Summary
Background
Detection and validation of inhibitors (antibodies) to hemophilia treatment products are important for clinical care, evaluation of product safety, and assessment of population trends.
Methods
Centralized monitoring for factor VIII (FVIII) inhibitors was conducted for patients in the Hemophilia Inhibitor Research Study using a previously reported modified Nijmegen-Bethesda clotting assay (NBA), a chromogenic Bethesda assay (CBA), and a novel fluorescence immunoassay (FLI).
Results
NBA and CBA were performed on 1005 specimens and FLI on 272 specimens. CBA was negative on 880/883 specimens (99.7%) with Nijmegen-Bethesda units (NBU)<0.5 and positive on 42/42 specimens (100%) with NBU≥2.0 and 43/80 specimens (53.8%) with NBU 0.5–1.9. Among specimens with positive NBA and negative CBA, 58.1% were FLI-negative, 12.9% had evidence of lupus anticoagulant, and 35.5% had non-time-dependent inhibition. CBA and FLI were positive on 72.4% and 100% of 1.0–1.9 NBU specimens and 43.1% and 50.0% of 0.5–0.9 NBU specimens. FLI detected antibodies in 98.0% of CBA-positive and 81.6% of NBA-positive specimens (P=0.004). Among 21 new inhibitors detected by NBA, 5 (23.8%) with 0.7–1.3 NBU did not react in CBA or FLI. Among previously positive patients with 0.5–1.9 NBU, 7/25 (28%) were not CBA or FLI positive. FLI was positive on 36/169 NBU-negative specimens (21.3%).
Conclusions
FVIII specificity could not be demonstrated by CBA or FLI for 26% of inhibitors of 0.5–1.9 NBU; such results must be interpreted with caution. Low titer inhibitors detected in clot-based assays should always be repeated, with consideration given to evaluating their reactivity with FVIII using more specific assays.
Keywords: factor VIII inhibitor, Nijmegen-Bethesda assay, chromogenic factor VIII assay, fluorescence immunoassay
Introduction
Inhibitors to infused factor VIII (FVIII) are the most significant complication of hemophilia treatment today. FVIII inhibitors are antibodies, usually IgG, that react with FVIII in a time and temperature dependent manner [1]. They are detected primarily through their ability to neutralize FVIII activity in a clot-based assay. Lupus anticoagulants (LA) can mimic FVIII inhibitors in clot-based assays and are reported to be relatively common in hemophilia patients [2, 3]. Additional confounders are the occurrence in healthy children of non-specific inhibitors of coagulation, which inhibit mixing studies but are not corrected by phospholipid [4] and the presence of heparin contamination due to sampling from central lines and ports [5]. Alternative inhibitor measurement methods such as an enzyme-linked immunosorbent assay (ELISA) [6] or chromogenic assay [3], have been proposed to avoid the confounding effects of LA and heparin contamination. More recently, fluorescence immunoassays have also been described [7, 8]. Non-neutralizing antibodies that bind to FVIII but have no impact on in vitro clotting tests have been demonstrated with immunoprecipitation [9], ELISA [10–12], and fluorescence-based immunoassays [8, 13]. Some studies have suggested that these antibodies have clinical significance [11, 12], while others have found no such effect [14, 15].
There is lack of consensus as to what constitutes a positive inhibitor, including the appropriate cutoff for inhibitor measurement using the Bethesda assay (BA) or Nijmegen-Bethesda assay (NBA) and the means for evaluation of clinical significance. Some inhibitors are transient and disappear without treatment; however, it is not possible at the time of onset to predict which low titer inhibitors will progress to higher titers and which will not [16]. A better understanding of these issues is crucial for accurate detection and validation of FVIII inhibitors in clinical care, evaluation of product safety, and surveillance.
We report here the results of a comparison of three FVIII inhibitor measurement methods in the Hemophilia Inhibitor Research Study (HIRS), conducted by the Centers for Disease Control and Prevention (CDC) at 17 U.S. hemophilia treatment centers with centralized inhibitor monitoring.
Materials and Methods
Subjects
People with hemophilia A and hemophilia B having FVIII or factor IX activity <50 International Units per deciliter (IU dL−1) were enrolled from 2006–2012 at 17 U.S. Hemophilia Treatment Centers in a study of prospective monitoring for inhibitors, which is described in detail elsewhere [17]. Demographic data and information on previous inhibitor history were collected from the enrolling site using standardized data collection tools. Treatment product exposure records were collected prospectively from the time of enrollment. Inhibitor measurements were performed centrally at CDC at study entry, annually, prior to any planned product switch, or for clinical indication of an inhibitor. The protocol was approved by the investigational review boards of CDC and each participating site, and all participants or parents of minor children gave informed consent. For this report, 702 subjects with hemophilia A were studied, of whom 55% had severe (< 1 unit/deciliter (U dL−1)), 20% had moderate (1–5 U dL−1), and 25% had mild (5–50 U dL−1) disease. Age of study subjects ranged from 3 months to 84 years. A previous history of an inhibitor was reported by the enrolling site for 17.4% of subjects.
Specimen Collection
Blood was collected into evacuated siliconized glass tubes (Becton Dickinson, Franklin Lakes, NJ) containing 3.2% sodium citrate in a ratio of 1:9 with blood and centrifuged at 1,600 x g for 20 minutes at 4°C, followed by repeat centrifugation of the separated plasma at 1,600 x g for 20 minutes at 4°C in polypropylene tubes. Blood processing was completed within two hours. The separated plasma and cell pellet were shipped to CDC overnight on cold packs. At CDC, the plasma samples were aliquoted and stored in polypropylene tubes at −70°C. Cell pellets were used for DNA analysis.
Measurement Methods
Nijmegen-Bethesda Assay (NBA)
The NBA [18] was performed with the modifications previously described [19], including heating of patient plasma to 56°C for 30 minutes and centrifugation prior to testing. Using the NBA, one Nijmegen-Bethesda unit (NBU) was defined as the amount of inhibitor per milliliter (mL) of patient plasma which inactivates 50% of the FVIII activity in 1mL of normal plasma during a 2 hour incubation at 37°C. A positive inhibitor by NBA was defined as ≥0.5 NBU, based on previous assay validation [19]. To detect non-time-dependent inhibition, the NBA was performed immediately upon mixing and compared with that after 2 hour incubation.
Chromogenic Bethesda Assay (CBA)
Chromogenic inhibitor testing was performed by the NBA method described [19], except that FVIII was measured using a chromogenic substrate method (Factor VIII Chromogenic Assay, Dade Behring, Marburg, Germany). Plasma diluted 1:31 in imidazole buffer was incubated with bovine factor X, bovine factor IXa, bovine thrombin, CaCl2, and phospholipid for 90 seconds at 37°C. Factor Xa substrate with a thrombin inhibitor and a stopping buffer was added. The change in absorbance per minute was read at 405 nanometers. One Chromogenic-Bethesda unit (CBU) was defined as the amount of inhibitor per mL of patient plasma which inactivates 50% of the FVIII activity in 1mL of normal plasma during a 2 hour incubation at 37°C. A positive inhibitor by CBA was defined as ≥0.5 CBU.
Fluorescent Immunoassay (FLI)
Recombinant FVIII (Kogenate FS, Bayer Healthcare, Tarrytown, NY) was buffer-exchanged into phosphate buffered saline (PBS) using Micro Bio-Spin 6 columns (Bio-Rad Laboratories, Hercules, CA) and coupled to SeroMAP Microspheres (Luminex Corporation, Austin, TX) using the Bio-Plex amine coupling kit (Bio-Rad Laboratories). Plasma samples were diluted 1:50 in PBS containing 1% dried milk (PBSM) and incubated with FVIII coupled microspheres for one hour at room temperature while shaking in a 96-well filter plate (Millipore, Billerica, MA). The microspheres were washed twice with PBSM, re-suspended in 50Ol PBSM containing 2Og/ml biotinylated anti-human IgG/M (Jackson ImmunoResearch Laboratories, West Grove, PA), and shaken at room temperature for 30 minutes. The beads were then washed again, re-suspended in 50Ol PBSM containing 2Og/ml R-phycoerythrin-conjugated streptavidin (Jackson ImmunoResearch Laboratories), and shaken for 15 minutes. Following two additional washes, the beads were re-suspended in 100Ol PBSM, shaken for two minutes, and read on a Bio-Plex 200 suspension array system (Bio-Rad Laboratories). Results were expressed in median fluorescence intensity units (MFIU) and converted to fluorescence intensity units (FLIU). One FLIU was defined as 1 MFIU/1000, to make the unitage more similar to that of the other assays.
Dilute Russell’s Viper Venom Time (DRVVT)
DRVVT was quantitated using DVVtest and DVVconfirm reagents (American Diagnostica, Stamford, CT). Heparin was quantitated using an anti-factor Xa assay (Liquid Anti-Xa Assay, Diagnostica Stago, Parsippany, NJ).
Statistical Analysis
Comparisons using Fisher’s exact test, Spearman correlation coefficient, and linear regression of dilution curves were calculated using GraphPad Prism, Version 5 (GraphPad Software Inc., San Diego, CA). Results were considered significant at the 0.05 level.
Results
Comparison of the Modified Nijmegen-Bethesda Method and the Chromogenic Bethesda Assay
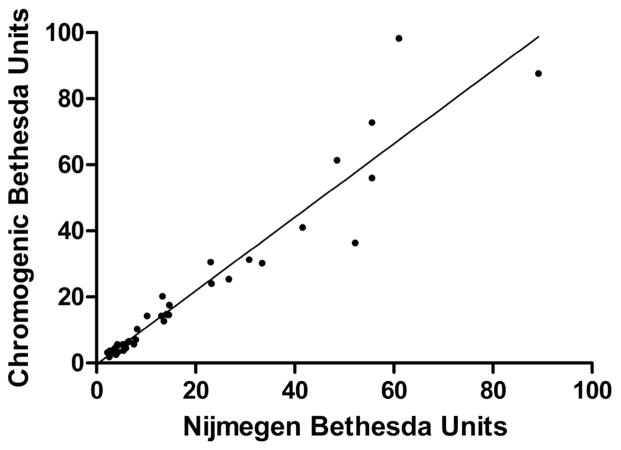
NBA and CBA were performed in parallel on 1005 specimens from 702 patients. As shown in Table 1, 880 (99.7%) of 883 specimens negative by NBA (<0.5NBU) were also negative by CBA (<0.5CBU). Three specimens (0.3%) with 0.3–0.4 NBU had positive CBU of 0.5–0.6 CBU. Of 122 NBA-positive specimens, 85 (69.7%) were also CBA-positive and were termed concordant. All specimens with NBU ≥ 2.0 (n=42) were concordant, with excellent correlation between NBU and CBU (r= 0.98, P<0.0001), as shown in Figure 1. Thirty-seven NBA-positive specimens (30.3%) had negative CBU and were termed discordant, including 8 of 29 specimens (27.6%) with 1.0–1.9 NBU and 29 of 51 specimens (56.9%) with 0.5–0.9 NBU. Overall, discordant results were seen in 40 of 1005 specimens (4.0%) from 28 of 702 patients (4.0%).
Table 1.
Comparison of results of the chromogenic Bethesda assay (CBA) and the fluorescence immunoassay (FLI) with the Nijmegen-Bethesda assay (NBA). Results shown in bold are discordant, with positive NBA results and negative CBA or FLI results or with negative NBA results and positive CBA or FLI results.
| Nijmegen-Bethesda Units (NBU) | Chromogenic Inhibitor Units (CBU) n (%)
|
Fluorescence Immunoassay Units (FLIU) n (%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | <0.5 | 0.5–0.9 | 1.0–1.9 | 2.0–4.9 | ≥5.0 | n | <0.47 | 0.47–0.99 | 1.00–1.99 | 2.00–4.99 | ≥5.00 | |
|
|
|
|||||||||||
| <0.5 | 883 | 880 (99.7) | 3 (0.3) | 0 | 0 | 0 | 169 | 133 (78.7) | 29 (17.2) | 6 (3.6) | 1 (0.6) | 0 |
| 0.5–0.9 | 51 | 29 (56.9) | 19 (37.3) | 2 (3.9) | 1 (2.0) | 0 | 36 | 18 (50.0) | 6 (16.7) | 3 (8.3) | 7 (19.4) | 2 (5.6) |
| 1.0–1.9 | 29 | 8 (27.6) | 10 (34.5) | 6 (20.7) | 5 (17.2) | 0 | 29 | 0 | 13 (44.8) | 8 (27.6) | 5 (17.2) | 3 (10.3) |
| 2.0–4.9 | 12 | 0 | 0 | 1 (8.3) | 10 (83.3) | 1 (8.3) | 13 | 1 (7.7) | 3 (23.1) | 2 (15.4) | 5 (38.5) | 2 (15.4) |
| ≥5.0 | 30 | 0 | 0 | 0 | 2 (6.7) | 28 (93.3) | 25 | 0 | 0 | 3 (12.0) | 10 (40.0) | 12 (48.0) |
Figure 1.
Correlation of Nijmegen-Bethesda Units (NBU) and Chromogenic Bethesda Units (CBU) in specimens with ≥2.0 NBU. Two points were outside the range of the graph at NBU 207.6, CBU 108.8 and NBU 688.0, CBU 512.0.
Comparison of the Modified Nijmegen-Bethesda Method and the Fluorescence Immunoassay
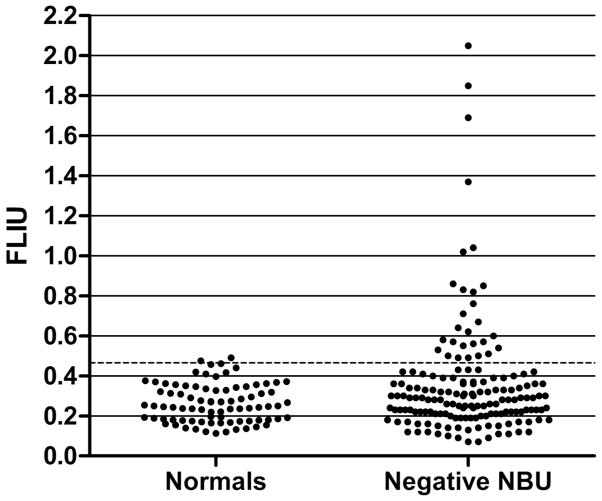
FLI was performed on a subset of 272 specimens (Table 1). A positive FLI was defined as >2 standard deviations above the mean of 83 normal subjects (Figure 2), or >0.466 FLIU. FLI was positive in 84 of 103 NBA-positive specimens (81.6%) and 50 of 51 CBA-positive specimens (98.0%) (P=0.004). NBU and FLIU were significantly correlated (r=0.54, P<0.0001), as were CBU and FLIU (r=0.65, P<0.0001). FLI was also positive on 36 (21.3%) of 169 specimens with negative NBU, indicating the presence of “non-neutralizing” antibodies (Figure 2). Of those 36, 33 were from patients with no inhibitor detected previously, and 3 were from previously positive patients.
Figure 2.
Results of fluorescence immunoassay in fluorescence immunoassay units (FLIU) in normal individuals (n=83) and hemophilia patients with negative Nijmegen-Bethesda assay results (n=169).
Comparison of All Methods
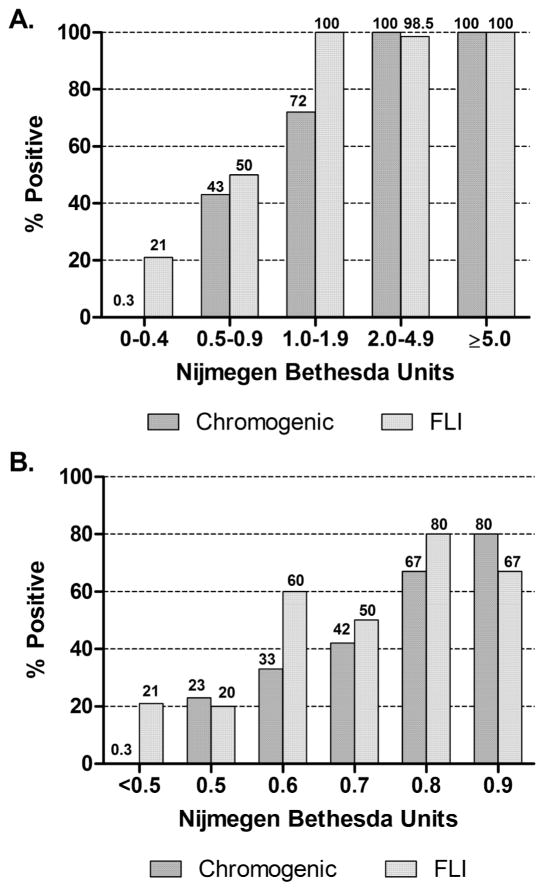
Figure 3A shows comparison of proportions of positive results for the NBA, CBA, and FLI. For NBU ≥2.0, 100% of specimens were CBA positive and 98.5% were FLI positive. For 1.0–1.9 NBU, 72.4% were CBA positive and 100% were FLI positive. CBA and FLI were positive in only 43.1% and 50% of specimens with 0.5–0.9 NBU, respectively. A breakdown of the 0.5–0.9 NBU specimen results into 0.1 NBU increments (Figure 3B) shows that the CBA positive results (n=22) increase with increasing NBU from 23% at 0.5 to 80% at 0.9, with none reaching 100%; the FLI results (n=18) were similar. The CBA was positive in significantly more of the 0.5 NBU specimens (23%) than in the negative NBU specimens (0.3%) (P<0.0001), suggesting the presence of some true positives at 0.5 NBU. At no point in the 0.5–0.9 NBU range were positive NBA results consistently confirmed by CBA and FLI.
Figure 3.
Comparison of positivity rates of Chromogenic Bethesda Assay and Fluorescence Immunoassay (FLI) with Nijmegen-Bethesda Units. A. All specimens. B. Specimens with NBU<1.0.
Assay Sensitivity
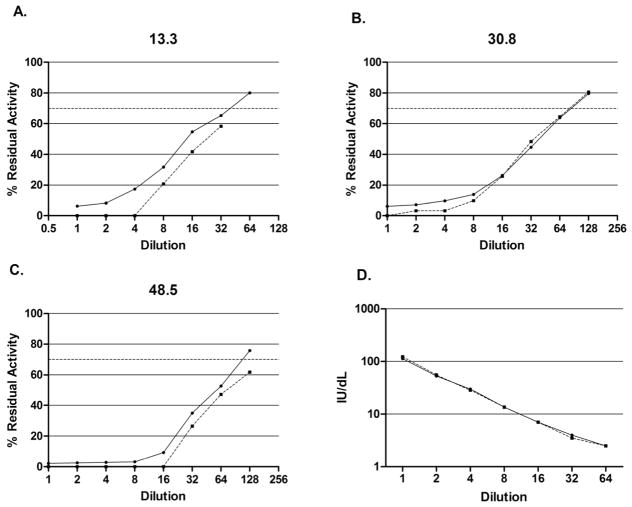
To evaluate the relative ability of the assays to detect low amounts of inhibitor, NBA and CBA dilution curves of known inhibitor plasmas were compared (Figure 4). Inhibitors of 13.6, 30.8, and 48.5 NBU showed linearity and parallelism in the region between 25% and 75% residual activity, the range in which the inhibitor titer is calculated. With each inhibitor, the CBA showed complete inhibition at one dilution higher than the NBA, indicating a two-fold greater sensitivity to inhibition. Dilution curves of the chromogenic and one-stage FVIII assays were also compared (Figure 4D), showing parallelism and similar sensitivity. Figure 5 shows FLI dilution curves of the 13.6 and 48.5 NBU inhibitors and two NBA-negative specimens. The 13.6 NBU curve crosses the threshold of positivity (0.466 FLIU) at a dilution of 1:400, suggesting a sensitivity equivalent to 0.03 NBU, and the 48.5 NBU curve remains above the threshold at 1:1600, showing a sensitivity of greater than 0.03 NBU.
Figure 4.
Dilution curves for inhibitors of 13.3 Nijmegen-Bethesda units (NBU) (A), 30.8 NBU (B), and 48.5 NBU (C). Solid lines represent the Nijmegen-Bethesda Assay. Dashed lines represent the Chromogenic Bethesda Assay with the cutoff for positivity (dotted line) at 70% residual activity (0.5 NBU). D. Dilution curve of factor VIII (FVIII) activity assay. Solid line represents FVIII clotting assay. Dashed line represents FVIII chromogenic assay.
Figure 5.
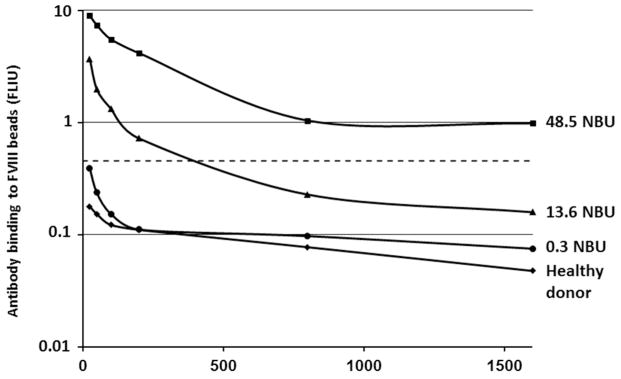
Dilution curves of the fluorescence immunoassay. The dotted line represents the cutoff for assay positivity at fluorescence immunoassay units (FLIU) =0.466.
Other Causes of Inhibition
Causes for the assay discrepancies observed were investigated in 51 specimens with a positive NBA of <2.0 NBU (Table 2). DRVVT, used as an indicator of the presence of LA, was positive in 4 specimens from 4 patients (7.8%); 3 had NBU of 0.5, CBU of 0, and negative FLI; one had NBU of 1.4, CBU of 0.3 and FLI of 2.57 FLIU, suggesting the presence of both LA and anti-FVIII antibodies. All had previous history of inhibitor with peak titers ranging from 2.1 to 819. Fourteen DRVVT-negative specimens (27.4%) from 9 patients showed immediate FVIII inhibition and minimal increase with incubation, or non-time-dependent inhibition (NTDI). Absence of the time dependence seen with the classic FVIII inhibitor might be interpreted as non-specific inhibition; however, all had previous history of inhibitor, and 13 of 14 were FLI positive. Specimens not reported to have been drawn peripherally were tested for heparin. No heparin contamination was detected (data not shown).
Table 2.
Comparison of patient groups with positive Nijmegen-Bethesda Units (NBU) <2.0 and positive Chromogenic Bethesda Units (CBU) results (concordant) or negative CBU results (discordant)
| Concordant (Positive NBU and Positive CBU) | Discordant (Positive NBU and Negative CBU) | |
|---|---|---|
| Patients: | ||
| n | 15 | 23 |
| Previous inhibitor | 12 (80%) | 18 (78.3%) |
| Specimens: | ||
| n | 20 | 31 |
| Positive FLI | 20 (100%) | 13 (41.9%) |
| Positive DRVVT | 0 | 4 (12.9%) |
| Non-time-dependent inhibition | 3 (15%) | 11 (35.5%) |
Inhibitor Kinetics
Low titer inhibitor plasmas, when diluted in the NBA, exhibited two different types of dilution curves, as shown in Figure 6. Concordant inhibitors (Figure 6A) showed increasing residual activity with dilution and non-zero slopes, except for a single patient who showed a slope approaching zero on four specimens drawn over a 4 month period with NBU of 0.6–0.8 and positive FLI. He had a previous peak inhibitor titer of 512 BU and was undergoing ITI during part of that period. Discordant specimens (Figure 6B) were heterogeneous; some had the expected change with dilution and positive slopes, while others had slopes that were not significantly different from zero. Among 9 inhibitors with positive slopes, NTDI was seen in 1, which was also DRVVT positive; 8 of 9 inhibitors with near zero slopes showed NTDI, one with a positive DRVVT. Inhibition that does not change with dilution suggests the presence of an interfering substance affecting all dilutions. For 3 of these, the NBA repeated with bovine serum albumin substituted for FVIII-deficient plasma as diluent gave the same results, indicating that there was not a reaction between the patient plasma and the FVIII-deficient plasma.
Figure 6.
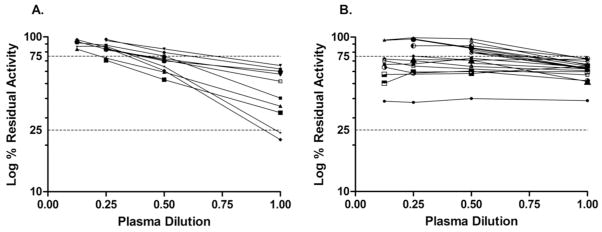
Nijmegen-Bethesda Assay (NBA) dilution curves of inhibitor plasmas. A: Concordant specimens [positive NBA and positive Chromogenic Bethesda Assay (CBA)].B: Discordant specimens (positive NBA and negative CBA). Dashed lines at 75% and 25% residual factor VIII activity define the interval within which NBU are calculated, representing the 0.4 and 2.0 NBU levels, respectively.
Patient Characteristics
Discordance among the assays often appeared to be patient specific. Among the 8 discordant specimens with 1.0–1.9 NBU, 4 were from the same patient over a four year period showing NBU of 1.4–1.9, CBU of 0–0.3, negative FLI, negative DRVVT, and NTDI. This patient had a previously diagnosed inhibitor with a peak titer of 39 Bethesda units and was treated with a by-passing agent. Among patients with 0.5–0.9 NBU, 5 had two discordant specimens at least one year apart. One was DRVVT-positive on the first specimen and FLI-negative on both; 2 were FLI-positive, DRVVT-negative, and NTDI-positive; and one was negative on all tests other than the NBA. When patients with NBU of 0.5–1.9 with and without history of inhibitor at study entry (HI) were compared (Table 3), patients with positive HI were less likely to have inhibitors confirmed by both CBA and FLI (28.0% vs.61.5%) and more likely to show positive FLI but negative CBA (32.0% vs. 0). Only patients with previous HI had positive DRVVT (16.0 %) and NTDI (48.0%). Of 25 patients with positive HI who had inhibitors in this range on study, seven (28%) were not confirmed by CBA and/or FLI or explained by LA.
Table 3.
Comparison of results in patients with 0.5–1.9 NBU by history of inhibitor
| Patients | History of Inhibitor Prior to Study Entry n (%)
|
|
|---|---|---|
| Negative | Positive | |
| n | 13 | 25 |
| Positive CBA and FLI | 8 (61.5) | 7 (28.0) |
| Negative CBA and FLI | 5 (38.5) | 10 (40.0) |
| Negative CBA and Positive FLI | 0 | 8 (32.0) |
| Positive DRVVT | 0 | 4 (16.0) |
| Non-time-dependent inhibition | 0/10 | 12 (48) |
During the study, 23 patients with no HI were detected by the central laboratory to have a positive NBA (Table 4). Two had insufficient specimen to be studied. Eight had NBU ranging from 2.6 to 688.2 with comparable CBU and positive FLI (Table 4A). Eight had concordant NBU and CBU titers between 0.5 and 1.8, 7 of the 8 with positive FLI (Table 4B). Five patients had positive NBA but negative CBA and FLI (Table 4C). Of the 16 concordant inhibitors, 10 persisted, 8 of them treated with by-passing agents and/or ITI and 2 requiring no treatment change. Two severe patients, age 2 and 5, had transient inhibitors which disappeared in less than one year and required no change in treatment. One patient had an inhibitor which was detected in two specimens one month apart and was treated with a by-passing agent but was not followed subsequently. Three patients were not retested. Of the 5 discordant patients, a 5-year-old had a persistent low-titer discordant inhibitor for more than 16 months and was treated with a by-passing agent; one moderate and one mild patient each reported no exposure to factor products prior to detection of the discordant inhibitors, suggesting false positive NBA results; one severe patient had a subsequent negative specimen within one month, suggesting a false positive test or a transient inhibitor; and one has not been retested. None had positive DRVVT or heparin contamination. Thus, 5 of 21 new inhibitors (23.8%) detected by NBA, ranging from 0.7 to 1.3 NBU, were not confirmed by either CBA or FLI.
Table 4.
Characteristics of patients with newly detected inhibitors
| Patient | Age | Severity | Race* | Mutation | Peak NBU | Peak CBU | FLI | DRVVT | Persistence | Treatment Change | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Patients with Positive NBU > 2.0 and Positive Chromogenic Bethesda Units (CBU) | |||||||||||
| 1 | 1.5 | Severe | AS | Deletion del exons 7–9 | 688.0 | 512.0 | 4.25 | N | >3 years | By-passing agent, ITI | Persistent |
| 2 | 18 | Mild | WNH | Missense, Arg1941Gln | 55.6 | 72.8 | 1.84 | N | > 3years | By-passing agent | Persistent |
| 3 | 3 | Severe | OTH | Nonsense, Arg427Stop | 54.4 | 54.7 | - | - | > 10 months | By-passing agent | Persistent |
| 4 | 1 | Severe | WNH | Inversion 22 | 18.7 | 12.4 | 7.89 | N | < 7 months | By-passing agent, ITI | Persistent |
| 5 | 2.9 | Severe | OTH | Frameshift51–52delTTGT | 14.1 | 14.8 | 3.54 | N | Unknown | By-passing agent | Persistent |
| 6 | 4 | Moderate | BNH | Inversion 22 | 6.5 | 6.5 | 2.89 | - | > 1 year | By-passing agent, ITI | Persistent |
| 7 | 5 | Severe | WNH | Inversion 22 | 3.9 | - | 1.47 | - | Unknown | Unknown | ? |
| 8 | 2 | Severe | WNH | Frameshift, 2154delAA | 2.6 | 2.9 | 0.54 | - | <9 months | ITI | Persistent |
|
| |||||||||||
| B. Patients with Positive NBU < 2.0 and Positive CBU | |||||||||||
| 9 | 12 | Mild | WNH | Missense Arg593Cys | 1.8 | 0.9 | 6.17 | N | > 5 months | By-passing agent | Persistent |
| 10 | 61 | Severe | WNH | Nonsense 2229Stop | 1.5 | 0.8 | 0.58 | N | > 2 years | No change | Persistent |
| 11 | 2 | Severe | WH | Inversion 22 | 1.3 | 0.9 | 0.50 | N | > 1 year | No change | Persistent |
| 12 | 5 | Severe | WH | Inversion 22 | 1.7 | 0.7 | 0.54 | N | < 1 year | No change | Transient |
| 13 | 2 | Severe | WNH | Frameshift833ins13 | 0.7 | 0.5 | 3.06 | N | < 3 months | No change | Transient |
| 14 | 46 | Mild | WNH | Missense Ser535Gly | 1.7 | 3.3 | 6.40 | N | > 1 month | By-passing agent | ? |
| 15 | 41 | Moderate | WNH | Frameshift1424delAAGA | 0.7 | 0.9 | 2.35 | N | Unknown | By-passing agent, ITI | ? |
| 16 | 2 | Severe | WH | Inversion 22 | 0.5 | 0.7 | - | N | Unknown | Unknown | ? |
|
| |||||||||||
| C. Patients with Positive NBU and Negative CBU | |||||||||||
| 17 | 5 | Severe | WNH | Frameshift 1615delA | 0.7 | 0.3 | 0.36 | N | > 1 year | By-passing agent | Persistent |
| 18 | 18 | Moderate | WNH | Missense Val326Ala | 1.3 | 0 | - | N | < 1 year | No prior product | False positive |
| 19 | 29 | Mild | WNH | Missense Arg593Cys | 0.9 | 0.3 | 0.19 | N | Unknown | No prior product | False positive |
| 20 | 15 | Severe | WNH | Frameshift1194delA | 0.7 | 0.1 | 0.03 | N | < 1 month | No change | False-positive or transient |
| 21 | 10 | Severe | WNH | Frameshift 1049delGA | 0.8 | 0 | 0.34 | N | Unknown | Unknown | ? |
AS=Asian, BNH=Black non-Hispanic, OTH=other, WH=White Hispanic, WNH=White non-Hispanic
Discussion
In clinical practice, the diagnosis of an inhibitor, with the concomitant decision to alter a patient’s treatment to an inhibitor regimen, is usually based on more than a single positive inhibitor titer; it includes the patient’s historical response to therapy and often pharmacokinetic studies. In clinical trials and surveillance programs, however, there is risk of miscounting of cases and mislabeling of patients due to false-positive inhibitor tests. As a means of determining how to define and validate a true positive inhibitor, we have examined the prospectively collected data of HIRS, which included over 1,000 patients and 3,000 specimens. In the present study, the clot-based method previously described [19] was compared with alternative methods of inhibitor measurement. Chromogenic and fluorescence-based assays were chosen because of their potential to improve on the specificity and sensitivity of clot-based assays.
Chromogenic assays are appealing for FVIII inhibitor detection, because they have been shown to be insensitive to heparin, lupus anticoagulants, and other non-specific inhibitors of coagulation [20, 21]. Their use in the Bethesda assay has been reported previously [3, 10, 22]. Chromogenic and clot-based assays differ in several key characteristics. Chromogenic assays, performed in two steps, depend upon FVIII activation by a standard amount of thrombin and generation of FXa in an artificial system, while one-stage clotting assays depend upon thrombin generation and the formation of a fibrin clot in a system containing many plasma components. Differences between the assays have been noted in their ability to measure recombinant versus native FVIII [24] and baseline FVIII levels in hemophilia patients with certain mutations [25], due to the longer reaction time in the chromogenic assays, which more closely resemble the two stage clotting assay [24]. The chromogenic assay used in this study was chosen because in its first stage activation time of 90 seconds, it more closely resembles a one-stage assay than those assays with up to 5 minute activation times. It has also been shown to be less sensitive to FVIII variants with one-stage/two-stage assay discrepancies [27]. Its kinetics appear to be similar to those of the clot-based FVIII assay.
The FLI measures antibodies that bind to the specific FVIII molecule attached to polystyrene beads. A FLI method was originally described for simultaneous measurement and epitope mapping of FVIII inhibitors in inhibitor-positive patients [7]. Subsequently, a FLI was described for measurement of anti-FVIII antibodies in nanomoles of protein through comparison with a locally prepared immunoglobulin calibrator [8]. We defined a FLI measurement method more adaptable to clinical practice with a result more readily correlated with results obtained from traditional inhibitor assays. The FLI described is more sensitive than either the NBA or CBA, and it is not surprising that it detects antibodies in specimens without detectable inhibition of FVIII in clotting assays. Using our method, 21.3% of 169 NBA-negative specimens had positive antibody binding to FVIII. Similarly, Krudysz-Amblo et al [8] found 33.3% of 39 inhibitor-negative patients to be positive, and Zakarija et al [13] found 15.9% of 44 inhibitor-negative patients to have antibodies above the levels detected in normal subjects using the FLI. Frequencies of antibodies to FVIII observed in hemophilia patients with negative BA were 16–39% by ELISA [11, 12] and 25% by immunoprecipitation [9]. Whether these antibodies are qualitatively different from those detected in the BA or NBA and truly fail to neutralize FVIII or whether the clot-based assays are simply less sensitive at detecting these low titer antibodies remains to be determined, as does their clinical significance.
Only 4% of specimens showed discordance between NBA and CBA results. Agreement was excellent among NBA-negative specimens (99.7%) and those with NBU≥2.0 (100%). Discordant specimens had 0.5–1.9 NBU and were more likely than concordant specimens to be FLI-negative, LA-positive, or NTDI. The FLI detected antibodies in 98.0% of CBA-positive specimens but only 81.6% of NBA-positive specimens (P=0.004). Both CBA and FLI were negative in approximately 50% of specimens with 0.5–0.9 NBU. These results are similar to those using an immunoprecipitation test, in which 50% of inhibitors <1.0 BU also failed to react [9]. Specimens with titers as low as 0.5 NBU, however, sometimes gave positive results by both confirmatory tests, indicating that altering the established assay cut-off could exclude true positives. The data suggest that inhibitor results of 0.5–1.9 NBU should be interpreted with caution.
Potential causes for the discrepancies observed between the NBA and CBA are false negative CBA results, false-positive NBA results, the presence of non-FVIII inhibitors, and atypical FVIII inhibitors; they are likely to be heterogeneous. The hypothesis that the CBA is simply a less sensitive method than the NBA is not supported by the dilution curve data. The kinetics of the NBA and the CBA appeared to be similar for proven inhibitors. Overall, the FLI, which was the most sensitive method tested, showed better agreement with the CBA than the NBA. It is possible, however, that differences in the epitope specificity of individual inhibitors might cause differences in the rate of FVIIIa generation, to which the CBA is more sensitive, or differences in the reactivity to bovine proteins, leading to false negative results. Since the FVIII being measured is from the added normal plasma rather than from the patient, differences due to the patient’s FVIII defect would not be expected to play a role.
Sources of false positive results in clot-based assays are well known. LA in hemophilia patients have been described [2, 3]. Non-specific inhibitors have been reported to occur in up to 35% of healthy children undergoing pre-surgical screening [4] and might appear in patients undergoing frequent immune stimulation. The false positive NBA results seen in two adults with no product exposure, who were DRVVT and NTDI negative, are more likely to be due to technical issues. Addition of the heating step to the NBA [19] could potentially cause false-positive results; however, 883 NBA-negative specimens were not affected. The heating step offers the benefit of allowing inhibitor measurement in treated patients, which in our view outweighs the risk of rare false positives, particularly if there are means to identify the errors by performing confirmatory tests. Assay variability could also account for false-positive results, as discussed previously [19]. There is also the possibility of specimen mix-up at the study site or the central laboratory. Redraw of all patients with unexpectedly positive inhibitor results is recommended.
Some discrepant inhibitors observed in this study could be directed against FVIII but atypical in their presentation. Time-dependent inhibition is one of the diagnostic criteria for FVIII inhibitors, but it is possible that some low titer inhibitors may not show this characteristic. Others may have dependence on phospholipid binding of FVIII for their reaction and more closely resemble LA in their kinetics. Inhibitors that fail to react in the FLI may be directed against epitopes not present in the single product used in the FLI and might react with other products. Because the FLI used only anti-IgG and IgM antibodies, an IgA inhibitor, rarely seen in hemophilia, might be missed. Additional limitations of this study include insufficient data on some patients who entered the study late or withdrew and were not available for follow up. Only small specimen volumes were available on some patients precluding additional testing.
In summary, FVIII specificity could not be demonstrated for 26% of inhibitors <2.0 NBU detected in the modified NBA. Low titer inhibitors ascertained in clot-based assays should always be repeated. If they are still present, consideration should be given to evaluating them with tests that more directly demonstrate their reactivity with FVIII, such as those described in this study. The clinical significance of low titer inhibitors with various characteristics needs to be defined in further studies.
Acknowledgments
The work was supported by the CDC Foundation through grants from Pfizer Pharmaceuticals and Baxter Healthcare. The authors wish to thank the patients who participated and the study coordinators and administrators at the sites.
Addendum
The Hemophilia Inhibitor Research Study Investigators include authors from the following study sites: Thomas C. Abshire, Amy L. Dunn, and Christine L. Kempton, Emory University, Atlanta GA; Paula L. Bockenstedt, University of Michigan Hemophilia and Coagulation Disorders, Ann Arbor, MI; Doreen B. Brettler, New England Hemophilia Center, Worcester, MA; Jorge A. Di Paola, Mohamed Radhi, and Steven R. Lentz, University of Iowa Carver College of Medicine, Iowa City, IA; Gita Massey and John C. Barrett, Virginia Commonwealth University, Richmond, VA; Anne T. Neff, Vanderbilt University Medical Center, Nashville, TN; Amy D. Shapiro, Indiana Hemophilia and Thrombosis Center, Indianapolis, IN; Michael Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; Brian M. Wicklund, Kansas City Regional Hemophilia Center, Kansas City, MO; Marilyn J. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, University of Colorado and The Children’s Hospital, Aurora, CO; Christine Knoll, Phoenix Children's Hospital Hemophilia Center, Phoenix, AZ; Miguel A. Escobar, Gulf States Hemophilia and Thrombophilia Center, Houston, TX; M. Elaine Eyster, Hemophilia Center of Central Pennsylvania, Hershey, PA; Joan C. Gill, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; Cindy Leissinger, Louisiana Center for Bleeding and Clotting Disorders, New Orleans, LA; Hassan Yaish, Primary Children’s Medical Center, Salt Lake City, UT.
J.M.Soucie and C.H.Miller designed the research project. C.H. Miller analyzed the data and wrote the paper. Anne S. Rice and Brian Boylan performed testing and analyzed data. A.D. Shapiro, S.R. Lentz, B.M. Wicklund, and the other HIRS Investigators provided data and patient specimens and revised the manuscript. F.M. Kelly coordinated the research project and collected data. All authors edited and approved the final paper.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Biggs R, Bidwell E. A method for the study of antihaemophilic globulin inhibitors with reference to six cases. Br J Haematol. 1959;5:379–95. doi: 10.1111/j.1365-2141.1959.tb04049.x. [DOI] [PubMed] [Google Scholar]
- 2.Nuss R, Jacobson L, Hathaway WE, Manco-Johnson M Recombinate PUP Study Group. Evidence for antiphospholipid antibodies in children with factor VIII inhibitors. Thromb Haemost. 1999;82:1559–60. [PubMed] [Google Scholar]
- 3.Blanco AN, Peirano AA, Grosso SH, Gennari LC, Perez Blanco R, Lazzari MA. A chromogenic substrate method for detecting and titrating anti-factor VIII antibodies in the presence of lupus anticoagulants. Haematologica. 2002;87:271–8. [PubMed] [Google Scholar]
- 4.Shaw PH, Reynolds S, Gunawardena S, Krishnamurti L, Ritchey AK. The prevalence of bleeding disorders among healthy pediatric patients with abnormal preprocedural coagulation studies. J Pediatr Hematol Oncol. 2008;30:135–41. doi: 10.1097/MPH.0b013e31815d8915. [DOI] [PubMed] [Google Scholar]
- 5.Manco-Johnson MJ, Nuss R, Jacobson LJ. Heparin neutralization is essential for accurate measurement of factor VIII activity and inhibitor assays in blood samples drawn from implanted venous access devices. J Lab Clin Med. 2000;136:74–9. doi: 10.1067/mlc.2000.107299. [DOI] [PubMed] [Google Scholar]
- 6.Blanco AN, Peirano AA, Grosso SH, Gennari LC, Perez Blanco R, Lazzari MA. An ELISA system to detect anti-factor VIII antibodies without interference by lupus anticoagulants. Haematologica. 2000;85:1045–50. [PubMed] [Google Scholar]
- 7.Lavigne-Lissalde G, Tarrade C, Lapalud P, Chtourou S, Schved JF, Granier C, Villard-Saussine S. Simultaneous detection and epitope mapping of anti-factor VIII antibodies. Thromb Haemost. 2008;99:1090–1096. doi: 10.1160/TH07-08-0497. [DOI] [PubMed] [Google Scholar]
- 8.Krudysz-Amblo J, Parhami-Seren B, Butenas S, Brummel-Ziedins KE, Gomperts ED, Rivard GE, Mann KG. Quantitation of anti-factor VIII antibodies in human plasma. Blood. 2009;113:2587–94. doi: 10.1182/blood-2008-08-174987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinge J, Auerswald G, Budde U, Klose H, Kreuz, Lenk H, Scandella D The Paediatric Inhibitor Study Group of the German Society on Thrombosis and Haemostasis. Detection of all anti-factor VIII antibodies in haemophilia by the Bethesda assay and a more sensitive immunoprecipitation assay. Haemophilia. 2001;7:26–32. doi: 10.1046/j.1365-2516.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- 10.Gilles JGG, Arnout J, Vermylen J, Saint-Remy J-MR. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993;82:2452–61. [PubMed] [Google Scholar]
- 11.Batlle J, Gomez E, Rendal E, Torea J, Loures E, Couselo M, Vila P, Sedano C, Tusell X, Magallon M, Quintana M, Gonzalez-Boullosa R, Lopez-Fernandez MF. Antibodies to factor VIIII in plasma of patients with hemophilia A and normal subjects. Ann Hematol. 1996;72:321–6. doi: 10.1007/s002770050179. [DOI] [PubMed] [Google Scholar]
- 12.Dazzi F, Tison T, Vianello F, Radossi P, Zerbinati P, Carraro P, Poletti A, Girolami A. High incidence of anti-FVIII antibodies against non-coagulant epitopes in haemophilia A patients: a possible role for the half-life of transfused FVIII. Br J Haematol. 1996;93:688–93. doi: 10.1046/j.1365-2141.1996.d01-1705.x. [DOI] [PubMed] [Google Scholar]
- 13.Zakarija A, Harris S, Rademaker AW, Brewer J, Krudysz-Amblo J, Buenas S, Mann KG, Green D. Alloantibodies to factor VIII in haemophilia. Haemophilia. 2011;17:636–40. doi: 10.1111/j.1365-2516.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 14.Mondorf W, Klinge W, Luban NLC, Bray G, Saenko E, Scandella D The Recombinate PUP Study Group. Low factor VIII recovery in haemophilia A patients without inhibitor titre is not due to the presence of anti-factor VIII antibodies undetectable by the Bethesda assay. Haemophilia. 2001;7:13–9. doi: 10.1046/j.1365-2516.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- 15.Kempton CL, Meeks SL, Donald Harvey R, Abshire TC. Evaluation of factor VIII pharmacokinetics and anti-factor VIII antibodies in four boys with haemophilia A and a poor clinical response to factor VIII. Haemophilia. 2011;17:155–6. doi: 10.1111/j.1365-2516.2010.02345.x. [DOI] [PubMed] [Google Scholar]
- 16.Caram C, de Souza RG, de Sousa JC, Pereira TA, Cerqueira AM, van der Bom JG, Rezende SM. The long-term course of factor VIII inhibitors in patients with congenital haemophilia A without immune tolerance induction. Thromb Haemost. 2011;105:59–65. doi: 10.1160/TH10-04-0231. [DOI] [PubMed] [Google Scholar]
- 17.Soucie JM, Miller CH, Kelly FM, Payne AB, Creary M, Bockenstedt PL, Kempton CL, Manco- Johnson MJ, Neff AT the Hemophilia Inhibitor Research Study Investigators. A pilot study of prospective surveillance for inhibitors among persons with hemophilia in the United States. Haemophilia. 2013 doi: 10.1111/hae.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbruggen B, Novakova I, Wessels H, Boezeman, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:c inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- 19.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM the Hemophilia Inhibitor Research Study Investigators. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10:1055–61. doi: 10.1111/j.1538-7836.2012.04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Maistre E, Wahl D, Perrett-Guillaume C, Regnault V, Clarac S, Briquel ME, Andre E, Lecompte T. A chromogenic assay allows reliable measurement of factor VIII levels in the presence of strong lupus anticoagulants. Thromb Haemost. 1998;79:237–8. [PubMed] [Google Scholar]
- 21.Chandler WL, Ferrell C, Lee J, Tun T, Kha H. Comparison of three methods for measuring factor VIII levels in plasma. Am J Clin Pathol. 2003;120:34–9. doi: 10.1309/C8T8-YNB4-G3W4-5PRF. [DOI] [PubMed] [Google Scholar]
- 22.Hutton RA, Kamiguti Yamaga A, Matthews KB, Woodhams BJ. The use of a chromogenic assay for factor VIII in patients with factor VIII inhibitors or von Willebrand’s disease. Thromb Res. 1991;63:189–93. doi: 10.1016/0049-3848(91)90281-z. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard AR, Bevan SA, Weller LJ. Potency estimation of recombinant factor VIII: effect of assay method and standard. Brit J Haematol. 2001;113:533–6. doi: 10.1046/j.1365-2141.2001.02761.x. [DOI] [PubMed] [Google Scholar]
- 24.D’Orion R, Pipe SW, Jacquemin M. Mild/moderate haemophilia A: new insights into molecular mechanisms and inhibitor development. Haemophilia. 2008;14(Suppl 3):138–46. doi: 10.1111/j.1365-2516.2008.01730.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers SE, Duncan EM, Sobieraj-Teagure M, Lloyd JV. Evaluation of three automated chromogenic FVIII kits for the diagnosis of mild discrepant haemophilia A. Int Jnl Lab Hem. 2009;31:180–8. doi: 10.1111/j.1751-553X.2007.01021.x. [DOI] [PubMed] [Google Scholar]