Abstract
Dental-derived mesenchymal stem cells (MSCs) provide an advantageous therapeutic option for tissue engineering due to their high accessibility and bioavailability. However, delivering MSCs to defect sites while maintaining a high MSC survival rate is still a critical challenge in MSC-mediated tissue regeneration. Here, we tested the osteogenic and adipogenic differentiation capacity of dental pulp stem cells (DPSCs) in a thermoreversible Pluronic F127 hydrogel scaffold encapsulation system in vitro. DPSCs were encapsulated in Pluronic® F-127 hydrogel and stem cell viability, proliferation and differentiation into adipogenic and osteogenic tissues were evaluated. The degradation profile and swelling kinetics of the hydrogel were also analyzed. Our results confirmed that Pluronic F-127 is a promising and non-toxic scaffold for encapsulation of DPSCs as well as control human bone marrow MSCs (hBMMSCs), yielding high stem cell viability and proliferation. Moreover, after 2 weeks of differentiation in vitro, DPSCs as well as hBMMSCs exhibited high levels of mRNA expression for osteogenic and adipogenic gene markers via PCR analysis. Our histochemical staining further confirmed the ability of Pluronic F-127 to direct the differentiation of these stem cells into osteogenic and adipogenic tissues. Furthermore, our results revealed that Pluronic F-127 has a dense tubular and reticular network morphology, which contributes to its high permeability and solubility, consistent with its high degradability in the tested conditions. Altogether, our findings demonstrate that Pluronic F-127 is a promising scaffold for encapsulation of DPSCs and can be considered for cell delivery purposes in tissue engineering.
Introduction
Mesenchymal stem cells (MSCs) present an advantageous treatment option where tissue regeneration is required, in comparison to traditional treatment modalities available in medicine and dentistry. Autografts procedures have well known disadvantages, such as additional surgery to remove tissue from the patient’s own body [1]. Besides the amount of tissue available may be modest, autograft harvesting is associated with increased patient pain and high risk of donor site morbidity [1]. In contrast, studies have confirmed that MSC-based tissue regeneration strategies offer advantages including the avoidance of donor-site harvesting and associated morbidity, high-quality regeneration of damaged tissues, and low risk of disease transmission or autoimmune rejection [2–4]. MSCs are multipotent cells that are capable of differentiation into multiple lineages depending on the nature of the environmental signals that they receive. It is well known that MSCs can be identified in a wide range of postnatal tissues, including the dental and orofacial tissues [5–10]. Our previous studies and others have shown that dental MSCs are proliferative postnatal stem cells capable of differentiating into odontogenic, adipogenic, and osteogenic tissues [11–16]. Furthermore, it has been reported that these MSCs might have superior proliferation and differentiation capacities compared to bone marrow mesenchymal stem cells (hBMMSCs), which are the current gold standard for MSC therapy [5, 7, 11, 17]. Therefore, in combination with an appropriate scaffold, dental MSCs might be considered promising candidates for applications in regenerative medicine and dentistry. Among the different types of dental MSCs that have been identified so far, dental pulp stem cells (DPSCs) are of special interest as they are readily accessible in the oral environment, and also can be readily found in discarded tissue samples. Several studies have reported the multilineage differentiation capacity of these stem cells in vitro and in vivo, confirming their regenerative capacities are suitable for applications in medicine and dentistry [18–22].
It is well known that the cell delivery vehicle plays an important role in the in vivo performance of MSCs and strongly contributes to the success of the regenerative therapy. Several studies have reported that providing a suitable microenvironment for MSC proliferation and differentiation in response to exogenous stimuli and growth factors is a critical step towards clinical applications in regenerative medicine [23]. Several types of scaffolds have been used to support growth and differentiation of progenitor cells for tissue regeneration, such as natural and synthetic polymers [19, 21, 22]. Hydrogels are of specific importance as they enable noninvasive surgical procedures using stem cell therapy principles. In addition, synthetic hydrogel materials that mimic natural stem cell microenvironments may be powerful tools to control stem cell functions [24].
Pluronic® F-127 (poloxamer 407) is a synthetic hydrogel made of amphiphilic copolymers consisting of units of ethylene oxide (PEO) and polypropylene oxide (PPO). It is injectable and has a reversible mechanism of gelation [25]. Moreover, Pluronic F-127 hydrogel has favorable properties such as non-toxicity, biocompatibility, and biodegradability. A unique characteristic of Pluronic F-127 is its thermosensitivity, which enables it to hold encapsulated cells in its structure and favors initial cell adhesion inside the defect site [26, 27]. Additionally, it has been shown that Pluronic F-127 enhances cell attachment and collagen formation, leading to improved levels of angiogenesis [28, 29]. Therefore, it has been reported that this type of hydrogel biomaterial may be a promising candidate for encapsulating MSCs to promote the regeneration of poorly vascularized tissues, such as cartilage, tendons, epithelial tissues or even adipose and bony tissues [26, 30–33]. Brunet-Maheu et al. [31] have shown that Pluronic F-127 has an optimized cell distribution in the liquid state with easy manipulation in its solid state. Moreover, the FDA has approved it for use in humans. Pluronic F-127 has been widely used in drug delivery, controlled release, and MSC encapsulation applications [34]. The thermosensitive properties of Pluronic F-127 make it an attractive biomaterial for regenerative applications in dentistry or medicine. However, a literature search revealed no reports assessing the application of dental MSCs (e.g., DPSCs) encapsulated in Pluronic F-127 for tissue engineering purposes. Therefore, in the present study, we aimed to demonstrate the osteogenic and adipogenic differentiation capacity of DPSCs and hBMMSCs in a 3D in vitro environment using Pluronic F-127 hydrogel scaffold as a potential MSC carrier for tissue regeneration/repair of craniofacial tissues. This approach was designed to optimize tissue regeneration for potential applications in the repair of soft and hard tissues. Considering the fact that DPSCs can often be obtained as discarded biological samples or can be easily harvested from the oral cavity, this MSC source could be ideal for stem cell banking purposes provided DPSCs show promise in MSC-based tissue regeneration.
Materials and methods
Progenitor cell isolation and culture
Teeth were obtained from twenty healthy male patients (18–25 years old) undergoing third molar extractions with IRB approval from the University of Southern California. Only subjects without any history of periodontal disease were included in this study. Human DPSCs were isolated and cultured according to previously published procedures [5]. Briefly, teeth were then cracked opened in the laminar flow hood and the dental pulp tissue was minced and digested in a solution of 4 mg/ml dispase and 3 mg/ml type I collagenase for 30–60 min at 37 °C. Single cells suspensions were obtained by passing cells through a 70 μm cell strainer. Human bone marrow mesenchymal stem cells (hBMMSCs) used as controls were purchased from Poetic Technologies (Gaithersburg, MD, USA). DPSCs and hBMMSCs were cultured in α-MEM (Invitrogen, Grand Island, NY) supplemented with 15 % Fetal Bovine Serum (FBS) (Invitrogen, San Diego, CA, USA), 100 units/ml penicillin (Invitrogen) and 100 μg/ml streptomycin (Invitrogen). Cells were incubated at 37 °C in a humid atmosphere containing 5 % CO2. In all the experiments, passage four cells were used. For the colony forming unit–fibroblastic (CFU-F) assay, 0.1 × 106 cells were seeded in a culture dish and cultured for 14 days, then stained with toluidine blue. A count of more than 50 cells in one colony was counted as positive on the CFU-F assay.
Flow cytometric analysis
Approximately 5 × 105 DPSCS or hBMMSCs from passages two to six were incubated with specific PE- or FITC-conjugated mouse monoclonal antibodies for human CD45 (as a non-MSC associated marker), CD146 (as a positive mesenchymal stem cell marker) (BD Biosciences, San Jose, CA, USA), or isotype-matched control IgGs (Southern Biotechnology Associates, Birmingham, AL, USA) and subjected to flow cytometric analysis using a Beckman Coulter flow cytometer and FACScan program (BD Biosciences).
Biomaterial fabrication and cell encapsulation
Pluronic® F-127 (Sigma Aldrich, St Louis, MO, USA; poloxamer 407) is liquid at 4 °C and semi-solid at 37 °C. The protocol reported by Vashi et al. [35] was followed. Briefly, Pluronic F-127 was slowly added to complete cell culture medium (α-minimum essential medium, supplemented with 15 % fetal bovine serum, 2 mM l-glutamine, 100 μ/ml penicillin and 100 μg/ml streptomycin) (all from GIBCO/Invitrogen, Grand Island, NY, USA) and 100 μM L-ascorbic acid-2-phosphate (Sigma Aldrich) at the ratio of 1:5 w/v by stirring on ice for 12 h. Before use in cell cultures, the polymer solution was filtered using a 0.22 μm pore size bottle-top filter and kept at 4 °C overnight. Hydrogel was kept on ice when manipulated inside the laminar flow hood.
Next, DPSCs or hBMMSCs were re-suspended in Pluronic F-127 on ice. A cell-hydrogel mixture containing 1 × 106 cells/ml was used in the experiments. After seeding, the plates were kept in an incubator at 37 °C and 5 % CO2 for 5 min to induce gel formation. Homogeneous cell encapsulation was confirmed by light microscopy. Regular culture media was overlaid on each plate and transferred to an incubator at 37 °C in a humid atmosphere containing 5 % CO2.
Cell viability
A live/dead assay kit (Invitrogen) was utilized to assess the viability of the encapsulated stem cells in Pluronic F-127 hydrogel after 1, 3 and 7 days of culturing in regular culture media. A fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan) was used to observe the cells. Five specimens per group were examined and the numbers of green (live) and red (dead) cells were counted in each bead. The percentage of live cells and live cell density were determined from five independent specimens for each experimental group using the Image-J software (Version 1.64, NIH, Bethesda, MD, USA). Additionally, in order to measure stem cell viability in Pluronic F-127 hydrogel in vitro, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (Invitrogen) was utilized according to methods in the literature [16]. The MTT absorbance was obtained at different time intervals (1, 3 and 7 days), and normalized to the absorbance of Pluronic F-127 hydrogel containing the same type of stem cells measured at day 1 (N = 4).
In vitro osteogenic differentiation
To study the osteogenic differentiation capacity of DPSCs and hBMMSCs encapsulated in Pluronic F-127 hydrogel in vitro, 1 × 106 DPSCs or hBMMSCs were encapsulated in the hydrogel and cultured in 6-well plates in osteogenic culture medium containing α-MEM, 15 % FBS, 10,000 μ/ml penicillin and streptomycin, 2 mM l-glutamine, 0.1 mM ascorbic acid phosphate, 1.8 mM potassium dihydrogen phosphate (KH2PO4) (Sigma Aldrich) and dexamethasone (10−8 M). The medium was changed twice per week. MSCs seeded in the Pluronic F-127 in regular culture media were used as controls.
After 4 weeks of culture under osteogenic induction conditions, the differentiation of the DPSCs and hBMMSCs was evaluated using Alizarin red staining. Briefly, the cultures were washed twice with PBS, fixed for 1 min in 60 % isopropanol, and rehydrated with distilled water. Samples were stained with Alizarin red solution for 5 min and excess dye was removed by washing twice with deionized water. Retained Alizarin red was quantified using NIH ImageJ software by determining the percentage of stained area out of the total area.
In vitro adipogenic differentiation
In order to study the adipogenic differentiation capacity of DPSCs and hBMMSCs encapsulated in Pluronic F-127 hydrogel in vitro, 1 × 106 of each type of MSC were encapsulated in the hydrogel and cultured in adipogenic induction medium containing α-MEM, 15 % FBS, 10,000 μ/ml penicillin and streptomycin, 2 mM l-glutamine, 0.1 mM ascorbic acid phosphate, 0.5 mM hydrocortisone, 10 mg/ml insulin, 50 mM isobutylmethylxanthin and 6 mM indomethacin (Sigma Aldrich). Cells were cultured in this medium for 4 weeks. MSCs seeded in the Pluronic F-127 in regular culture media were used as controls.
Following 4 weeks of adipogenic differentiation, the specimens were stained with Oil Red O (Sigma Aldrich) according to methods in the literature. The specimens were stained with Oil Red O solution (in 60 % isopropyl alcohol) for 45 min, fixed with 60 % isopropyl alcohol, and washed. The samples were then analyzed under a light microscope. Four specimens were tested from each group.
RNA isolation, reverse transcription and real time PCR
After 2 weeks of osteogenic or adipogenic differentiation, RNA was extracted from the encapsulated MSCs using Trizol reagent (Invitrogen) according to the manufacturer’s recommendations. Next, single-stranded cDNA was synthesized with 100 ng total RNA using a Superscript III cDNA synthesis kit (Invitrogen). The relative production of each mRNA was determined and normalized to the expression of the internal housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Primer and probe sequences are described in Table 1.
Table 1.
Oligonucleotide primers used in RT-PCR analysis
| Gene | Sequence | Product (bp) |
|---|---|---|
| Runt-related transcription factor 2 (Runx2) | Sense: 5′-CAGTTCCCAAGCATTTCATCC-3′; Antisense: 5′-TCAATATGGTCGCCAAA CAG-3′ |
289 |
| Osteocalcin (OCN) | Sense: 5′-CATGAGAGCCCTCACA-3′; Antisense: 5′-AGAGCGACACCCTAGAC-3′ |
292 |
| Peroxisome proliferatoractivated receptor-g2 (PPAR g2) | Sense: 5′-CT CCTATTGACCCAGAAAGC-3′; Antisense: 5′-GTAGAGCTGAGTCTTCTCAG-3′ |
351 |
| Lipoprotein lipase (LPL) | Sense: 5′-ATGGAGAGCAAAGCCCTGCTC-3′; Antisense, 5′-GTTAGGTCCAGCTGGATCGAG-3′ |
198 |
| Glyceraldehyde 3-phosphate dehydrogenase (GADPH) | Sense: 5′-AGCCGCATCTTCTTTTGCGTC-3; Antisense: 5′-TCATATTTGGCAGGTTTTT CT-3′ |
418 |
Degradation profile and swelling kinetics characterization
In a thermostatic bath, 500 μl of Pluronic F-127 was incubated in a 1.5 ml micro-tube until gelation. The microtubes were filled with 1 ml of regular culture media and the amount of weight loss after recovery of the samples was measured at different time intervals (1 h to 1 week). At each time interval the samples were centrifuged at 10.000 g for 5 min at room temperature, then specimens were dried and weighed. Microtubes were then stored at 37 °C. The degradation profile was evaluated by measuring the weight loss (%) at each time interval, as reported previously [36]. In addition, in order to evaluate the water content of the Pluronic F-127, freeze-dried specimens were incubated in PBS (pH 7.4) at 37 °C and swelling kinetics of the hydrogel biomaterials were analyzed for up to 10 h. At each time interval, the weight of the swollen specimens was measured after the excess surface solution was removed by filter paper. The swelling ratio (SR) was calculated according to the following formula:
where Wt and W0 are the weights of the swollen and dried specimens, respectively. In addition, the effect of increasing the temperature on the swelling ratio of the hydrogels was determined between 4 and 37 °C.
Scanning electron microscopy (SEM)
In order to characterize the morphology and structure of Pluronic F-127 hydrogel, scanning electron microscopy (SEM) (JEOL 5300, Peabody, MA, USA) was utilized. Sections of semi-solid hydrogel measuring 3 × 3 × 1 mm were frozen in liquid nitrogen to obtain mechanically fractured surfaces [37]. Next, specimens were freeze-dried for 24 h to keep the microporosities open. Lyophilized specimens were mounted on metallic stubs and sputter-coated in gold (30 mA, 30 s, 10−2 vacuum) (SCD 050, BAL-TEC, Furstentum, Liechtenstein) before visualization under the microscope.
Statistical analysis
Quantitative data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to compare multiple groups at a significance level of α = 0.05.
Results
Characterization and comparison of DPSCs versus hBMMSCs
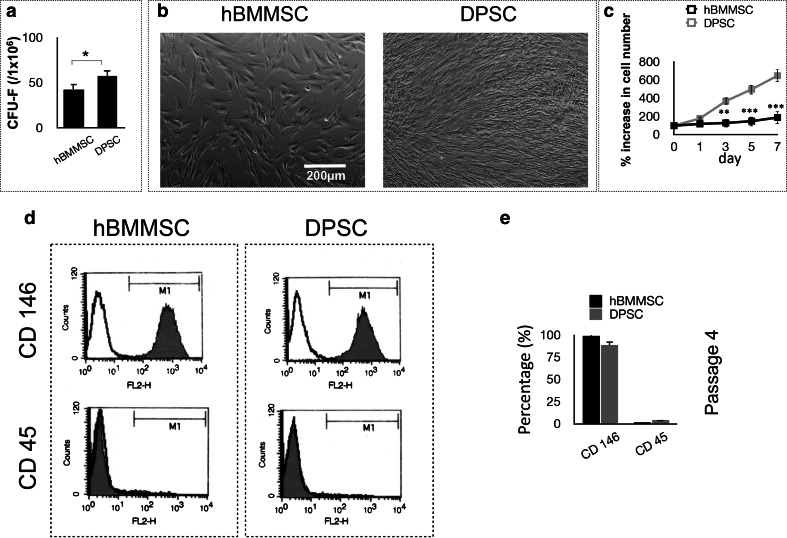
In order to analyze the colony-forming ability of the newly isolated stem cells, a CFU-F assay was utilized. Our data confirmed that DPSCs formed significantly higher numbers of single-colony clusters (CFU-F) than hBMMSCs (Fig. 1a). In addition, the in vitro growth properties of DPSCs were analyzed and compared to those of hBMMSCs (Fig. 1b). Both types of tested stem cells exhibited MSC-like fibroblast CFUs, organized as single CFUs, and adhered to culture dishes. DPSCs showed significantly higher proliferation rates than hBMMSCs (Fig. 1c). FACS analysis confirmed that human DPSCs display CD 146 (MSC marker), while not expressing CD 45 (non-MSC lineage marker) (Fig. 1d, e) confirming the stemness of DPSCs.
Fig. 1.
Characterization of stem cells used in the current study: a generation of colony-forming units in cultures containing either dental pulp stem cells (DPSCs) or human bone marrow mesenchymal stem cells (hBMMSCs) at a low density after 10 days (P value = 0.032). b Morphological characteristics of hBMMSCs and DPSCs after 72 h of culturing, showing superior growth properties of DPSCs in comparison to hBMMSCs c Percentual increase in the cell number after 1, 3, 5 and 7 days, confirming the morphological findings. d Flow cytometric analysis demonstrating the expression of cell surface markers on DPSCs and hBMMSCs (passage 4). e Quantification of the percentage of cells expressing specific stem cell markers. The results are representative of at least five independent experiments from passage four. *P < 0.05, **P < 0.01, ***P < 0.001, NS not significant
Pluronic F-127 hydrogel maintains DPSC viability
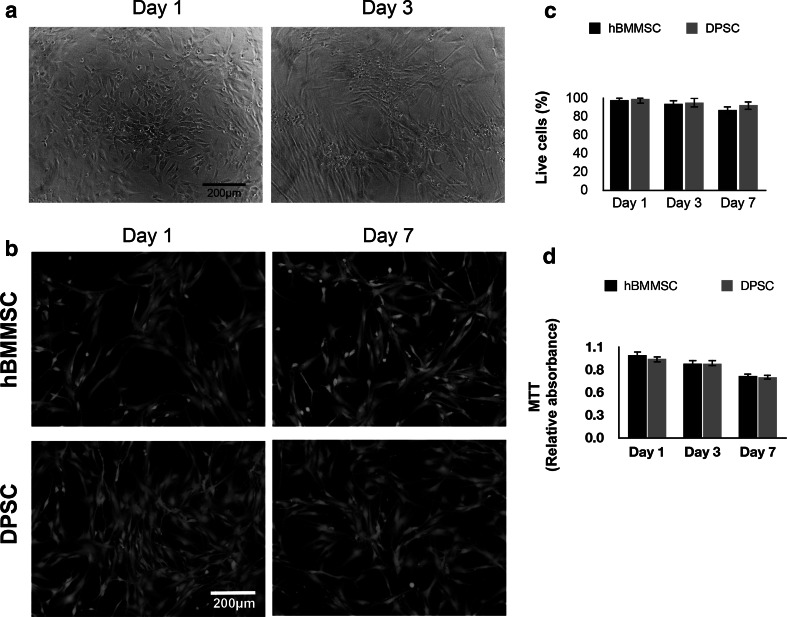
In the current study we investigated the application of Pluronic F-127 hydrogel as an injectable thermosensitive vehicle for DPSCs. As a positive control, hBMMSCs encapsulated in alginate were used (Fig. 2a). A live/dead-staining assay was performed and the results exhibited a high degree of viability of encapsulated MSCs in Pluronic F-127 hydrogel after 1 week of in vitro culturing (Fig. 2b). In this assay, live cells were stained green with calcein-acetoxymethyl, indicating intracellular esterase activity, and dead cells were stained red with ethidium homodimer-1, confirming the loss of plasma membrane integrity. Quantitatively, no significant difference was observed between the percentages of live cells in the DPSC and hBMMSC groups (P > 0.05) (Fig. 2c).
Fig. 2.
Development of stem cell delivery vehicle based on Pluronic F-127 hydrogel maintaining high stem cell viability: a microscopic images of cells (DPSCs) encapsulated in Pluronic F-127 hydrogel after 1 and 3 days of culturing in regular culture media. b Live/dead staining of the stem cells after 1 week of culturing (scale bar = 200 mm). c Percentages of viable encapsulated stem cells in Pluronic F-127 hydrogel. d 3-(4,5-Di- methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of metabolic activity of cells showing no significant difference between the two stem cell groups at each time interval. NS not significant
In order to assess the cytotoxicity and metabolic activity of MSCs encapsulated in Pluronic F127 hydrogel in vitro, the MTT assay was utilized. All encapsulated MSCs showed high metabolic activity and viability after up to 1 week of culturing in regular media (Fig. 2d), confirming the suitability of Pluronic F-127 hydrogel for encapsulation of DPSCs as well as hBMMSCs.
Osteogenic and adipogenic differentiation of DPSCs in vitro
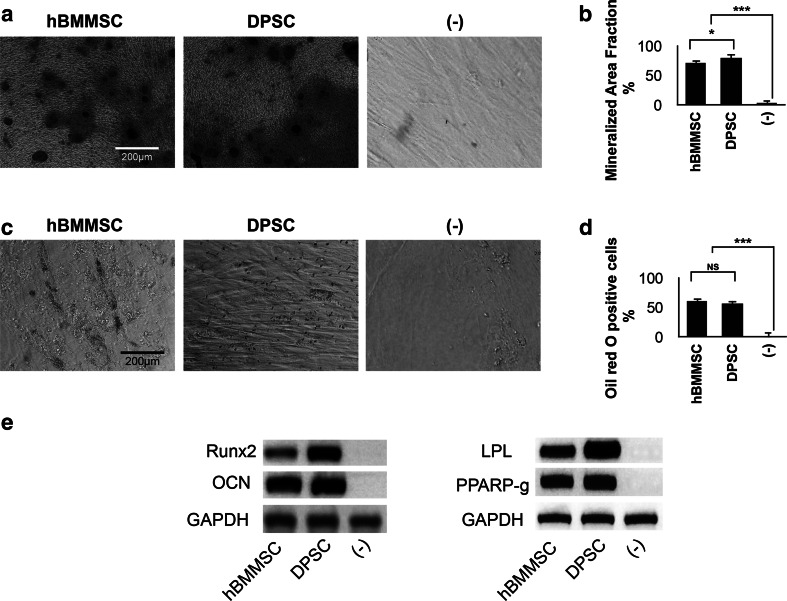
For tissue engineering applications, it is necessary to analyze and verify the multilineage differentiation potential of encapsulated DPSCs in vitro. DPSCs after passage 4 were encapsulated in Pluronic F-127 hydrogel and the culture medium was changed either to osteogenic or adipogenic differentiation media. After 4 weeks of culturing in the osteogenic media, DPSCs and hBMMSCs showed positive Alizarin red staining (Fig. 3a). As expected, DPSCs exhibited significantly greater mineralized area in comparison to hBMMSCs (P < 0.05) (Fig. 3b), confirming the superior osteogenic potential of DPSCs. Moreover, osteogenesis-related genes such as Runt-related transcription factor 2 (Runx2) and Osteocalcin (OCN) were highly expressed in both lineages after the induction period (Fig. 3e).
Fig. 3.
In vitro Osteo- and adipo-differentiation of encapsulated stem cells. a Alizarin red staining. b Mineralization area fraction was significantly higher for DPSCs in comparison to hBMMSCs. c Oil Red O staining. d No significant difference was observed in the amounts of adipogenesis of DPSCs and hBMMSCs. e PCR analysis of osteogenic and adipogenic gene expression after 2 weeks of differentiation. GAPDH (housekeeping) gene expression was used as the control. *P < 0.05, ***P < 0.001, NS not significant (Color figure online)
Results of the Oil Red O staining showed that both DPSCs and hBMMSCs within the hydrogel were intensively stained (Fig. 3c). Lipid droplets were observed to be clustered or randomly distributed for both cell lineages. No significant difference in the amount of adipogenic tissue was observed between DPSCs and hBMMSCs (P > 0.05) (Fig. 3d). The adipogenesis-related genes including Peroxisome proliferators activated receptor γ2 (PPAR γ2) and lipoprotein lipase (LPL) were highly expressed for both cell lineages (Fig. 3e).
Degradation profile, swelling kinetics, and morphological characterization
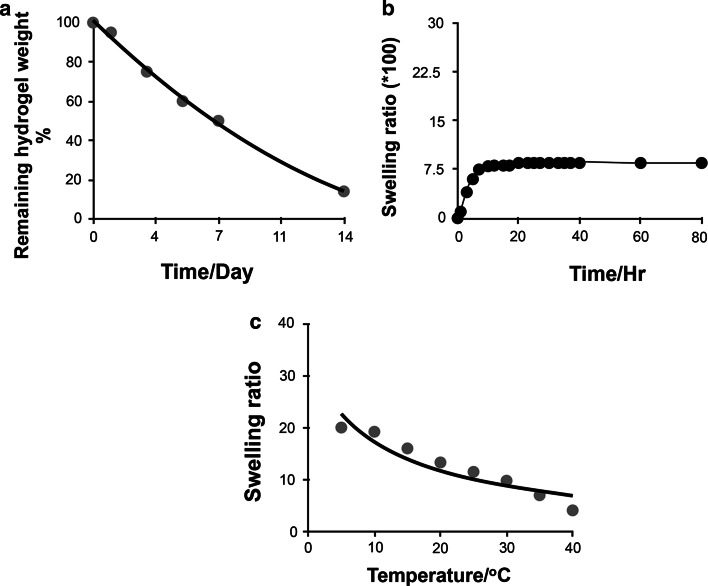
The results of the degradation profile characterization showed that Pluronic F-127 hydrogel degraded rapidly. After 1 week of culturing, the hydrogel was fragmented into many small pieces. Pluronic F-127 hydrogel lost more than 85 % of its initial weight after 2 weeks of incubation in regular culture media (Fig. 4a). The swelling kinetics of Pluronic F-127 hydrogel as a function of time is plotted in Fig. 4b. The results showed that the examined hydrogel swelled rapidly in PBS and reached equilibrium within the first 5 h of incubation. Moreover, as presented in Fig. 4c, a decrease in the swelling ratio of the hydrogel was observed at elevated temperatures. This phenomenon is attributed to the thermo-sensitive properties of the hydrogel.
Fig. 4.
Characterization of the degradation behavior of Pluronic F-127 hydrogel. a Degradation profile of Pluronic F-127 hydrogel in PBS (pH 7.4) at 37 °C. b Swelling behavior of the hydrogel as a function of time. c Swelling ratios of Pluronic F-127 hydrogel in PBS buffer (pH 7.4) at different temperatures
Figure 5 shows SEM photographs of cross-sectional morphology of Pluronic F-127 hydrogel, demonstrating dense tubular and reticular network morphology with well interconnected pores within the matrix of the hydrogel. These morphological characteristics contribute to its high permeability and solubility, consistent with its high degradability in the tested conditions.
Fig. 5.
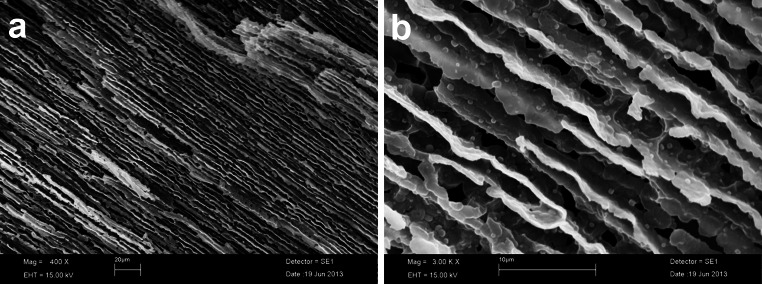
Morphological characterization of Pluronic F-127 hydrogel. SEM images of Pluronic F-127 hydrogel at a low and b high magnification. The images show the morphology of the hydrogel, confirming a porous structure
Discussion
It has been shown that the cell delivery material plays a vital role in the success of MSC therapies in regenerative dentistry and medicine. In the current study, we investigated an injectable and biodegradable thermosensitive hydrogel as a 3D dental MSC delivery vehicle for potential application in tissue engineering [38–40]. We demonstrated that this system supported the viability, metabolic activity and osteogenic and adipogenic differentiation capacity of encapsulated DPSCs in vitro.
This is the first study to investigate Pluronic F-127 thermosensitive hydrogel encapsulating human dental MSCs for potential applications in tissue engineering. Here, we reported based on in vitro studies that Pluronic F-127 hydrogel appears to be suitable for encapsulation of DPSCs. Our data confirmed that both DPSCs and hBMMSCs favorably adhered and proliferated within the biomaterial and also differentiated into osteogenic or adipogenic tissues in vitro. Our results agree with previous studies available in literature reporting favorable cell response and growth upon encapsulation in Pluronic F-127 [31, 35]. Additionally, the current study confirmed that DPSCs have superior growth properties compared to hBMMSCs, and also showed greater osteogenic differentiation capacity. Furthermore, in comparison to hBMMSCs, DPSCs showed higher cell viability in the first days after encapsulation, which might be attributed to less sensitivity of DPSCs (in comparison to hBMMSCs) to the components of the Pluronic F-127 hydrogel or the temperature changes during the gelation of the biomaterial.
Microscopic evaluation of the encapsulated stem cells showed that cell clustering within the biomaterial happened within the first few days of culturing. Additionally, during osteogenic and adipogenic differentiation, aggregation resulted in the formation of macroscopic mineralized and adipose tissues of several microns in diameter. The osteogenic and adipogenic differentiation capacities of the DPSCs as well as hBMMSCs encapsulated in F-127 hydrogel were evaluated in vitro. Our histochemical and PCR analysis confirmed that both of the tested stem cells are promising candidates for bone and adipogenic tissue regeneration. These data correlate well with previous reports, which found that Pluronic F-127 provided a suitable environment for MSCs to differentiate into osteogenic and adipogenic tissues [4, 27, 32, 35, 41]. The data obtained here are specifically important for DPSCs as they are readily accessible in the oral environment and can be readily found in discarded tissue samples as well. These unique characteristics make DPSCs promising candidates for tissue engineering applications.
Several studies have shown that adding Pluronic F-127 to differentiation media significantly increased alkaline phosphatase levels during the osteogenic differentiation of human tooth germ cells [42, 43]. Other studies also corroborate with our results showing that Pluronic F-127 facilitated MSC growth and differentiation into osteoblasts on their interpore granules [26]. Additionally, the association of Pluronic F-127, PGE2 and platelet-rich plasma is reported to support osteoblast phenotype maintenance with minimal effects on osteoblast viability.
SEM analysis confirmed that Pluronic F-127 had a dense well-oriented tubular appearance and reticular networks. These morphological features are of utmost importance as the oriented channel pores are desirable for providing nutrients and oxygen to the encapsulated cells [37]. Moreover, the degree of crosslinking and swelling of the hydrogel produces morphologies that can increase or decrease the rate of diffusion and permeation of biomaterials [44]. In this way, tubular morphologies appear to lead to higher rates of diffusion and permeability of drugs than spheroidal morphologies [37]. Furthermore, a very fast degradation profile was observed for Pluronic F-127. Within the first week of immersion in PBS solution, the material lost more than 85 % of its initial weight. This attribute, in addition to the thermosensitivity of this biomaterial, makes Pluronic F-127 a promising candidate for fast release of stem cells and other sensitive bioactive molecules in vivo [45]. However, it has to be mentioned that fast degradation might be disadvantageous for some tissue engineering applications where a longer-lasting material is advantageous. Studies have confirmed that slower degradation can be achieved by mixing Pluronic F-127 with different additives (e.g. polymers [46–48] or hydroxyapatite [27]). Modifications such as UV curing would make this hydrogel even more versatile for clinical applications [4]. These modifications may benefit the purpose of a specific graft, such as enhanced mechanical properties and greater drug sustained release [46–48].
Although the presence of body fluids reduces the hydrogel concentration, resulting in rather fast disintegration of the gel in vivo [49], this property might be of importance for regenerating/repairing avascular or poorly vascularized tissues, such as cartilage or dense connective tissues (tendons, ligaments). The lack of microvasculature may retard the degradability of the biomaterial, but not as much as it impairs the tissue neogenesis due to the persistence of non-degradable materials in the defect site [4, 27, 30, 32, 41]. The thermo-responsive properties of these hydrogels makes them attractive biomaterials for encapsulation and delivery of cells and other sensitive bioactive molecules from the lab to the warmer environment of the oral cavity and the rest of the human body.
Further in vivo studies should be conducted to investigate the feasibility of Pluronic F-127 as a cell delivery scaffold for tissue engineering purposes. In the future, we plan to use this material in an animal model to study the ability of encapsulated DPSCs and other dental-derived MSCs to differentiate upon implantation in vivo. However, prior to undertaking this in vivo study, it is desirable to optimize the degradability and osteoconductivity of the biomaterial further. The results of these endeavors will be reported in due course.
Conclusion
In the current study, dental pulp stem cells were encapsulated in a 3-D thermosensitive biodegradable, and injectable hydrogel, Pluronic F-127. This material is a promising and non-toxic scaffold for encapsulation of DPSCs, yielding high stem cell viability and proliferation. The findings of this in vitro study demonstrated for the first time that immobilization of DPSCs in Pluronic F-127 hydrogel provides a promising strategy for tissue engineering. Further in vivo studies should be conducted to investigate the feasibility of Pluronic F-127 as a cell delivery scaffold for tissue engineering purposes, and specifically, for oral applications.
Acknowledgments
This work was supported by Grants from the National Institute of Dental, Craniofacial Research and FAPESP (K08DE023825 to A.M., R01 DE017449 to S.S., and 2013/17268-6 to M.M.).
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933–938. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 4.Lippens E, Vertenten G, Gironès J, Declercq H, Saunders J, Luyten J, et al. Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng Part A. 2010;16:617–627. doi: 10.1089/ten.tea.2009.0418. [DOI] [PubMed] [Google Scholar]
- 5.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSC) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent post-natal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 8.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24(2):155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitrano TI, Grob MS, Carrion F, Nova-Lamperti E, Luz PA, Fierro FS. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–925. doi: 10.1902/jop.2010.090566. [DOI] [PubMed] [Google Scholar]
- 12.Lindroos B, Maenpaa K, Ylikomi T, Oja H, Suuronen R, Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329–335. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 13.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 14.Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, et al. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285–3294. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 15.Moshaverinia A, Xu X, Chen C, Akiyama K, Snead ML, Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9:9343–9350. doi: 10.1016/j.actbio.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, et al. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A. 2014;20:611–621. doi: 10.1089/ten.tec.2013.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 18.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 19.Kim NR, Lee Dh, Chung PH, Yang HC. Distinct differentiation properties of human dental pulp cells on collagen, gelatin and chitosan scaffolds. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Oral Endod. 2009;108:e94–e100. doi: 10.1016/j.tripleo.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89(8):842–847. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2011;18:174–184. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Zhao Y, Jia W, Yang J, Ge L. Preliminary study on dental pulp stem cell-mediated pulp regeneration in canine immature permanent teeth. J Endod. 2013;39:195–201. doi: 10.1016/j.joen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 24.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54:13–36. doi: 10.1016/S0169-409X(01)00240-X. [DOI] [PubMed] [Google Scholar]
- 25.Schmolka IR. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972;6:571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 26.Huang JW, Chen WJ, Liao SK, Yang CY, Lin SS, Wu CC. Osteoblastic differentiation of rabbit mesenchymal stem cells loaded in a carrier system of Pluronic F127 and Interpore. Chang Gung Med J. 2006;29:363–372. [PubMed] [Google Scholar]
- 27.Chen WJ, Huang JW, Niu CC, Chen LH, Yuan LJ, Lai PL, et al. Use of fluorescence labeled mesenchymal stem cells in pluronic F127 and porous hydroxyapatite as a bone substitute for posterolateral spinal fusion. J Orthop Res. 2009;27:1631–1636. doi: 10.1002/jor.20925. [DOI] [PubMed] [Google Scholar]
- 28.Fowler EB, Cuenin MF, Hokett SD, Peacock ME, McPherson JC, 3rd, Dirksen TR, et al. Evaluation of pluronic polyols as carriers for grafting materials: study in rat calvaria defects. J Periodontol. 2002;73:191–197. doi: 10.1902/jop.2002.73.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/S0142-9612(02)00618-X. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Yang X, Cheng J, Wang X, Shen SG. Distraction osteogenesis combined with tissue-engineered cartilage in the reconstruction of condylar osteochondral defect. J Oral Maxillofac Surg. 2011;69:e558–e564. doi: 10.1016/j.joms.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Brunet-Maheu JM, Fernandes JC, de Lacerda CA, Shi Q, Benderdour M, Lavigne P. Pluronic F-127 as a cell carrier for bone tissue engineering. J Biomater Appl. 2009;24:275–287. doi: 10.1177/0885328208096534. [DOI] [PubMed] [Google Scholar]
- 32.Jung HH, Park K, Han DK. Preparation of TGF-β1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J Control Release. 2010;147:84–91. doi: 10.1016/j.jconrel.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Heilmann S, Küchler S, Wischke C, Lendlein A, Stein C, Schäfer-Korting M. A thermosensitive morphine-containing hydrogel for the treatment of large-scale skin wounds. Int J Pharm. 2013;444:96–102. doi: 10.1016/j.ijpharm.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O’Connor AJ, Cooper-White JJ, Thompson EW. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29:573–579. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Gao C, Liu M, Chen J, Zhang X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym Degrad Stab. 2009;94:1405–1410. doi: 10.1016/j.polymdegradstab.2009.05.011. [DOI] [Google Scholar]
- 37.Silva R, Oliveira MG. Effect of the cross-linging degree on the morphology of poly (NIPA Am-co-AAc) hydrogels. Polymer. 2007;48:4114–4122. doi: 10.1016/j.polymer.2007.05.010. [DOI] [Google Scholar]
- 38.Ansari S, Moshaverinia A, Pi SH, Han A, Abdelhamid AI, Zadeh HH. Functionalization of scaffolds with chimeric anti-BMP-2 monoclonal antibodies for osseous regeneration. Biomaterials. 2013;34:10191–10198. doi: 10.1016/j.biomaterials.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moshaverinia A, Xu X, Chen C, Ansari S, Zadeh HH, Snead ML, et al. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials. 2014;35:2642–2650. doi: 10.1016/j.biomaterials.2013.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moshaverinia A, Chen C, Xu X, Ansari S, Zadeh HH, Schricker SR, et al. Regulation of the stem cell–host immune system interplay using hydrogel coencapsulation system with an anti-inflammatory drug. Adv Func Mater. 2015. doi:10.1002/adfm.201500055. [DOI] [PMC free article] [PubMed]
- 41.Mylonas D, Vidal MD, De Kok IJ, Moriarity JD, Cooper LF. Investigation of a thermoplastic polymeric carrier for bone tissue engineering using allogeneic mesenchymal stem cells in granular scaffolds. J Prosthodont. 2007;16:421–430. doi: 10.1111/j.1532-849X.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 42.Doğan A, Yalvaç ME, Şahin F, Kabanov AV, Palotás A, Rizvanov AA. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int J Nanomed. 2012;7:4849–4860. doi: 10.2147/IJN.S31949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taşlı PN, Yalvaç ME, Sofiev N, Sahin F. Effect of F68, F127, and P85 pluronic block copolymers on odontogenic differentiation of human tooth germ stem cells. J Endod. 2013;39:1265–1271. doi: 10.1016/j.joen.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Khare AR, Peppas NA. Swelling/deswelling of anionic copolymer gels. Biomaterials. 1995;16:559–567. doi: 10.1016/0142-9612(95)91130-Q. [DOI] [PubMed] [Google Scholar]
- 45.Cortiella J, Nichols JE, Kojima K, Bonassar LJ, Dargon P, Roy AK, et al. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006;12:1213–1225. doi: 10.1089/ten.2006.12.1213. [DOI] [PubMed] [Google Scholar]
- 46.Oh SH, Kim TH, Lee JH. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials. 2011;32:8254–8260. doi: 10.1016/j.biomaterials.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Cohn D, Sosnik A, Levy A. Improved reverse thermo-responsive polymeric systems. Biomaterials. 2003;24:3707–3714. doi: 10.1016/S0142-9612(03)00245-X. [DOI] [PubMed] [Google Scholar]
- 48.Sosnik A, Cohn D. Ethoxysilane-capped PEO-PPO-PEO triblocks: a new family of reverse thermo-responsive polymers. Biomaterials. 2004;25:2851–2858. doi: 10.1016/j.biomaterials.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 49.Dumortier G, Groissord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]