Abstract
Purpose
To evaluate the prognostic factors in locally advanced cervical cancer limited to the pelvis and develop nomograms for 2-year progression-free survival (PFS), 5-year overall survival (OS), and pelvic recurrence.
Patients and Methods
We retrospectively reviewed 2,042 patients with locally advanced cervical carcinoma enrolled onto Gynecologic Oncology Group clinical trials of concurrent cisplatin-based chemotherapy and radiotherapy. Nomograms for 2-year PFS, five-year OS, and pelvic recurrence were created as visualizations of Cox proportional hazards regression models. The models were validated by bootstrap-corrected, relatively unbiased estimates of discrimination and calibration.
Results
Multivariable analysis identified prognostic factors including histology, race/ethnicity, performance status, tumor size, International Federation of Gynecology and Obstetrics stage, tumor grade, pelvic node status, and treatment with concurrent cisplatin-based chemotherapy. PFS, OS, and pelvic recurrence nomograms had bootstrap-corrected concordance indices of 0.62, 0.64, and 0.73, respectively, and were well calibrated.
Conclusion
Prognostic factors were used to develop nomograms for 2-year PFS, 5-year OS, and pelvic recurrence for locally advanced cervical cancer clinically limited to the pelvis treated with concurrent cisplatin-based chemotherapy and radiotherapy. These nomograms can be used to better estimate individual and collective outcomes.
INTRODUCTION
Numerous prognostic factors are associated with survival for patients with cervical cancer. Previous Gynecologic Oncology Group (GOG) studies have addressed risk factors for recurrence and survival. In 1990, Delgado et al1 prospectively evaluated patients who had undergone radical hysterectomy and node dissection for stage IB cervical cancer. Among the 645 who had undergone pelvic and para-aortic (PA) lymphadenectomy and radical hysterectomy, five risk factors were significantly associated with pelvic lymph node metastasis: depth of invasion, parametrial involvement, capillary-lymphatic space invasion, tumor grade, and gross-versus-occult primary tumor. In 1991, Stehman et al2 evaluated prognostic factors in locally advanced cervical cancer treated with radiation therapy in three clinical trials. In these three trials, 626 patients underwent pretreatment operative assessment of the PA lymph nodes. Patients received standardized external radiation to the pelvis or to the pelvis and PA lymph nodes followed by one or two brachytherapy applications. Pooled data and multivariable analysis identified patient age, performance status, PA lymph node status, tumor size, and pelvic node status to be significantly associated with progression-free survival (PFS). When modeling for survival, all these factors as well as clinical stage and bilateral parametrial tumor extension were significant.
In 1999, the National Cancer Institute (NCI) released a clinical announcement stating strong consideration should be given to adding chemotherapy to radiation therapy in the treatment of invasive cervical cancer.3 This was based on five clinical trials, three of which were conducted solely by the GOG,4–6 one of which the GOG participated in with the Southwest Oncology Group,7 and one of which the Radiation Therapy Oncology Group conducted solely.8 Because different chemotherapy regimens were used in the studies, the NCI announcement stated that although the best chemotherapy regimen for cervical cancer had not been determined, “significant results were seen using cisplatin alone or cisplatin in combination with FU [fluorouracil] and other agents.”3 Collectively, these trials demonstrated that the use of cisplatin-based chemotherapy concurrently with radiation therapy decreased the risk of recurrence or death by 30% to 50%. As a result of this clinical impact, and at the strong recommendation of the NCI clinical announcement, cisplatin-based chemoradiotherapy became a National Comprehensive Cancer Network guideline standard for the management of locally advanced cervical cancer.9
The current GOG ancillary data study was undertaken to evaluate prognostic factors for locally advanced cervical cancer treated in the era of cisplatin-based chemoradiotherapy. Second, we sought to develop nomograms for 2-year PFS, 5-year overall survival (OS), and pelvic recurrence for these patients.
PATIENTS AND METHODS
We retrospectively analyzed data from GOG trials 85, 120, 123, 165, 191, and 219.4–6,10–12 All patients provided written informed consent before study entry in compliance with all local institutional review boards and federal guidelines. These trials have been reported previously and included patients with stage IB2 disease (tumors limited to cervix measuring > 4 cm) in GOG trials 123, 191, and 219; stage IIA disease in GOG trials 191 and 219; and stage IIB to IVA disease in GOG trials 85,120, 165, 191, and 219. In GOG trials 85 and 120, patients underwent surgical staging to exclude PA nodal metastases, and pelvic nodal dissection was optional, whereas in GOG trials 123, 165, 191, and 219, surgical staging was optional and performed in 7.5%, 18%, 17.3%, and 11.1% of patients, respectively. All patients were treated with a combination of external radiation and brachytherapy per protocol guidelines. The duration of external radiotherapy for GOG trials 85, 120, and 123 required external radiation treatment to be administered over 10 weeks. GOG trials 165, 191, and 219 required external radiation treatment to be administered over 8 weeks. All patient tumors underwent central pathologic review for confirmation of histology and tumor grade. In univariable analysis, categorical variables were compared using the Pearson χ2 test13 and continuous variables using the Wilcoxon Mann-Whitney test.14 Survival was estimated using the Kaplan-Meier method.15 The Cox proportional hazards model was used to evaluate independent prognostic factors and estimate their covariate-adjusted effects on PFS and OS.16 Some missing values of tumor size (< 1%) and method of negative PA node evaluation (approximately 14%) warranted interpolation by multiple imputation while considering all the model variables at once. Under the assumption of data missing at random, we created a complete imputed data set using predictive mean matching, and the Cox models were fitted to the imputed data set. The nonlinearity of the effect of continuous variables was assessed using restricted cubic splines. All statistical tests were two tailed, with the significance level set at α = 0.05. Statistical analyses were performed using the R programming language and environment (http://www.r-project.org/).
Prognostic variables including histology, race, performance status, tumor size, International Federation of Gynecology and Obstetrics stage, tumor grade, pelvic nodal status, and cisplatin-based chemotherapy treatment were used to create nomograms to predict 2-year PFS, 5-year OS, and pelvic recurrence. A 2-year PFS was chosen because 82% of the patients who experienced disease progression did so within 2 years. Patients with subgroups of adenocarcinoma and adenosquamous carcinoma were combined and compared with patients with squamous cell carcinoma. This combination of adenocarcinoma and adenosquamous carcinoma was based on the fact that these two entities had similar patterns of failure, PFS, and OS.17 Starting from full Cox models for PFS and OS containing all prognostic factors, we removed factors not meeting a certain threshold (in this case, using Akaike's information criterion as stopping rule: χ2 for variables tested > 2× df) by fast backward elimination18 and kept the resulting model as the basis for the nomograms. Validation of each nomogram included two procedures. First, model discrimination was measured quantitatively with the concordance index, which is a measure of classification accuracy similar to the area under the receiver operating characteristic curve but for censored data.19 Possible values of the concordance index ranged from 0.5 (random classification) to 1.0 (perfect classification). Bootstrapping provided a relatively unbiased estimate of the concordance index.20 (Bootstrapping is a method of repetitive resampling for calculating bias in statistical estimators, which allows us to correct for high estimates of the classification accuracy of a model resulting from potential overfitting. When we say that the corrected estimate is relatively unbiased, we mean as compared with the true classification accuracy of the model.) Second, calibration was assessed through grouping patients by their nomogram-predicted probabilities, then comparing the group mean with the observed Kaplan-Meier estimate of OS; bootstrapping was again used for bias correction.
We also evaluated the demographic and clinicopathologic factors associated with pelvic recurrence in a logistic model comparing patients with pelvic recurrence with those who did not experience recurrence, and the resulting model became the basis for the nomogram. Validation of the nomogram proceeded as previously described, with model discrimination measured by the concordance index and calibration assessed by comparing nomogram-predicted probabilities with observed probabilities.
RESULTS
A total of 2,042 patients treated in the GOG studies were analyzed. Demographic and clinical characteristics of all patients, patients treated with cisplatin-based chemoradiotherapy, and patients treated with radiotherapy alone or a noncisplatin regimen are listed in Table 1. The majority of patients had squamous cell carcinoma (88.7%), had a performance status of 0 (72.1%), and were white (60.8%). Comparing cisplatin- and noncisplatin-treated patients, there was no statistical difference in age, race, performance status, histology, or hydronephrosis. However, patients who received cisplatin-based chemoradiotherapy tended to have more advanced disease stage, smaller tumor size, and more poorly differentiated tumors. Treatments are listed in Appendix Table A1 (online only). Sixty-five percent of patients received cisplatin-based chemoradiotherapy, with the once-per-week cisplatin regimen most commonly used (36.4% of all patients).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | All Patients (N = 2,042) |
RT Plus Cisplatin (n = 1,325) |
RT Plus Other (n = 717) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years (N = 2,042) | .206* | ||||||
| Median | 46.6 | 46.7 | 46.5 | ||||
| Range | 39.0-55.9 | 39.2-56.0 | 38.9-55.7 | ||||
| Race/ethnicity (N = 2,042) | .251† | ||||||
| White | 1,242 | 60.8 | 805 | 60.8 | 437 | 60.9 | |
| Black | 459 | 22.5 | 284 | 21.4 | 175 | 24.4 | |
| Hispanic | 210 | 10.3 | 148 | 11.2 | 62 | 8.6 | |
| Asian | 75 | 3.7 | 52 | 3.9 | 23 | 3.2 | |
| other | 56 | 2.7 | 36 | 2.7 | 20 | 2.8 | |
| Performance status (N = 2,042) | .122† | ||||||
| Normal, asymptomatic | 1,473 | 72.1 | 962 | 72.6 | 511 | 71.3 | |
| Symptomatic, ambulatory | 503 | 24.6 | 328 | 24.8 | 175 | 24.4 | |
| Symptomatic, in bed | 66 | 3.2 | 35 | 2.6 | 31 | 4.3 | |
| Negative PA nodes found (n = 1,760) | < .001† | ||||||
| Pathology | 971 | 55.2 | 608 | 49.1 | 363 | 69.7 | |
| Radiography | 789 | 44.8 | 631 | 50.9 | 158 | 30.3 | |
| Histology (N = 2,042) | .212† | ||||||
| Squamous | 1,811 | 88.7 | 1,164 | 87.8 | 647 | 90.2 | |
| Adenosquamous | 117 | 5.7 | 84 | 6.3 | 33 | 4.6 | |
| Adenocarcinoma | 114 | 5.6 | 77 | 5.8 | 37 | 5.2 | |
| Tumor size, cm (n = 2,028) | .004* | ||||||
| Median | 6.0 | 6.0 | 6.0 | ||||
| Range | 5.0-7.0 | 5.0-7.0 | 5.0-7.5 | ||||
| Tumor size, cm (n = 2,028) | .004† | ||||||
| < 5.0 | 375 | 18.5 | 274 | 20.8 | 101 | 14.3 | |
| 5.0-6.0 | 406 | 20.0 | 261 | 19.8 | 145 | 20.5 | |
| 6.0-7.0 | 494 | 24.4 | 316 | 23.9 | 178 | 25.1 | |
| ≥ 7.0 | 753 | 37.1 | 469 | 35.5 | 284 | 40.1 | |
| FIGO stage (N = 2,042) | < .001† | ||||||
| IB | 410 | 20.1 | 235 | 17.7 | 175 | 24.4 | |
| IIA | 23 | 1.1 | 23 | 1.7 | 0 | 0.0 | |
| IIB | 960 | 47.0 | 632 | 47.7 | 328 | 45.7 | |
| IIIA | 23 | 1.1 | 12 | 0.9 | 11 | 1.5 | |
| IIIB | 566 | 27.7 | 384 | 29.0 | 182 | 25.4 | |
| IVA | 60 | 2.9 | 39 | 2.9 | 21 | 2.9 | |
| Grade (N = 2,042) | < .001† | ||||||
| Good | 126 | 6.2 | 82 | 6.2 | 44 | 6.1 | |
| Moderate | 1,244 | 60.9 | 766 | 57.8 | 478 | 66.7 | |
| Poor | 628 | 30.8 | 451 | 34.0 | 177 | 24.7 | |
| Not graded | 44 | 2.2 | 26 | 2.0 | 18 | 2.5 | |
| Hydronephrosis (n = 1,671) | .06† | ||||||
| None | 1,490 | 89.2 | 836 | 87.6 | 654 | 91.2 | |
| Unilateral | 143 | 8.6 | 92 | 9.6 | 51 | 7.1 | |
| Bilateral | 38 | 2.3 | 26 | 2.7 | 12 | 1.7 | |
| Parametrial involvement (N = 2,042) | .017† | ||||||
| None | 477 | 23.4 | 288 | 21.7 | 189 | 26.4 | |
| Unilateral | 907 | 44.4 | 586 | 44.2 | 321 | 44.8 | |
| Bilateral | 658 | 32.2 | 451 | 34.0 | 207 | 28.9 | |
| Pelvic nodes (N = 2,042) | < .001† | ||||||
| Positive | 286 | 14.0 | 209 | 15.8 | 77 | 10.7 | |
| Negative | 1,285 | 62.9 | 848 | 64.0 | 437 | 60.9 | |
| Unknown | 471 | 23.1 | 268 | 20.2 | 203 | 28.3 | |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; PA, para-aortic; RT, radiotherapy.
Wilcoxon test.
Pearson's test.
Multivariable Cox modeling was used to evaluate independent prognostic factors and estimate their effects on PFS and OS for all patients and for patients treated with and without concurrent cisplatin. The nonlinearity of the effect of continuous variables (age and tumor size) was tested using restricted cubic splines, which were subsequently deemed unnecessary for lack of significant nonlinearity. Among all patients, race was significant for both PFS and OS. This was largely because of poorer PFS and OS outcomes among African Americans and statistical improvement in OS for Asians. Among all patients, poorer performance status (2 to 3 and 1 v 0) was associated with poorer OS, and compared with well-differentiated tumors, poorly differentiated tumors were associated with worse PFS. On the basis of the Cox model, each 10% increase in tumor size was associated with a 3% increase in risk of disease progression and a 3% increase in risk of death. Appendix Table A2 (online only) lists the actual 5-year survival rates and 95% CIs for patients treated in these studies by stage and use of concurrent cisplatin during radiotherapy. To account for the importance of other tumor and clinical prognostic variables, including tumor size, histology, grade, pathologically confirmed pelvic node status, patient performance status, and race/ethnicity, nomograms were developed. Nomograms for 2-year PFS, 5-year OS, and pelvic recurrence were created as visualizations of the Cox proportional hazards regression models. The 2-year PFS nomogram (Fig 1) had a bootstrap-corrected concordance index of 0.62 and was well calibrated (Fig 2). The 5-year OS nomogram (Fig 3) had a bootstrap-corrected concordance index of 0.64 and was well calibrated (Fig 4).
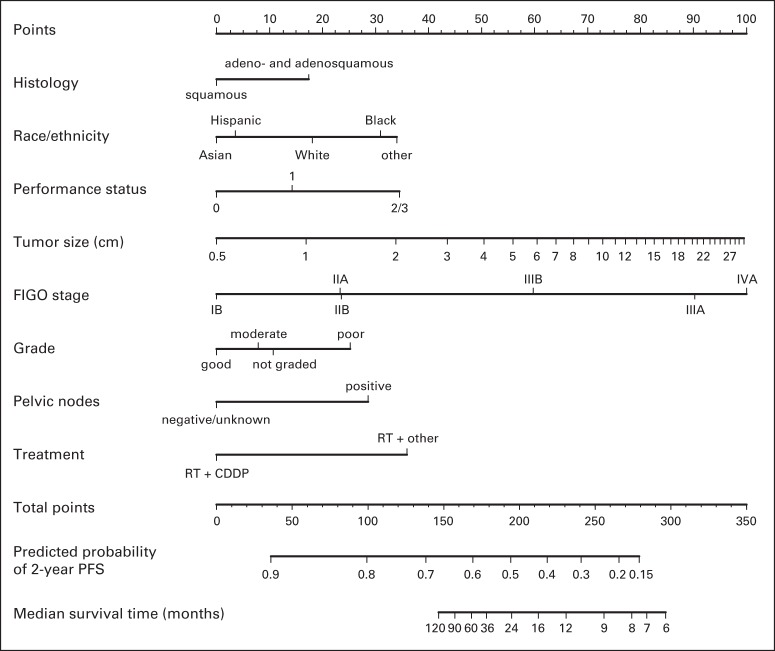
Fig 1.
Nomogram for predicting 2-year progression-free survival (PFS). To use, find patient's histology on histology axis, then draw straight line upward to points axis to determine how many points toward progression patient receives for histology. Do this again for other axes, each time drawing straight line upward toward points axis. Sum points received for each predictor, and find sum on total points axis. Draw straight line down to survival-probability axis to find patient's probability of no progression of cervical cancer at 2 years. For cohort of women exactly like patient, we would expect between (predicted probability [PP] − 0.10) × 100% and (PP + 0.10) × 100% of them to remain free of disease after 2 years. For example, patient receives following number of points for each specific characteristic: histology, adenocarcinoma/adenosquamous carcinoma (17 points); race, white (18 points); performance status, 2 to 3 (35 points); tumor size, 6 cm (60 points); International Federation of Gynecology and Obstetrics (FIGO) stage, IIA (23 points); grade, poor (25 points); pelvic nodes, negative (0 points); and treatment: radiotherapy (RT) plus cisplatin (CDDP; 0 points). Points total is 17 + 18 + 35 + 60 + 23 + 25 + 0 + 0 = 178, and straight line drawn down from total points axis at 178 crosses PP axis at approximately 0.55. Therefore, patient's PP of 2-year PFS is within 0.55 ± 0.10 = 0.55 (95% confidence limits, 0.45 to 0.65).
Fig 2.
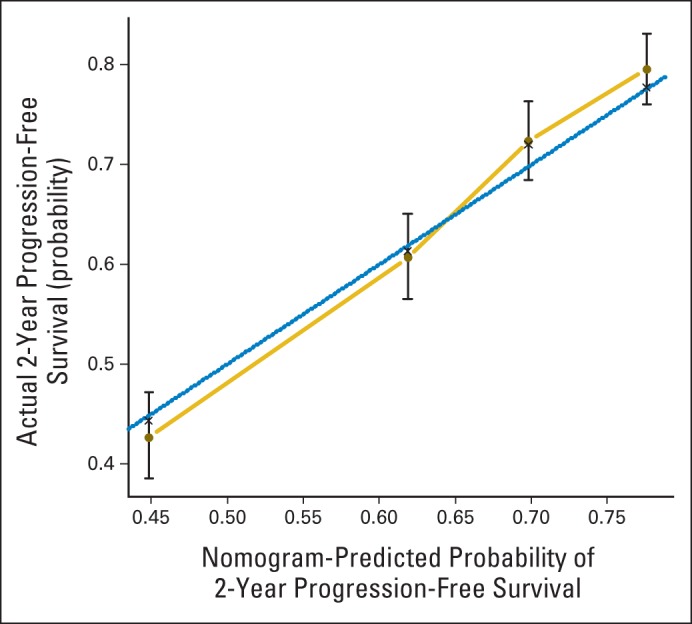
Calibration curve for progression-free survival nomogram model. Dashed line represents ideal nomogram, and solid line represents observed nomogram. Vertical bars indicate 95% CIs, and crosses indicate bias-corrected estimates.
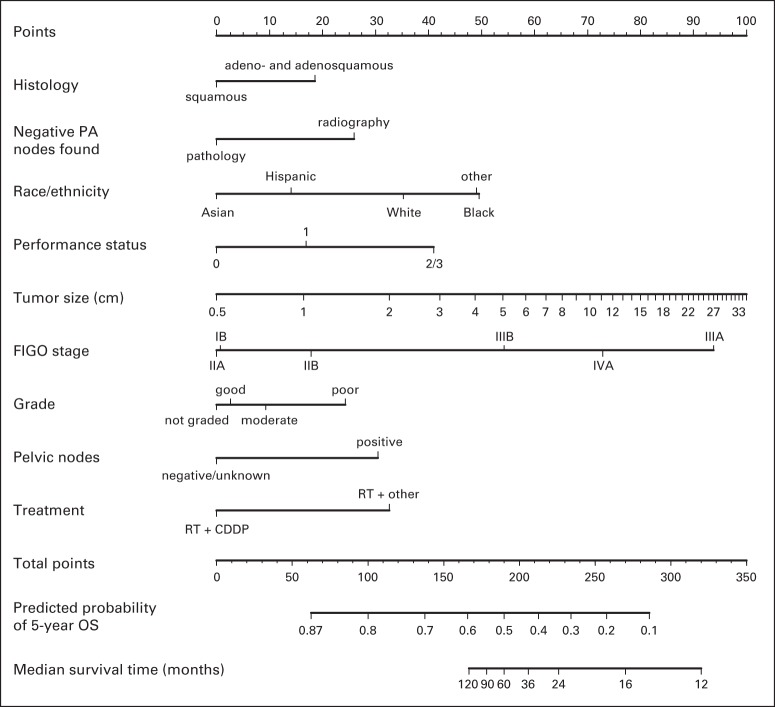
Fig 3.
Nomogram for predicting 5-year overall survival (OS). To use, find patient's histology on histology axis, then draw straight line upward to points axis to determine how many points toward death patient receives for histology. Do this again for other axes, each time drawing straight line upward toward points axis. Sum points received for each predictor, and find sum on total points axis. Draw straight line down to survival-probability axis to find patient's probability of surviving cervical cancer for 5 years. For cohort of women exactly like patient, we would expect between (predicted probability [PP] − 0.10) × 100% and (PP + 0.10) × 100% of them to survive for 5 years. CDDP, cisplatin; FIGO, International Federation of Gynecology and Obstetrics; PA, para-aortic; RT, radiotherapy.
Fig 4.
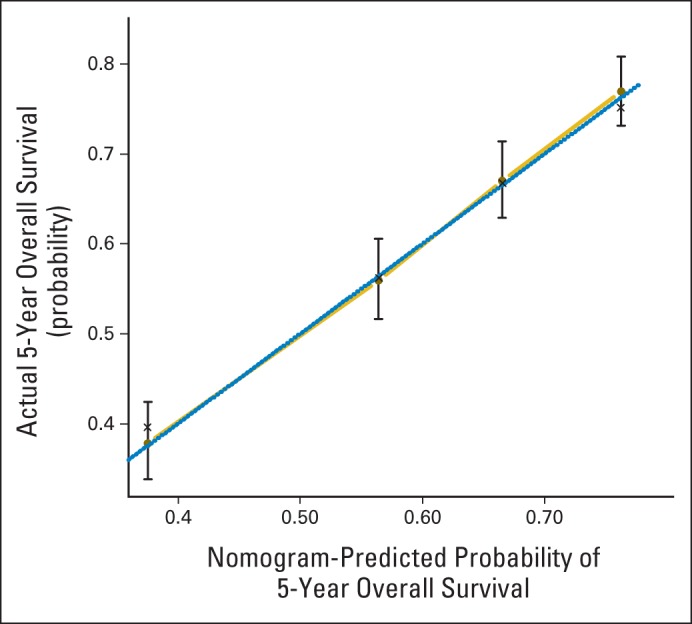
Calibration curve for overall survival nomogram model. Dashed line represents ideal nomogram, and solid line represents observed nomogram. Vertical bars indicate 95% CIs, and crosses indicate bias-corrected estimates.
How to use the nomograms is described in the figure captions. For example, in the nomogram for predicting 2-year PFS, the user should find the patient's histology on the histology axis, then draw a straight line upward to the points axis to determine how many points toward progression the patient receives for histology. This should be done again for the other axes, with the user each time drawing a straight line upward toward the points axis. The points received for each predictor are then summed and the sum found on the total points axis. The user should then draw a straight line down to the survival-probability axis to find the patient's probability of no progression of cervical cancer at 2 years. In Figure 2, the confidence limits at the several predicted probabilities of progression are wide, covering up to approximately ± 0.10, which allows us to roughly describe the accuracy of a nomogram predicted probability (PP): Given a cohort of women exactly like the patient, we would expect between (PP − 0.10) × 100% and (PP + 0.10) × 100% of them to remain free of disease after 2 years.
We also evaluated the demographic and clinicopathologic factors associated with pelvic recurrence in a logistic model comparing patients with pelvic recurrence (n = 331) with patients who did not experience recurrence (n = 1,210). Starting from the logistic model containing all prognostic factors, we removed some statistically insignificant factors (P > .05) and kept the resulting model as the basis for the nomogram. Validation of the nomogram proceeded as previously described, with model discrimination measured by the concordance index and calibration assessed by comparing nomogram-predicted probabilities with observed probabilities. The pelvic recurrence nomogram (Fig 5) had a bootstrap-corrected concordance index of 0.73 and was well calibrated.
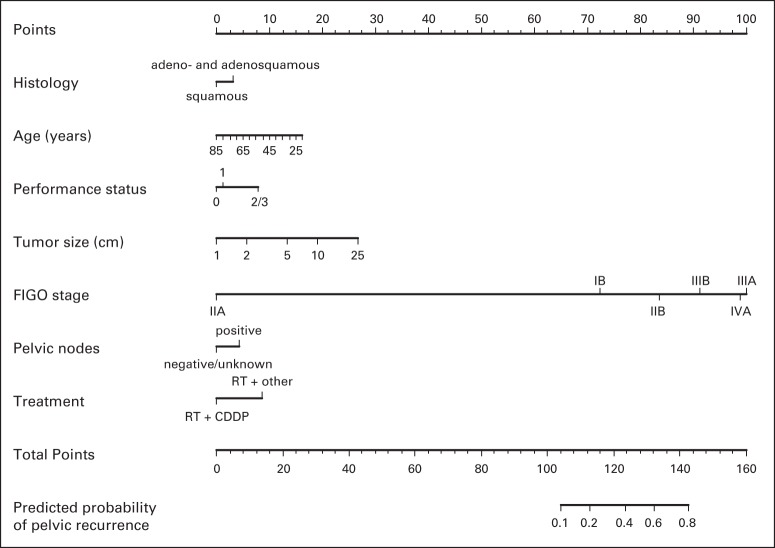
Fig 5.
Nomogram for predicting pelvic recurrence. To use, find patient's cell type on histology axis, then draw straight line upward to points axis to determine how many points toward pelvic recurrence patient receives for cell type. Do this again for other axes, each time drawing straight line upward toward points axis. Sum points received for each predictor, and find sum on total points axis. Draw straight line down to recurrence-probability axis to find patient's probability of pelvic recurrence. CDDP, cisplatin; FIGO, International Federation of Gynecology and Obstetrics; RT, radiotherapy.
DISCUSSION
In this study, we evaluated the prognostic factors for locally advanced cervical cancer treated with radiotherapy and concurrent cisplatin-based chemotherapy and their impact on PFS, OS, and pelvic recurrence. Similar to the report by Stehman et al,2 we found that clinical stage, tumor size, pelvic node status, and performance status were significantly associated with PFS and OS. Other prognostic factors identified in our study included tumor histology, race/ethnicity, tumor grade, and radiation treatment with concurrent cisplatin-based chemotherapy. Patients with PA lymph node involvement either surgically or radiologically documented were excluded from the trials in our study. Therefore, we were unable to assess the impact of PA lymph node status. In contrast to the study by Stehman et al, we did not find age to be a significant risk factor for recurrence or survival.
Accurate estimation of survival for patients receiving a cancer diagnosis based on patient and tumor characteristics permits critical stratification in clinical trials and offers the possibility of tailoring the aggressiveness of treatment to the individual situation. Previous reports of survival in cervical cancer have been based on disease stage. However, for both our PFS and OS models, the adequacy index of stage alone accounted for only approximately 60% of the prognostic information, with the other factors we considered accounting for the rest.21 Our study demonstrates that there are numerous other prognostic factors, including histology, race/ethnicity, performance status, tumor size, tumor grade, pelvic node status, and treatment, that significantly affect PFS and survival. Therefore, we sought to develop nomograms that would include these prognostic factors for PFS, survival, and pelvic recurrence. The nomograms were validated for 2-year PFS, 5-year OS, and pelvic recurrence. Therefore, if patients met the eligibility criteria for entry into this study based on performance status and organ function, the nomogram could be used to estimate their 2-year PFS, 5-year OS, and pelvic recurrence rates.
In addition, a benchmark of estimated survival is important in cancer quality assurance. These nomograms were developed from patients with locally advanced cervical cancer participating in multicenter clinical trials. Because the nomograms were developed from multicenter clinical trials, the results should be more applicable to the general population than if they had been developed from a single institution. However, the clinical trials excluded patients with a performance status of 4; this exclusion biases the estimates of the nomograms, because patients participating clinical trials tend to have a better performance status than the general population of patients with locally advanced cervical cancer. In addition, patients participating in clinical trials might be more motivated to aggressively treat their cancer. For example, 60% of patients with stage ≥ IIB disease underwent operative PA nodal dissection before initiation of therapy; this may or may not have occurred outside of the clinical trial. Lastly, patients participating in a clinical trial are potentially more compliant with the treatments that are prescribed.22 This may also affect the results when comparing outcomes with the general cervical cancer population.
Strengths of this analysis include large sample size, data collected prospectively and quality controlled, few missing data elements, and diversity of the population (39% nonwhite). Because all patients entered into these trials were analyzed, weaknesses of the study include possible variations in compliance with prescribed treatment, including chemotherapy, total radiation treatment times, and completion of brachytherapy. In addition, the trials included in our analyses were limited to chemotherapy being administered concurrently with radiotherapy. The ongoing OUTBACK trial is investigating the role of postchemoradiotherapy chemotherapy. The results of the trial are pending, but if they were to be positive, and postchemoradiotherapy chemotherapy should become the new treatment paradigm, then revised nomograms reflecting this approach would be needed.
In summary, our multivariable analysis identified numerous prognostic factors that affect PFS and OS in locally advanced cervical cancer primarily treated with radiation therapy. These prognostic factors allowed development of nomograms predicting 2-year PFS, 5-year OS, and pelvic recurrence rates.
Glossary Terms
- cisplatin:
an inorganic platinum agent (cis-diamminedichloroplatinum) with antineoplastic activity. Cisplatin forms highly reactive, charged, platinum complexes, which bind to nucleophilic groups such as GC-rich sites in DNA, inducing intrastrand and interstrand DNA cross-links as well as DNA-protein cross-links. These cross-links result in apoptosis and cell growth inhibition. Carboplatin and oxaliplatin are other members of this class.
- overall survival:
the duration between random assignment and death.
- progression-free survival:
time from random assignment until death or first documented relapse, categorized as either locoregional (primary site or regional nodes) failure or distant metastasis or death.
Appendix
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Medical Center, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group, University of California at Los Angeles, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical Center, University of California Medical Center at Irvine, Tufts–New England Medical Center, Rush-Presbyterian-St Luke's Medical Center, SUNY Downstate Medical Center, Eastern Virginia Medical School, Johns Hopkins Cancer Center, State University of New York at Stony Brook, Eastern Pennsylvania Gynecology/Oncology Center, Southwest Oncology Group, Cooper Hospital/University Medical Center and Columbus Cancer Council, University of Oklahoma, Wayne State University, Ellis Fischel Cancer Center, Tampa Bay/H. Lee Moffitt Cancer Center, New York Hospital/Cornell Medical Center, University of Kentucky, Case Western Reserve University, Stanford University Medical Center, Tacoma General Hospital, University of Washington/Puget Sound Oncology Consortium, Cleveland Clinic Foundation, Fox Chase Cancer Center, Women's Cancer Center, University of Massachusetts Medical Center, University of Chicago, University of Minnesota Medical School, Emory University Clinic, Community Clinical Oncology Program, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, National Cancer Institute of Cancer, MD Anderson Community Clinical Oncology Program, Thomas Jefferson University Hospital, University of Virginia, University of Texas-Galveston, and Mayo Clinic.
Table A1.
Patient Treatments (N = 2,042)
| Treatment | No. | % |
|---|---|---|
| RT | 199 | 9.7 |
| RT plus FU | 155 | 7.6 |
| RT plus hydroxyurea | 363 | 17.8 |
| RT plus cisplatin | 743 | 36.4 |
| RT, cisplatin, and FU | 176 | 8.6 |
| RT, cisplatin, FU, and hydroxyurea | 171 | 8.4 |
| RT, cisplatin, and rHuEPO | 54 | 2.6 |
| RT, cisplatin, and tirapazamine | 181 | 8.9 |
Abbreviations: FU, fluorouracil; rHuEPO, recombinant human erythropoietin; RT, radiotherapy.
Table A2.
Survival Rates
| Stage | All |
RT Plus Cisplatin |
RT Plus Other |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | 2-Year PFS |
5-Year OS |
No. of Patients | 2-Year PFS |
5-Year OS |
No. of Patients | 2-Year PFS |
5-Year OS |
|||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||||
| IB | 410 | 73 | 69 to 78 | 72 | 68 to 77 | 235 | 79 | 74 to 84 | 78 | 72 to 83 | 175 | 65 | 59 to 73 | 66 | 59 to 73 |
| IIA | 23 | 77 | 61 to 97 | 72 | 54 to 97 | 23 | 77 | 61 to 97 | 72 | 54 to 97 | — | — | — | ||
| IIB | 960 | 69 | 66 to 72 | 63 | 59 to 66 | 632 | 73 | 70 to 77 | 68 | 64 to 72 | 328 | 61 | 56 to 66 | 53 | 48 to 59 |
| IIIA | 23 | 28 | 14 to 55 | 27 | 14 to 54 | 12 | 46 | 24 to 87 | 46 | 24 to 87 | 11 | 9 | 1 to 59 | 9 | 1 to 59 |
| IIIB | 566 | 53 | 49 to 57 | 48 | 44 to 52 | 384 | 57 | 52 to 62 | 52 | 47 to 57 | 182 | 44 | 37 to 52 | 38 | 32 to 46 |
| IVA | 60 | 27 | 18 to 41 | 35 | 24 to 50 | 39 | 27 | 16 to 45 | 34 | 21 to 54 | 21 | 29 | 15 to 56 | 37 | 21 to 65 |
Abbreviations: OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
Footnotes
Supported by National Cancer Institute Grants No. CA 27469 to the Gynecologic Oncology Group Administrative Office and No. CA 37517 to the Gynecologic Oncology Group Statistical and Data Center and by NRG Oncology Grant No. 1 U10 CA180822.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Peter G. Rose, Frederick B. Stehman, Rachelle Lanciano
Provision of study materials or patients: Peter G. Rose, Charles W. Whitney, Frederick B. Stehman, Rachelle Lanciano, Gillian M. Thomas, Paul A. DiSilvestro
Collection and assembly of data: Peter G. Rose, James Java, Charles W. Whitney, Frederick B. Stehman, Rachelle Lanciano, Paul A. DiSilvestro
Data analysis and interpretation: Peter G. Rose, James Java, Frederick B. Stehman, Rachelle Lanciano, Gillian M. Thomas
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nomograms Predicting Progression-Free Survival, Overall Survival, and Pelvic Recurrence in Locally Advanced Cervical Cancer Developed From an Analysis of Identifiable Prognostic Factors in Patients From NRG Oncology/Gynecologic Oncology Group Randomized Trials of Chemoradiotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Peter G. Rose
No relationship to disclose
James Java
No relationship to disclose
Charles W. Whitney
No relationship to disclose
Frederick B. Stehman
No relationship to disclose
Rachelle Lanciano
Stock or Other Ownership: Philadelphia Cyberknife
Honoraria: Alliance Oncology (lecture)
Speakers' Bureau: Philadelphia Cyberknife
Research Funding: Philadelphia Cyberspace
Travel, Accommodations, Expenses: Philadelphia Cyberknife
Gillian M. Thomas
No relationship to disclose
Paul A. DiSilvestro
Honoraria: Hologic Corporation
Speakers' Bureau: Hologic Corporation
REFERENCES
- 1.Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 2.Stehman FB, Bundy BN, DiSaia PJ, et al. Carcinoma of the cervix treated with radiation therapy: I. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–2785. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. NCI issues clinical announcement on cervical cancer: Chemotherapy plus radiation improves survival. http://www.nih.gov/news/pr/feb99/nci-22.htm.
- 4.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 8.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN guideline for locally advanced cervical cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 10.Lanciano R, Calkins A, Bundy BN, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiSilvestro PA, Ali S, Craighead PS, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: A Gynecologic Oncology Group study. J Clin Oncol. 2014;32:458–464. doi: 10.1200/JCO.2013.51.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine Series 5. 1900;50:157–175. [Google Scholar]
- 14.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Chen JL, Cheng JC, Kuo SH, et al. Outcome analysis of cervical adenosquamous carcinoma compared with adenocarcinoma. Acta Obstet Gynecol Scand. 2012;91:1158–1166. doi: 10.1111/j.1600-0412.2012.01420.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawless JF, Singhal K. Efficient screening of nonnormal regression models. Biometrics. 1978;34:318–327. [Google Scholar]
- 19.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Lee KL. A comparison of the discrimination of discriminant analysis and logistic regression under multivariate normality. In: Sen PK, editor. Biostatistics: Statistics in Biomedical, Public Health and Environmental Sciences. Amsterdam, the Netherlands: North-Holland; 1985. pp. 333–343. [Google Scholar]
- 22.Abu-Rustum NR, Lee S, Correa A, et al. Compliance with and acute hematologic toxic effects of chemoradiation in indigent women with cervical cancer. Gynecol Oncol. 2001;81:88–91. doi: 10.1006/gyno.2000.6109. [DOI] [PubMed] [Google Scholar]