have occurred prior to hospitalization; however we did not have adequate information to identify all of these separately. Similarly, we were not able to assess VTE occurring after discharge from the hospital. Also, we did not have information regarding administration of VTE prophylaxis, which may have reduced the likelihood of VTE among hospitalized patients, and thus could not account for this. Similarly, we could not account for therapy, including glucocorticoids, or other treatment received by patients for autoimmune diseases that could have affected their disease condition and thus could have affected VTE occurrence. We also could not account for the status of autoimmune disease, e.g. acute state or under control. It was also possible that other factors could have affected or modified the associations between autoimmune diseases and VTE, but assessment for such was beyond the scope of the current study. Strengths of our study include that we were able to assess the association between VTE and the four autoimmune diseases in a large sample of hospitalizations from across the US. We were also able to control for selected demographic characteristics and comorbidities during the analysis.
The autoimmune diseases, AIHA, ITP, RA, and SLE may be associated with an increased risk of VTE among hospitalized patients. The risk of VTE among patients with SLE may vary by sex and by age-group. Current VTE prevention guidelines of the American College of Chest Physicians indicate antiphospholipid syndrome as a risk factor for VTE when assessing risk level among hospitalized medical patients. Such guidelines can help in clinical decision making regarding patients for consideration for VTE prophylaxis [31]. Also, additional research can help develop a more comprehensive understanding of the effect of autoimmune diseases on VTE risk among hospitalized patients.
Appendix
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes used
Diagnosis codes used:
DVT 451.1, 451.11, 451.19, 451.81, 451.83, 451.89, 453.2, 453.4, 453.40, 453.41, 453.42, 453.82, 453.84, 453.85;
PE 415.1, 415.11, 415.19.;
Autoimmune hemolytic anemia 283.0;
Immune thrombocytopenic purpura 287.31;
Rheumatoid arthritis 714, 714.0;
Systemic lupus erythematosus 710.0;
Cancer 140.xx–208.xx, 209.0x–209.3x;
Heart failure 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx;
Stroke 431, 434.xx, 436;
Infection 001.xx–139.xx, 460.xx-466.xx, 480.xx-488.xx;
Long bone fracture 812.xx, 813.xx, 820.xx, 821.xx, 823.xx.
Procedure code used:
Venous catheterization 38.93.
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official policy of the Centers for Disease Control and Prevention.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Hussain R. Yusuf, Email: hyusuf@cdc.gov, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd., N.E., MS-E64, Atlanta, GA 30333, USA
W. Craig Hooper, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd., N.E., MS-E64, Atlanta, GA 30333, USA.
Michele G. Beckman, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd., N.E., MS-E64, Atlanta, GA 30333, USA
Qing C. Zhang, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd., N.E., MS-E64, Atlanta, GA 30333, USA
James Tsai, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd., N.E., MS-E64, Atlanta, GA 30333, USA.
Thomas L. Ortel, Hemostasis and Thrombosis Center, Duke University Medical Center, Durham, NC, USA
References
- 1.Raskob GE, Silverstein R, Bratzler DW, et al. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(4):S502–S509. doi: 10.1016/j.amepre.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25 years population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman PC. Immune hemolytic anemia—selected topics. Hematol Am Soc Hematol Educ Program. 2009 doi: 10.1182/asheducation-2009.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Palatinus A, Adams M. Thrombosis in systemic lupus erythematosus. Semin Thromb Hemost. 2009;35:621–629. doi: 10.1055/s-0029-1242716. [DOI] [PubMed] [Google Scholar]
- 6.Matta F, Singala R, Yaekoub AY, Najjar R, Stein PD. Risk of venous thromboembolism with rheumatoid arthritis. Thromb Haemost. 2009;101:134–138. [PubMed] [Google Scholar]
- 7.Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2011;152(3):360–362. doi: 10.1111/j.1365-2141.2010.08418.x. [DOI] [PubMed] [Google Scholar]
- 8.Pullarkat V, Ngo M, Iqbal S, et al. Detection of lupus anticoagulant identifies patients with autoimmune haemolytic anaemia at increased risk for venous thromboembolism. Br J Haematol. 2002;118(4):1166–1169. doi: 10.1046/j.1365-2141.2002.03729.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune diseases: record linkage study. BMC Med. 2011;9:1–8. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoller B, Li X, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;21:244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 11.Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2(3):171–183. [PMC free article] [PubMed] [Google Scholar]
- 12.Cogo A, Bernardi E, Prandoni P, et al. Acquired risk factors for deep-vein thrombosis in symptomatic outpatients. Arch Intern Med. 1994;154:164–168. [PubMed] [Google Scholar]
- 13.Spencer FA, Emery C, Lessard D, et al. The Worcester venous thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Genintern Med. 2006;21:722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heit JA, Melton LJ, 3rd, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102–1110. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]
- 15.Stein PD, Matta F. Epidemiology and incidence: the scope of the problem and risk factors for development of venous thromboembolism. Clin Chest Med. 2010;31:611–628. doi: 10.1016/j.ccm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30(1):5–6. [PubMed] [Google Scholar]
- 17.Sangle NA, Smock KJ. Antiphospholipid antibody syndrome. Arch Pathol Lab Med. 2011;135:1092–1096. doi: 10.5858/2010-0325-RSR.1. [DOI] [PubMed] [Google Scholar]
- 18.de Groot PG, Lutters B, Derksen RH, et al. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3(9):1993–1997. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 19.George D, Erkan D. Antiphospholipid syndrome. Rev Prog Cardiovasc Dis. 2009;52(2):115–125. doi: 10.1016/j.pcad.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case–control study. JAMA Intern Med. 2013;173(9):743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 21.van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483–2493. doi: 10.1111/j.1538-7836.2010.04034.x. [DOI] [PubMed] [Google Scholar]
- 22.Calvo-Alén J, Toloza SM, Fernández M, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXV. Smoking, older age, disease activity, lupus anticoagulant, and glucocorticoid dose as risk factors for the occurrence of venous thrombosis in lupus patients. Arthritis Rheum. 2005;52(7):2060–2068. doi: 10.1002/art.21149. [DOI] [PubMed] [Google Scholar]
- 23.Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935–943. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 24.Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743–2750. doi: 10.1210/jc.2009-0290. [DOI] [PubMed] [Google Scholar]
- 25.Falanga A, Marchetti M. Anticancer treatment and thrombosis. Thromb Res. 2012;129(3):353–359. doi: 10.1016/j.thromres.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382(9894):809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 27.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Johannesdottir SA, Schmidt M, Horváth-Puhó E, Sørensen HT. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10(5):815–821. doi: 10.1111/j.1538-7836.2012.04666.x. [DOI] [PubMed] [Google Scholar]
- 29.Mok CC, Ho LY, Yu KL, To CH. Venous thromboembolism in southern Chinese patients with systemic lupus erythematosus. Clin Rheumatol. 2010;29(6):599–604. doi: 10.1007/s10067-009-1364-z. [DOI] [PubMed] [Google Scholar]
- 30.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–67. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]


