Abstract
Objectives
The objective of our study was to determine whether angular vestibulo-ocular reflex (aVOR) gains correlated with vertigo control after intratympanic gentamicin treatment for Meniere’s disease.
Methods
We conducted a prospective study of 18 subjects with unilateral Meniere’s disease treated with intratympanic gentamicin injection and followed all subjects for 1 year. We measured the gain of the aVOR elicited by rapid rotary head thrusts in each of the canal planes for each subject before and after treatment with intratympanic gentamicin by using magnetic search coils to record eye movements.
Results
During the follow-up period, 11 subjects (“single-treatment group”; 61%) had control of their vertigo with a single gentamicin injection. The remaining 7 subjects (“multiple-treatment group”; 39%) experienced recurrent vertigo that required a second injection of gentamicin at a mean of 6 months after the first treatment. The 11 subjects in the single-treatment group had significantly greater reduction of labyrinthine function after the first treatment, as measured by change in ipsilateral horizontal canal gain, than did the 7 subjects with vertigo recurrence. Changes in caloric asymmetry did not correlate with vertigo control.
Conclusions
Our results suggest that successful treatment of Meniere’s disease is closely related to attenuation of semicircular canal function as measured by horizontal canal aVOR gains.
Keywords: angular vestibulo-ocular reflex gain, intratympanic gentamicin, Meniere’s disease
INTRODUCTION
Intratympanic injection of gentamicin is increasingly used to control vertigo that is refractory to medical treatment in unilateral Meniere’s disease. Since Schuknecht1 first introduced intratympanic aminoglycosides as a treatment for Meniere’s disease in 1956, myriad treatment regimens have been proposed. These regimens have varied in the drug concentration, as well as in the number and frequency of doses. There does not appear to be a consensus on which regimen most effectively controls vertigo.2 In a titration-based protocol previously used at our institution.3 patients were given weekly injections of intratympanic gentamicin until they developed clinical signs of vestibular hypofunction. Of these patients, recurrence of vertigo occurred at 6 months or more after treatment in 22%. Kaplan et al4 treated their series of patients with 3 injections of gentamicin over days. Nineteen percent of the patients in their series required re-treatment for vertigo control at a mean of months after the initial treatment. Harner et al5 gave 1 intratympanic injection of gentamicin and found that 41% of their patients required additional treatments. In all of these recent case series, it is unclear why there is recurrence of vertigo in some patients but not others, or what clinical signs could be used to predict the recurrence of vertigo.
The goal of our study was to identify the factors that correlate with successful control of vertigo in patients treated with 1 intratympanic injection of gentamicin. We used magnetic search coil testing to measure the angular vestibulo-ocular reflex (aVOR) elicited by rapid rotary head thrusts in the planes of the semicircular canals in subjects tested before and after treatment. These measurements were then compared between subjects who, during a 1-year follow-up period, had control of their vertigo (“single-treatment group”) and those who required additional injections for recurrent vertigo (“multiple-treatment group”).
The aVOR for any canal can be measured with magnetic search coil recordings of eye and head movements during rapid head thrusts in the plane of the canal being tested. Normal subjects are able to maintain visual fixation on an object during rapid head thrusts and hence have gain values (ratio of eye velocity to head velocity) close to 1.0.6 Asymmetries in the aVOR evoked by high-frequency, high-acceleration head movements occur after loss of vestibular function in one ear. The gain of the aVOR evoked by rapid head movements exciting the canal on the side of the lesion is lower than that evoked by rotations exciting the coplanar canal on the intact side.7 Patients with surgical unilateral vestibular destruction have markedly reduced gain values (0.2 to 0.3) when head thrusts are directed toward any of the ipsilateral canals.8 Carey et al9 demonstrated that a single intratympanic injection of gentamicin in patients with Meniere’s disease was often sufficient to reduce gains for ipsilateral canals to values in the range of 0.35 to 0.45. The patients with surgical unilateral vestibular destruction in the same study showed aVOR gains of only 0.2 to 0.3. These results suggest that a single intratympanic injection of gentamicin most likely does not cause complete hair cell destruction and that the remaining hair cell function may account for the higher gains in the gentamicin-treated patients. For the present study, the issue to be resolved was whether greater reduction of vestibular function as measured by quantitative aVOR gains and by the qualitative clinical head thrust test would be associated with a greater degree of vertigo control.
METHODS
Eighteen subjects with unilateral Meniere’s disease as defined by the 1995 criteria of the American Academy of Otolaryngology–Head and Neck Surgery were treated with intratympanic gentamicin for vertigo that was refractory to medical treatment (salt-restricted diet, diuretics, vestibular suppressant, and antiemetic medications).10 All subjects gave informed consent for the aVOR recordings through a protocol approved by the Institutional Review Board at the Johns Hopkins University School of Medicine, the institution at which treatment and testing were performed. Pretreatment and posttreatment gain data for several of the subjects have previously been included in an earlier publication.9
Protocol for Gentamicin Treatments
Gentamicin was administered as we have previously described.3 The mid-posterior aspect of the tympanic membrane was anesthetized and punctured, and the middle ear was filled with a buffered gentamicin solution (26.7 mg/mL gentamicin, 0.4 mL typically injected). The patients remained supine with the head angled slightly down and turned to the contralateral side for 30 minutes to continually bathe the round window with gentamicin solution. The solution was then aspirated from the external canal.
A single injection of intratympanic gentamicin was given, and the subjects were assessed approximately 3 weeks later to determine whether they bad the symptoms and signs expected from the ablation of unilateral vestibular function. The subjects were also instructed to call or return if they experienced any vertigo attacks. The treatment was considered complete after 1 injection if the subjects through 1 year of follow-up had no vertigo attacks or if they had vertigo that they judged to be far less severe (not requiring medication or interruption of activities) and far less frequent (<10%) than their pretreatment attacks (single-treatment group, n = 11). Subjects who had recurrent vertigo attacks that required medication or disrupted activities were defined as not having control of their Meniere’s disease. These subjects were treated with additional injections of gentamicin and were categorized in the multiple-treatment group (n = 7). The subjects in the multiple-treatment group had a second treatment at a mean of 6.0 ± 2.9 months (mean ± SD) after the first injection. Three subjects in the multiple-treatment group continued to have recurrent vertigo after the second injection and received additional treatments. (The numbers of total injections received were 5 injections for subject M2, 3 injections for subject M6, and 3 injections for subject M7.)
Audiograms were obtained before and after each treatment. Our previous experience with this protocol has shown that further hearing loss is noted in approximately 17% of patients when they are tested 6 months or more after intratympanic gentamicin treatment.”3,11 These long-term hearing changes following intratympanic gentamicin have not differed from the natural history of hearing loss in patients with Meniere’s disease who have not received intratympanic gentamicin.12
Caloric Tests
Caloric tests were performed on subjects with Meniere’s disease with a temperature switch irrigation technique for 30.5°C and 43.5°C.13 Horizontal eye movements were recorded with electro-oculography, and the maximum velocity of the slow-phase component of nystagmus was analyzed for unilateral weakness and directional preponderance as determined by conventional formulas.14 An ice water caloric test was performed when there was no response to warm or cold irrigation of the affected ear (100% asymmetry). If nystagmus was noted in response to the ice water test, the subject was turned from supine to prone to see whether the nystagmus reversed direction as expected on the basis of a convective mechanism.15,16
Head Thrust Tests and Gain Calculation
Pretreatment head thrust tests were performed on all subjects on the same day as treatment except for subjects S8 and M3 (in whom they were performed 3 and 196 days, respectively, before treatment). All posttreatment head thrust tests were performed 2 to 11 weeks after treatment except for that performed on subject M7, whose test was done 4 months after treatment. There was no significant difference in the mean time from treatment to testing between the two groups (single-treatment group, 40 ± 15 days; multiple-treatment group, 51 ± 36 days; p = .66).
The subject, seated with the head centered in the magnetic field, was instructed to gaze at a light-emitting diode located 124 cm directly forward at eye level. The examiner stood behind the subject and grasped the head over the temporoparietal area. The head was kept stationary in a comfortable, “upright” position before each head thrust. This position placed Reid’s stereotactic line (inferior orbital rim to superior external auditory canal) 7° ± 7° nose-up from the earth-horizontal plane. From this position the examiner rapidly rotated the head by 10° to 20° in 1 of 3 planes: the earth-horizontal plane, the plane containing the left anterior and right posterior canals, or the plane containing the right anterior and left posterior canals. For horizontal head thrusts, the head was turned to the left to excite the left horizontal canal or to the right to excite the right horizontal canal. For left anterior–right posterior head thrusts, the head was turned down and counterclockwise (with respect to the subject) in the left anterior–right posterior plane to excite the left anterior canal or up and clockwise to excite the right posterior canal. For right anterior–left posterior bead thrusts, the head was turned down and clockwise to excite the right anterior canal or up and counterclockwise to excite the left posterior canal. The subject knew which plane would be stimulated in each trial, but could not predict the direction of the rotation. Approximately 10 to 20 head thrusts were performed in each canal plane.
The instrumentation and technique for recording eye and head movements during these head thrusts with magnetic search coils have been described in detail elsewhere.17 Our protocol for measuring aVOR reflexes elicited by manual head thrusts and for calculating gains bas also previously been described.9 In brief, monocular or binocular eye movements were recorded in 3 dimensions at 500-Hz sampling rates with magnetic search coils embedded in scleral contact lenses (Skalar, Delft, the Netherlands). Head movements were recorded with magnetic search coils attached to a Plexiglas plate coated with hardened dental impression compound and molded to the subject’s dental occlusion. Eye and head positions in 3 dimensions were expressed as rotation vectors and used to derive the angular velocities of the eye and head. For each head thrust, we calculated the aVOR gain by dividing the eye velocity by the bead velocity, using the components of these velocities in the plane being stimulated. The highest gain value that occurred in a 30-ms period before peak head velocity was taken as the gain for a given trial. The reported results are mean gain values obtained from multiple trials performed in each canal plane.
Clinical Head Thrust Tests
The clinical head thrust test is a qualitative visual assessment of the aVOR in the horizontal canals with the same head thrust stimulus that is used for the quantitative aVOR tests. Gross failure of the aVOR is indicated by the appearance of a corrective eye movement following the head movement. Clinical head thrust tests were performed on all subjects before and after treatment. The examiner stood in front of the seated subject and grasped the subject’s head over the temporoparietal area. The subject was asked to look at the examiner’s nose. The head was then rapidly rotated by 10° to 20° in the earth-horizontal plane toward one side. The subject was noted to have a “head thrust sign” if the examiner observed a corrective eye movement following the head movement. Clinical head thrust tests were performed before quantitative testing, so that the decision as to whether the subject had a head thrust sign was independent of the results of the quantitative testing.
Data Analysis
For within-subject comparisons of gain values before and after treatment, t-tests assuming unequal variances were used. Gains, gain asymmetries, and caloric asymmetries between the single-and multiple-treatment groups were compared with the Mann-Whitney test. The Fisher exact test was used to determine the significance of a positive clinical head thrust test in differentiating subjects in the single- and multiple-treatment groups. Data are reported as mean ± SD.
RESULTS
Eighteen subjects with unilateral Meniere’s disease refractory to medical therapy were treated with a single intratympanic injection of gentamicin and followed for 1 year. Eleven subjects (61%; single-treatment group) had control of their vertigo with this treatment alone at the 1-year follow-up. Seven subjects (39%; multiple-treatment group) had recurrent vertigo that required a second treatment at an average of 6.0 ± 2.9 months after the first treatment. Nine of the 11 subjects in the single-treatment group had no vertigo attacks in this follow-up period. One subject in the single-treatment group (S1) had a single episode of mild vertigo 3 months after the treatment, but no vertigo thereafter. The other subject (S4) had 3 episodes of mild vertigo 4 months after treatment, but no vertigo in the subsequent follow-up period. The number of vertigo attacks during the 6 months before and the 6 months after treatment is summarized in the Table.14 The single-treatment group subjects had experienced vertigo, ipsilateral hearing loss, tinnitus, and/or aural fullness for 7.7 ± 7.7 years (range, 1 to 25 years) before treatment, and the subjects in the multiple-treatment group had had symptoms for 4.7 ± 4.9 years (range, 0.6 to 15 years, p = .32). The subjects in the single-treatment group were 51 ± 11 years old (range, 32 to 71 years), and the subjects in the multiple-treatment group were 50 ± 15 years old (range, 30 to 75 years). The mean pretreatment gain was not significantly different (p > .10) between the two groups for any of the canals.
SUMMARY OF MEAN AVOR GAINS AND CLINICAL DATA BEFORE AND AFTER INTRATYMPANIC GENTAMICIN TREATMENT
| Subject | IHC Gain | IAC Gain | IPC Gain | Clinical HTT Signa |
% Caloric Asymmetryb |
No. of Vertigo Attacksc |
Additional Injectionsd |
|---|---|---|---|---|---|---|---|
| S1 | 0.84/0.34 | 0.78/0.42 | 0.57/0.42 | −/+ | 9/0 | 12/1 | |
| S2 | 1.11/0.53 | 0.89/0.26 | 0.92/0.21 | −/+ | 25/88 | 78/0 | |
| S3 | 0.86/0.45 | 0.89/0.47 | 0.74/0.35 | −/+ | 4/3 | 10/0 | |
| S4 | 0.86/0.39 | 0.99/0.37 | 0.82/0.30 | −/+ | 23/92 | 70/3 | |
| S5 | ND/0.27 | ND/0.29 | ND/0.18 | −/+ | 52/100e | 3/0 | |
| S6 | 0.73/0.32 | 0.70/0.28 | 0.82/0.48 | −/+ | 15/42 | 20/0 | |
| S7 | 0.58/0.31 | 0.34/0.37 | 0.99/0.32 | +/+ | 100/100 | 52/0 | |
| S8 | 1.31/0.38 | 0.80/0.30 | 0.88/0.29 | −/+ | 32/31 | 39/0 | |
| S9 | 0.94/0.20 | 0.96/0.39 | 0.91/0.21 | −/+ | 33/82 | 52/0 | |
| S10 | 0.74/0.74 | 0.63/0.70 | 0.62/0.68 | +/+ | 17/22 | 15/0 | |
| S11 | 1.04/0.61 | 0.85/0.56 | 0.77/0.67 | −/+ | 8/11 | 12/0 | |
| M1 | 0.90/0.75 | 0.48/0.36 | 0.65/0.41 | −/+ | 24/100e | 6/0 | 7 |
| M2 | 0.88/0.57 | 0.69/0.57 | 0.65/0.40 | −/− | 17/18 | 45/4 | 3, 5, 7, 11 |
| M3 | 0.84/0.90 | 0.93/0.83 | 0.89/0.88 | −/− | 1/1 | 15/2 | 12 |
| M4 | 1.00/0.30 | 1.05/0.44 | 0.74/0.31 | −/+ | 6/ND | 11/4 | 5 |
| M5 | 0.76/0.50 | 0.83/0.53 | 0.72/0.31 | +/+ | 16/44 | 7/3 | 4 |
| M6 | 0.59/0.77 | 0.63/0.61 | 0.77/0.87 | −/+ | 37/ND | 21/3 | 3, 5 |
| M7 | 0.82/1.26 | 0.87/0.66 | 0.57/0.50 | −/+ | 4/18 | 12/22 | 7, 9 |
Data before slash are pretreatment; those after slash are posttreatment.
aVOR — angular vestibulo-ocular reflex; S — single-treatment group; M — multiple-treatment group: IHC — ipsilateral horizontal canal; IAC — ipsilateral anterior canal; IPC — ipsilateral posterior canal; HTT — head thrust test; ND — not done.
Plus sign indicates appearance of relaxation saccade on HTT, suggesting to clinical examiner deficit in horizontal aVOR; minus sign indicates no refixation saccade, indicating clinically normal horizontal aVOR.
Unilateral vestibular weakness calculated by formula of Jongkees et al.14
Data are 6 months pretreatment (before slash) and either 6 months posttreatment or until second treatment (after slash).
Data are number of months after initial treatment until subjects received additional treatments.
Subject had no response to ice-water irrigation.
Measurements of aVOR Before and After First Intratympanic Gentamicin Treatment
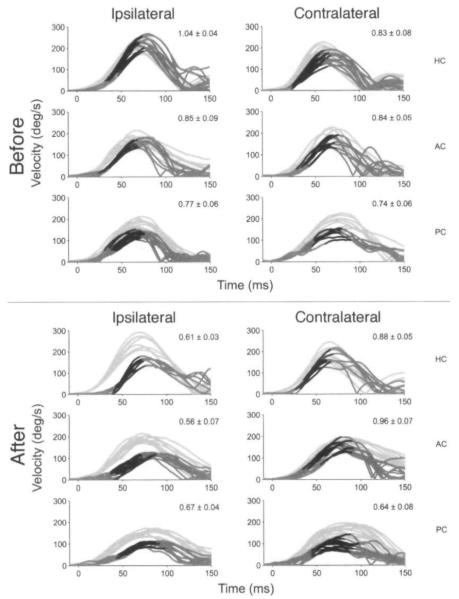
Figure 1 shows the head and eye velocity traces of a subject in the single-treatment group (S11) during rapid rotary head thrusts before and 70 days after treatment. The subject was a 49-year-old man with a 15-year history of attacks of left fluctuating hearing loss, aural fullness, and vertigo. Just before treatment of the left ear with a single intratympanic gentamicin injection, the attacks occurred approximately 2 to 3 times per month despite medical therapy. After treatment, the subject developed a positive head thrust sign, and he had no vertigo attacks during the 1-year follow-up period.
Fig 1.
Angular vestibulo-ocular reflex (aVOR) head and eye velocity traces for subject in single-treatment group (S11) before and after treatment with intratympanic gentamicin. Light gray lines represent head velocity, dark gray lines represent eye velocity, and black segments denote 30 ins before peak bead velocity when gain was calculated. Values in upper right corner of each panel represent aVOR gain (mean ± SD). HC — horizontal canal, AC — anterior canal, PC — posterior canal.
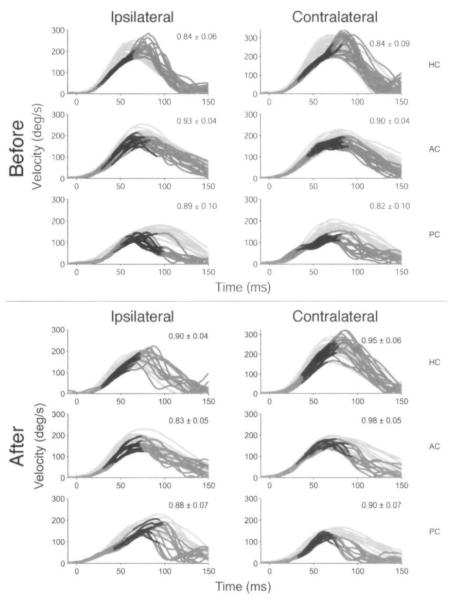
Figure 2 shows the traces for a subject in the multiple-treatment group (M3, a 30-year-old man) before and 28 days after the first treatment with intratympanic gentamicin. Before the first injection of the right ear with intratympanic gentamicin, his attacks were refractory to medical therapy and were characterized by right aural fullness, fluctuating hearing loss, and vertigo that would last from 12 to 36 hours. After the first gentamicin treatment, he did not develop a head thrust sign. Nine months after treatment, he again began to have attacks of right aural fullness, fluctuating hearing loss, and vertigo lasting several hours. He described these attacks as being identical to his previous ones. He had 3 attacks approximately 2 weeks apart before being treated with a second injection of gentamicin.
Fig 2.
aVOR head and eye velocity traces for subject in multiple-treatment group (M3) before and after treatment with intratympanic gentamicin. Light gray lines represent head velocity, dark gray lines represent eye velocity, and black segments denote 30 ms before peak head velocity when gain was calculated. Values in upper right corner of each panel represent aVOR gain (mean ± SD). HC — horizontal canal, AC — anterior canal, PC — posterior canal.
The Table displays the mean aVOR gains collected from the 18 subjects before and after treatment with the first intratympanic injection of gentamicin. After 1 treatment, gain for the ipsilateral horizontal canal (IHC) decreased significantly (p < .05) for 9 of 10 single-treatment subjects who had both pretreatment and posttreatment tests available. (The exception was S10.) In contrast, only 3 of the 8 multiple-treatment subjects (M2, M4, M5) had significant decreases in IHC gain. Significant (p < .05) decreases in gain for the ipsilateral anterior canal (IAC) were seen after treatment for all subjects from both groups except for S7 and S10, and significant decreases in gain for the ipsilateral posterior canal (IPC) were also seen in all subjects from both groups except for M3, M6, and S10.
Significantly Greater Change in IHC Gain From Pretreatment to Posttreatment in Single-Treatment Group Than in Multiple-Treatment Group
Figure 3 shows the mean change in gain from pretreatment to posttreatment for the IHC. IAC, and IPC in the single-treatment and multiple-treatment groups. The mean change in IHC gain for the single-treatment group was significantly greater than that in the multiple-treatment group (single-treatment, −0.47 ± 0.25, versus multiple-treatment. −0.11 ± 0.37; p = .03 by Mann-Whitney). The high SD for mean IHC gain change in the multiple-treatment group is primarily attributable to subject M7, who had an increase in gain after treatment of +0.44 (change from 0.82 to 1.26). Re-analysis of the data hypothetically assuming that subject M7 had no change in IHC gain from pretreatment to posttreatment (ie, posttreatment gain remains at 0.82) does not affect the significance (p = .036).
Fig 3.
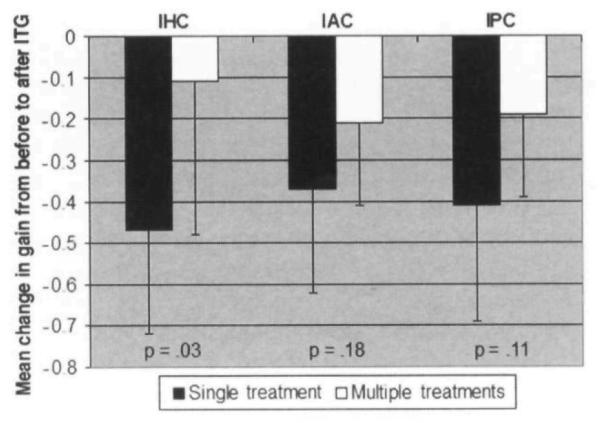
Mean change in gain, before versus after intratympanic gentamicin (ITG) treatment, for ipsilateral horizontal canal (IHC), ipsilateral anterior canal (IAC), and ipsilateral posterior canal (IPC). Error bars denote 1 SD; p values denote significance of difference in mean change in gain, before versus after ITG treatment, between multiple- and single-treatment groups (Mann-Whitney test). Data from subject S5 were not used in analysis, because pretreatment data were not available.
The gain changes for the IAC and IPC were greater in the single-treatment group than in the multiple-treatment group, but these results did not reach statistical significance (IAC single versus multiple, −0.37 ± 0.25 versus −0.21 ± 0.20. p = . 18; IPC single versus multiple, −0.41 ± 0.28 versus −0.19 ± 0.20, p = .11).
Similar results were found when gain asymmetry data were compared between the two groups. The mean change in IHC gain asymmetry, but not IAC or IPC gain asymmetry, was significantly greater in the single- than in the multiple-treatment group (data not shown).
Correlation of Vertigo Recurrence With Failure to Develop Head Thrust Sign After Treatment
The clinical head thrust test is an indicator of diminished gain in the IHC. A head thrust sign signifies the examiner’s observation of a refixating eye movement or “catch-up saccade” after the head is rotationally thrust in the plane of the horizontal canal toward a hypofunctional side.18 Nine of the 11 subjects in the single-treatment group and 6 of the 7 subjects in the multiple-treatment group did not have a head thrust sign before treatment. A head thrust sign developed after treatment in all 9 subjects in the single-treatment group but in only 4 of the 6 subjects in the multiple-treatment group (see Table). The association between failure to develop a head thrust sign and the need for future re-treatment did not reach statistical significance (Fisher exact test, p = .14).
Mean Change in Caloric Asymmetry Is Not Significantly Different Between Multiple- and Single-Treatment Groups
Data on pretreatment and post-treatment caloric asymmetry for the 18 subjects are presented in the Table. The mean pretreatment caloric asymmetry was not significantly different between the two groups (single-treatment. 28.9 ± 27.3, versus multiple-treatment, 15.0 ± 12.7; p = .22). The mean change in caloric asymmetry from pretreatment to posttreatment was not significantly different between the subjects in the two groups who had both pretreatment and posttreatment caloric data measured (single-treatment. 25.3 ± 30.0, versus multiple-treatment, 23.8 ± 31.3; p = .80). The data from subject S7 were not used, because the subject had a pretreatment caloric asymmetry of 100%.
DISCUSSION
In this study, we investigated whether aVOR gain changes correlated with vertigo control in subjects with unilateral Meniere’s disease treated with a fixed-dose regimen of 1 intratympanic injection of gentamicin. Our results indicate that control of vertigo in unilateral Meniere’s disease is closely associated with a reduction in semicircular canal function as measured by horizontal canal aVOR gain. We first discuss the implications of these findings for our understanding of gentamicin’s effect on the labyrinth. We then discuss the clinical implications of our findings for the treatment of vertigo in Meniere’s disease with intratympanic gentamicin.
Effects of Gentamicin on Labyrinth
Work in our laboratory using a chinchilla model has recently shed light on the potential nature of the lesion caused by conservative use of intratympanic gentamicin.19 A single dose of the drug at the concentration used in the present study was given into the left otic bullae of chinchillas, and the right ears served as controls. Vestibular nerve afferent physiology and semicircular canal crista histology were examined after 5 to 25 days (early) or 90 to 115 days (late). Afferent recordings demonstrated that spontaneous afferent firing persisted at both time periods in the treated ear and that all classes of discharge regularity were preserved. Nevertheless, the majority of treated afferents did not measurably respond to tilt or rotation (82% in the early group and 76% in the late group), and those that did respond had vestibular sensitivities that were fractions of those found on the control sides (p < .001). In contrast, sensitivities to galvanic currents, which may act directly on the vestibular afferents, were unaffected by the treatment. Intratympanic gentamicin treatment reduced the histologic density of type I hair cells by 99%; type II hair cell density was not significantly reduced. These findings suggest that conservative intratympanic gentamicin treatment causes partial damage and loss of vestibular hair cells (particularly type I hair cells), does not damage the afferent spike initiation zones, and spares enough hair cell synaptic activity to drive the spontaneous activity of vestibular afferents.
The results of the present study support the hypothesis that successful intratympanic gentamicin treatment reduces vestibular sensory function in the treated ear. In this study, we quantitatively and qualitatively assessed vestibular function with aVOR gain and gain asymmetry, caloric asymmetry, and the clinical head thrust test. Subjects who had control of their vertigo after a single intratympanic injection of gentamicin had a greater degree of vestibular hypofunction as measured by the quantitative head thrust test, and had a significantly greater change in mean pretreatment to posttreatment IHC gain and gain asymmetry than did subjects who needed subsequent (multiple) injections.
Our data from the qualitative clinical head thrust test, although not reaching clinical significance (p = .14), are consistent with these results. A head thrust sign indicates ipsilateral vestibular hypofunction in the horizontal canal.18 Both subjects (M2 and M3) who started without a head thrust sign before treatment and who failed to develop this sign after treatment had recurrent vertigo. Although small numbers may have limited the ability of a qualitative test with a binary outcome (refixation saccade versus no refixation saccade) to reach statistical significance, these results are consistent with both the quantitative IHC gain data and the physiologic mechanism of the head thrust test.
Another hypothesis for how gentamicin may affect the labyrinth is the “dark cell hypothesis.” which is based on evidence that aminoglycosides may be directly toxic to dark cells, the principal endolymph-producing cells in the labyrinth. Damage to these cells would presumably prevent production of excess endolymph, limiting hydrops and subsequent vertigo, Pender20 demonstrated dark cell damage in cats treated with daily intratympanic injections of gentamicin. Dark cell damage has also been shown in guinea pigs and chicks treated with subcutaneous streptomycin.21-23 However, in a more recent study involving middle ear instillation of gentamicin or streptomycin in chinchillas, scanning electron microscopy revealed only cochlear and vestibular neuroepithelial cell damage and negligible damage to the dark cells.24
Our results primarily support the hypothesis that gentamicin exerts its beneficial effect on vertigo through reduced semicircular canal function. However, the dark cell hypothesis cannot be discounted in all cases. For example, subject S10 had no substantial gain changes for the 3 canals, but remained vertigo-free at the 1-year follow-up. This finding may also simply be due to the recognized high spontaneous remission rate of Meniere’s disease.
Significant increases in IHC canal gains were noted after gentamicin treatment in subjects M3, M6, and M7. An increase in gain suggests the possibility that gentamicin may have had an initial irritative effect on semicircular canal function. Indeed, Parnes and Riddell25 have previously described 3 patients who developed an irritative (ipsilateral) nystagmus that persisted for more than a month after intratympanic gentamicin treatment. Robertson et al26 have also described possible otolithic irritation after treatment with intratympanic gentamicin.
After 1 injection of gentamicin, the single-treatment group had a significantly greater pretreatment-to-posttreatment change in IHC gain, but not in IAC or IPC gain, than did the multiple-treatment group (Fig 3). The significance of the IHC results is not solely from the effect of subject M7, whose increase in IHC gain from pretreatment to posttreatment lessened the mean decrease in IHC gain seen in the multiple-treatment group relative to the single-treatment group. Reanalysis of the data assuming no change in IHC gain from pretreatment to posttreatment for M7 minimally affects the significance of the results (p = .036). The lack of significance for the IAC and IPC may reflect that our technique has less power to detect gain changes in the vertical canals than in the horizontal canals. In a previous study,9 we found that it was more difficult to thrust the head in the planes of the vertical canals than it is to do so horizontally, and that peak head thrust velocities were smaller for the vertical canals than for the horizontal canals. Thus, the stimulus for the vertical canals may simply not be as dynamic as that for the horizontal canals, and small changes in vertical canal gain may not be as easily detected.
Caloric asymmetry was inadequate to predict vertigo control in this study. This finding is not surprising, given that there was no consistent correlation between caloric and aVOR gain asymmetry data. Before treatment with gentamicin, 8 subjects had abnormal (>20%) pretreatment caloric asymmetries, but only 2 subjects had abnormal IHC gain asymmetries. Based on our data, one possible explanation for the lack of correlation between caloric asymmetry and aVOR gain asymmetry is that Meniere’s disease may have a differential impact on vestibular nerve afferents in the affected ear such that responses to low-velocity, low-acceleration stimuli (caloric endolymph flow) are reduced more than responses to high-velocity, high-acceleration stimuli (head thrusts). Therefore, although the caloric test may be a very sensitive indicator of subtle loss of function, caloric responses may become too impaired before treatment to show substantial changes after treatment. Another possible explanation for the lack of correlation between caloric and aVOR testing is that responses to rapid head movements tested with the aVOR may adapt better to vestibular hypofunction than caloric responses. Rapid angular head movements are encountered in everyday situations, and therefore the necessary signals are provided for central adaptation. However, for the caloric stimulus, there are no direct physiologic correlates in terms of head movements. Without adequate signals for adaptation, caloric responses become rapidly impaired with any reduction in vestibular function.
Clinical Implications
Remaining within the therapeutic window for intratympanic gentamicin to avoid excess hearing or vestibular loss is made difficult by the widely varying response to treatment. It is unclear why some subjects have a marked decrease in gain after treatment whereas other subjects have little to no response after the same treatment. Silverstein et al27 have previously suggested that round window obstruction may affect the diffusion of medication into the perilymph. The round window niche has been found to be obstructed by a second “false” membrane in up to 20% of human temporal bones.28,29 Moreover, the thickness of the round window membrane may increase significantly after prior middle ear inflammation. Hellstrom et al30 found that the rat round window membrane increased five-fold in thickness after purulent otitis media. Despite positioning the patient with the contralateral ear down for 30 minutes after the injection, it is unclear how much gentamicin is lost through the eustachian tube or whether an air bubble may block the gentamicin solution’s meniscus from reaching the round window membrane. Finally, certain subjects may have increased susceptibility to the vestibulotoxic effects of gentamicin on a genetic basis, analogous to the increased cochleotoxic susceptibility conferred by identified mitochondrial mutations.31
Previously, there has not been an effective way to determine when to conclude intratympanic gentamicin therapy. In our study, caloric data were not adequate to predict which patients would have control of vertigo. The results of this study suggest that quantitative aVOR measurements may provide an endpoint for treatment. Although magnetic search coil testing is not in widespread use, rapid advances in video-oculography and other techniques may soon provide quantitative measurements of the aVOR without the need for search coils. Although our study did not have sufficient power to determine the precise posttreatment aVOR levels needed for vertigo control, larger studies in the future may be able to delineate whether a certain level of a VOR function correlates with vertigo control.
Footnotes
Presented in part at the Mid-Winter Meeting of the Association for Research in Otolaryngology, St Petersburg, Florida, January 27-31, 2002.
REFERENCES
- 1.Schuknecht HF. Ablation therapy for the relief of Meniere’s disease. Laryngoscope. 1956;66:859–70. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Blakley BW. Update on intratympanic gentamicin for Meniere’s disease. Laryngoscope. 2000;110:236–40. doi: 10.1097/00005537-200002010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Minor LB. Intratympanic gentamicin for control of vertigo in Meniere’s disease: vestibular signs that specify completion of therapy. Am J Otol. 1999;20:209–19. [PubMed] [Google Scholar]
- 4.Kaplan DM, Nedzelski JM, Chen JM, Shipp DB. Intratympanic gentamicin for the treatment of unilateral Meniere’s disease. Laryngoscope. 2000;110:1298–305. doi: 10.1097/00005537-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Harner SG, Driscoll CL, Facer GW, Beatty CW, McDonald TJ. Long-term follow-up of transtympanic gentamicin for Meniere’s syndrome. Otol Neurotol. 2001;22:210–4. doi: 10.1097/00129492-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol. 1996;76:4009–20. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- 7.Aw ST, Halmagyi GM, Haslwanter T, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. II. Responses in subjects with unilateral vestibular loss and selective semicircular canal occlusion. J Neurophysiol. 1996;76:4021–30. doi: 10.1152/jn.1996.76.6.4021. [DOI] [PubMed] [Google Scholar]
- 8.Cremer PD, Halmagyi GM, Aw ST, et al. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain. 1998;121:699–716. doi: 10.1093/brain/121.4.699. [DOI] [PubMed] [Google Scholar]
- 9.Carey JP, Minor LB, Peng GC, Delia Santina CC, Cremer PD, Haslwanter T. Changes in the three-dimensional angular vestibulo-ocular reflex following intratympanic gentamicin for Meniere’s disease. J Assoc Res Otolaryngol. 2002;3:430–43. doi: 10.1007/s101620010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology–Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 11.Wu IC, Minor LB. Long-term hearing outcome in patients receiving intratympanic gentamicin for Meniere’s disease. Laryngoscope. 2003;113:815–20. doi: 10.1097/00005537-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Santos PM, Hall RA, Snyder JM, Hughes LF, Dobie RA. Diuretic and diet effect on Meniere’s disease evaluated by the 1985 Committee on Hearing and Equilibrium guidelines. Otolaryngol Head Neck Surg. 1993;109:680–9. doi: 10.1177/019459989310900408. [DOI] [PubMed] [Google Scholar]
- 13.Proctor L, Dix R, Hughes D, Rentea R. Stimulation of the vestibular receptor by means of step temperature changes during continuous aural irrigation. Acta Otolaryngol (Stockh) 1975;79:425–35. doi: 10.3109/00016487509124707. [DOI] [PubMed] [Google Scholar]
- 14.Jongkees LBW, Maas JPM, Philipszoon AJ. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 1962;24:65–93. [PubMed] [Google Scholar]
- 15.Paige GD. Caloric responses after horizontal canal inactivation. Acta Otolaryngol (Stockh) 1985;100:321–7. doi: 10.3109/00016488509126555. [DOI] [PubMed] [Google Scholar]
- 16.Minor LB, Goldberg JM. Influence of static head position on the horizontal nystagmus evoked by caloric, rotational and optokinetic stimulation in the squirrel monkey. Exp Brain Res. 1990;82:1–13. doi: 10.1007/BF00230832. [DOI] [PubMed] [Google Scholar]
- 17.Straumann D, Zee DS, Solomon D, Lasker AG, Roberts DC. Transient torsion during and after saccades. Vision Res. 1995;35:3321–34. doi: 10.1016/0042-6989(95)00091-r. [DOI] [PubMed] [Google Scholar]
- 18.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 19.Hirvonen TR, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–55. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 20.Pender DJ. Gentamicin tympanoclysis: effects on the vestibular secretory cells. Am J Otolaryngol. 1985;6:358–67. doi: 10.1016/s0196-0709(85)80013-2. [DOI] [PubMed] [Google Scholar]
- 21.Park JC, Cohen GM. Vestibular ototoxicity in the chick: effects of streptomycin on equilibrium and on ampullary dark cells. Am J Otolaryngol. 1982;3:117–27. doi: 10.1016/s0196-0709(82)80042-2. [DOI] [PubMed] [Google Scholar]
- 22.Park JC, Cohen GM. Further observations of vestibular ototoxicity in the chick: effects of streptomycin on the ampullary sensory epithelium. Am J Otolaryngol. 1984;5:387–93. doi: 10.1016/s0196-0709(84)80053-8. [DOI] [PubMed] [Google Scholar]
- 23.Ge X, Shea JJ. Intramuscular streptomycin effect on dark cells of utricle in guinea pigs. Am J Otol. 1993;14:74–8. [PubMed] [Google Scholar]
- 24.Chen JM, Kakigi A, Hirakawa H, Mount RJ, Harrison RV. Middle ear instillation of gentamicin and streptomycin in chinchillas: morphologic appraisal of selective ototoxicity. J Otolaryngol. 1999;28:121–8. [PubMed] [Google Scholar]
- 25.Parnes LS, Riddell D. Irritative spontaneous nystagmus following intratympanic gentamicin for Meniere’s disease. Laryngoscope. 1993;103:745–9. doi: 10.1288/00005537-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Robertson DD, Garber LZ, Ireland DJ. Ocular torsion monitoring in chemical labyrinthectomy. J Otolaryngol. 1996;25:171–7. [PubMed] [Google Scholar]
- 27.Silverstein H, Rowan PT, Olds MJ, Rosenberg SI. Inner ear perfusion and the role of round window patency. Am J Otol. 1997;18:586–9. [PubMed] [Google Scholar]
- 28.Alzamil KS, Linthicum FH., Jr Extraneous round window membranes and plugs: possible effect on intratympanic therapy. Ann Otol Rhinol Laryngol. 2000;109:30–2. doi: 10.1177/000348940010900105. [DOI] [PubMed] [Google Scholar]
- 29.Schachern PA, Paparella MM, Duvall AJ, III, Choo YB. The human round window membrane. An electron microscopic study. Arch Otolaryngol. 1984;110:15–21. doi: 10.1001/archotol.1984.00800270019005. [DOI] [PubMed] [Google Scholar]
- 30.Hellstrom S, Johansson U, Anniko M. Structure of the round window membrane. Acta Otolaryngol Suppl (Stockh) 1989;(suppl457):33–42. doi: 10.3109/00016488809138882. [DOI] [PubMed] [Google Scholar]
- 31.Prezant TR, Agapian JV, Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–94. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]