Abstract
Objectives
Late presentation to HIV/AIDS services compromises treatment outcomes and misses opportunities for biomedical and behavioral prevention. There has been significant heterogeneity in how the term “late presentation” (LP) has been used in the literature. In 2011, a consensus definition was reached using CD4 counts to define and measure late presenters and while it is useful for clinical care, the consensus definition has several important limitations that we discuss in this article.
Methods
Using the spectrum of engagement in HIV care presented by Gardner and colleagues, this article highlights issues and opportunities associated with use of the consensus definition.
Results
The consensus definition is limited by three principal factors: 1) The CD4 threshold of 350 is being increasingly questioned as the biomedical justification grows for earlier initiation of treatment; 2) CD4 evaluations are conducted at multiple services providing HIV care, thus it remains unclear to which service the patient is presenting late; and 3) The limited availability of CD4 evaluations restricts its use in determining the prevalence of LP in many settings.
Conclusions
The consensus definition is useful because it describes the level of disease progression and allows for consistent evaluation of prevalence and determinants of LP. Suggestions are provided for improving the application of the consensus definition in future research.
Keywords: late presentation, HIV/AIDS, testing, treatment, CD4 evaluations
INTRODUCTION
Studies in high and low-income countries continue to document delays in the utilization of HIV/AIDS services, from testing, to receiving and adhering to effective antiretroviral therapy (ART), and access to continued care (1). Delayed utilization of HIV/AIDS services is associated with compromised treatment response and missed opportunities for preventing HIV transmission. The term ‘late presentation to care’ (LP) has been widely used in the peer-reviewed literature but, until recently, there has been little agreement as to how LP should be defined (2–8).
In response to the heterogeneity of approaches to characterizing LP, the European Late Presenter Group (ELPG), consisting of 51 experts from 13 countries, published a consensus statement in 2011 proposing a definition of LP based on CD4 count or clinical symptoms: “Persons presenting for care with a CD4 count below 350 cells/mm3 or presenting with an AIDS defining event, regardless of the CD4 cell count.” In addition, the ELPG defined presentation with advanced HIV disease as “Persons presenting for care with a CD4 count below 200 cells/mm3 or presenting with an AIDS-defining event, regardless of CD4 cell count” (9).
The consensus definition describes the level of disease progression and is therefore useful from a clinical perspective. Further, it allows for consistent evaluation of the prevalence and determinants of LP. However, from a public health perspective, several issues remain. This article uses the spectrum of engagement in HIV care proposed by Gardner and colleagues to evaluate the implications of the consensus definition and to highlight issues and provide suggestions to improve its application going forward. Doing so will help ensure research, programs and providers can more effectively address the clinical and public health concerns related to LP.
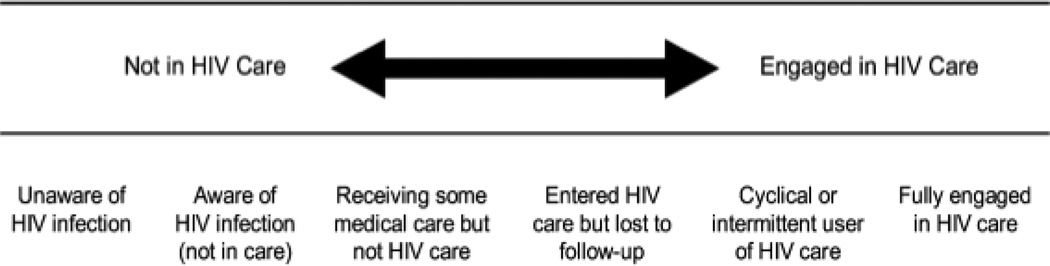
THE SPECTRUM OF ENGAGEMENT IN HIV CARE
Several different models have been developed to highlight stages in the continuum of HIV/AIDS care (10, 11). Recently, Gardner and colleagues presented the ‘spectrum of engagement in HIV care’ (hereafter referred to as the ‘cascade’) to highlight the range of barriers to achieving optimal treatment outcomes (1). This model has subsequently been referenced in several high-impact peer-reviewed journals (12–15) and has been highlighted for clarifying the range of services that should be considered to improve the uptake of HIV testing and subsequent linkage to care. Though this model was developed in a high-income context, the range of services highlighted is relevant in other economic settings, although the provision of these services may differ based on the existing infrastructure of the health system. This model therefore highlights important considerations with respect to individual health and how the provision of services can best improve the health outcomes of HIV-infected patients. The cascade is shown in Figure 1 and has been described extensively elsewhere (15).
Figure 1.
The Cascade Showing the Spectrum of Engagement in HIV Care from Gardner and Colleagues (1)
The cascade provides a useful framework to determine how the ELGP measure will work in practice and highlights important concerns with respect to individual health and the role of health services. The cascade highlights two types of information important: First, CD4 count is important because it describes the level of disease progression. This, however, is not sufficient to characterize the nature of delays in testing and treatment services more generally because CD4 thresholds represent immune system assessments of a particular individual at one specific point in time, and do not contain information on health seeking history or other relevant information. The second key piece of information is the time between access to services along the various stages on the cascade. The timing of events is not only important from a clinical perspective, but also critically affects the individual’s knowledge about the disease, as well as the risk of transmission to their partners, especially as individuals are highly infectious in early and late stages of disease. Therefore the following analysis will use the cascade to consider the limitations of using the consensus definition and propose suggestions to improve its application going forward.
OPPORTUNITIES AND ISSUES RELATED TO THE CONSENSUS DEFINITION
The consensus proposed by the ELPG allows for consistency in the definition of late presentation and facilitates comparative studies across districts and countries. In the past, definitions have often employed different levels of immunological competence to determine what ‘late ’means, and therefore differentially consider the size of the problem. For example, some studies report the number of HIV cases, while others report AIDS cases. Johnson’s review of late testing identified two studies using AIDS cases and eight studies using HIV cases as the denominator (16). It is clear that unless measures are consistent, such as including all HIV cases or alternatively only AIDS cases, it is not possible to determine whether the scale of the problem is due to differences in individuals presenting to services or simply differences in defining late presentation. The consensus definition brought clarity by suggesting all individuals diagnosed positive for HIV should be considered in the analysis of late presentation. This helps avoid confusion as AIDS cases can be defined differently across contexts (17, 18). So, while the consensus definition is helpful in some respects, several issues remain.
Issue 1: The CD4 threshold of 350 is being increasingly questioned as the biomedical justification grows for earlier initiation of treatment
The ELPG use of CD4 threshold 350 is based on empirical evidence demonstrating the low “risk of disease progression” at this level. However, there is some evidence in favor of an earlier ART initiation. With respect to HIV-related morbidity, studies have demonstrated that earlier treatment initiation improves health by preventing or limiting damage related to the viral replication of HIV, especially during the early stages of infection (19–25). With respect to the risk of transmission for HIV, a Phase III, two-arm, multi-site, randomized trial demonstrated that the transmission of HIV was reduced by 96% to HIV-negative partners among individuals starting ART with a CD4 count >350 cells per mm3 (26) and thus provides evidence to initiate treatment at earlier stages of disease progression to promote the prevention of transmission. Several months following the publication of these results, the US guidelines were revised to recommend treatment for all HIV infected patients (17). However a 2013 report showed that although studies demonstrated a greater CD4 cell recovery with earlier initiation of therapy during primary infection, the studies did not define a clear clinical benefit for such early treatment (27, 28). The results of the a new Phase IV study, conducted in 30 countries and enrolling 4,000 HIV-infected individuals, will aim to provide definitive evidence of the risks and benefits of early treatment initiation (29). If the biomedical justification grows for treatment initiation at higher CD4s, then CD4 count may become less important.
Issue 2: CD4 evaluations are conducted at multiple services providing HIV care, thus it remains unclear to which service the patient is presenting late
The ELPG defines presentation for care as “attendance at a health care facility that is able to monitor progression of HIV infection and initiate appropriate medical care, including ART, as appropriate. Diagnosis of HIV infection alone does not signify presentation for care.” In many instances, however, this constellation of services may be available in different locations and at different stages of care depending on the local economic and health system context. For example in Kenya, a country where ART has been available since early 2000, individuals enrolling in HIV/AIDS care have CD4 evaluations conducted at the facility where they are tested (30). In this context, the ELPG definition would capture barriers associated with testing and initial engagement in care. As a result, a more limited range of problems could be contributing to the prevalence of late presentation, such as ineffective testing campaigns and weak support for linkage to care. In Brazil, one of the first countries to make ART free and universally available, guidelines call for CD4 evaluations to be conducted once an individual is enrolled in care (31). However to obtain a CD4 count the patient must go to a separate laboratory, have their blood drawn, and then must return for the results, thus increasing the effort required by the individual to pursue treatment and continued care. In this scenario, late presentation could represent late presentation to testing, initial engagement or potentially through continued care.
Issue 3: The limited availability of CD4 evaluations restricts its use in determining the prevalence of LP in many settings
The availability of CD4 evaluations can also present several challenges. While it is largely assumed that systems for CD4 evaluation are widely available where ART is provided, several studies in the US and in Europe, in addition to one study in Vietnam, have highlighted the frequency of missing CD4 count data (32–39). When no CD4 is recorded, it is often assumed the individual was not sick enough to justify an evaluation of their CD4 count. Nonetheless it is possible that these patients died before receiving a CD4 count evaluation. Exclusion of patients without a CD4 count could lead to underestimating the proportion of patients who are late presenters. More often, studies tend to include these patients in the denominator of any calculations but make the assumption that in the absence of a measured low CD4 cell count, these patients are above the threshold of interest, which would also contribute to underestimating the proportion of patients who are late presenters. This would therefore minimize the scope of the problem and diminish potential resources that could be directed to address LP. The consensus definition does not advise as to how patients without CD4 evaluations should be categorized and therefore reduces the comparability of data.
CONSIDERATIONS GOING FORWARD
There are several ways that the issues raised above can be clarified in the application of the consensus definition, all of which would help to enhance the comparability of data and augment further research and programmatic work in this area. Table 1 provides a summary of key action points that can potentially address the limitations highlighted. To begin with, as noted in the consensus definition, the existing CD4 threshold of 350 should continue to be reported, even if other CD4 thresholds are introduced to allow for more consistent comparisons across studies. Second, it is necessary to specify where the CD4 evaluation was conducted: at the testing facility; where treatment is provided; or at a separate laboratory. This will help clarify the range of services to which individuals may be presenting late. Third, the percentage of patients for whom CD4 evaluations are not available should be documented. This will not help determine the direction of bias but will at least make the scope of missing data known.
Table 1.
Suggestions for Improving Use of the Consensus Definition
| ACTION POINTS | ||
|---|---|---|
| Issue | Potential Solution | |
| 1 | The CD4 threshold of 350 is being increasingly questioned as the biomedical justification grows for earlier initiation of treatment. | Document the existing CD4 threshold of 350, even if other CD4 thresholds are introduced, to allow for more consistent comparisons across studies. |
| 2 | CD4 evaluations are conducted at multiple services providing HIV care, thus it remains unclear to which service the patient is presenting late. | Specify that the consensus definition evaluates “late presentation to CD4 evaluation” and report where the CD4 evaluation was conducted to clarify the range of services to which individuals may be presenting late. |
| 3 | The limited availability of CD4 evaluations restricts its use in determining the prevalence of LP in many settings. | Report the percentage of patients for which CD4 evaluations was not available. Doing so will establish the scope of missing data. |
CONCLUSIONS
The ELPG consensus definition for late presentation represents an important step forward and has the potential to enhance the comparability of data and the feasibility and effectiveness of research in this area. As a next step, researchers should give attention to the issues raised to improve our understanding of LP and its implications. Further, the engagement of public health authorities is critical to ensuring CD4 data (where available) are collated in central surveillance systems to monitor and evaluate LP. Going forward, more detailed measures will be needed as the consensus definition does not take into consideration the time between two sequential stages on the cascade. The consensus definition should be applied in ways so that it can be clear whether the noted delay is the result of barriers to testing, enrollment, treatment, or continued engagement in care. This is important not only because of the implications for the definition of LP but also because the time between infection and treatment affects risk of transmission to sexual partners, especially as individuals are highly infectious in early and late stages of disease progression. In the future, collecting data on the time between HIV diagnosis and subsequent services will allow for a more comprehensive evaluation of the barriers to prompt HIV treatment and to ensure services are used in a timely manner by all who need them.
ACKNOWLEDGEMENTS
This publication resulted from research supported by the training grant entitled “HIV and Other Infectious Consequences of Substance Abuse (T32DA13911-12). In addition, this publication was made possible with help from the Lifespan/Tufts/Brown Center for AIDS Research (P30AI042853) from the National Institute Of Allergy And Infectious Diseases.
Works Cited
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, WJ B. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony MN, Gardner L, Marks G, Anderson-Mahoney P, Metsch LR, Valverde EE, et al. Factors associated with use of HIV primary care among persons recently diagnosed with HIV: examination of variables from the behavioural model of health-care utilization. AIDS Care. 2007 Feb;19(2):195–202. doi: 10.1080/09540120600966182. [DOI] [PubMed] [Google Scholar]
- 3.Hocking JS, Rodger AJ, Rhodes DG, Crofts N. Late presentation of HIV infection associated with prolonged survival following AIDS diagnosis--characteristics of individuals. Int J STD AIDS. 2000 Aug;11(8):503–508. doi: 10.1258/0956462001916407. [DOI] [PubMed] [Google Scholar]
- 4.Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo ZoneEthiopia: a case-control study. AIDS Res Ther. 2011;8:8. doi: 10.1186/1742-6405-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010 Jun 1;50(11):1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonjour MA, Montagne M, Zambrano M, Molina G, Lippuner C, Wadskier FG, et al. Determinants of late disease-stage presentation at diagnosis of HIV infection in Venezuela: a case-case comparison. AIDS Res Ther. 2008;5:6. doi: 10.1186/1742-6405-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009 Oct 1;52(2):280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojumdar K, Vajpayee M, Chauhan NK, Mendiratta S. Late presenters to HIV care and treatment, identification of associated risk factors in HIV-1 infected Indian population. BMC Public Health. 2010;10:416. doi: 10.1186/1471-2458-10-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011 Jan;12(1):61–64. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 10.Janssen RS, Holtgrave DR, Valdiserri RO, Shepherd M, Gayle HD, KM DC. The serostatus approach to fighting the HIV epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91(7):1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MO, Rose CD, Dilworth SE, Neilands TB. Advances in the conceptualization and measurement of Health Care Empowerment: development and validation of the Health Care Empowerment inventory. PLoS One. [Research Support, N.I.H., Extramural] 2012;7(9):e45692. doi: 10.1371/journal.pone.0045692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallant JE, Adimora AA, Carmichael JK, Horberg M, Kitahata M, Quinlivan EB, et al. Essential components of effective HIV care: a policy paper of the HIV Medicine Association of the Infectious Diseases Society of America and the Ryan White Medical Providers Coalition. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. [Practice Guideline Research Support, Non-U.S. Gov't] 2011 Dec;53(11):1043–1050. doi: 10.1093/cid/cir689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. Journal of Acquired Immune Deficiency Syndromes. [Research Support, N.I.H., Extramural] 2012 Jan 1;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;10(26):1735–1738. doi: 10.1097/QAD.0b013e328355d67b. [DOI] [PubMed] [Google Scholar]
- 16.Johnson M, Sabin C, Girardi E. Definition and epidemiology of late presentation in Europe. Antiviral Therapy. 2010;15(1):3–8. doi: 10.3851/IMP1522. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Bethesda: 2011. [Google Scholar]
- 18.British HIV Association. Guidelines for the treatment of HIV-1 positive adults with antiretroviral therapy. HIV Med. 2012;13(2):1–85. doi: 10.1111/j.1468-1293.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 19.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53(9):927–935. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reekie J, Gatell JM, Yust I, Bakowska E, Rakhmanova A, Losso M, et al. Fatal and nonfatal AIDS and non-AIDS events in HIV-1-positive individuals with high CD4 cell counts according to viral load strata. AIDS. 2011;25(18):2259–2268. doi: 10.1097/QAD.0b013e32834cdb4b. [DOI] [PubMed] [Google Scholar]
- 21.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8(5):522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 22.Estrella M, Fine DM, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, et al. HIV type 1 RNA level as a clinical indicator of renal pathology in HIV-infected patients. Clin Infect Dis. 2006;43(3):377–380. doi: 10.1086/505497. [DOI] [PubMed] [Google Scholar]
- 23.Atta MG, Gallant JE, Rahman MH, Nagajothi N, Racusen LC, Scheel PJ, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant. 2006;21(10):2809–2813. doi: 10.1093/ndt/gfl337. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, PE K. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16(8):2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 25.Kalayjian RC, Franceschini N, Gupta SK, Szczech LA, Mupere E, Bosch RJ, et al. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22(4):481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le T, Wright EJ, Smith DM. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The SPARTAC Trial Investigators. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368:207–217. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover K. NIH Study Examines Best Time for Healthy HIV-infected People to Begin Antiretrovirals. Bethesda: National Institute of Allergy and Infectious Diseases; 2011. [cited 2013 April 4]. [Google Scholar]
- 30.Ferguson L, Lewis J, Grant AD, Watson-Jones D, Vusha S, Ong'ech JO, et al. Patient attrition between diagnosis with HIV in pregnancy-related services and long-term HIV care and treatment services in Kenya: a retrospective study. J Acquir Immune Defic Syndr. 2012;60(3):90–97. doi: 10.1097/QAI.0b013e318253258a. [DOI] [PubMed] [Google Scholar]
- 31.Saude Md, Saude SdVe Departamento de DST AeHV. Recomendacoes para Profilaxia da Transmissao Vertical do HIV e Terapia Antirretroviral em Gestantes. Brasilia: 2010. [Google Scholar]
- 32.Grigoryan A, Hall HI, Durant T, Wei X. Late HIV diagnosis and determinants of progression to AIDS or death after HIV diagnosis among injection drug users, 33 US States, 1996–2004. PLoS One. 2009;4(2):e4445. doi: 10.1371/journal.pone.0004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobrino-Vegas P, Garcia-San Miguel L, Caro-Murillo AM, Miro JM, Viciana P, Tural C, et al. Delayed diagnosis of HIV infection in a multicenter cohort: prevalence, risk factors, response to HAART and impact on mortality. Curr HIV Res. 2009 Mar;7(2):224–230. doi: 10.2174/157016209787581535. [DOI] [PubMed] [Google Scholar]
- 34.Yang B, Chan SK, Mohammad N, Meyer JA, Risser J, Chronister KJ, et al. Late HIV diagnosis in Houston/Harris County, Texas, 2000–2007. AIDS Care. 2010 Jun;22(6):766–774. doi: 10.1080/09540120903431348. [DOI] [PubMed] [Google Scholar]
- 35.Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis and consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000–2004) AIDS. 2006 Nov 28;20(18):2371–2379. doi: 10.1097/QAD.0b013e32801138f7. [DOI] [PubMed] [Google Scholar]
- 36.Delpierre C, Cuzin L, Lauwers-Cances V, Marchou B, Lang T. High-Risk groups for late diagnosis of HIV infection: a need for rethinking testing policy in the general population. AIDS Patient Care STDS. 2006 Dec;20(12):838–847. doi: 10.1089/apc.2006.20.838. [DOI] [PubMed] [Google Scholar]
- 37.Delpierre C, Cuzin L, Lert F. Routine testing to reduce late HIV diagnosis in France. BMJ. 2007 Jun 30;334(7608):1354–1356. doi: 10.1136/bmj.39225.458218.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hacker MA, Leite IC, Renton A, Torres TG, Graceie R, Bastos FI. Reconstructing the AIDS epidemic among injecting drug ssers. Cad Saude Publica. 2006;22(4):751–760. doi: 10.1590/s0102-311x2006000400014. [DOI] [PubMed] [Google Scholar]
- 39.Nhac-Vu HT, Giard M, Phong ND, Vanhems P. Risk factors for delayed HIV diagnosis at the Hospital of Tropical Diseases in Ho Chi Minh City, Vietnam. Int J STD AIDS. 2010 Dec;21(12):802–805. doi: 10.1258/ijsa.2010.010045. [DOI] [PubMed] [Google Scholar]