Abstract
Recent literature suggests that sEcad exerts pro-oncogenic effects, possibly acting as a ligand for the human epidermal growth factor family. Here we show that sEcad is a novel candidate protein for drug targeting since it is increased in human and mouse HER2-positive (HER2+) breast tumors, MMTV-PyMT bodily fluids and human cell culture systems. Mechanistically, we show that endogenous sEcad, and to a lesser extent membrane-bound E-cadherin, associates with HER1, HER2, and HER3 in human and MMTV-PyMT mouse HER2+ tumors and with HER1 in triple negative breast cancer (TNBC) specimens. Furthermore, addition of exogenous recombinant human E-cadherin/Fc chimeric protein (rhEcad/Fc; sEcad) to HER2+ MCF-7, SKBR3, and HER2-negative MDA-MB-231 TNBC cells, resulted in sEcad-HER receptor family interactions, activation of HER1–4 and downstream pro-survival signaling, including the MAPK-PI3K/Akt/mTOR pathways and IAP family members. Lastly, we demonstrate that sEcad exerts pro-oncogenic effects via HER signaling, and acts additively with the HER ligand EGF to promote HER2+ breast cancer proliferation and migration, as well as TNBC invasion. Because sEcad associates and activates many of the oncogenic pathways that tumors utilize for growth and survival and serum levels in patients correlates with clinical response, suggests that targeted therapy against sEcad in combination with other therapies may potentially offer a novel therapeutic strategy for the treatment of breast cancers.
Keywords: sEcad, HER1–4, PI3K, Akt, mTOR, IAPs
INTRODUCTION
Breast cancer invasiveness, tumor grade, and poor prognosis are associated with down-regulation of membrane-bound E-cadherin. E-cadherin, a transmembrane protein that mediates homotypic cell–cell adhesions, contains a proteolytic cleavage site proximal to the transmembrane region, which mediates the shedding of its soluble ectodomain fragment, termed sEcad into the intercellular milieu [1,2]. This proteolytic processing of intact E-cadherin is mediated by metalloproteinases (MMPs) or members of the membrane-anchored family of metalloproteinases (a disintegrin and metalloproteinase; ADAMs) [2–6]. Shedding of sEcad occurs constitutively at low levels in normal epithelial cells, but is significantly increased in cancer cells in vitro and primary prostate tumor sites and metastatic foci in vivo [2–7]. However, expression levels of sEcad in human and mouse HER2+ or triple negative breast cancer (TNBC) primary breast tumors or HER2+MMTV-PyMT mouse bodily fluids, have yet to be explored. Nonetheless, in breast and other cancers, heightened sEcad levels correlate with tumor size, response to chemotherapy and predict a shorter disease-free interval [8,9], suggesting that sEcad may play a central role in tumor growth and survival.
The human epidermal growth factor receptors (HER1–4) are transmembrane glycoproteins that play a fundamental role in breast carcinogenesis. Within the tumor mileu, more than a dozen EGF receptor ligands differentially bind to the HER family of receptors to promote HER homo- or heterodimerization. Within this epidermal growth factor receptor ligand family, EGF, transforming growth factor-α (TGFα), and amphiregulin bind HER1, neuregulins-1 and -2 bind to either HER3 or HER4, heparin binding EGF (HB-EGF), betacellulin (BTC), and epiregulin bind to either HER1 or HER4, and neuregulins-3 and -4 are specific for HER4 [10–12]. In contrast, no direct high-affinity ligand has been identified for HER2, although it is the preferred heterodimerization partner for all HER family members [10–12]. After ligand binding, HER dimerization results in the phosphorylation of intracellular tyrosine residues, and subsequent activation of the mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathways [10–12]. Induction of these pathways culminates in many cellular responses, including proliferation, survival, angiogenesis, and metastasis [10–12]. As such, overexpression or constitutive activation of HER1 or HER2 is associated with a poor clinical prognosis in breast, lung, head and neck, and other cancers [13], and accordingly drugs that target the HER receptors and/or MAPK, PI3K, and mTOR are now in active development for breast cancer.
Another gene family regulating tumor cell survival and that are actively being targeted in breast cancer are the inhibitor of apoptotic proteins (IAPs), including survivin, human inhibitor of apoptosis protein-1 and -2 (cIAP-1 and cIAP-2), X-linked inhibitor of apoptosis (XIAP) and livin [14–16]. The smallest IAP family member survivin is undetectable in most adult tissues, but is selectively expressed in human breast and other cancers, and correlates with a poor clinical outcome [17–19]. Similarly, in invasive ductal breast cancers with a triple-negative phenotype, expression of XIAP correlated with a more aggressive phenotype and was associated with a decreased overall and disease-free survival [20]. Overexpression of IAP proteins has further been shown to confer protection against a number of pro-apoptotic stimuli and to play a critical role in resistance to chemotherapeutic agents [21,22]. A functional link between HER2 and survivin has also been recently described. Specifically, Xia et al. [23] demonstrated that genetic or pharmacologic knockdown of HER2, via PI3K but not MAPK signaling, significantly reduced survivin levels and induced apoptosis in HER2-overexpressing breast cancer cell lines. Moreover, patients with HER2-overexpressing tumors, treated with the HER1/HER2 inhibitor Lapatinib, exhibited a marked reduction in survivin protein levels, providing a clinical validation of the mouse studies [23]. In breast cancer, a regulatory mechanism of HER2 and downstream PI3K/Akt signaling in the expression of survivin protein has also been described [24]. Therefore, these studies suggest that the HER family members, via downstream PI3K signaling, may activate IAPs to promote mitogenesis and survival in breast tumorigenesis.
Recent literature suggests that sEcad enhances tumor cell proliferation, migration, and invasion, possibly acting as a ligand for the HER. Specifically, Najy et al. [4] demonstrated endogenous sEcad interacting with HER2 and HER3 in E-cadherin+ MCF-7 breast cancer cells, and exogenous sEcad associating with HER2 in E-cadherin− SKBR3 cells, the latter resulting in HER2–HER3 heterodimerization, HER3 phosphorylation, and ERK activation. In normal Madin–Darby canine kidney (MDCK) cells, exogenous sEcad promoted cell survival via activation of HER1, PI3K, Akt, and ERK1/2 signaling [25]. Additionally, Zuo et al. [26] demonstrated that HER1 activation promoted SCC10A migration and invasion by promoting MMP-9-mediated degradation of E-cadherin into sEcad. However, whether sEcad activates other HER receptors or downstream IAP family members in HER2+ and TNBC breast cancers has not been previously shown. Moreover, the functional effects of sEcad on these distinct breast cancer phenotypes have yet to be fully elucidated.
In the present study, we investigated whether endogenous sEcad is overexpressed in HER2+ human and mouse breast cancer specimens, human TNBC tumors and cell culture systems. We then used HER2+ and TNBC cell lines to explore whether sEcad may exert pro-oncogenic effects via modulation of the HER-PI3K/Akt/mTOR-IAP axis and/or by synergizing with the HER ligand EGF.
METHODS
Animal Studies
Wild-type (WT) and MMTV-PyMT mouse breeders were obtained from Jackson Laboratories, Bar Harbor, ME and mated according to vendor’s specifications. At 90-d of age, tumors, blood and urine were collected and stored until assayed. Overnight urine collections were obtained from wild-type (WT) and 90-d-old MMTV-PyMT transgenic mice using specialized metabolic cages (Tecniplast USA Inc., Exton, PA) with food and water supplied ad libitum.
Patients and Tissues
HER2+ human breast cancer specimens were obtained from the NCI Cooperative Human Tissue Network (CHTN) and from Proteogenex (Culver City, CA). Tumors from CHTN included 7 HER2+ infiltrating ductal carcinomas with an age range of 52–77 yr with corresponding adjacent normal breast tissues from reduction mammoplasty tissue (n = 7). TNBC human tumor specimens and adjacent normal breast tissue specimens were obtained from Proteogenex (n = 3).
Drug and Inhibitor Treatments
Cells were pretreated for 2 h with the following inhibitors from LC Laboratories (Woburn, MA): pan-HER (1 µM; Canertinib), HER1/HER2 (5 µM; Lapatinib), HER1 (5 µM; Gefitinib), HER2 (20 µM; Mubritinib), and then incubated with or without rhEcad/Fc (sEcad) for 22–24 h. Cell proliferation, migration or invasion were measured as described below. Recombinant human E-cadherin Fc chimera (sEcad), purchased from R&D Systems, Minneapolis, MN, represents the cDNA sequence encoding the extracellular domain of human E-cadherin (amino acid residues 155–707 or EC1-EC5 domains of the E-cadherin extracellular domain) fused by means of a polypeptide linker to the Fc region of human IgG1 that is 6× histidine-tagged at the C-terminus. GM6001 was purchased from Biomol (Ann Arbor, MI). Here, MCF-7 cells were incubated with or without 10 µM of GM6001 in serum free culture medium for 24 h, and the conditioned media and cell lysates were collected and analyzed for sEcad levels. Recombinant human EGF (rhEGF) was purchased from Enzo Life Sciences (Farmingdale, NY). In this set of experiments, cells were treated with 10 or 20 µg/ml of rhEcad/Fc, equimolar concentrations of EGF (500 ng or 1 µg/ml) alone or EGF in combination with rhEcad/Fc for the designated time periods, and then assessed for cell proliferation, migration, invasion and/or HER and downstream signaling.
Cell Culture and Antibodies
Human MCF-7, SKBR3, MDA-MB-231, and MCF-10A cells were purchased from ATCC, and cultured according to ATCC recommendations. Primary antibodies used in this study were as follows: for immunoprecipitation, EGFR (Ab-15), HER2 (Ab-17), HER3 (Ab-6), HER4 (Ab-1) (Thermo Fisher Scientific, Rockford, IL), and E-cadherin ectodomain-specific (H108, Santa Cruz Biotchnology, Dallas, Tx); for Western blot, EGFR (4267), HER2 (2165), HER3 (4754), HER4 (4795), pEGFR (3777), pHER2 (2243), pHER3 (4561), pHER4 (4757), pPI3K (4228), pAkt (4060), pmTOR (5536), p4E-BP1(2855), pp70S6K (9234), survivin (2808), cIAP-1 (4952), XIAP(2045), and livin (5471) (Cell Signaling, Danvers, MA); E-cadherin (H-108), β-actin (C4) (Santa Cruz); G3PDH (AM4300, Ambion, Grand Island, NY), and His-Tag (ab9108, Abcam, Cambridge, MA).
Immunoprecipitation and Immunoblotting
Protein extraction in cells was performed on ice using total protein extraction buffer: 20 mM Tris pH7.5; 137 mM NaCl; 100 mM NaF; 10% glycerol;1% NP40; 1 mM PMSF and protease inhibitor cocktail (Sigma, St. Louis, MO). For normal and tumor tissues, sEcad was extracted in an aqueous solution (PBS) and not in the protein extraction buffer. Protein concentration was measured using a BCA Protein Assay Kit (Pierce, Rockford, IL). Protein samples (50–100 µg) were denatured at 95°C and subsequently separated by 4–15% SDS–PAGE. After transfer to nitrocellulose membrane and blocking with 5% nonfat milk, samples were probed with primary antibodies. Western blot images were captured using HP Scanjet G4050 and analyzed relative to G3PDH or actin using NIH Scion Image. Immunoprecipitation assays were carried out by harvesting tissues or cells with immunoprecipitation lysis buffer [20 mM Tris–HCl, pH 7.5; 137 mM NaCl; 100 mM NaF; 10% glycerol (v/v); 1% (v/v) Nonidet P-40; 1 mM PMSF and protease inhibitor cocktail (Sigma)]. After brief sonication, lysates were cleared by centrifugation at 4°C. Supernatants were precleared and incubated with EGFR/HER1, HER2, HER3, HER4, or E-cadherin ectodomain-specific antibodies for 4 h and protein A/G plus agarose beads (Santa Cruz, sc-2003) for 2 h at 4°C. The immunocomplexes were washed three times, boiled in sample buffer [60 mM Tris–Cl, pH 6.8; 2% SDS (v/v); 10% glycerol (v/v); 5% β-mercaptoethanol (v/v); and 0.01% bromophenol blue (v/v)], and loaded on SDS–PAGE for protein analysis.
ELISA Assay
Levels of sEcad in the serum and urine of MMTV-PyMT mice, or conditioned media of serum starved cells, were quantified using human E-cadherin Quantikine ELISA Kits (R&D Systems), according to the manufacturer’s recommendations. Serum, urine and conditioned media were diluted appropriately to fall within the standard range of the assay. Urinary sEcad results were corrected for urine creatinine concentrations using the QuantiChrom Creatinine Assay Kit (DICT-500, BioAssay Systems, Hayward, CA). Each experiment was performed in triplicate.
Immunofluorescence
Cells cultured on chamber slides (no. 177437, Nalge Nunc International, Naperville, IL) were fixed with 4% formaldehyde or 100% methanol, blocked in PBS containing 1% (w/v) BSA and incubated in an anti-BrdU antibody (ab2284, Abcam), as previously described [27]. For staining of actin, microtubules and double labeling, MCF-7, SKBR3 or MDA-MB-231 cells were fixed with 100% (v/v) methanol at −20°C and blocked in 1% (w/v) BSA: 0.4% (v/v) Triton X-100. Cells were incubated with Alexa 594-conjugated phalloidin (Invitrogen, Grand Island, NY) or with monoclonal anti-α-tubulin (Invitrogen) overnight at 4°C, followed by incubation with corresponding fluorescence-conjugated secondary antibodies (Invitrogen). Nuclei were counterstained with 2 µg/ml Hoechst 33342 (Invitrogen).
Cell Proliferation, Migration, and Invasion Assays
For BrdU staining of human cell lines, cells were incubated with 10 µM BrdU for 2 h prior to fixation and processed for immunofluorescence microscopy as previously described [27]. For BrdU analyses, cells were counted from 6 low-power fields (100×) per section. BrdU incorporation was also analyzed by a cell proliferation ELISA 5-bromo-2’-deoxyuridine (BrdU; colorimetric) kit (Roche, Stockholm, Sweden), according to the manufacturer’s instructions. Migration and invasion of MCF-7, SKBR3, and MDA-MB-231 cells were measured using 8.0 µmpore BD BioCoat Control Insert 24-well plates (no. 354578) and Matrigel Invasion Chamber 24-well Plates (no. 354480), respectively (BD Bioscience, San Jose, CA). Cells were collected, washed, and 2 × 105 cells were plated in 0.4% FBS medium in the top chamber, with 0–20 µg/ml of rhEcad/Fc in the bottom chamber. After 22 h, cells on the top were removed using a cotton swab. Migrated or invaded cells on the lower surface were fixed with methanol, stained with 0.5% crystal violet, examined by bright field microscopy and photographed. Migration or invasion was quantitated by counting migrated or invaded cells in at least ten random high-power fields per insert and expressed as averages. Results are presented as fold change of the number of migrated/invaded cells to the untreated controls in triplicate experiments.
Statistical Analysis
Data are shown as the mean – SEM. To determine statistical differences between means, Student’s t-test was used where applicable. Statistical significance is indicated in figures as * P < 0.05, ** P < 0.01, or *** P < 0.001.
Study Approval
This study was conducted in accordance with NIH guidelines for the use of experimental animals. Protocols were approved by the Institutional Animal Care and Use Committee and the use of human tissues was according to the Institutional Review Board at Stony Brook University.
RESULTS
Enhanced sEcad in Human and Mouse Breast Cancers and Cell Culture Systems
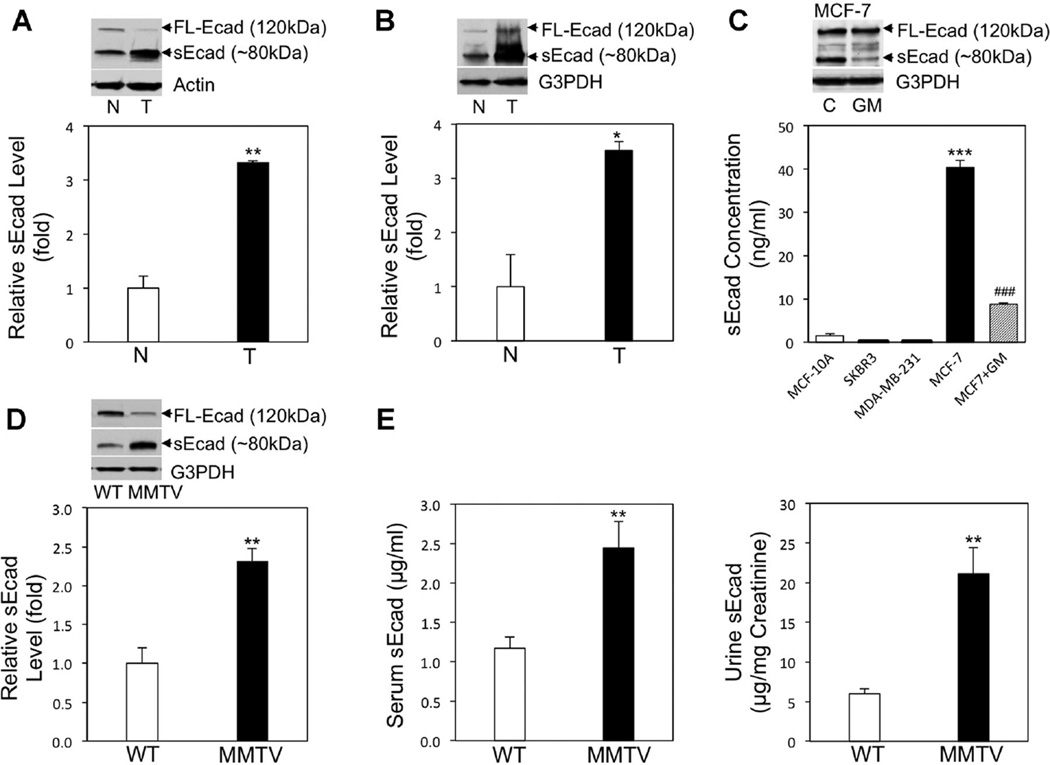
As sEcad upregulation has been previously reported in the serum of breast cancer patients [8], we set out to determine whether endogenous sEcad levels are increased in HER2+ and TNBC breast cancer specimens harvested from human patients and from breast cancer laboratory (animal and tissue culture) models. To this end, we assessed the level of sEcad expression in HER2+human breast tumor specimens and human TNBC tumor specimens and found sEcad levels to be significantly higher than normal human mammary tissue specimens (Figure 1A and B). In human cell culture studies, sEcad levels were found to be significantly increased in the conditioned media of MCF-7 breast cancer cells compared to normal MCF-10A breast epithelial cells, and were significantly decreased in the presence of the broad spectrum MMP inhibitor GM6001 (Figure 1C). In contrast, no appreciable level of sEcad was found in HER2+ SKBR3 and TNBC MDA-MB-231 cells, which can be attributed to the lack of E-cadherin expression in these cell lines. In the MMTV-PyMT mice bearing HER2+ breast tumors, increased amounts of endogenous sEcad levels were exhibited in resected breast tumors, as well as heightened levels of sEcad in the serum and urine compared to wild-type controls (Figure 1D and E). Taken together, our data demonstrate that sEcad levels are increased in resected HER2+ and TNBC human tumors, human breast cancer cell lines and HER2+ MMTV-PyMT mouse breast tumors and bodily fluids.
Figure 1.
Enhanced cleavage of the sEcad ectodomain fragment in breast carcinogenesis. (A) Western blot analysis and representative immunoblots of endogenous sEcad levels in normal human mammary tissues (N) from reductive mammoplasty and resected human HER2+ breast specimens (T). Protein levels were normalized to β-actin and quantified using NIH Scion Image. **P < 0.01 versus N. (B) Western blot analysis and representative immunoblots of endogenous sEcad levels in resected human TNBC specimens (T) with adjacent normals (N). Protein levels were normalized to G3PDH and quantified using NIH Scion Image. *P < 0.05 versus N. (C) Analysis of sEcad levels in the conditioned medium of normal mammary cells (MCF-10A), SKBR3, MDA-MB-231 and MCF-7 cells (in the presence or absence of 10 µM of GM6001) by ELISA after normalization for cell number. Insert: Immunoblot of intact E-cadherin and sEcad in cell lystes from MCF-7 cells treated with or without GM6001. ***P < 0.001 versus normal mammary cells MCF-10A; ###P < 0.001 versus MCF-7 without GM6001. (D) Western blot analysis of endogenous sEcad levels in resected normal mouse mammary tissues (WT) and MMTV-PyMT breast tumors (MMTV) relative to G3PDH. (E) Concentration of sEcad in the serum and urine of WT and MMTV-PyMT mice as measured by ELISA. Urinary levels of sEcad were normalized to creatinine. Data are presented as Mean ± SEM. *P < 0.05, **P < 0.01 versus corresponding WT controls.
Endogenous and Exogenous sEcad Associates With HER Receptor Family Members
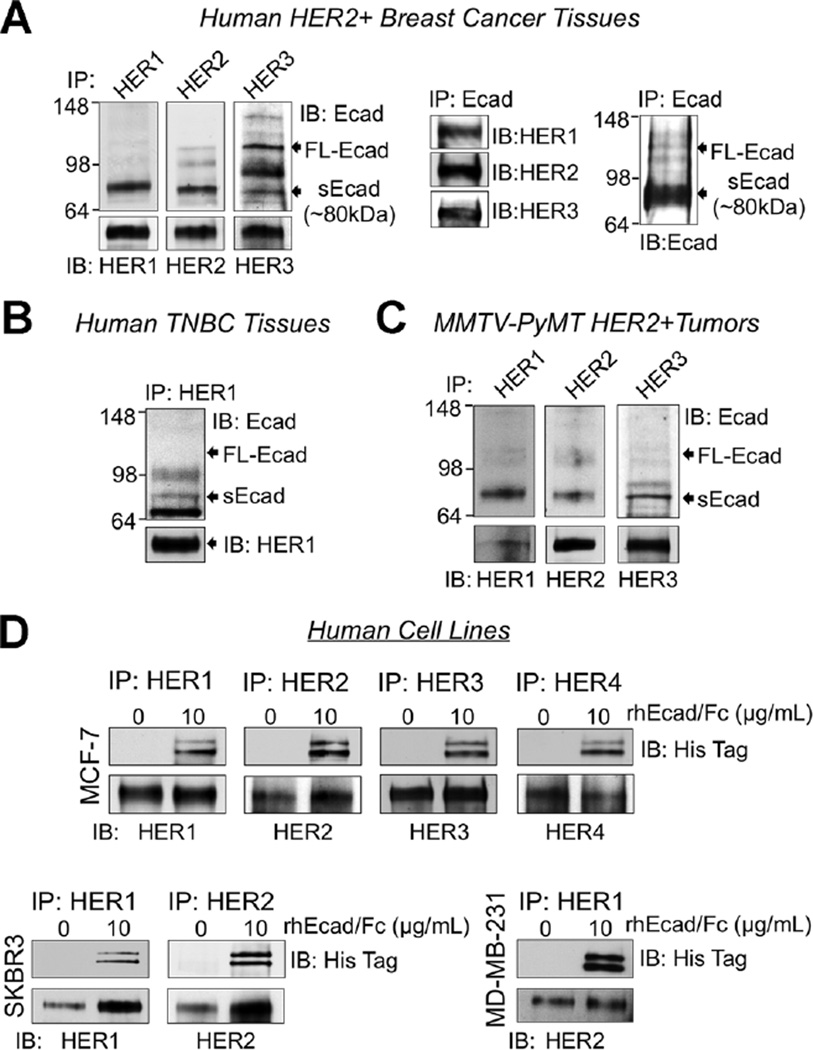
One prior paper by Najy et al. [4], demonstrated that exogenous sEcad associated with HER2 and HER3 in MCF-7 and SKBR3 cells to promote breast cancer migration. However, whether sEcad associates with the HER receptor family in vivo or with other HER family members, has yet to be elucidated. Therefore, we next determined whether sEcad associates with the HER receptor family in HER2+ and TNBC breast tumor samples by co-immunoprecipitation and Western blot assays. Of note, in the HER2+ breast tumor specimens we focused on HER1, HER2, and HER3 as we could not detect HER4. In TNBC tumor tissues, we focused on HER1 as these tumors typically lack HER2. Here, extracts prepared from HER2+ human ductal adenocarcinomas, human TNBC tumor specimens and resected HER2+ mouse MMTV-PyMT tumors were immunoprecipitated using antibodies against HER1, HER2, and/or HER3 and subsequently immunoblotted with an anti-E-cadherin ectodomain-specific antibody that recognizes both membrane-bound intact E-cadherin and sEcad. We found that HER1 and HER2 predominately associated with sEcad and minimally with membrane-bound E-cadherin in HER2+ human breast tumors (Figure 2A). In contrast, HER3 associated with both intact E-cadherin and sEcad in this tumor subtype (Figure 2A). Reverse co-immunoprecipitation studies whereby we IP E-cadherin (ectodomain-specific antibody) and immunoblotted with HER1–3 confirmed these results. Of note, this E-cadherin ectodomain-specific antibody predominately precipitated out the sEcad fragment. In human TNBC tumors, HER1 associated with sEcad but not the 120 kDa intact E-cadherin protein (Figure 2B). Similarly, in MMTV-PyMT resected tumors, which express HER1–3 and minimal levels of HER4, HER1–3 associated with sEcad but minimally with intact E-cadherin (Figure 2C). To validate that sEcad, but not membrane-bound E-cadherin, associates with the HER receptor family in vitro, extracts from E-cadherin-positive MCF-7 breast cancer cells (express HER1–4), HER2+ E-cadherin-negative SKBR3 and TNBC HER1 + MDA-MB-231 cells were treated with exogenous recombinant human E-cadherin Fc chimera (rhEcad/Fc). Depending on the cell-type, extracts were then immunoprecipitated with HER1–4-specific antibodies and subsequently analyzed by Western blotting with an antibody against the rhEcad/Fc’s His-tag epitope. Again, we detected sEcad-HER1–4, sEcad-HER1–2, and sEcad-HER1interactions, in MCF-7, SKBR3, and MDA-MB-231 cell lines, respectively (Figure 2D). Together, these results demonstrate that sEcad physically interacts with HERs in human HER2+ and TNBC breast tumors and cells, as well as in HER2+ mouse MMTV-PyMT tumors.
Figure 2.
sEcad associates with the HER family of receptors in human and mouse breast tumors and cells. (A) HER1, HER2, and HER3 were immunoprecipitated (IP) from human ductal adenocarcinoma lysates and precipitates were immunoblotted (IB) using anti-E-cadherin extracellular domain, anti-HER1, anti-HER2, and anti-HER3 antibodies. sEcad-HER association was confirmed by reverse immunoprecipitation, whereby HER1, HER2, and HER3 were detected by immunoblot analysis following immunoprecipitation of E-cadherin using an E-cadherin ectodomain-specific antibody. (B) HER1 was IP from human triple negative breast carcinoma (TNBC) specimens and precipitates were immunoblotted using anti-HER1 and anti-E-cadherin extracellular domain antibodies. (C) HER1, HER2, and HER3 were IP from resected MMTV-PyMT mouse tumors and precipitates were immunoblotted using anti-E-cadherin extracellular domain, anti-HER1, anti-HER2, and anti-HER3 specific antibodies. (D) Lysates from MCF-7, SKBR3, and MDA-MB-231 cells in the presence or absence of 10 µg/ml of recombinant E-cadherin ectodomain-Fc chimeric protein (rhEcad/Fc; sEcad) fused with a C-terminal His Tag were IP with anti-HER1, anti- HER2, anti-HER3, or anti-HER4 specific antibodies, followed by immunoblotting with an anti-His Tag antibody.
sEcad Activates the HER-MAPK and PI3K/Akt/mTOR Signaling Pathways
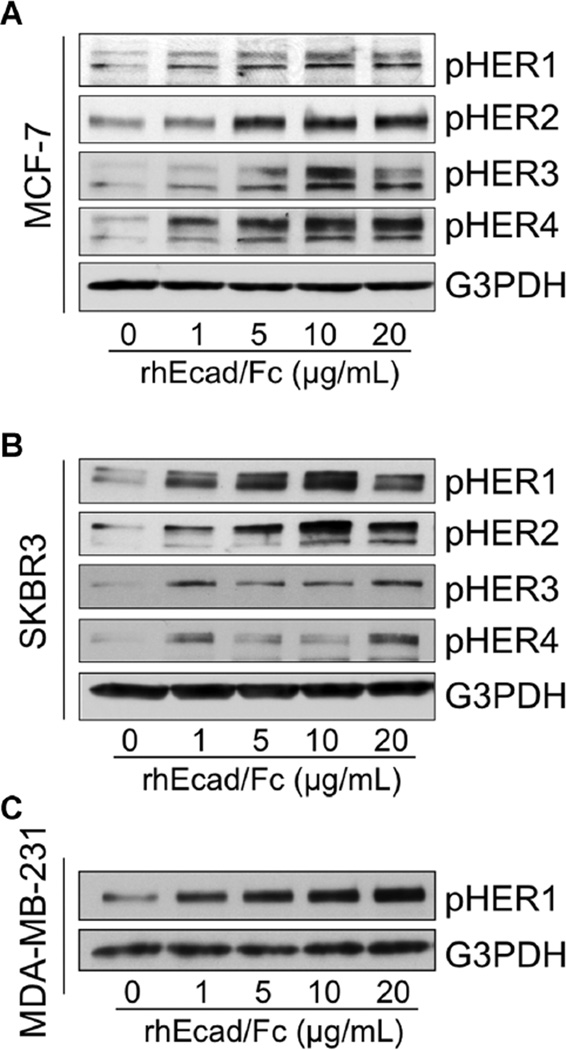
Because ligand binding to the HER family of receptors induces the formation of homo- and heterodimers, resulting in phosphorylation on specific tyrosine residues within the cytoplasmic domain [11], we next examined the effects of sEcad on HER phosphorylation. HER1–4 was markedly increased by rhEcad/Fc in HER2+/E-cadherin+ MCF-7 cells (Figure 3A). In the HER2+/E-cadherin− SKBR3 cell line, a dose-dependent increase in phospho- HER1 and HER2 was detected in the presence of rhEcad/Fc, whereas the rhEcad/Fc-induced HER3 and HER4 phosphorylation was increased but not dose-dependent (Figure 3B). In the MDA-MB-231 TNBC cell line, which lacks high expression and/or amplification of HER2, HER3 or HER4, exogenous rhEcad/Fc induced a dose-dependent increase in the expression of HER1, 26 h after stimulation (Figure 3C).
Figure 3.
sEcad activates the HER family of receptors in vitro Cells were incubated with rhEcad/Fc (0–20 µg/ml) under serum free condition for 26 h. (A) Western blotting showing the effects of rhEcad/Fc (0–20 µg/ml) on HER1–4 activation in E-cadherin-positive MCF-7 cells. (B) Immunoblots showing the effects of rhEcad/Fc (0–20 µg/ml) on HER1–4 activation in E-cadherin-negative SKBR3 cells. (C) Immunoblots showing the effects of rhEcad/Fc (0–20 µg/ml) on HER1 activation in E-cadherin-negative MDA-MB-231 cells. An antibody to G3PDH was used as a loading control in all panels. Images are representative of two independent experiments.
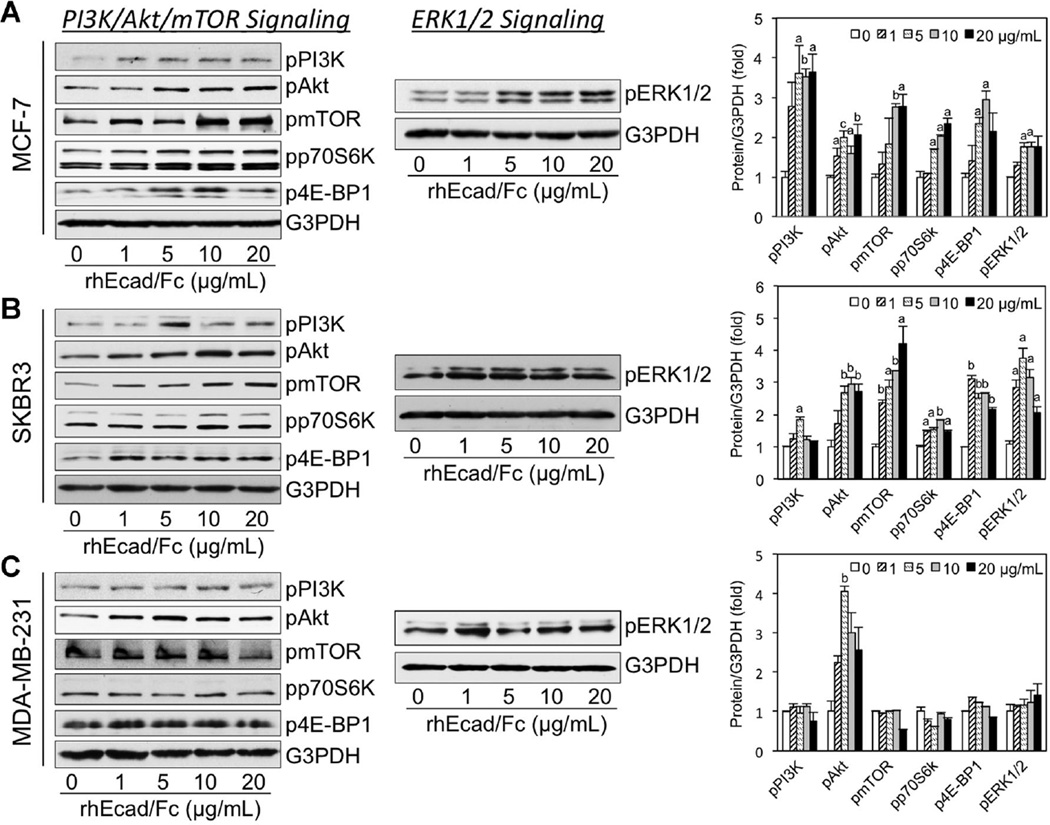
The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) network plays a key regulatory function in tumor cell survival, and is an important mediator of HER receptor signaling [28]. Therefore, we next evaluated whether sEcad-induced HER activation modulates this signaling pathway in the aforementioned E-cadherin+ and E-cadherin− human breast cancer cell lines. Western blot analyses showed activation of PI3K/Akt and mTOR in rhEcad/Fc stimulated MCF-7 and SKBR3 cells (Figure 4A and B). As the p70S6 kinase (p70S6K) and eIF4E binding protein 1 (4E-BP1) are among the most well characterized targets of mTOR [28,29], we next studied the phosphorylation of p70S6K at Thr-389 and 4E-BP1 at Thr-37 by Western blotting. Not surprisingly, phosphorylated p70S6K and 4E-BP1 levels were higher in rhEcad/Fc treated MCF-7 and SKBR3 cells versus untreated controls (Figure 4A and B). In contrast, in rhEcad/Fc stimulated MDA-MB-231 cells, only Akt phosphorylation levels were markedly increased (Figure 4C). Because there is considerable cross-talk/feedback between the PI3K/Akt/mTOR and the MAPK pathways [29], we next assessed the MAPK cascade in our cell culture system. As anticipated, sEcad-induced a dose-dependent increase in the phosphorylation of ERK1/2 in MCF-7 cells (Figure 4A). In the E-cadherin−/HER2+ SKBR3 cell line, rhEcad/Fc also increased the phosphorylation of ERK1/2, in a bell-shaped curve, with a maximal effect at 5 µg/ml (Figure 4B). In contrast, in E-cadherin− MDA-MB-231 cells, exogenous sEcad did not show as much of a significant effect on phospho-ERK1/2 as in the other two cells lines tested (Figure 4C). Altogether, these results demonstrate that sEcad is capable of activating the PI3K/Akt/mTOR and/or ERK1/2 MAPK signaling pathways, but this effect is cell-type dependent.
Figure 4.
sEcad activates downstream PI3K/Akt/mTOR and MAPK signaling in vitro Immunoblots of protein lysates showing phospho-PI3K, Akt, mTOR, p70S6K, 4E-BP1, and ERK1/2 expression in (A) MCF-7, (B) SKBR3, and (C) MDA-MB-231 cells treated with rhEcad/Fc (0–20 µg/ml) for 26 h. An antibody to G3PDH was used as a loading control in all panels. Images are representative of two independent experiments. Corresponding protein levels were normalized to G3PDH and quantified using NIH Scion Image. Results are presented as Mean ± SEM. aP < 0.05, bP < 0.01, cP < 0.001 versus corresponding 0 µg/ml of rhEcad/Fc controls.
sEcad Activates IAP Family Members
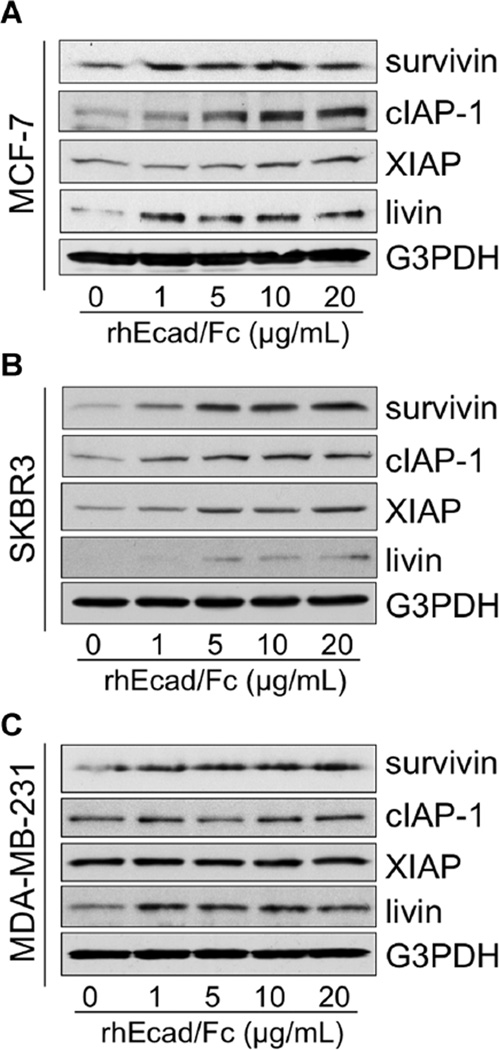
As increasing evidence indicates that the PI3K/Akt pathway can regulate the IAP family of proteins [24], and that IAP members are associated with de novo and acquired resistance [30], we next examined the effects of rhEcad/Fc on the levels of IAPs in E-cadherin-negative and -positive cells using Western blot analysis. To the best of our knowledge, this study is the first to show that exogenous rhEcad/Fc upregulated many of the IAP family members, including survivin, cIAP-1, XIAP, and livin in both MCF-7 and SKBR3 cells (Figure 5A and B). However, inMDA-MB-231 cells only survivin and livin protein expression levels were increased in the presence of sEcad (Figure 5C). These findings provide evidence that sEcad modulates IAP family members, although to varying degrees, in both HER2+ and HER2− TNBC cells that express or lack E-cadherin.
Figure 5.
sEcad activates IAP family members in vitro Equal amounts of protein from whole-cell lysates of (A) MCF-7, (B) SKBR3, or (C) MDA-MB-231 cells after treatment for 26 h with rhEcad/Fc (0–20 µg/ml) were separated by SDS–PAGE and immunoblotted for survivin, cIAP-1, XIAP, and livin. G3PDH served as a control for equal loading of protein. Images are representative of two independent experiments.
sEcad Exerts Pro-Oncogenic Effects
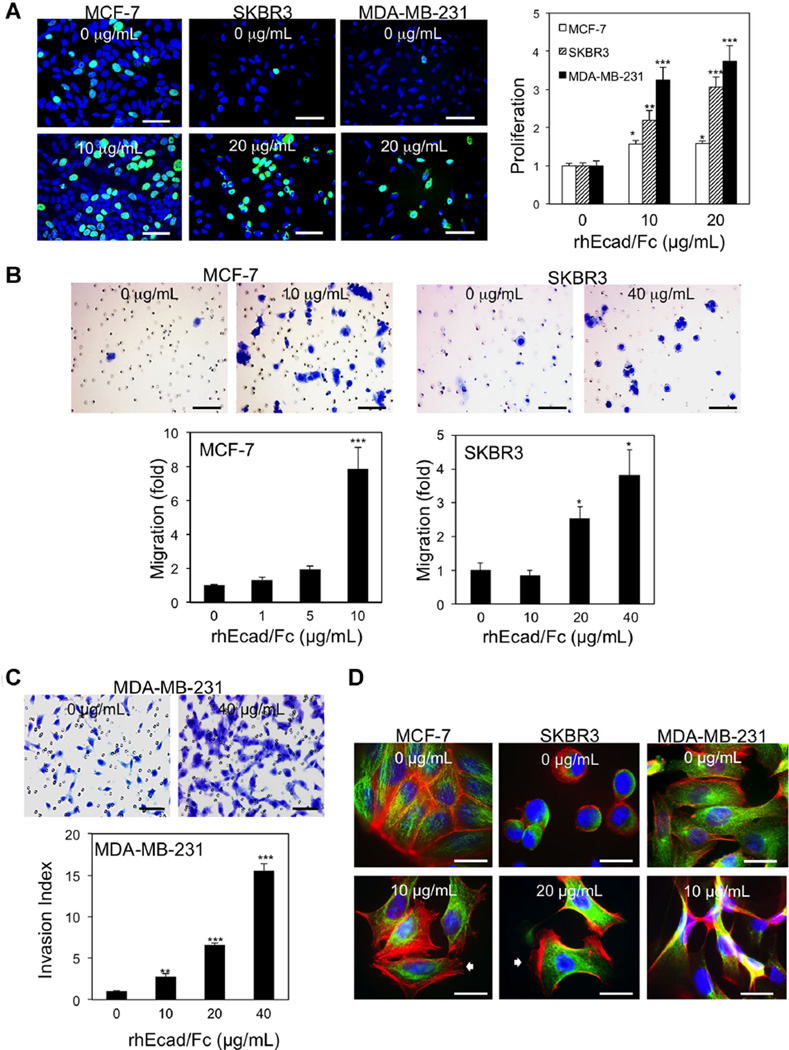
Shed proteins can be inert or biologically active. To assess the functional consequences of sEcad in the tumor microenvironment, we examined the effects of exogenous sEcad on breast cancer cell proliferation, migration, invasion, and the formation of microtubules, stress fibers and focal adhesions in serum starved cell culture systems. In the MCF-7, SKBR3, and MDA-MB-231 cell lines, BrdU incorporation was significantly increased with 10 and 20 µg/ml of rhEcad/Fc, albeit to varying levels depending on the cell type (Figure 6A). This result is in agreement with recent findings showing that exogenous sEcad increased proliferation in SKBR3 cells [4]. The effects of sEcad on migration and invasion were then examined in MCF-7, SKBR3, and MDA-MB-231 cells. Using Transwell plates, rhEcad/Fc treatment of MCF-7 and SKBR3 cells induced a significant dose-dependent increase in migration compared to untreated controls (Figure 6B). In contrast, addition of exogenous sEcad to MDA-MB-231 cells had no effect on cell migration (data not shown). To assess whether sEcad promotes cell invasion, we assayed the ability of serum-starved cells treated with rhEcad/Fc to invade through Transwell inserts coated with a thin layer of Matrigel. MDA-MB-231 cells treated with rhEcad/Fc exhibited a dramatic increase in invasion through Matrigel compared to untreated controls (Figure 6C). Of note, baseline MCF-7 and SKBR3 cells exhibited minimal invasive characteristics (data not shown). As cytoskeletal rearrangement and formation of newly branched F-actin and microtubules have been suggested to play a role in regulating cell migration [31], we next assessed actin polymerization and microtubule assembly in cells stimulated for 24 h with rhEcad/Fc. A noticeable change in cell shape associated with lamellipodia and stress fibers, were noted in both rhEcad/Fc treated MCF-7 and SKBR3 cells whereas serum-starved MDA-MB-231 cells displayed the presence of spindle-shaped cells (Figure 6D). Moreover, all cell types displayed increased intercellular separation 24 h after stimulation (unpublished observations). Altogether, these data suggest that sEcad in the tumor microenvironment acts in an autocrine and/or paracrine manner to facilitate tumor cell proliferation, migration, and invasion.
Figure 6.
Exogenous sEcad promotes tumor cell proliferation, migration, and invasion. (A) Immunofluorescence (IF) microscopy showing BrdU incorporation in MCF-7, SKBR3, and MDA-MB-231 cells in the presence or absence of 10 or 20 µg/ml of rhEcad/Fc. The proportion of BrdU-positive cells was determined relative to Hoechstpositive cells (n = 3). (B) Representative transwell migration images and analysis of 0.5% crystal violet stained MCF7 and SKBR3 cells treated with rhEcad/Fc (0–40 µg/ml), respectively. (C) Matrigel invasion images and analysis of 0.5% crystal violet stained MDA-MB-231 cells in the presence of rhEcad/Fc (0–40 µg/ml). (D) Representative fluorescent images of MCF-7, SKBR3, and MDA-MB-231 cells stained with anti-α-tubulin antibody (green; microtubules) and Alexa Fluor 594 phalloidin (red; actin microfilaments) in the presence or absence of 10 or 20 µg/ml of rhEcad/Fc. Nuclei were counterstained with Hoechst 33342 blue dye. Data are presented as Mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding untreated groups. Scale bars = 400 µm.
sEcad Promotes Breast Cancer Proliferation, Migration and Invasion Through HER Signaling
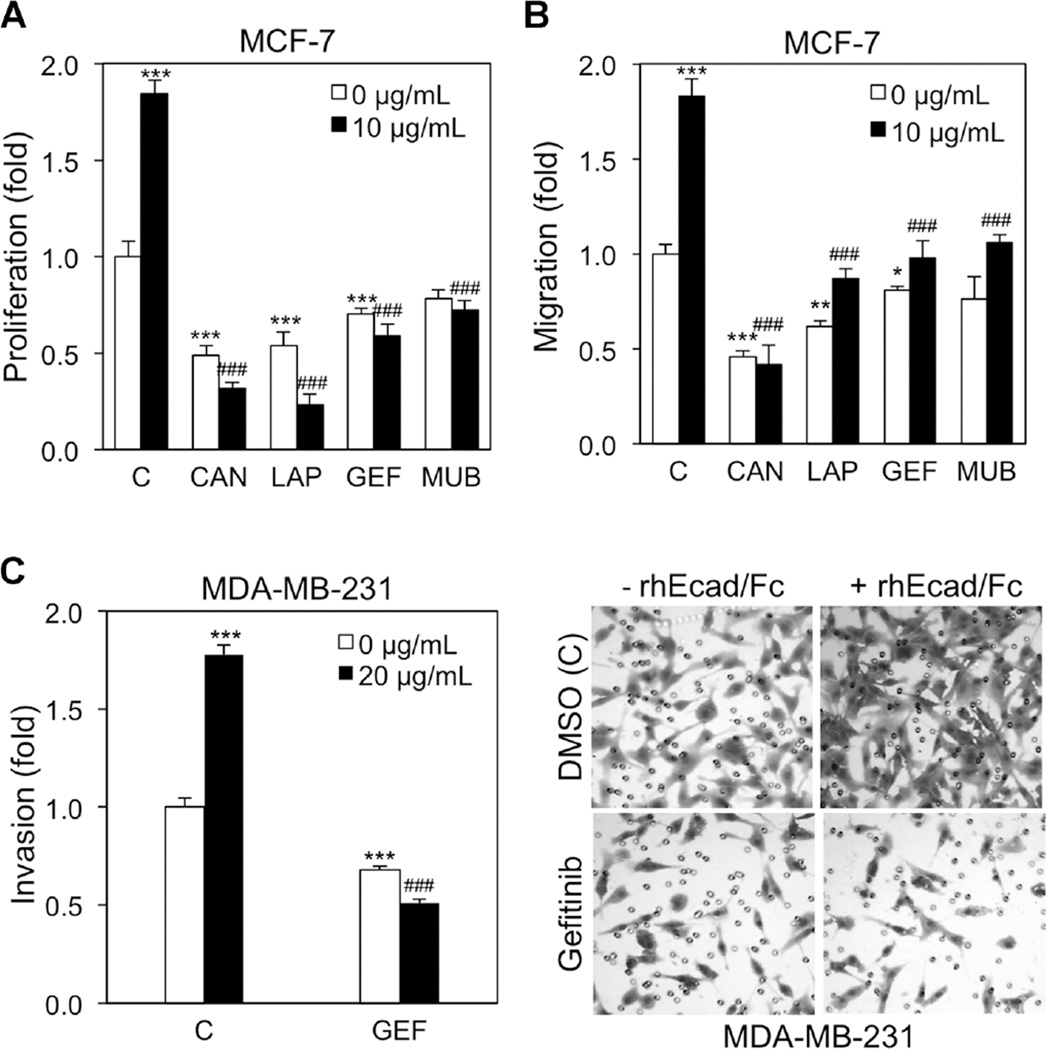
Because sEcad associates and activates the HER axis, we next used specific HER receptor tyrosine kinase inhibitors to determine whether these sEcad-induced pro-oncogenic effects were mediated via HER signaling. Here, the pan HER inhibitor Canertinib (1 µM), the HER1/HER2 inhibitor Lapatinib (5 µM), and the HER1 inhibitor Gefitinib (5 µM) significantly reduced both basal and rhEcad/Fc-induced BrdU incorporation, whereas Mubritinib (5 µM) only abolished rhEcad/Fc-induced proliferation (Figure 7A). Next, MCF-7 cells in the presence or absence of rhEcad/Fc were treated with the aforementioned inhibitors and analyzed by Transwell migration assays. Here, Canertinib exhibited the most potent reduction in MCF-7 basal and rhEcad/Fc-induced migration (Figure 7B). In addition, Lapatinib and Gefitinib exhibited a similar decrease on basal and rhEcad/Fc-mediated migration, whereas Mubritinib again only blocked rhEcad/Fc-induced migration. Next, in an effort to evaluate invasion, we used the highly aggressive MDA-MB-231 cell line that exhibits a high invasion capacity and overexpresses HER1, but minimal levels of HER2–4 [32]. In the presence of Gefitinib, both basal and rhEcad/Fc-induced invasion was significantly reduced compared to untreated controls (Figure 7C).
Figure 7.
sEcad exerts pro-oncogenic effects via the HER axis (A) MCF-7 cells were pre-treated with DMSO (C), Canertinib (CAN, 1 µM), Lapatinib (LAP, 5 µM), Gefitinib (GEF, 5 µM), or Mubritinib (MUB, 20 µM) for 2 h, and then incubated with or without 10 µg/ml of rhEcad/Fc for 24 h. Two hours before the end of incubation, 10 µMof BrdU was added and cellular proliferation was performed using an ELISA BrdU (colorimetric) assay. (B) MCF-7 cells were seeded onto uncoated filters in the presence of DMSO (C), Canertinib (CAN, 1 µM), Lapatinib (LAP, 5 µM), Gefitinib (GEF, 5 µM), or Mubritinib (MUB, 20 µM) for 2 h, and then vehicle or 10 µg/ml of rhEcad/Fc were added to the lower chambers. Twenty-two hours later, migrating cells were stained and counted. (C) MDA-MB-231 cells were seeded onto Matrigel-coated filters in the presence of DMSO (C) or Gefitinib (GEF, 5 µM) for 2 h, and then vehicle or 10 µg/ml of rhEcad/Fc were added to the lower chambers. Twenty-two hours later, invading cells were stained and counted using ImageJ software. Representative 0.5% crystal violet staining of MDA-MB-231 cells treated with or without rhEcad/Fc in the presence or absence of Gefitinib (5 µM) invading through Matrigel. Data are presented as Mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding 0 µg/ml of rhEcad/Fc without inhibitors; ###P < 0.001 versus corresponding 10 µg/ml of rhEcad/Fc without inhibitors.
sEcad and EGF Exert Additive Effects on Tumor Proliferation, Migration, and Invasion
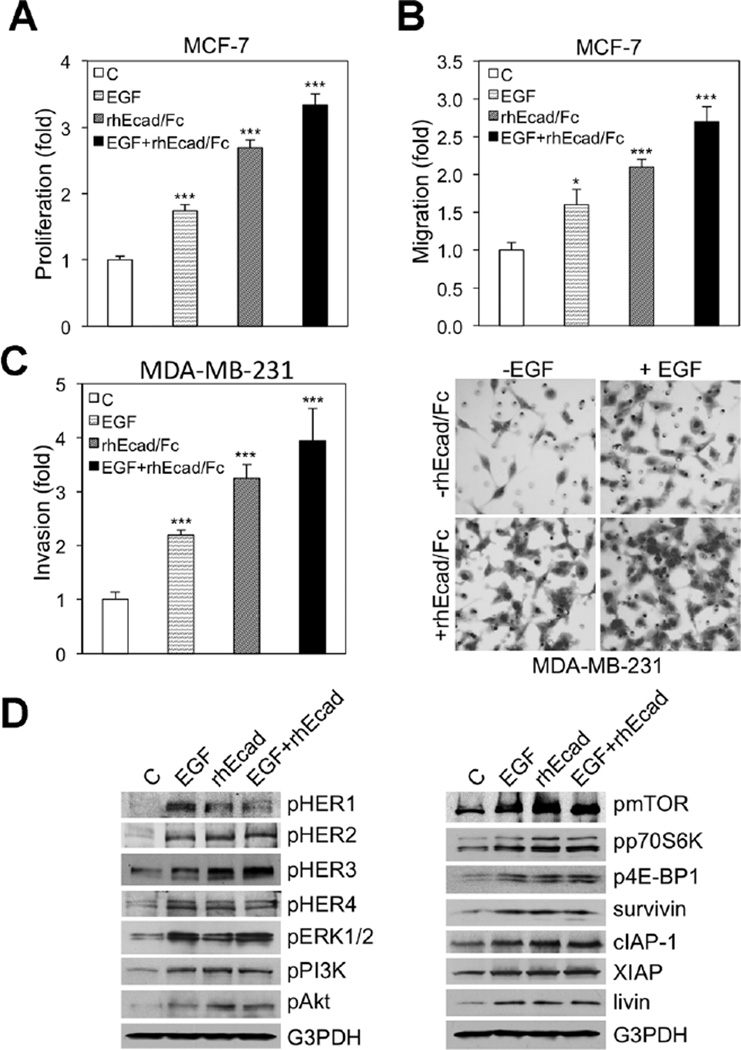
Because the tumor mileu likely contains HER-related ligands that could potentially compete, act additively or synergize with sEcad for binding, we next investigated whether exogenous ligands such as EGF could alter the pro-oncogenic effects induced by sEcad. Here, cells were treated for 24 h with equimolar concentrations of EGF, sEcad alone, or sEcad in combination with EGF, and then proliferation, migration, and invasion were assessed. Treatment of cell cultures with rhEGF or rhEcad/Fc alone, significantly increased proliferation, migration, and invasion, but these pro-oncogenic effects were more potent with exogenous sEcad (Figure 8A–C). Strikingly, combination treatment with equimolar rhEGF and rhEcad/Fc resulted in a partial, but statistically significant, additive effect on proliferation, migration, and invasion compared to sEcad or EGF treatments alone (Figure 8A–C). Next, in order to examine HER and downstream signaling, we evaluated this axis in the presence or absence of equimolar concentrations of rhEGF, rhEcad/Fc and rhEGF and rhEcad/Fc in combination. As anticipated, rhEGF alone exhibited more potent effects on HER1 and ERK1/2 phosphorylation compared to rhEcad/Fc alone (Figure 8D). In contrast, exogenous rhEcad/Fc demonstrated enhanced phospho-HER3, Akt, and less of an increase on phospho-mTOR expression levels. In combination, no significant additive or synergistic effects were noted (Figure 8D). Together, these data provide evidence that sEcad acts additively with EGF to promote breast cancer oncogenicity.
Figure 8.
sEcad exerts additive effects with EGF to promote oncogenicity (A) MCF-7 cells were starved for 4 h in serum-free medium and treated with vehicle control (C), rhEGF (500 ng/ml), rhEcad/Fc (10 µg/ml), or rhEGF and rhEcad/Fc in combination for 24 h, and cellular proliferation was assessed using an ELISA BrdU (colorimetric) assay. Results are presented as mean ± SEM. (B) Effect of 22-h incubation of rhEGF (500 ng/ml), rhEcad/Fc (10 µg/ml), and rhEGF + rhEcad/Fc on MCF-7 migration. Cells that migrated through the control insert were fixed, stained, photographed and counted in ten random high-power fields per insert. Results are presented as mean ± SEM of fold change in rhEGF, rhEcad/Fc, and rhEGF + rhEcad/Fc treated cells compared with vehicle control (C). All quantitative data were generated from a minimum of three replicates. (C) Invasion of MDA-MB-231 cells in the presence or absence of equimolar concentrations of rhEGF (1 µg/ml), rhEcad/Fc (20 µg/ml), or rhEGF and rhEcad/Fc in combination for 22 h. Cells that invaded the Matrigel layer were fixed, stained, photographed and counted in ten random high-power fields per insert. Results are presented as mean ± SEM of fold change in rhEGF, rhEcad/Fc, and rhEGF + rhEcad/Fc treated cells compared with vehicle control (C). All quantitative data were generated from a minimum of three replicates. Insert: Representative 0.5% crystal violet staining of MDA-MB-231 cells treated with Vehicle, rhEGF, rhEcad/Fc, or rhEGF + rhEcad/Fc. (D) Immunoblotting of phospho-HER-MAPK-PI3K/Akt/mTOR and IAP family members in MCF-7 cells after incubation with equimolar concentrations of rhEGF (500 ng/ml), rhEcad (10 µg/ml), and rhEGF and rhEcad/Fc in combination for 24 h.
DISCUSSION
The tumor microenvironment is enriched with factors that nurture the growth and survival of tumors, and as such, is an indispensable player in malignant growth. Contained within this functional space are abundant proteases that regulate the proteolytic cleavage of membrane proteins into bioactive soluble ectodomain fragments. It is well established that E-cadherin is subject to this kind of proteolysis, wherein MMPs or the ADAM family of proteases, cleave E-cadherin in the extracellular region proximal to the transmembrane domain, releasing the sEcad fragment into the extracellular space [2–6]. Furthermore, in ovarian and prostate cancer cells, the MMP or ADAM-induced sEcad fragment can act in a paracrine or autocrine manner to stimulate tumor cell growth and survival [3,6]. Accordingly, levels of sEcad in breast cancer patients correlates with tumor size, response to chemotherapy and predicts a shorter disease-free interval [8]. In the present study, we found that endogenous sEcad expression levels were significantly elevated in human HER2+ and TNBC tumor specimens, as well as in MMTV-PyMT mouse mammary tumors, serum and urine. Prior reports have demonstrated elevated sEcad expression levels in gastric tumors, primary prostate tumor tissues and metastatic foci [7,33], but we are unaware of any studies showing this in human and mouse breast tumor specimens and bodily fluids in vivo. In addition, although membrane-bound intact E-cadherin has been shown to be downregulated in TNBC tumors and levels correlate with overall survival [34], there are no prior studies reporting enhanced sEcad expression in this tumor type. In cell culture assays, we further show that endogenous sEcad levels were increased in the E-cadherin-positive MCF-7 cell line, but not E-cadherin-negative SKBR3 and MDA-MB-231 monolayers, and that sEcad levels but not intact E-cadherin were inhibited by the general MMP inhibitor GM6001, which is in agreement with prior studies [6].
Although the mechanisms of action of sEcad are not well understood, both HER1 and HER2 overexpression and enhanced sEcad predict for a poor clinical outcome in breast cancer, making it tempting to speculate that the two interact. Along these lines, one recent study reported that sEcad acts as a ligand for HER2 and HER3 family members in vitro [4]. Specifically, Najy et al. [4] demonstrated endogenous sEcad binding preferentially to HER2 and HER3 in MCF-7 cells and exogenous sEcad (rhEcad/Fc) binding to HER2 and HER3, enhancing HER2-HER3 heterodimerization, HER3 phosphorylation and activating downstream ERK1/2 without affecting HER2 phosphorylation. However, we are unaware of any reports showing this same association in human and mouse tumor specimens in vivo, or demonstrating sEcad-HER1 interactions in vivo or in vitro. Here, we show that endogenous sEcad, but less membrane-bound E-cadherin, associates with HER1, HER2, and HER3 in human and mouse HER2+ breast tumors and show sEcad-HER1 interactions in human TNBC specimens. Consistent with the in vivo findings, exogenous sEcad induced sEcad-HER1, HER2, HER3 and HER4 interactions in human E-cadherin+ MCF-7 cells, sEcad-HER1, and sEcad-HER2 interactions in the E-cadherin-negative SKBR3 cells, as well as sEcad-HER1 interactions in the MDA-MB-231 TNBC cell line.
The fact that sEcad can interact with HER1–4 proteins, prompted us to test whether this sEcad-HER association could result in the transactivation of HER1–4 and downstream signaling. Under in vitro conditions, sEcad induces the phosphorylation of all HER1–4 family members in MCF-7 and SKBR3 human breast cancer cells. In MDA-MB-231 cells, which express HER1 and low levels of HER2–4 (unpublished observations), a dose-dependent increase in HER1 activation was noted in the presence of sEcad. Intriguingly, this sEcad-mediated HER transactivation was accompanied by enhanced phospho-PI3K/Akt/mTOR and ERK1/2 signaling in MCF-7 and SKBR3 cells, and Akt signaling in MDA-MB-231 cells. Moreover, our results are the first to show an increase in the sEcad-induced phosphorylation of mTOR and two of the most well characterized targets of mTOR [34], p70s6K and 4E-BP1 in HER2+ but not TNBC cells. This is in line with a previous finding in normal MDCK cells, wherein exogenous sEcad promoted cell survival via activation of HER1, PI3K, Akt, and ERK1/2 signaling [25]. A similar sEcad-induced activation of ERK was described in SKBR3 breast cancer cells [4]. Altogether, this activation of multiple HER family members and downstream PI3K/Akt/mTOR signaling by sEcad in varying breast cancer cell types, is especially intriguing, because breast carcinomas typically express multiple HERs, each of which can regulate any of these downstream signal transduction pathways to promote oncogenesis or de novo and acquired resistance.
A large body of data demonstrates elevated expression of IAP proteins in many human malignancies, including HER2+ breast cancers and TNBCs [14–16]. Besides contributing to tumorigenesis, their expression levels also correlate with disease progression, chemoresistance and poor patient survival [14–16]. These IAP family members, including survivin, cIAP-1, cIAP-2, XIAP, and livin exhibit potent anti-apoptotic properties, and as such, have become valid targets for anti-cancer drugs [16]. Survivin, the smallest IAP family member is highly expressed in dividing cells and cancer-derived cell lines and appears to be selectively upregulated in tumors, but not differentiated adult tissues [18]. Importantly, inhibition of survivin by YM155 led to complete regression of subcutaneously established tumors, reduced spontaneous metastases and prolonged survival in mice TNBC xenografts [35], implicating survivin in TNBC growth and survival. Similarly, inhibition of survivin using ribozyme and a dominant-negative mutant resulted in spontaneous apoptosis and a decrease in the growth rates of MCF-7 breast cancer cells [36,37]. Surprisingly, there are few studies on XIAP, cIAP-1, cIAP-2, and livin in breast cancer, but in vivo expression of XIAP was shown to correlate with tumor grade [38] and anti-livin antibodies were found to be significantly increased in breast cancer patients compared with healthy donors [39]. Recent studies also suggest that interactions between IAP family members and the HER-PI3K/Akt pro-survival pathways may prevent apoptosis, promote cell survival and serve as a potential mechanism for chemoresistance [30,40–42]. Specifically, survivin was shown to be regulated by both HER1 and HER2 via PI3K/Akt signaling and overexpression of survivin was demonstrated to abrogate the inhibition of cell growth induced by Herceptin, a HER2-targeted monoclonal antibody for HER2-amplified breast cancers [24,43,44]. Here, we show a previously unrecognized activation of IAP family members by sEcad. Specifically, we demonstrate that sEcad induced the expression of survivin, cIAP-1, XIAP and livin, although this effect was cell-type dependent. Interestingly, in MDA-MB-231 TNBC cells exogenous sEcad activated survivin and livin, but not other IAP family members, suggesting that in our model system sEcad has the potential to signal through both IAPs and alternate pathways such as Akt to promote breast cancer survival. The detailed molecular mechanism by which sEcad stimulates these biologic responses in HER2+ and TNBC breast cancer cells that lack membrane bound intact E-cadherin, is not clear. However, tumors exist in a dynamic microenvironment whereby sEcad can be cleaved from membrane-bound E-cadherin found in many interdependent cell types, including monocytes, tumor-associated macrophages and dendritic cells [45,46]. Moreover, within the tumor mileu, these and other cell-types secrete MMPs and a plethora of cytokines, all of which can enhance the generation of sEcad from the intact E-cadherin glycoprotein [47,48].
Previous studies have demonstrated a functional role for the shed sEcad ectodomain fragment in a wide array of epithelial-derived cancers. Specifically, sEcad has been shown to disrupt cell–cell adhesion and enhanced migration and invasion in ovarian, prostate and transformed MDCK cancer cell lines, whereas immunodepletion of sEcad from the conditioned media reversed these effects [2–6]. Using a wound channel migration assay, Najy et al. [4] demonstrated that knockdown of ADAM15, which mediated the endogenous generation of sEcad, decreased migration compared to MCF-7 scrambled controls and also reported that exogenous sEcad enhanced SKBR3 proliferation. In addition, knockdown of MMP-9 in squamous cell carcinoma cells (SCC10A) inhibited the degradation of intact E-cadherin into sEcad, and reduced migration and invasion [26]. Here, we show that sEcad enhances breast cancer proliferation, migration, and invasion in HER2+ and/or TNBC breast cancer cells and that these functional effects were associated with cytoskeletal reorganization, formation of lamellopodia and alterations in cellular morphology, processes typically involved with tumor cell motility [31].
Because sEcad, the HER family of receptors and their cognate growth factor ligands all regulate several key mechanisms underlying tumor pathogenesis and progression, including proliferation, migration and tissue invasion, suggested that sEcad may signal via this receptor tyrosine kinase family to promote these functional effects. Here, we are the first to report that sEcad signals via HERs to promote these cellular functions in breast cancer. Specifically, in the presence of the selective HER inhibitors Canertinib, Lapatinib, Gefitinib, and Mubritinib, we demonstrate that sEcad signals predominately through HER1 and HER2 to promote breast cancer proliferation/migration and invasion in a HER2 positive and a TNBC cell line, respectively. However, the tumor microenvironment also contains HER ligands that they may compete or act additively/synergistically with sEcad for binding, and hence impact tumor cell migratory and invasive properties. Since HER1 and HER2 were the predominant receptor subtypes expressed in our human HER2+ and TNBC tumor specimens and there is no direct high-affinity ligand for HER2, we chose to use EGF since it is one of the ligands specific for the HER1 receptor [49]. As previously reported by others, EGF is not only capable of activating HER1 but also other HER family members in trans via heterodimerization with HER1 [49]. In the current study, we found that combinatorial treatment with equimolar concentrations of rhEGF and rhEcad/Fc resulted in a partial, but significant additive effect on cell proliferation, migration, and invasion. As far as HER activation and downstream signaling, our previous time course studies (30 min–48 h) with rhEcad/Fc demonstrated a bimodal effect in MCF-7, SKBR3, and MDA-MB-231 cells. That is, we observed an early (30 min–2 h) and late (24–26 h) rhEcad/Fc-induced activation of this axis, but the latter exhibited a more heightened and sustained effect (data not shown). Therefore, we utilized this 24–26 h time point to investigate these signaling pathways in this study. Thus, when we examined HER phosphorylation and downstream signaling, EGF stimulated higher levels of HER1 and ERK1/2 phosphorylation compared with exogenous sEcad, but both exhibited similar levels of phospho-HER2, HER4, PI3K, and expression levels of specific IAPs. In contrast, independent administration of sEcad resulted in higher levels of HER3, Akt, and mTOR phosphorylation, suggesting that ligand binding or the geometry of the receptor dimers induced by EGF and sEcad may be subtly different. Of note, combination treatment with EGF and sEcad, did not result in an additive effect on HER and downstream signaling compared to either agent alone. This discrepancy by which combination treatment exerted additive functional effects, without a similar additive effect on signaling may be related to the 26 h time point chosen. Typically, phosphorylation of these receptors by EGF occurs within minutes and future studies using these early time points are needed to address this dichotomy.
Taken together, we show that sEcad protein expression levels are increased in resected human HER2+ and TNBC breast tumor tissues, mouse HER2+ breast tumors and bodily fluids and corresponding human cell culture systems. Our data further demonstrate that sEcad associates, activates and signals via HER family members, to enhance MAPK, PI3K/Akt/mTOR, and IAP signaling and pro-oncogenic functions in both HER2+ and TNBC breast cancer cell lines. Lastly, in vitro we demonstrate that sEcad acts additively with the EGF ligand to promote breast cancer proliferation, migration, and invasion. Altogether, the abundance of sEcad in tumor tissues and bodily fluids, together with its pro-oncogenic properties and correlation with disease-free survival and therapeutic response, suggests that sEcad may be a potential novel candidate protein for drug targeting in breast cancer.
ACKNOWLEDGMENTS
The authors would like to thank Liqun Wang for her technical assistance. This work was supported by the Susan G. Komen Breast Cancer Career Catalyst grant KG081308 (to S.B.).
Abbreviations
- MMPs
metalloproteinases
- ADAMs
a disintegrin and metalloproteinase
- TNBC
triple negative breast cancer
- HER
human epidermal growth factor receptors
- MAPK
mitogen-activated protein kinase
- p70S6K
p70S6 kinase
- 4E-BP1
eIF4E binding protein 1
- IAPs
inhibitor of apoptotic proteins
- XIAP
X-linked inhibitor of apoptosis
- MDCK
Madin–Darby canine kidney
- rhEGF
recombinant human EGF
- mTOR
mammalian target of rapamycin
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES
- 1.Berx G, Van Roy F. The E-cadherin/catenin complex: An important gatekeeper in breast cancer tumorigenesis and malignant progression. Cancer Res. 2001;3:289–293. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maretzky T, Reiss K, Ludwig A, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: A key mechanism in hepatocyte growth factor/scatter factor induced cell-cell dissociation and in vitro invasion. Clin Cancer Res. 2001;7:3289–3297. [PubMed] [Google Scholar]
- 4.Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noë V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Symowicz J, Adley BP, Gleason KJ, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 7.Kuefer R, Hofer MD, Gschwend JE, et al. The role of an 80 kDa fragment of E-cadherin in the metastatic progression of prostate cancer. Clin Cancer Res. 2003;9:6447–6452. [PubMed] [Google Scholar]
- 8.Hofmann G, Dandachi N, Balic M, et al. Quantitative measurement of soluble E-cadherin in sera of breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy and its predictive and prognostic value. J Clin Oncol. 2005;23:768. [Google Scholar]
- 9.Katayama M, Hirai S, Kamihagi K, et al. Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer. 1994;69:580–585. doi: 10.1038/bjc.1994.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holbro T, Beerli RR, Maurer F, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 12.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network or growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 14.Salvesen GS, Duckett CS. IAP proteins: Blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 15.Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115:2673–2678. doi: 10.1172/JCI26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: Implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 17.Altieri DC. Validating survivin as a cancer therapeutic target. Nature Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 19.Hinnis AR, Luckett JCA, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007;96:639–645. doi: 10.1038/sj.bjc.6603616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Liu Y, Ji R, Gu Q, Zhao X, Sun B. Prognostic value of the X-linked inhibitor of apoptosis protein for invasive ductal breast cancer with triple-negative phenotype. Hum Pathol. 2010;41:1186–1195. doi: 10.1016/j.humpath.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Schimmer AD, Welsh K, Pinilla C, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 22.Vucic D, Fairbrother WJ. The Inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Bisi J, Strum J, et al. Regulation of survivin by ErbB2 signaling: Therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 24.Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018–11025. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 25.Inge LJ, Barwe SP, D'Ambrosio J, et al. Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res. 2011;317:838–848. doi: 10.1016/j.yexcr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Zuo JH, Zhu W, Li MY, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112:2508–2517. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 27.Brouxhon S, Kyrkanides S, O'Banion MK, et al. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: Loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/Akt pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 29.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, et al. Inhibitor of apoptosis (IAP) survivin is indispensable for survival of HER2 gene-amplified breast cancer cells with primary resistance to HER1/2-targeted therapies. Biochem Biophys Res Commun. 2011;407:412–419. doi: 10.1016/j.bbrc.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–2111. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Sahin O, Fröhlich H, Löbke C, et al. Modeling ERBB receptor-regulated G1/S transition to find novel targets for de novo trastuzumab resistance. BMC Syst Biol. 2009;3:1–20. doi: 10.1186/1752-0509-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gofuku J, Shiozaki H, Doki Y, et al. Characterization of soluble E-cadherin as a disease marker in gastric cancer patients. Br J Cancer. 1998;78:1095–1101. doi: 10.1038/bjc.1998.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashiwagi S, Yashiro M, Takashima T, et al. Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer. 2010;103:249–255. doi: 10.1038/sj.bjc.6605735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanaka K, Nakata M, Kaneko N, et al. YM155, a selective survivin suppressant, inhibits tumor spread and prolongs survival in a spontaneous metastatic model of human triple negative breast cancer. Int J Oncol. 2011;39:569–575. doi: 10.3892/ijo.2011.1077. [DOI] [PubMed] [Google Scholar]
- 36.Choi KS, Lee TH, Jung MH. Ribozyme-mediated cleavage of the human survivin mRNA and inhibition of antiapoptotic function of survivin in MCF-7 cells. Cancer Gene Ther. 2003;10:87–95. doi: 10.1038/sj.cgt.7700531. [DOI] [PubMed] [Google Scholar]
- 37.Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffer S, Orta L, Sunkara S, Sabo E, Burstein DE. Immunohistochemical detection of antiapoptotic protein X-linked inhibitor of apoptosis in mammary carcinoma. Hum Pathol. 2007;38:864–870. doi: 10.1016/j.humpath.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Yagihashi A, Ohmura T, Asanuma K, et al. Detection of autoantibodies to survivin and livin in sera from patients with breast cancer. Clin Chim Acta. 2005;362:125–130. doi: 10.1016/j.cccn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Feng Q, Kim JM, et al. Human ovarian cancer and cisplatin resistance: Possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–380. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 41.Asselin E, Wang Y, Tsang BK. X-linked inhibitor of apoptosis protein activates the phosphatidylinositol 3-kinase/Akt pathway in rat granulosa cells during follicular development. Endocrinology. 2001;142:2451–2457. doi: 10.1210/endo.142.6.8080. [DOI] [PubMed] [Google Scholar]
- 42.Asselin E, Mills GB, Tsang BK. Xiap regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- 43.Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates antiapoptotic proteins survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC Cancer. 2008;8:129. doi: 10.1186/1471-2407-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H, Zhang G, Wang Y, et al. Inhibition of ErbB2 by Herceptin reduces survivin expression via the ErbB2-b-catenin/TCF4-survivin pathway in ErbB2-overexpressed breast cancer cells. Cancer Sci. 2012;101:1156–1162. doi: 10.1111/j.1349-7006.2010.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riedl E, Stöckl J, Majdic O, Scheinecker C, Knapp W, Strobl H. Ligation of E-cadherin on in vitro-generated immature Langerhans type dendritic cells inhibits their maturation. Blood. 2000;96:4276–4284. [PubMed] [Google Scholar]
- 46.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated Colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maretzky T, Scholz F, Köten B, Proksch E, Saftig P, Reiss K. ADAM10-mediated E-cadherin release is regulated by proinflammatory cytokines and modulates keratinocyte cohesion in eczematous dermatitis. J Invest Dermatol. 2008;128:1737–1746. doi: 10.1038/sj.jid.5701242. [DOI] [PubMed] [Google Scholar]
- 48.Pittard AJ, Banks RE, Galley HF, Webster NR. Soluble E-cadherin concentrations in patients with systemic inflammatory response syndrome and multiorgan dysfunction syndrome. Br J Anaesth. 1996;76:629–631. doi: 10.1093/bja/76.5.629. [DOI] [PubMed] [Google Scholar]
- 49.Riese DJ, Komurasakii T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]