Abstract
Autism Spectrum Disorders (ASDs) are neurodevelopmental syndromes characterised by repetitive behaviours and restricted interests, impairments in social behavior and relations, and in language and communication. These symptoms are also observed in a number of developmental disorders of known origin, including Fragile X Syndrome, Rett syndrome, and Fetal Anticonvulsant Syndrome. While these conditions have diverse etiologies, and poorly understood pathologies, emerging evidence suggests that they may all be linked to dysfunction in particular aspects of GABAergic inhibitory signalling in the brain. We review evidence from genetics, molecular neurobiology and systems neuroscience relating to the role of GABA in these conditions. We conclude by discussing how these deficits may relate to the specific symptoms observed.
Keywords: GABA, autism spectrum disorders, GABAA receptor, inhibitory interneurons, autism, Fragile X syndrome, Rett syndrome, Fetal Anticonvulsant Syndrome
1. Introduction
Autism spectrum disorders (ASDs) are a group of common neurodevelopmental syndromes. ASDs are diagnosed on the basis of qualitative behavioral abnormalities in three domains: social interaction, language and communication, and repetitive or restricted interests or behaviors (American Psychiatric Association, 2000).
ASD symptoms are also observed in a number of neurodevelopmental disorders of known etiology, including Fragile X syndrome, Rett syndrome, and Fetal Anticonvulsant Syndrome. While these disorders have very different underlying etiologies, their overlapping symptom profiles suggest that they may share a final common neurobiological pathway, which may also be present in idiopathic ASDs. However, the nature of this pathophysiology remains unclear.
Emerging evidence suggests that this pathway critically involves impairments in particular aspects of inhibitory gamma-aminobutyric acid (GABA) neurotransmission. In this paper, we review the evidence of abnormalities in GABAergic neurons and synapses in neurodevelopmental disorders characterised by a shared symptomatology of ASD symptoms. A number of previous reviews have discussed selected aspects of this topic in detail, such as the evidence from molecular neurobiology and animal models (D’Hulst and Kooy, 2007; Pizzarelli and Cherubini, 2011; Rossignol, 2011; Sgado et al., 2011). However, few authors have attempted to integrate this literature with the evidence from studies of human patients. The question of the mechanisms by which the hypothesized GABA deficits give rise to the characteristic symptoms of these disorders in humans, has likewise rarely been raised.
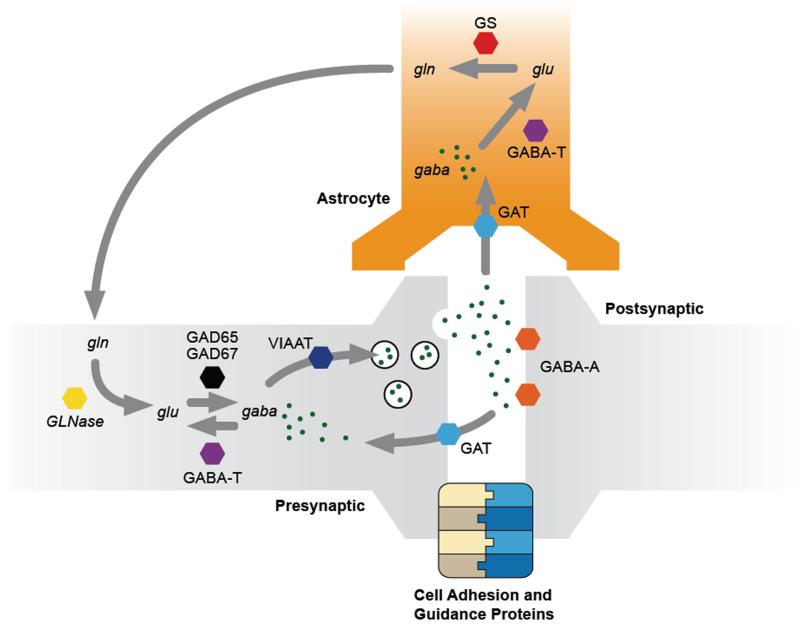
In this paper, we begin by presenting a concise overview of the neurobiology of the human GABA system. See Figure 1 for a graphical overview of the main components of this system. We then examine the evidence relating to the hypothesis that ASD and related disorders are characterised by particular abnormalities in this system. We conclude by discussing how these abnormalities might relate to the particular clinical features seen in these syndromes.
Figure 1.
A simplified illustration of the major synaptic pathways responsible for GABA synthesis, breakdown, release and reuptake. Abbreviations: gaba = gamma-aminobutyric acid; glu = glutamate; gln = glutamine. GLNase = glutaminase. GAD = glutamate decarboxylase. GABA-T = GABA transaminase. VIAAT = vesicular inhibitory amino acid transporter. GAT = GABA transporter. GABA-A = GABAA receptor (note: there are numerous subtypes of this receptor, but these are not shown, for simplicity.) GS = glutamine synthetase. Not pictured: GABAB receptors; GABAA or GABAB autoreceptors.
2. The GABA System
2.1. GABA Metabolism, Release and Reuptake
GABA (gamma-aminobutyric acid) is the primary inhibitory neurotransmitter in the adult human brain. An amino acid transmitter, GABA is synthesised from the excitatory neurotransmitter glutamate via the action of glutamate decarboxylase (GAD) enzymes, of which there are two main isoforms, GAD65 and GAD67.
Release of synaptic GABA depends upon the loading of GABA into synaptic vesicles, which is performed by the vesicular inhibitory amino acid transporter (VIAAT) (Gasnier, 2004). Following release, reuptake of extracellular GABA into the presynaptic interneurons is performed by the GABA transporter GAT1, and to a lesser extent by the related GAT2 and GAT3 transporters (Madsen et al., 2009). Glial cells, such as astrocytes, are also involved in GABA reuptake that escapes the synapse and this is primarily via GAT3 (Madsen et al., 2009). Following reuptake the breakdown of GABA into glutamate is performed by GABA transaminase (GABA-T) (Madsen et al., 2008).
In the central nervous system, GABA is produced and released by inhibitory interneurons. These are cells of typically small dimensions and small projection fields, although small populations of longer range GABA projections have been reported in the cortex (Tamamaki and Tomioka, 2010). GABAergic interneurons are diverse, and can be differentiated on the basis of either their cytoarchitecture (basket, chandelier, stellate, and others) or their molecular properties (parvalbumin-expressing (PV+), calretinin+, calbindin+, and others). These subtypes have distinct electrophysiological and functional properties (De Marco Garcia et al., 2011; Fu et al., 2011). In addition, the cerebellum contains a distinct set of GABAergic cells, including the very large Purkinje cells (Bailey et al., 1998) and others.
In addition to those proteins involved in GABA synthesis, release and breakdown, as discussed above, many other molecules play a role in the formation and stabilization of GABAergic synapses, and numerous transcription factors exert indirect effects on GABA function through the regulation of gene expression, receptor trafficking, and downstream signalling pathways (Luscher et al., 2011). The GABA system is therefore vulnerable to genetic perturbation, even if the mutation in question does not directly relate to GABA.
2.2. GABA Receptors
GABA acts on two main classes of membrane-bound receptors - ionotropic GABAA receptors, which are ligand-gated chloride channels, and metabotropic GABAB receptors.
The GABAA receptor is composed of five subunits arranged around a central pore (Nutt and Malizia, 2001), derived from a family of different genes which generates a large degree of receptor diversity, with different combinations of subunits giving rise to receptors with specific properties. GABA binding to the GABAA receptor enhances conductance through the receptor ionophore, which conducts anions, particularly chloride and bicarbonate. The resultant increase of these negatively charged ions within the cell hyperpolarizes the membrane resting potential, thus leading to the inhibition of cell firing under most physiological conditions.
The generation of transgenic mice with point mutations of the benzodiazepine binding site on specific subunits has highlighted the significance of the individual α subunit subtypes in the actions of the benzodiazepines (Rudolph and Mohler, 2004). The α1 subunit receptors appear to be responsible for sedative effects of positive allosteric modulators of the GABAA system, such as diazepam, α2 and α3 receptors for anxiolytic effects (Low et al., 2000; McKernan et al., 2000; Mohler, 2006; Rudolph et al., 1999) and α5 receptors for cognitive and memory deficits (Collinson et al., 2006; Crestani et al., 2002). The α1, α 2, and α 3 subtypes are located in synaptic processes, whereas the α5 subtype is located extrasynaptically, where they regulate tonic inhibition along with another receptor type that contains the α4 and δ subunits.
By contrast, GABAB receptors are involved with second messenger systems through the binding and inhibition of guanine nucleotide-binding proteins (G proteins) (Bettler et al., 2004). These were first observed on presynaptic terminals, where they influence transmitter release by curbing neuronal calcium (Ca2+) conductance, but were subsequently also found on postsynaptic neurons where their activation can result in both increased conduction and neuronal hyperpolarisation (Bowery et al., 2002). The GABAB receptor comprises a homodimer with similar subunits, designated 1 and 2- t (Bowery et al., 2002).
Although GABA is an inhibitory transmitter in the adult brain, it is believed to act as an excitatory (depolarizing) neurotransmitter during neurodevelopment (Sibilla and Ballerini, 2009) and in adults under under certain pathological conditions (Beaumont and Maccaferri, 2011). GABAergic interneurons mature relatively late, with the migration and differentiation of these cells occurring during late gestation and into early infancy in humans (Xu et al., 2011).
Some of the subunits of the GABAA receptor system appear to have functional roles in neurodevelopment. For instance, in rodents, α1 subunit containing receptors play a key role in defining the critical period for plasticity in the developing visual cortex (Fagiolini et al., 2004).
2.3. GABA In Neuropsychiatric Disorders
GABAergic dysfunction has been implicated in anxiety (Durant et al., 2011), epilepsy (Macdonald et al., 2010) and learning impairment, amongst other conditions. A detailed discussion of this topic is outside the scope of this paper, however. Drugs which either act a direct agonists at the GABAA receptor, or those which act as positive allosteric modulators, for example, benzodiazepines, have hypnotic, anxiolytic, anticonvulsant, and sedative effects. These effects are, to some degree, fractionable, with subunit-specific drugs having more selective effects (Rowlett et al., 2005).
Other anticonvulsant drugs with anti-anxiety effects act by indirectly increasing GABAA transmission by promoting the synthesis of GABA or inhibiting its reuptake or breakdown (Madsen et al., 2009). These include vigabatrin, which blocks GABA catabolism by inhibition of GABA-transaminase, and tiagabine, which blocks the reuptake of GABA. Conversely, drugs which either antagonize the GABAA receptor (Fernandez et al., 2007) or inhibit GABA synthesis (Horton and Meldrum, 1973) cause central nervous system stimulation, anxiety and, in high doses, seizures. This is of interest given the high comorbidity of both epilepsy and anxiety disorders in ASD (see Section 3.1).
3. Autism Spectrum Disorders
3.1. Clinical Features
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders, with a population prevalence of approximately 0.5-1.5% (Baron-Cohen et al., 2009; Brugha et al., 2011; Fountain et al., 2010). ASDs are more common in males than in females, with a gender ratio of approximately 4:1 (Baron-Cohen et al., 2009).
ASDs are characterised by a triad of impairments in three domains: social interaction, language and communication, and repetitive and restricted interests and behaviors (Rutter, 1978). For a diagnosis of ASD to be made, these symptoms must be present from before the age of 36 months (World Health Organization, 1993).
Individuals with an ASD may also have an intellectual disability. However, at least 25% of cases show normal or superior intellectual function – at least in some elements (Rutter, 1983). ASDs are described as a spectrum of disorders because the severity of these symptoms, and the associated functional impairment, vary between individuals. The terms “autism” and “childhood autism” refers specifically to cases characterised by delays in the acquisition of expressive language. “Asperger’s Syndrome” is used to describe cases with normal language development and no intellectual disability. Finally “Atypical Autism” and “Pervasive Developmental Disorder – Not Otherwise Specified” are terms used in individuals who show many, but not all, of the symptoms required for a diagnosis of autism or Asperger’s Syndrome (American Psychiatric Association, 2000).
Epilepsy is observed in up to 25% of people with an ASD (Amiet et al., 2008; Bolton et al., 2011), a high rate when compared with a population prevalence of less than 1%. Epilepsy in ASD is correlated with having a low IQ, and with more severe symptoms, and is also more common in females (Bolton et al., 2011). The age of onset of seizures varies from infancy through to early adulthood, with a peak in childhood at age 5-10 (Bolton et al., 2011). Given the close involvement of GABA dysfunction in epilepsy (see above), this is suggestive evidence for the presence of a GABA system deficit in ASD, in at least some cases.
3.2. Etiology
ASDs are highly heritable, with estimates of heritability ranging from about 0.5 to 0.9 (Freitag et al., 2010; Ronald and Hoekstra, 2011). However, most cases of ASD are idiopathic i.e. not associated with a discernable genetic or environmental cause. This stands in contrast to the syndromes discussed below, such as Fragile X Syndrome (Section 4) and Rett Syndrome (Section 5), that are known to be caused by a single gene mutation.
It is likely that the etiology of idiopathic ASDs involves complex interactions between heritable and environmental factors. Heritable changes that influence gene expression can be attributed to DNA sequence variation as well as epigenetic modifications (including DNA methylation, post-translational modifications of histone proteins, and RNA-based silencing), and certain heritable genetic factors have been associated with abnormalities in GABA, which in turn have been linked with epigenetic findings. While the literature on genetic studies of neurodevelopmental disorders is extensive, findings from epigenetic studies are still in their infancy, but are showing promising initial findings (Grafodatskaya et al.; James et al., 2004; James et al.; Schanen, 2006; Williams et al., 2001).
Large genome-wide studies have implicated a number of single-nucleotide polymorphisms (SNPs) (Weiss et al., 2009) and chromosomal linkage regions (Weiss et al., 2009) in the causality of ASDs. However, thus far, known SNPs only account for a small proportion of the risk for ASD. This suggests that ASDs are genetically heterogeneous with no single variant accounting for a large proportion of cases.
Emerging evidence suggests, however, that many cases of ASD are caused by microdeletions or microduplications, collectively known as copy-number variations (CNVs). CNVs are submicroscopic chromosomal abnormalities ranging in size from a few bases up to about 500 kilobases (Feuk et al., 2006). Whole-genome CNV scans have only been in widespread use for less than 5 years, yet they have revealed candidate loci for ASD (Christian et al., 2008; Schaaf and Zoghbi, 2011; Sebat et al., 2007) as well as other neuropsychiatric disorders (Guilmatre et al., 2009; International Schizophrenia Consortium, 2008).
3.3. ASD and GABA
3.3.1. Genetics
One of the most commonly reported chromosomal loci where microdeletion / microduplication CNVs have been observed in ASD is the chromosome 15q11-q13 region. This site contains a number of genes coding for particular subunits of the GABAA receptor, namely GABRB3, GABRA5 and GABRG3, encoding for the β3, α5, and γ3 subunits, respectively.
Deletions spanning this region result in either Angelman’s syndrome (AS) or Prader-Willi Syndrome (PWS). Deletions affecting the maternally inherited copy of this region cause AS, while paternal deletions cause PWS - an example of genomic imprinting (Knoll et al., 1989).
By contrast, duplications of the 15q11-13 locus have been observed in patients with ASD in numerous studies (Buxbaum et al., 2002; Cook et al., 1998; McCauley et al., 2004; Menold et al., 2001; Sebat et al., 2007; Shao et al., 2003). Maternally derived duplications predominate, suggesting genomic imprinting, just as in the case of deletions. The prevalence of 15q11-13 duplications in the population of individuals with idiopathic ASD has been estimated at 0.5-3%, although not everyone with the duplication meets criteria for ASD (Bolton et al., 2001). Epilepsy, suggestive of an inhibitory signalling deficit, is also common in 15q11-13 duplication (Simon et al., 2009).
It might seem that duplication of a region containing GABA receptor subunits ought to lead to excessive inhibitory neurotransmission. However, in vitro studies of a human neuronal cell line carrying a maternal 15q duplication showed that this variant leads to reduced GABRB3 expression via impaired homologous pairing (Meguro-Horike et al., 2011), and may also affect cell migration and maturation Furthermore, a mutation in the GABRB3 gene was associated with a 3- to 6-fold increased risk of ASD (with epilepsy in some cases), especially when maternally inherited (Delahanty et al., 2009). Other GABAA subunit variants, including GABRA4 and GABRB1, have also been linked to ASD (Ma et al., 2005)
Further, although the majority of cases of idiopathic autism do not carry mutations at this locus, abnormal expression of proteins encoded by 15q11-13 genes have also been reported in autism. In the normal human brain, GABRB3, GABRA5 and GABRG3 are biallelically expressed. However, Hogart et al (2007) reported that in four out of eight cases of idiopathic ASD, the maternal copies of these genes predominated, and levels of these proteins, especially GABRB3, were reduced (Hogart et al., 2007). This suggests that the 15q11-13 locus is commonly implicated in autism and that it can be disrupted, either directly by mutation, or indirectly, perhaps by epigenetic factors.
As discussed in the introduction, GABAergic dysfunction could also be a downstream consequence of mutations in genes not directly involved in GABA transmission because the proper development and function of GABA synapses relies on numerous signalling and scaffolding proteins. Several putative ASD mutations disrupt genes known to be involved in synaptic formation and signalling, and have been shown in mouse models to have a specific effect on inhibitory signalling. For example, deletions of contactin-associated protein 2 (CNTNAP2) have been linked to autism (Gregor et al., 2011; Nord et al., 2011; Stephan, 2008) and common variants are associated with communication and language difficulties (Stein et al., 2011; Whitehouse et al., 2011). This gene encodes a protein in the neurexin family of cell adhesion molecules required for neural development. In mice, CNTNAP2 deficiency leads to specific deficits in inhibitory signalling with reduced expression of GAD1 and of parvalbumin+ inhibitory interneurons (Penagarikano et al., 2011).
Similar findings were observed in mice lacking another ASD candidate gene, CADPS2 (Sadakata et al., 2007) – reduced cortical parvalbumin+ GABA interneurons, as well as cerebellar Purkinje cells. Likewise, mice lacking the murine version of PLAUR, another gene mutated in some ASD individuals, show epilepsy, anxiety and impaired social behaviors, as well as reduced GABAergic interneurons in regions of the cortex, and altered GABAA expression (Eagleson et al., 2011). This led some authors to propose a “common circuit defect of excitatory-inhibitory balance in mouse models of autism”(Gogolla et al., 2009).
Most recently, whole-exome sequencing of idiopathic ASD cases has revealed an over-representation of deleterious de novo single nucleotide variants impacting known genes in ASD (Iossifov and al., 2012; Neale et al., 2012; O’Roak et al., 2012; Sanders et al., 2012). However, these variants were widely distributed over the genome rather than being associated with particular genes. Although the functional impact of these diverse de novo variants remains to be determined, and is probably different in each case, it is interesting that affected genes were over-represented among Fragile X Mental Retardation Protein (FMRP) interaction partners (Iossifov and al., 2012). Because FMRP knockout is known to create an inhibitory signalling defect in mice (see Section 4) it is plausible that mutations may cause ASD if they (directly or indirectly) impact inhibitory signalling. However, this is speculative and much more work is needed.
3.3.2. Gene Expression
The previous section discussed evidence for an association between mutations affecting GABA gene function and ASD. Other studies have reported reduced expression of GABAergic genes and reduced density of GABA-related proteins in postmortem brain samples from individuals with an ASD.
GAD65 and GAD67 proteins have been reported as reduced in the cerebellum and parietal cortex (Fatemi et al., 2002), while GAD67 messenger RNA (mRNA) was reduced in cerebellar Purkinje cells (Yip et al., 2007), indicating reduced expression of this gene. In the hippocampus (Blatt et al., 2001) and anterior and posterior cingulate cortices, GABAA receptor binding was reduced (Oblak et al., 2009; Oblak et al., 2010). Recent studies showed reduced GABRB3 expression in the cingulate cortex (Thanseem et al., 2011) and the cerebellar vermis (Fatemi et al., 2011) in ASD, consistent with the genetic evidence for the involvement of this gene in ASD, as discussed in Section 3.3.1.
By contrast, increased expression of the excitatory glutamate receptor AMPA, and of glutamate transporter proteins were found, most notably in the cerebellum (Purcell et al., 2001). Pilot evidence points to an association between the GAD65 gene and ASD (Johnston et al., 2008)
Several post mortem studies have also reported decreases in the number of the large GABAergic Purkinje cells in the cerebellar cortex. A 2004 review found that 21 out of 29 ASD specimens examined showed this reduction (Palmen et al., 2004), although a subsequent study showed abnormalities in only 3 of 6 (Whitney et al., 2008).
The studies discussed above focussed on particular genes, but a recent post mortem study used transciptomic analysis to discover abnormalities in the expression of genes across the genome in the autistic brain. This study provides strong convergent evidence for an inhibitory deficit. In two separate samples, they found reduced cortical expression of mRNA in M12, a cluster of genes including CNTNAP2, which is highly expressed in parvalbumin+ GABA interneurons (Voineagu and al., 2011). Interestingly, one of the ASD subjects in this study was found to carry a 15q11-13 duplication, but their pattern of mRNA expression was similar to that of the other ASD cases, consistent with the view that this syndrome is a useful model of idiopathic ASD.
In summary, there is extensive evidence of abnormal patterns of expression of GABA-A receptor genes, the GABA synthesis enzymes GAD65 and GAD67, and other genes known to be expressed in GABA interneurons, in idiopathic ASD. However, little is known about the underlying molecular mechanisms which are responsible for these differences. Gene expression in the brain is controlled by numerous regulatory pathways and epigenetic influence (REFS). Research should examine this…
3.3.3. In Vivo Human Studies
In contrast to the large number of studies using animal models and post-mortem techniques, relatively few studies have attempted to measure GABA or GABA-related proteins in living ASD subjects. This is likely due to the technical difficulties in measuring GABA function in vivo.
For example, proton magnetic resonance spectroscopy ([1H]MRS) is a technique widely used to quantify key neural metabolites in vivo, including glutamate, glutamine, and others. However, while there have been several ([1H]MRS) investigations of the brain in ASD (see (DeVito et al., 2007; Friedman et al., 2006; Page et al., 2006)), these studies were not able to estimate concentrations of GABA because of the difficulties in distinguishing GABA from other similar molecules in the magnetic resonance spectrum.
However, advances in J-edited [1H]MRS (Rothman et al., 1993) have made it possible to measure GABA and this technique has recently been used to investigate GABA function in healthy volunteers (Near et al., 2011; Stagg et al., 2011). There has only been one [1H]MRS investigation of GABA in ASD, and this reported that GABA concentration was reduced in the frontal cortex of children (Harada et al., 2011). By contrast, no differences were seen in the basal ganglia. However, this study was relatively small, with 12 patients and 10 controls, and most subjects were sedated with trichlofos twenty minutes before the scan, potentially biasing the results. Further work using this promising technique is therefore required – and in medication-free individuals.
Using Positron Emission Tomography (PET) or Single Photon Emission Computed Tomography (SPECT) with selective radioligands, it is possible to quantify the density of neurotransmitter receptors in the human brain in vivo. For GABA, the ligands flumazenil and flunitrazepam bind GABAA receptors, and the novel Ro154513 is largely selective for the α5 subtype of GABAA receptors (Lingford-Hughes et al., 2010).
Two in vivo studies investigating the GABAA receptor in ASD have recently been conducted, suggesting that GABAA is reduced in both adults and children with ASD: (Mori et al., 2011) found reduced accumulation of the GABAA-benzodiazepine ligand [123I]iomazenil using SPECT (Single Photon Emission Computed Tomography) in children with ASD. A recent pilot study from our group (Mendez et al., 2012) accepted for publication, confirmed reduced GABAA in 3 adult males with ASD. Further work is called for to confirm these results.
Other researchers have attempted to indirectly probe brain GABA function. Transcranial Magnetic Stimulation (TMS) offers one such approach (Farzan et al., 2011). TMS uses strong pulsed localized magnetic fields to stimulate cortical neural activity. Depending upon the cortical region affected this can cause, for example, muscular contraction. When two pulses are administered in quick succession (2 ms), the effect of the second is inhibited and this is believed to reflect GABAA signalling (intracortical inhibition).
Three small studies have examined intracortical inhibition in ASD. Two showed reduced inhibition only in some subgroups of patients (Enticott et al., 2010)(Oberman et al., 2011) although the most recent, and largest, study found no significant differences in ASD adults (Enticott et al., 2012)..These studies therefore provide mixed evidence for a cortical inhibitory deficit in some cases of ASD, but the small sample size makes interpretation difficult, and the hypothesis that TMS measures of “cortical inhibition” are proxies for cortical GABA concentration has recently been questioned (Stagg et al., 2011).
Finally, it is possible to measure GABA and related molecules in blood plasma. Increased GABA in the plasma of individuals with ASD has been found in two studies (Dhossche et al., 2002; El-Ansary et al., 2011). However, decreased platelet GABA has also been observed (Rolf et al., 1993). Similarly, increased glutamate and decreased glutamine in plasma has been reported (Aldred et al., 2003; Moreno-Fuenmayor et al., 1996; Shimmura et al., 2011; Shinohe et al., 2006). The interpretation of such findings in terms of brain function is complicated, since neither glutamate nor GABA cross the blood-brain barrier under normal conditions (Smith, 2000).
In summary, there has been little work examining the GABA system in living human brains, owing to technical limitations, but in the past two years such studies have begun to appear and provide preliminary evidence consistent with a GABA defect. However, a challenge for the future will be to relate these findings to the genetic and postmortem gene-expression data, discussed previously (Sections 3.3.1 and 3.3.2), in order to understand the underlying basis of these changes.
One approach that holds great promise is the in vitro culture of neurons via the generation of human induced pluripotent stem cells (iPSCs) from adult somatic cell samples. This technique has been successfully used to investigate gene expression changes in patients with schizophrenia (Brennand et al., 2011) and Timothy syndrome, a rare neurodevelopmental disorder (Pasca et al., 2011), revealing abnormal expression of genes linked to neurotransmission and neuronal connectivity. There have been no published studies using this technique to investigate idiopathic ASD but this approach could provide important insights into the cellular mechanisms behind neural function in ASD, including GABA system abnormalities.
4. Fragile X Syndrome
4.1. Clinical Features and Etiology
Fragile X Syndrome (FXS) is a monogenetic neurodevelopmental disorder, caused by an abnormal expansion of a CGG trinucleotide repeat within the promoter region of the gene FMR1 on the X chromosome (Wisniewski et al., 1991). FXS has an estimated prevalence of 1/4000 in males and 1/6000 in females (Turner et al., 1996). Common symptoms of FXS include intellectual disability, a distinctive physical phenotype, and symptoms of social and communication impairment and repetitive behaviours similar to those seen in ASD.
Around 20% of those with FXS meet formal criteria for an ASD (Clifford et al., 2007; Harris et al., 2008; Hatton et al., 2006; Wang et al., 2010), with others showing milder degrees of autistic symptoms. In comparison to several other monogenetic neurodevelopmental syndromes, including Cri-du-Chat, Smith-Magenis and Angelman Syndrome, FXS was the condition most associated with autistic features, and was the only one in which repetitive behaviors and interests were prominent (Oliver et al., 2010).
Healthy individuals carry less than 55 repeats of the CGG sequence in the FMR1 promoter region. Copy numbers exceeding 200 cause gene silencing by hypermethylation, and this leads to FXS. Copy numbers ranging between 55-200, termed premutations, do not cause FXS, but may cause milder neurological and neuropsychiatric presentations, particularly in males. Fragile X mental retardation protein (FMRP) is an mRNA-binding protein involved in the regulation of the translation of mRNA into proteins (Laggerbauer et al., 2001). Recent evidence suggests that FMRP is involved in neuronal activity-dependent trafficking of mRNA to synapses (Dictenberg et al., 2008), and that it is especially associated with transcripts involved in synapse formation and implicated in ASD (Darnell et al., 2011).
Epilepsy, which responds well to anticonvulsants, is observed in approximately 20-25% of cases of FXS, implicating a cortical inhibitory deficit (Wisniewski et al., 1991). The most distinctive neuropathological marker of FXS is abnormal neuronal morphology, in the form of long, thin dendritic spines of pyramidal neurons (Penzes et al., 2011; Portera-Cailliau, 2011; Wisniewski et al., 1991). This phenotype has been attributed to the overexpression of metabotropic glutamate receptors of the mGluR5 subtype, and it has been proposed that mGluR5 antagonists might rescue some of the deficits associated with this disorder (Levenga et al., 2011; Wang et al., 2010). However, abnormalities in the function and biology of the GABA system are also emerging as important in FXS.
4.2. GABA and Fragile X
An early study reported regional alterations in brain tissue GABA levels in male juvenile, but not adult, mice lacking a functional copy of the FMR1 gene (FMR1 knockout mice), with both increases and decreases seen in different regions (Gruss and Braun, 2001). However, it is hard to interpret differences in GABA levels without consideration of the expression of GABA receptors and other proteins.
In terms of function, D’Antuono et al (D’Antuono et al., 2003) reported abnormal responses to the acetylcholine agonist carbachol in knockout mice. In wild-type mice, carbachol indirectly suppresses excitatory post-synaptic potentials (ePSPs) in the subiculum by promoting inhibitory GABA signalling, but in knockouts, it caused potentiation of ePSPs, mimicking the effects seen in wild-type mice after administration of a GABAA antagonist. Fragile X mice also show blunted cortical inhibitory signalling in response to the administration of a glutamate agonist (Paluszkiewicz et al., 2011). This suggests a functional GABAA signalling deficit in FXS.
Direct evidence for such a deficit has come from studies of the expression of GABAA subunit proteins or mRNA in FMR1 knockout mice. GABAA β subunit immunoreactivity was decreased to 60-70% of normal levels in several brain regions with the exception of the cerebellum, while GAD65 and GAD67 expression was increased (El Idrissi et al., 2005).
Furthermore, D’Hulst et al reported that mRNA encoding 8 of the 18 known GABA subunits (α1, α3 and α4; β1 and β2, γ1 and γ2, as well as δ) were significantly reduced in the cortex, but not in the cerebellum, of FMRP-lacking mice (D’Hulst et al., 2006; D’Hulst et al., 2009). GABAA δ mRNA is a binding target of FMRP and it is involved in activity-dependent regulation of this subunit (Dictenberg et al., 2008).
D’Hulst et al also found reduced expression of all three subunits of the Drosophila GABA receptor in Fragile X Drosophila (D’Hulst et al., 2006), suggesting an evolutionarily conserved role for FMRP in GABA receptor function. A separate study reported reduced GABAA β2 subunit in all areas (replicating D’Hulst et al, above) and reductions in β3 in the cortex, but increases were seen in subcortical areas (Hong et al., 2011). Similarly, Downregulation of GABAA α1, β2, and δ subunits, and GABA-T and SSADH were observed at different stages of postnatal development (Adusei et al., 2010).
However, FXS seems to affect the integrity of the GABA system in a regionally-specific fashion. As noted above, the cerebellum seems to be spared. Centonze et al (Centonze et al., 2008) found a 50% decrease in the number of GABA synapses in the striatum of FMR1 knockout mice, but also observed a paradoxical increase in the level of spontaneous GABA inhibitory transmission (iPSPs). By contrast, Curia et al (Curia et al., 2009) observed reduced tonic GABAA currents in the subiculum of FMR1 knockouts, with normal phasic GABA currents, and reduced expression of α5 and ∂ subunits – consistent with D’Antuono et al.’s finding of reduced inhibitory function in the same circuits (see above).
Profoundly reduced tonic and phasic inhibitory currents in the amygdala of FX mice have been reported, along with reduced GAD65 and GAD67 expression, reduced GABA, and a reduced number of GABAergic synapses (Olmos-Serrano et al., 2010); amygdala hyperexcitability was rescued by a GABAA δ agonist.
A marked reduction in cortical parvalbumin+ GABA interneurons together with abnormal morphology has also been observed (Selby et al., 2007). This finding is interesting, given that reduced parvalbumin+ interneuron density has been seen in other mouse models of ASD-associated genetic mutations (Gogolla et al., 2009), as previously mentioned (Section 3.3.1), and also in Fetal Anticonvulsant Syndrome (see Section 6).
By contrast, the GABAB system has not been reported as abnormal in FMR1 knockout mice, although GABAB agonists such as baclofen have been shown to alleviate some of the symptoms, including seizures, in animal models (Pacey et al., 2009).
In conclusion, there is growing evidence from animal models for the involvement of GABA in FXS, alongside the established role of glutamate. However, abnormalities appear to be regionally and temporally specific, with the cerebellum being spared.
4.3. Human Studies of GABA in Fragile X Syndrome
In contrast to the fairly extensive postmortem literature on idiopathic ASD (see Section 3.3.2 and 3.3.3), there have been no published investigations directly measuring GABA, GABA receptors or GABA system proteins in the brain of humans with Fragile X syndrome either post mortem, or in vivo. One small study used transcranial magnetic stimulation (TMS) to indirectly probe inhibitory signalling in Fragile X patients, finding no differences from healthy controls (Oberman et al., 2011). See Section 3.3.3 for more on TMS.
Given the strong evidence of GABA abnormalities from rodent models of Fragile X syndrome, the lack of human studies in this disorder is unfortunate. Future work should attempt to confirm whether the same abnormalities are seen in humans, using methods such as [1H]MRS, PET and SPECT as this could have direct clinical applications.
5. Rett Syndrome
Rett syndrome is a severe neurodevelopmental disorder caused by the loss of function of one copy of the methyl-CpG-binding protein 2 (MECP2) gene on the X chromosome. Clinically, Rett syndrome presents with developmental regression usually between the ages of 6-18 months, with the loss of speech, social and motor skills. Repetitive stereotyped movements, most notably hand flapping, emerge.
The syndrome is very rare in males, because it is lethal in those with only one X chromosome, and male fetuses with the mutation rarely survive to term (Renieri et al., 2003). However, mutations leading to only partial loss-of-function of the MECP2 gene have been associated with less severe neuropsychiatric disturbances including ASDs (Carney et al., 2003; Lam et al., 2000).
Epilepsy is comorbid in approximately 80% of cases of Rett syndrome, suggestive of an inhibitory signalling deficit (Jian et al., 2006). MECP2 is a transcriptional regulator which is highly expressed in GABAergic neurons in the brain (Akbarian et al., 2001), but its role in the function of the GABAergic system is unclear. It appears that MECP2 may regulate the number of GABAergic synapses in the thalamus and medulla.
GABAergic abnormalities in the thalamus (Zhang et al., 2010) and medulla (Medrihan et al., 2008) develop in Mecp2-null mice even prior to the onset of symptoms. Zhang et al (Zhang et al., 2010) demonstrated that GABAergic transmission is altered in opposite directions in interconnected GABAergic and glutamatergic neurons in Mecp2-null mice, implying that the cellular and molecular mechanisms underlying Mecp2-mediated regulation of GABAergic transmission are likely to be specific to the location and cell type. A reduction of the β3 subunit of the GABAA receptor has also been demonstrated in cortical samples of Rett syndrome patients and in the neocortex of Mecp2-deficient mice (Samaco et al., 2005). However, some recent studies have shown that MeCP2 deficiency resulted in an enhancement of GABAergic transmission in the neocortex (Dani et al., 2005).
There is preliminary evidence linking epigenetic events to GABA modulation in the context of neurodevelopmental disorders, including Rett syndrome. The MECP2 protein acts as a key mechanism by which DNA methylation regulates gene expression. MECP2 is critical for the normal functioning of cortical GABA-releasing neurons, which express 50% more MECP2 than non-GABAergic neurons (Chao et al., 2010)
A recent study (Chao et al., 2010) demonstrated that mice with a selective MECP2 deficiency in GABAergic neurons resulting in a 30–40% reduction in neurotransmitter release showed abnormalities that resembled Rett syndrome and several autistic features. The MECP2-deficient mice showed reductions in GAD65 and GAD67 mRNA levels (the rate-limiting enzymes of GABA synthesis) and an enrichment of MECP2 protein occupancy at both GAD promoters. As described previously, a post-mortem study observed reduced GAD65 and GAD67 expression in ASD (Fatemi et al., 2002).
As in the case of Fragile X Syndrome, there have been remarkably few studies of GABA in humans with Rett syndrome. An early study of five girls with the disorder found normal GABA in cerebrospinal fluid (Perry et al., 1988). However, reduced GABAA receptor density was observed using SPECT in three adult females in vivo (Yamashita et al., 1998). Post-mortem studies reported normal or increased GABA receptor and glutamate receptor density in girls with Rett syndrome below the age of 8, but reductions with increasing age (Blue et al., 1999a, b). Taken together, this suggests that the GABA deficits seen in animal model of Rett syndrome may not emerge in human patients until late childhood, implying that animal models do not provide an adequate model of Rett pathophysiology in very young patients. However, more studies to confirm this possibility.
6. Fetal Anticonvulsant and Alcohol Syndromes
Fetal exposure to anticonvulsant medication is linked to an increased rate of developmental defects (Holmes et al., 2001). The “Fetal Anticonvulsant Syndrome” (FACS) has varied manifestations, including a pattern of physical dysmorphic features, intellectual disability, and behavioral abnormalities, including autistic symptoms (Rasalam et al., 2005). In one study, approximately 60% of children with FACS displaying at least two autistic features and 10% had a diagnosis of an ASD, around 10 times the population prevalence (Moore et al., 2000).
The mechanism by which fetal anticonvulsants cause developmental disorders is unclear. Since the majority of anticonvulsants work by increasing GABA transmission (Vellucci and Webster, 1984), and given that GABA is a key neurodevelopmental signalling molecule, it is possible that the mechanism involves GABA. However, other mechanisms such as anoxia, secondary to fetal cardiac effects, have also been suggested (Danielsson et al., 2001).
Prenatal valproate exposure has been used in rats and mice as a model for FACS and also idiopathic ASD (Dufour-Rainfray et al., 2011). A single, high-dose injection of valproate given to the pregnant female at the period of embryonic neural tube closure, i.e. Embryonic Day 12 in rats, produces the most consistent behavioral effects (Kim et al., 2011).
Prenatal valproate exposure causes characteristic behavioral and neurobiological abnormalities in rodents. Behaviorally, exposed rats show fewer social behaviors from early life, along with motor incoordination, repetitive, stereotyped locomotor hyperactivity, and a reduced tendency to explore (Schneider and Przewlocki, 2005). These symptoms are similar to those seen in individuals with an ASD.
Neurobiologically, prenatal valproate-exposed rats have a marked reduction in the number of cortical GABA interneurons of the parvalbumin+ type (Gogolla et al., 2009), and characteristic EEG abnormalities, which are also seen in humans with ASD (Gandal et al., 2010). Reduced cerebellar Purkinje GABA cells are also seen, consistent with neuropathological evidence in humans with idiopathic ASD, as discussed previously (Ingram et al., 2000). In summary, prenatal exposure to anticonvulsants is a major risk factor for ASD symptoms. Although the teratogenic mechanism is unclear, there is emerging evidence that deficits in inhibitory interneuron development are a pathophysiological mechanism.
Fetal alcohol exposure is another major risk factor for neurodevelopmental problems, and has been called the largest preventable cause of these disorders. In severe cases, alcohol exposure causes fetal alcohol syndrome which is characterised by growth deficiency (below-normal weight and height), physically dysmorphic features, below-normal IQ, and behavioral disorders (Riley and McGee, 2005). However, the condition exists on a spectrum of severity with the term fetal alcohol spectrum disorders, used to cover the milder manifestations (Chudley et al., 2005).
It has been suggested that rates of ASD symptoms are raised in those with fetal alcohol syndrome (Dufour-Rainfray et al., 2011; Landgren et al., 2011). However, other studies suggest that there are key differences in the manifestations of the two disorders. Although children with fetal alcohol syndrome commonly show impairments in social skills and behavior, they do not show symptoms such as restrictive interests, lack of eye-contact, and lack of shared enjoyment in social activities (Bishop et al., 2007). Children with fetal alcohol syndrome have been described as friendly, loquacious and over-familiar, whereas autistic children are often aloof, shy and withdrawn (Bishop et al., 2007; Riley and McGee, 2005). Maternal alcohol consumption was not correlated with the risk of ASD in a general population study (Eliasen et al., 2010).
The paucity of research focussed on the role of GABA in fetal alcohol spectrum disorders is unfortunate, given that alcohol is known to act on GABA (Kumar et al., 2009). More work is required to investigate possible links between alcohol exposure and ASD.
7. Theoretical and Clinical Implications
We have discussed evidence supporting a role of GABA abnormalities in a number of neurodevelopmental disorders characterised by autistic features, including impaired social interaction and repetitive and restricted behaviors, and intellectual disability. However, this raises the question: why does this neurobiology lead to these particular symptoms?
7.1. Minicolumns and Feature Discrimination
A number of theoretical frameworks for understanding the development of ASDs and related neurodevelopmental disorders have been proposed. One influential theory, proposed on the basis of neuropathological data by Casanova et al (Casanova et al., 2003), is that inhibitory GABA signalling within and between cortical minicolumns is altered due to reductions in the neuropil which separates adjacent minicolumns. This, it is argued, leads to information-processing which tends to display stronger than normal discrimination between related stimuli rather than generalization across them. This may explain why individuals with ASD show a preference for exact sameness (e.g. the same daily routines, the same behaviors and interests), since only exactly the same stimuli would be recognised as similar. It might also explain sensory hypersensitivities and the occasional presence of superior ‘savant’ skills in a narrow domain.
7.2. Gamma Band Activity and Feature Binding
Another model focuses on gamma activity. Evoked gamma band electrical activity (i.e. high frequency >40 Hz) has been extensively studied using EEG and MEG. Theory suggests that these oscillations are produced within the cortex (as opposed to slower waves which likely originate subcortically), and crucially depend on GABA, specifically upon fast-spiking parvalbumin-expressing (PV+) cells (Carlen et al., 2011; Gulyas et al., 2010; Volman and al., 2011) which act to coordinate the firing of excitatory cortical pyramidal cells. Consistent with this view, a recent study using magnetoencephalography found that occipital cortex GABA levels measured with [1H]MRS, correlated with the peak frequency of the visually evoked gamma response in the visual cortex (Muthukumaraswamy et al., 2009) – increased GABA indicates a higher gamma frequency. Another recent MEG study found a similar picture in the motor cortex (Gaetz et al., 2011).
Gamma waves have been proposed as the “temporal solution to the binding problem” (Brock et al., 2002; Singer, 2001). The “binding problem” in cognitive neuroscience refers to the question as to how the brain is able to represent conjunctions of features, e.g. AB CD, but how does the brain represent the fact that A is paired with B, and C with D, if all are active? The proposed temporal solution is that conjunctions are represented by simultaneous activity over very short timescales, and gamma band activity is a leading candidate for the “synchronization wave” which does this.
According to some theorists, this temporal binding is impaired in ASD, leading to weak central coherence i.e. impaired gestalt perception of “wholes” as opposed to parts (Brock et al., 2002). While hypothetical at present, this account does offer a possible explanation for how genetic or environmental effects converging on impairments in certain GABA subtypes (particularly parvalbumin+ cells) could precipitate the symptoms of this disorder.
7.3. Clinical Implications
At present, there are no medications licensed for the specific treatment of ASD, Fragile X syndrome, or related disorders. A number of psychoactive medications are used for symptomatic relief e.g. antidepressants for obsessive and compulsive symptoms, and atypical antipsychotics for irritability and challenging behaviours (McPheeters et al., 2011; Pringsheim et al., 2011). However, the efficacy and tolerability of such treatments is modest (Carrasco et al., 2012; Sharma and Shaw, 2012) so there is an urgent need for more selective treatments targeted at the pathophysiology of these conditions.
The evidence reviewed here suggests that the GABA system offers a key therapeutic target. Although a full discussion of this issue is outside the scope of this review, the GABAB agonist arbaclofen (STX209) has recently been shown to help relieve symptoms of Fragile X Syndrome in a preliminary placebo-controlled trial and also showed benefits in an uncontrolled open-label trial in idiopathic ASD; further trials are underway (Seaside Therapeutics, 2011). Likewise, a recent open-label trial found benefits of the drug acamprosate in youth with ASD. Acamprosate acts as a GABAA agonist and excitatory glutamate antagonist (Erickson et al., 2012). These results are consistent with a GABA hypothesis of ASD symptoms but remain preliminary in the absence of double blind, placebo-controlled trials.
8. Conclusions
In this review, we have discussed the evidence that autism and related neurodevelopmental disorders are characterised by abnormalities in GABA function. Several converging lines of evidence – from genetics, epigenetics, animal models, and post-mortem studies of humans – point to a GABA deficit in autism, Fragile X syndrome, and Rett syndrome. Theoretical perspectives that may explain how these neurobiological abnormalities relate to the specific symptoms of these disorders were also discussed.
However, despite the growing interest in the GABA hypothesis of autism and related disorders, there have been very few studies to directly examine the GABA system in the brain of living human patients. We believe that such studies are urgently warranted as, if these abnormalities are present and measurable in humans, this would have important implications both from a purely scientific perspective and also for future drug development.
Other key challenges for the future include understanding how environmental, epigenetic and genetic factors interact to produce the complex features observed in neurodevelopmental disorders.
Footnotes
Funding and Conflict of Interest Statement: The authors declare no financial conflict of interest. There was no specific source of funding for this work.
References
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Chen RZ, Gribnau J, Rasmussen TP, Fong H, Jaenisch R, Jones EG. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784–791. doi: 10.1006/nbdi.2001.0420. [DOI] [PubMed] [Google Scholar]
- Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR., Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th Edition Text Revision ed. American Psychiatric Association; 2000. pp. xxxii–xxxii. [Google Scholar]
- Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, Mottron L, Cohen D. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry. 2008;64:577–582. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Brayne C. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Beaumont M, Maccaferri G. Is connexin36 critical for GABAergic hyper-synchronization in the hippocampus? J Physiol. 2011 doi: 10.1113/jphysiol.2010.201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. J Child Psychol Psychiatry. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol. 1999a;156:345–352. doi: 10.1006/exnr.1999.7030. [DOI] [PubMed] [Google Scholar]
- Blue ME, Naidu S, Johnston MV. Development of amino acid receptors in frontal cortex from girls with Rett syndrome. Ann Neurol. 1999b;45:541–545. doi: 10.1002/1531-8249(199904)45:4<541::aid-ana21>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M. Epilepsy in autism: features and correlates. Br J Psychiatry. 2011;198:289–294. doi: 10.1192/bjp.bp.109.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton PF, Dennis NR, Browne CE, Thomas NS, Veltman MW, Thompson RJ, Jacobs P. The phenotypic manifestations of interstitial duplications of proximal 15q with special reference to the autistic spectrum disorders. Am J Med Genet. 2001;105:675–685. doi: 10.1002/ajmg.1551. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011 doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, Bebbington P, Jenkins R, Meltzer H. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr., Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, Vance JM, Pericak-Vance MA. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Volkmar FR, Bloch MH. Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics. 2012;129:e1301–1310. doi: 10.1542/peds.2011-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, Bagni C. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Brune CW, Sudi J, Kumar RA, Liu S, Karamohamed S, Badner JA, Matsui S, Conroy J, McQuaid D, Gergel J, Hatchwell E, Gilliam TC, Gershon ES, Nowak NJ, Dobyns WB, Cookjr EH. Novel Submicroscopic Chromosomal Abnormalities Detected in Autism Spectrum Disorder. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ. 2005;172:S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology (Berl) 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr., Courchesne RY, Cox NJ, Lord C, Gonen D, Guter SJ, Lincoln A, Nix K, Haas R, Leventhal BL, Courchesne E. Linkage-disequilibrium mapping of autistic disorder, with 15q11-13 markers. Am J Hum Genet. 1998;62:1077–1083. doi: 10.1086/301832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Merlo D, Avoli M. Involvement of cholinergic and gabaergic systems in the fragile X knockout mice. Neuroscience. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson BR, Skold AC, Azarbayjani F. Class III antiarrhythmics and phenytoin: teratogenicity due to embryonic cardiac dysrhythmia and reoxygenation damage. Curr Pharm Des. 2001;7:787–802. doi: 10.2174/1381612013397744. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco Garcia NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011 doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty RJ, Kang JQ, Brune CW, Kistner EO, Courchesne E, Cox NJ, Cook EH, Jr., Macdonald RL, Sutcliffe JS. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, Nicolson R. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61:465–473. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8:PR1–6. [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev. 2011;35:1254–1265. doi: 10.1016/j.neubiorev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Durant C, Christmas D, Nutt D. The pharmacology of anxiety. Curr Top Behav Neurosci. 2011;2:303–330. doi: 10.1007/7854_2009_8. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Gravielle MC, Schlueter McFadyen-Ketchum LJ, Russek SJ, Farb DH, Levitt P. Genetic disruption of the autism spectrum disorder risk gene PLAUR induces GABAA receptor subunit changes. Neuroscience. 2011;168:797–810. doi: 10.1016/j.neuroscience.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary AK, Bacha AB, Ayahdi LY. Relationship between chronic lead toxicity and plasma neurotransmitters in autistic patients from Saudi Arabia. Clin Biochem. 2011;44:1116–1120. doi: 10.1016/j.clinbiochem.2011.06.982. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Eliasen M, Tolstrup JS, Nybo Andersen AM, Gronbaek M, Olsen J, Strandberg-Larsen K. Prenatal alcohol exposure and autistic spectrum disorders--a population-based prospective study of 80,552 children and their mothers. Int J Epidemiol. 2010;39:1074–1081. doi: 10.1093/ije/dyq056. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. GABAergic activity in autism spectrum disorders: An investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Rinehart NJ, Tonge BJ, Bradshaw JL, Fitzgerald PB. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol. 2010;52:e179–183. doi: 10.1111/j.1469-8749.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Early M, Stigler KA, Wink LK, Mullett JE, McDougle CJ. An open-label naturalistic pilot study of acamprosate in youth with autistic disorder. J Child Adolesc Psychopharmacol. 2012;21:565–569. doi: 10.1089/cap.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS-EEG study. J Neurophysiol. 2011;104:1339–1346. doi: 10.1152/jn.00279.2010. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken) 2011;294:1635–1645. doi: 10.1002/ar.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- Fountain C, King MD, Bearman PS. Age of diagnosis for autism: individual and community factors across 10 birth cohorts. J Epidemiol Community Health. 2010 doi: 10.1136/jech.2009.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R. Genetics of autistic disorders: review and clinical implications. Eur Child Adolesc Psychiatry. 2010;19:169–178. doi: 10.1007/s00787-009-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63:786–794. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- Fu C, Cawthon B, Clinkscales W, Bruce A, Winzenburger P, Ess KC. GABAergic Interneuron Development and Function Is Modulated by the Tsc1 Gene. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting gamma-Aminobutyric acid (GABA) concentration. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating gamma Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Sudhof T, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafodatskaya D, Chung B, Szatmari P, Weksberg R. Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry. 49:794–809. doi: 10.1016/j.jaac.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Gregor A, Albrecht B, Bader I, Bijlsma EK, Ekici AB, Engels H, Hackmann K, Horn D, Hoyer J, Klapecki J, Kohlhase J, Maystadt I, Nagl S, Prott E, Tinschert S, Ullmann R, Wohlleber E, Woods G, Reis A, Rauch A, Zweier C. Expanding the clinical spectrum associated with defects in CNTNAP2 and NRXN1. BMC Med Genet. 2011;12:106. doi: 10.1186/1471-2350-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss M, Braun K. Alterations of amino acids and monoamine metabolism in male Fmr1 knockout mice: a putative animal model of the human fragile X mental retardation syndrome. Neural Plast. 2001;8:285–298. doi: 10.1155/NP.2001.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, Layet V, Rosier A, Briault S, Bonnet-Brilhault F, Laumonnier F, Odent S, Le Vacon G, Joly-Helas G, David V, Bendavid C, Pinoit JM, Henry C, Impallomeni C, Germano E, Tortorella G, Di Rosa G, Barthelemy C, Andres C, Faivre L, Frebourg T, Saugier Veber P, Campion D. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009;66:947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T. Non-Invasive Evaluation of the GABAergic/Glutamatergic System in Autistic Patients Observed by MEGA-Editing Proton MR Spectroscopy Using a Clinical 3 Tesla Instrument. J Autism Dev Disord. 2011 doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr., Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, Ryan LM. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–1138. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- Hong A, Zhang A, Ke Y, El Idrissi A, Shen CH. Downregulation of GABA(A) beta Subunits is Transcriptionally Controlled by Fmr1p. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9531-5. [DOI] [PubMed] [Google Scholar]
- Horton RW, Meldrum BS. Seizures induced by allylglycine, 3-mercaptopropionic acid and 4-deoxypyridoxine in mice and photosensitive baboons, and different modes of inhibition of cerebral glutamic acid decarboxylase. Br J Pharmacol. 1973;49:52–63. doi: 10.1111/j.1476-5381.1973.tb08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008 doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I. De Novo Gene Disruptions in Children on the Autistic Spectrum. Neuron. 2012 doi: 10.1016/j.neuron.2012.04.009. al., E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Pavliv O, Trusty T, Lehman S, Seidel L, Gaylor DW, Cleves MA. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am J Med Genet B Neuropsychiatr Genet. 153B:1209–1220. doi: 10.1002/ajmg.b.31094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Bower C, Anderson A, Williamson S, Christodoulou J, Leonard H. Predictors of seizure onset in Rett syndrome. J Pediatr. 2006;149:542–547. doi: 10.1016/j.jpeds.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Johnston P, Ecker C, Daly E, Sinnwell P, Bolton P, Powel J, D.G. M. Linkage of GAD to autism and differences in brain anatomy, IMFAR. 2008 [Google Scholar]
- Kim KC, Kim P, Go HS, Choi CS, Yang SI, Cheong JH, Shin CY, Ko KH. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett. 2011 doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Knoll JH, Nicholls RD, Magenis RE, Graham JM, Jr., Lalande M, Latt SA. Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet. 1989;32:285–290. doi: 10.1002/ajmg.1320320235. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lam CW, Yeung WL, Ko CH, Poon PM, Tong SF, Chan KY, Lo IF, Chan LY, Hui J, Wong V, Pang CP, Lo YM, Fok TF. Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J Med Genet. 2000;37:E41. doi: 10.1136/jmg.37.12.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren M, Svensson L, Stromland K, Andersson Gronlund M. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics. 2011;125:e1178–1185. doi: 10.1542/peds.2009-0712. [DOI] [PubMed] [Google Scholar]
- Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RA, Pop AS, Gomezmancilla B, Nelson DL, Willemsen R, Gasparini F, Oostra BA. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Reid AG, Myers J, Feeney A, Hammers A, Taylor L, Rosso L, Turkheimer F, Brooks DJ, Grasby P, Nutt DJ. A [11c]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced {alpha}5 benzodiazepine receptors in limbic regions. J Psychopharmacol. 2010 doi: 10.1177/0269881110379509. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABA(A) Receptor Trafficking-Mediated Plasticity of Inhibitory Synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KK, Clausen RP, Larsson OM, Krogsgaard-Larsen P, Schousboe A, White HS. Synaptic and extrasynaptic GABA transporters as targets for anti-epileptic drugs. J Neurochem. 2009;109(Suppl 1):139–144. doi: 10.1111/j.1471-4159.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- Madsen KK, Larsson OM, Schousboe A. Regulation of excitation by GABA neurotransmission: focus on metabolism and transport. Results Probl Cell Differ. 2008;44:201–221. doi: 10.1007/400_2007_036. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- Meguro-Horike M, Yasui DH, Powell W, Schroeder DI, Oshimura M, Lasalle JM, Horike SI. Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum Mol Genet. 2011;20:3798–3810. doi: 10.1093/hmg/ddr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, Horder J, Myers J, Coghlan S, Stokes P, Erritzoe D, Howes OD, Lingford-Hughes A, Murphy DG, Nutt DJ. The brain GABA-benzodiazepine receptor alpha-5 subtype in autism spectrum disorder: A pilot [11C]Ro15-4513 positron emission tomography study. 2012 doi: 10.1016/j.neuropharm.2012.04.008. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR. Association analysis of chromosome 15 gabaa receptor subunit genes in autistic disorder. J Neurogenet. 2001;15:245–259. doi: 10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JC. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Fuenmayor H, Borjas L, Arrieta A, Valera V, Socorro-Candanoza L. Plasma excitatory amino acids in autism. Invest Clin. 1996;37:113–128. [PubMed] [Google Scholar]
- Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, Hashimoto T, Kagami S. Evaluation of the GABAergic nervous system in autistic brain: (123)I-iomazenil SPECT study. Brain Dev Epub Ahead of Print. 2011 doi: 10.1016/j.braindev.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, Depristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012 doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR Biomed. 2011 doi: 10.1002/nbm.1688. [DOI] [PubMed] [Google Scholar]
- Nord AS, Roeb W, Dickel DE, Walsh T, Kusenda M, O’Connor KL, Malhotra D, McCarthy SE, Stray SM, Taylor SM, Sebat J, King B, King MC, McClellan JM. Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, Picker J, Rotenberg A, Pascual-Leone A. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2011;2:26. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABA(A) receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2010;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C, Berg K, Moss J, Arron K, Burbidge C. Delineation of Behavioral Phenotypes in Genetic Syndromes: Characteristics of Autism Spectrum Disorder, Affect and Hyperactivity. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-1125-5. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Heximer SP, Hampson DR. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 2009;76:18–24. doi: 10.1124/mol.109.056127. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, Ambery F, McAlonan GM, Murphy KC, Murphy DG. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile x syndrome. J Neurophysiol. 2011 doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, Bernstein JA, Hallmayer J, Geschwind DH, Dolmetsch RE. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011 doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, Vanleeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, Dunn HG, Ho HH, Crichton JU. Cerebrospinal fluid values for monoamine metabolites, gamma-aminobutyric acid, and other amino compounds in Rett syndrome. J Pediatr. 1988;112:234–238. doi: 10.1016/s0022-3476(88)80060-x. [DOI] [PubMed] [Google Scholar]
- Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C. Which Comes First in Fragile X Syndrome, Dendritic Spine Dysgenesis or Defects in Circuit Plasticity? Neuroscientist. 2011 doi: 10.1177/1073858410395322. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Lam D, Patten SB. The Pharmacoepidemiology of Antipsychotic Medications for Canadian Children and Adolescents: 2005-2009. J Child Adolesc Psychopharmacol. 2011 doi: 10.1089/cap.2010.0145. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- Renieri A, Meloni I, Longo I, Ariani F, Mari F, Pescucci C, Cambi F. Rett syndrome: the complex nature of a monogenic disease. J Mol Med. 2003;81:346–354. doi: 10.1007/s00109-003-0444-9. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rolf LH, Haarmann FY, Grotemeyer KH, Kehrer H. Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand. 1993;87:312–316. doi: 10.1111/j.1600-0447.1993.tb03378.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011 doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. doi: 10.1155/2011/649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rutter M. Diagnosis and definition of childhood autism. J Autism Child Schizophr. 1978;8:139–161. doi: 10.1007/BF01537863. [DOI] [PubMed] [Google Scholar]
- Rutter M. Cognitive deficits in the pathogenesis of autism. J Child Psychol Psychiatry. 1983;24:513–531. doi: 10.1111/j.1469-7610.1983.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest. 2007;117:931–943. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, Dilullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Gunel M, Roeder K, Geschwind DH, Devlin B, State MW. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012 doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CP, Zoghbi HY. Solving the autism puzzle a few pieces at a time. Neuron. 2011;70:806–808. doi: 10.1016/j.neuron.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15(Spec No 2):R138–150. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Seaside Therapeutics . Seaside Therapeutics Reports Positive Data from Phase 2 Study of STX209 in Autism Spectrum Disorders. Seaside Therapeutics; 2011. [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412:227–232. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgado P, Dunleavy M, Genovesi S, Provenzano G, Bozzi Y. The role of GABAergic system in neurodevelopmental disorders: a focus on autism and epilepsy. Int J Physiol Pathophysiol Pharmacol. 2011;3:223–235. [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Cuccaro ML, Hauser ER, Raiford KL, Menold MM, Wolpert CM, Ravan SA, Elston L, Decena K, Donnelly SL, Abramson RK, Wright HH, DeLong GR, Gilbert JR, Pericak-Vance MA. Fine mapping of autistic disorder to chromosome 15q11-q13 by use of phenotypic subtypes. Am J Hum Genet. 2003;72:539–548. doi: 10.1086/367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Shaw SR. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: a meta-analysis. J Pediatr Health Care. 2012;26:291–299. doi: 10.1016/j.pedhc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H, Iwata K, Matsumoto K, Wakuda T, Kameno Y, Suzuki K, Tsujii M, Nakamura K, Takei N, Mori N. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One. 2011;6:e25340. doi: 10.1371/journal.pone.0025340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Sibilla S, Ballerini L. GABAergic and glycinergic interneuron expression during spinal cord development: dynamic interplay between inhibition and excitation in the control of ventral network outputs. Prog Neurobiol. 2009;89:46–60. doi: 10.1016/j.pneurobio.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Simon EW, Haas-Givler B, Finucane B. A longitudinal follow-up study of autistic symptoms in children and adults with duplications of 15q11-13. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:463–467. doi: 10.1002/ajmg.b.31000. [DOI] [PubMed] [Google Scholar]
- Singer W. Consciousness and the binding problem. Ann N Y Acad Sci. 2001;929:123–146. doi: 10.1111/j.1749-6632.2001.tb05712.x. [DOI] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130:1016S–1022S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011 doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Yang BZ, Chavira DA, Hitchcock CA, Sung SC, Shipon-Blum E, Gelernter J. A Common Genetic Variant in the Neurexin Superfamily Member CNTNAP2 Is Associated with Increased Risk for Selective Mutism and Social Anxiety-Related Traits. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan DA. Unraveling autism. Am J Hum Genet. 2008;82:7–9. doi: 10.1016/j.ajhg.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Tomioka R. Long-Range GABAergic Connections Distributed throughout the Neocortex and their Possible Function. Front Neurosci. 2010;4:202. doi: 10.3389/fnins.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanseem I, Anitha A, Nakamura K, Suda S, Iwata K, Matsuzaki H, Ohtsubo M, Ueki T, Katayama T, Iwata Y, Suzuki K, Minoshima S, Mori N. Elevated Transcription Factor Specificity Protein 1 in Autistic Brains Alters the Expression of Autism Candidate Genes. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vellucci SV, Webster RA. The role of GABA in the anticonflict action of sodium valproate and chlordiazepoxide. Pharmacol Biochem Behav. 1984;21:845–851. doi: 10.1016/s0091-3057(84)80063-5. [DOI] [PubMed] [Google Scholar]
- Voineagu I. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011 doi: 10.1038/nature10110. al., e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V. Downregulation of Parvalbumin at Cortical GABA Synapses Reduces Network Gamma Oscillatory Activity. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.3041-11.2011. al., E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7:264–274. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV, Ang QW, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011 doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202–206. [PubMed] [Google Scholar]
- Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–480. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- World Health Organization . ICD-10 International statistical classification of diseases and related health problems. Tenth Edition 1993. [Google Scholar]
- Xu G, Broadbelt KG, Haynes RL, Folkerth RD, Borenstein NS, Belliveau RA, Trachtenberg FL, Volpe JJ, Kinney HC. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Matsuishi T, Ishibashi M, Kimura A, Onishi Y, Yonekura Y, Kato H. Decrease in benzodiazepine receptor binding in the brains of adult patients with Rett syndrome. J Neurol Sci. 1998;154:146–150. doi: 10.1016/s0022-510x(97)00223-2. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Zak JD, Liu H. MeCP2 is required for normal development of GABAergic circuits in the thalamus. J Neurophysiol. 2010;103:2470–2481. doi: 10.1152/jn.00601.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]