Abstract
Purpose
The NCIC CTG PR3/MRC PR07 randomized phase III trial compared androgen-deprivation therapy (ADT) alone versus ADT with radiotherapy (RT) for patients with locally advanced prostate cancer. This article reports the health-related quality-of-life (HRQOL) outcomes of this trial.
Patients and Methods
A total of 1,205 patients were randomly allocated to either ADT alone or ADT with RT. HRQOL was assessed at baseline and every 6 months thereafter using the European Organisation for Research and Treatment of Cancer Core Questionnaire and a prostate cancer–specific checklist or the Functional Assessment of Cancer Therapy–Prostate questionnaire. Mean changes from baseline scores for five function domains and nine symptom domains were analyzed as those most relevant to ADT and RT. The proportions of patients with improved, stable, or worsened HRQOL scores according to instrument-specific minimal important differences were calculated.
Results
Baseline questionnaires were completed by 1,028 patients (88%). At 6 months, RT had a statistically significant impact on mean score for bowel symptoms (P = .02), diarrhea (P < .001), urinary function (P = .003), and erectile dysfunction (P = .008); by 3 years, however, there were no significant between-group differences in any domain. Generalized linear mixed modeling revealed no significant between-arm differences in any of the function scales but showed significant deterioration in both arms over time for Functional Assessment of Cancer Therapy–Prostate total score, treatment outcome index, and physical and functional well-being.
Conclusion
The addition of RT to ADT for patients with locally advanced prostate cancer significantly improved overall survival and had only modest and transient negative impact on relevant domains of HRQOL.
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer in men and a leading cause of cancer mortality in North America and the European Union.1,2 The role of radiotherapy (RT) in the setting of locally advanced disease was addressed by the NCIC CTG (NCIC Clinical Trials Group) PR3/MRC (Medical Research Council) PR07 clinical trial, which sought to determine the relative benefits and risks of adding RT to lifelong androgen-deprivation therapy (ADT).3 When this trial was launched in 1995, optimal management in this setting was controversial, in that many physicians treated with ADT alone, believing that the disease was incurable. RT was known to have associated toxicities, and the incremental benefit of adding RT to ADT was uncertain. The PR3/PR07 trial was thus designed with a primary end point of overall survival and secondary end points of disease-specific survival, toxicity, and quality of life; it successfully accrued 1,205 patients. The interim analysis, at median follow-up of 6.0 years, showed that the addition of RT to ADT significantly affected overall survival, reducing the risk of death resulting from any cause (hazard ratio, 0.77; 95% CI, 0.61 to 0.98; P = .033).3 In this report, we provide a detailed description of the patient-reported outcomes (PROs) of this trial and their relationship to treatment-related toxicity outcomes to further inform clinical decision making regarding the risks and benefits of RT as provided from the patients' perspective.
Health-related quality-of-life (HRQOL) outcomes, when properly executed, are valid evaluations of both harmful and beneficial effects of treatment from the patient's perspective.4,5 The objectives were to: describe the differential impact of RT in this setting by comparing PROs by trial arm, to describe the QOL of men treated with long-term ADT from diagnosis, and to assess the relative responsiveness of two PRO instruments (ie, Functional Assessment of Cancer Therapy–Prostate questionnaire [FACT-P]6 compared with European Organisation for Research and Treatment of Cancer [EORTC] Core Questionnaire [QLQ C30]7 plus prostate cancer–specific checklist). The third objective was undertaken as a substudy and will be reported separately.
PATIENTS AND METHODS
PR3/MRCPR07 was an open randomized controlled trial comparing ADT alone with ADT plus RT.3 Patients were eligible if they had T3 or T4 (N0 M0) disease or high-risk disease (prostate-specific antigen [PSA] > 40 μg/L or PSA ≥ 20 μg/L plus Gleason score ≥ 8). The primary trial outcome was overall survival. Secondary outcome measures included disease-specific survival, toxicity (reported using NCIC CTG Expanded Common Toxicity Criteria), and HRQOL. All patients provided written informed consent, and appropriate national and local regulatory and ethics review approvals were obtained.3
Protocol Treatments
Patients in both treatment arms received lifelong ADT (either bilateral orchiectomy or luteinizing hormone–releasing hormone [LHRH] agonist therapy with ≥ 2 weeks of antiandrogens [latter continued at investigator discretion]). RT was delivered using four-field box techniques in two phases (prostate, seminal vesicles, and pelvic nodes to 45 Gy in phase one and prostate gland with periprostatic tumor extension, if present, to 20 to 24 Gy, both in conventional fractionation). Patients with pathologically negative lymph nodes and those in whom the treating physician felt that pelvic RT was inappropriate were treated to only the prostate volume (65 to 69 Gy).
HRQOL Assessment
Two validated HRQOL instruments were used. In a substudy of instrument responsiveness, North American participating centers were randomly assigned to either the EORTC QLQ-C30, including a trial-specific PR19 checklist,7 or the FACT-P.6 MRC participating centers did not participate in the substudy and used only the FACT-P instrument. HRQOL was completed directly by the patient at baseline before RT, at the end of RT (if allocated), and at 6, 12, 18, and 24 months and yearly thereafter until the patient was off study protocol.
The EORTC QLQ-C30 and PR19 checklist consist of questions addressing five function domains (physical, role, emotional, cognitive, and social), global QOL, specific symptom scales, and individual symptom items. At the time of protocol design, there was no validated prostate cancer–specific EORTC QOL module; the early PR19 version used items addressing specific symptoms relevant to prostate cancer and its treatment. For function scales, high scores represent high functioning, whereas for symptom scales, high scores indicate greater symptom burden. A minimal important difference of 10.0 points was used as a conservative estimate of clinically meaningful change from baseline.8
The FACT-P comprises the FACT-G instrument and additional prostate cancer–specific items.6 It generates FACT-P total, trial outcome index (TOI), physical and functional well-being, and urinary domain scores. High function or TOI scores reflect better quality of life, whereas high symptom scores reflect higher symptom burden. A minimal important difference of 7.0 points was used for the function and symptom scales.9 FACT-P scales were primarily used to evaluate the secondary HRQOL outcomes of the trial. EORTC QLQ-C30 items are reported for long-term HRQOL and for domains where no comparable FACT-P item exists.
Statistical Methods
Compliance at baseline was the proportion of randomly assigned patients who completed an HRQOL assessment before random assignment; subsequently, compliance at each assessment was the proportion of patients whose questionnaires were received of those expected (not expected if patient had no baseline HRQOL assessment, experienced progressed, or died). Scoring of instruments was performed per the respective statistical manuals. All analyses were by intention to treat. The sample-size calculation for the HRQOL end points assumed the EORTC QOL scores to have a normal distribution, with standard deviation of 25 points; to have 80% power for detecting a 10-point difference in global QOL (using two-sided α of 5%), 202 patients were required for the EORTC QLQ-C30 measure. The corresponding sample size for the FACT-P scale was smaller (n = 150).
The first objective (differential impact of RT on HRQOL) was evaluated by calculating between-arm differences in mean change scores at specific time points for domains relevant to RT (null hypothesis tested using Wilcoxon rank sum test). A change was defined as post-treatment value minus baseline value for each scale. The distributions of both baseline and change scores had symmetric bell shapes; therefore, means and standard deviations were used to present the results. Between-arm differences at 3 years were tabulated as a summary of each domain at a time when late radiation toxicity was expected to have occurred. Between-arm differences for relevant domains at other time points were plotted graphically. In addition, we calculated the proportion of patients improving or deteriorating10 by the clinically minimal important differences from baseline. The second objective regarding the overall impact of ADT over time was evaluated using generalized linear mixed modeling to statistically evaluate the overall effects of time (by domain or scale item) and potential interaction between treatment allocation and time (by domain or scale item).
In an exploratory analysis, we sought to compare the apparent negative impact of treatments as determined by HRQOL scores versus as determined by treatment toxicity rates. For specific symptoms relevant to the impact of RT (EORTC PR19 diarrhea, rectal pain, and rectal bleeding and FACT-P urine function and erectile function), and for symptoms relevant to ADT (PR19 hot flushes and FACT-P impotence/libido, lethargy, and insomnia), patients' responses to single-item questions were tabulated. Because both instruments used the same format, consisting of a question stem (eg, “During the past week, have you had diarrhea?”) followed by a 4-point Likert scale of graded responses (“not at all” to “very much”), we calculated the proportion of patients responding with nonzero responses for each response category as follows: HRQOL grade 4 (“very much”), grade 3 (“quite a bit”), and so on. Similarly, we calculated the proportion of men having any toxicity (nonzero toxicity grades) on each Common Toxicity Criteria category that best corresponded to each patient-reported symptom.
No adjustments for missing data were made beyond the use of generalized linear mixed modeling. FACT-P data were not analyzed beyond 48 months because of dropping compliance rates. No adjustments to the interpretation of P values were made for multiple comparisons. All statistical tests were two sided, with significance level of α = 0.05. All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
RESULTS
Patients
A detailed description of the 1,205 patient participants is provided elsewhere.3 Briefly, 78% of patients were age ≥ 65 years, and 78% had an Eastern Cooperative Oncology Group performance of 0. The majority of patients had T3 (83%) or T4 (5%) disease, and 50% had PSA > 40 μg/L. As expected with random assignment, patient attributes were balanced between arms at baseline, including HRQOL scores.
Of the 603 patients randomly assigned to ADT plus RT, 17 patients did not receive RT, most (72%) received a two-phase RT plan that included pelvic lymph nodes, and 28% received treatment to the prostate alone. Nine patients randomly assigned to ADT alone received RT (≥ 50 Gy). LHRH agonists were administered in 1,105 patients (92%), and orchiectomy was performed in 93 patients (8%).
Instrument Completion and Compliance Rates
Figure 1 shows the flow of patients according to assigned HRQOL instrument. Baseline participation was > 88%.
Fig 1.
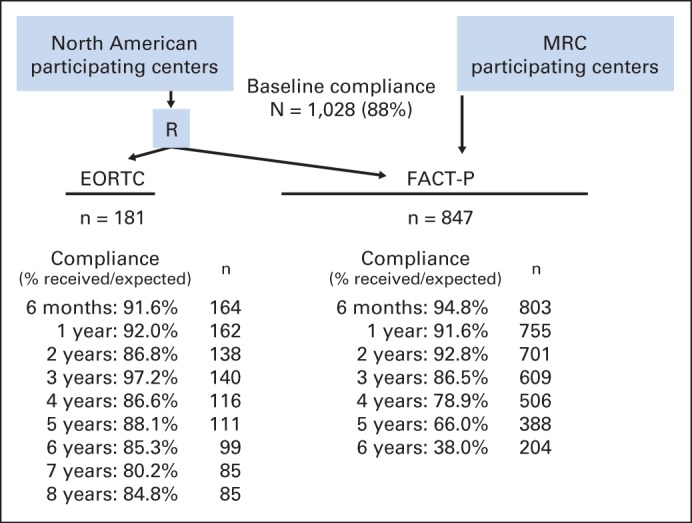
Health-related quality-of-life (HRQOL) data collection schema and compliance rates for each HRQOL instrument. EORTC, European Organisation for Research and Treatment of Cancer; FACT-P, Functional Assessment of Cancer Therapy–Prostate questionnaire; MRC, Medical Research Council; R, radiotherapy.
Mean HRQOL Scores at Baseline
Table 1 lists mean scores at baseline for the function scales and selected symptom items most relevant to toxicities. At baseline, bowel symptom scores were low (3.3 to 3.6), whereas urinary scores were moderate (28.7 to 29.7).
Table 1.
QOL Scores for Selected EORTC Core Questionnaire and FACT-P Domains at Baseline and Change From Baseline
| Domain | Baseline QOL Score |
Change From Baseline |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Months |
12 Months |
36 Months |
|||||||||||||||||
| ADT |
ADT + RT |
ADT |
ADT + RT |
P* | ADT |
ADT + RT |
P* | ADT |
ADT + RT |
P* | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Global and Function Scales | |||||||||||||||||||
| EORTC physical function | 92.5 | 11.9 | 91.5 | 15.9 | −3.5 | 14.3 | −3.8 | 14.7 | .73 | −5.1 | 12.9 | −5 | 18.6 | .79 | −9 | 18 | −10 | 18.5 | .63 |
| EORTC role function | 95.0 | 11.7 | 94.8 | 13.8 | −2.9 | 15.7 | −6.9 | 22.6 | .62 | −4.9 | 16.4 | −6 | 17 | .66 | −11.5 | 20.1 | −13.3 | 26.7 | .75 |
| EORTC emotional function | 85.3 | 14.2 | 83.1 | 17.7 | 0.2 | 12.7 | 3 | 17.9 | .31 | 1.7 | 14.2 | 4.4 | 19.1 | .13 | 0.3 | 14.9 | 0.8 | 21 | .62 |
| EORTC cognitive function | 87.2 | 15.9 | 91 | 11.1 | −1.8 | 16.3 | −2.6 | 16.2 | .9 | −2.2 | 17.3 | −4.7 | 18.2 | .43 | −4.6 | 22.2 | −6.7 | 20.2 | .41 |
| EORTC social function | 95 | 12.2 | 94.1 | 14.4 | −4.7 | 19.9 | −6.2 | 19.7 | .33 | −6 | 15.4 | −4.7 | 19.8 | .61 | −14 | 26.2 | −14 | 28.6 | .87 |
| EORTC global function | 77.8 | 18.2 | 77.4 | 17.5 | −1.8 | 14.4 | −9 | 21.4 | .03 | −5.7 | 16.9 | −7.9 | 20.6 | .78 | −10 | 16.8 | −11.1 | 18.9 | .73 |
| FACT-P physical well-being | 90.7 | 9.5 | 90.3 | 11.5 | −1.1 | 3.5 | −2.1 | 4 | .001 | −1.4 | 3.8 | −1.6 | 3.7 | .39 | −1.7 | 3.8 | −1.5 | 4 | .78 |
| FACT-P social/family | 80.4 | 17 | 80.7 | 16.4 | −0.5 | 4.7 | −0.5 | 4.6 | .85 | −0.9 | 4.8 | −0.6 | 4.5 | .41 | −1.4 | 5.1 | −0.9 | 5.1 | .53 |
| FACT-P emotional function | 81.3 | 16.7 | 81.8 | 16.1 | 0.9 | 3.1 | 0.8 | 2.9 | .88 | 0.9 | 3.5 | 1 | 3.4 | .69 | 0.6 | 3.2 | 1.1 | 3.3 | .15 |
| FACT-P functional well-being | 81.1 | 18 | 80.2 | 18.6 | −3.2 | 17.3 | −6.4 | 16.9 | .004 | −3.8 | 17 | −5.1 | 18.5 | .42 | −7.2 | 20.3 | −6.8 | 17 | .97 |
| FACT-P global assessment | 55.3 | 28.6 | 58.1 | 29.7 | 4.3 | 27.6 | −3 | 31 | .002 | 3.3 | 31.2 | −0.7 | 30.4 | .35 | 2.6 | 31.6 | −1.1 | 29.6 | .17 |
| FACT-P total score | 121.5 | 16.1 | 121.5 | 11.6 | −1.7 | 14.9 | −6.0 | 13.9 | .001 | −3.6 | 15.4 | −4.3 | 14.7 | .95 | −5.9 | 16.4 | −5.2 | 15.4 | .56 |
| FACT-P TOI | 82.7 | 12.3 | 82.5 | 13 | −2.1 | 11.8 | −6.4 | 11.7 | < .001 | −3.4 | 12 | −5 | 12.3 | .22 | −5 | 13.4 | −5.4 | 12.3 | .71 |
| FACT-P prostate cancer subscale | 34.7 | 7.1 | 34.8 | 7.1 | −0.1 | 6.7 | −2.4 | 6.9 | < .001 | −1 | 6.8 | −1.9 | 7.3 | .14 | −1.2 | 7.0 | −1.9 | 7.3 | .35 |
| Symptom/Organ Domains | |||||||||||||||||||
| EORTC bowel symptoms | 3.6 | 7.3 | 3.3 | 8.4 | −1.3 | 7.1 | 3.3 | 14.8 | .02 | −0.9 | 10.6 | 3.8 | 14.2 | .02 | −0.3 | 10.1 | 1.7 | 15.3 | .55 |
| EORTC diarrhea | 4.3 | 11.2 | 5.8 | 17.1 | −1.8 | 12 | 7.7 | 21.8 | < .001 | −2.2 | 9.9 | 3.3 | 20.6 | .03 | 1 | 16.8 | 1.7 | 19 | .32 |
| FACT-P urinary function | 28.7 | 24.8 | 29.7 | 23.5 | −6.1 | 22.2 | 0.1 | 25.3 | .003 | −5.8 | 25.4 | −5.5 | 24.9 | .97 | −5.2 | 25.6 | −5.2 | 25.5 | .89 |
| EORTC urinary frequency | 23.4 | 26.3 | 25.7 | 29.9 | −1.8 | 28.9 | 5.3 | 32.9 | .19 | −3.5 | 32 | −7 | 33.3 | .62 | −2 | 30.9 | 1.1 | 35.8 | .65 |
| EORTC urinary incontinence | 5 | 13.8 | 8.1 | 15.2 | 0 | 17.3 | 0.9 | 12.2 | .7 | 1.3 | 13.9 | 0 | 16.1 | .48 | 1 | 17.5 | 3.3 | 27 | .94 |
| FACT-P erections | 74.6 | 33 | 69.4 | 35.1 | 14.6 | 34.9 | 22.8 | 36 | .008 | 18 | 35 | 25 | 36.5 | .03 | 18 | 37.9 | 24.8 | 38 | .1 |
| EORTC fatigue | 14 | 13.8 | 14.3 | 18 | 4 | 17.7 | 7.6 | 23.6 | .06 | 7.1 | 17 | 6.7 | 18.9 | .75 | 9.1 | 15.9 | 10.6 | 24.9 | .45 |
| EORTC hot flashes | 3.6 | 11.5 | 3.1 | 14.1 | 47.7 | 29 | 38.7 | 32.4 | .05 | 43.6 | 33.8 | 37.7 | 37 | .24 | 38 | 33.5 | 33.3 | 31 | .36 |
| EORTC sleep | 15.3 | 23.8 | 16.1 | 24.8 | 11 | 35 | 8 | 31.4 | .39 | 8.7 | 29.8 | 7.5 | 31.5 | .8 | 13.1 | 29.2 | 11.7 | 28.7 | .81 |
Abbreviations: ADT, androgen-deprivation therapy; EORTC, European Organisation for Research and Treatment of Cancer; FACT-P, Functional Assessment of Cancer Therapy–Prostate questionnaire; QOL, quality of life; RT, radiotherapy; SD, standard deviation.
P values reflect between–treatment arm comparisons of mean change scores for each time point.
Assessment of Impact of RT on PROs
Change in mean scores by treatment allocation.
Table 1 lists change scores at 6 months and 1 and 3 years. Significant between-arm differences at 6 months were evident for bowel symptoms, diarrhea, urinary function, and erectile function. At 3 years, however, no clinically or statistically significant differences between arms were evident. Figure 2 shows these findings for the FACT and EORTC physical functioning scores as examples. Additional illustrations of function scale scores over time are provided in Appendix Figs A1 and A2 (online only). Mean scores over time for the symptoms most relevant to RT toxicity are shown in Figure 3.
Fig 2.
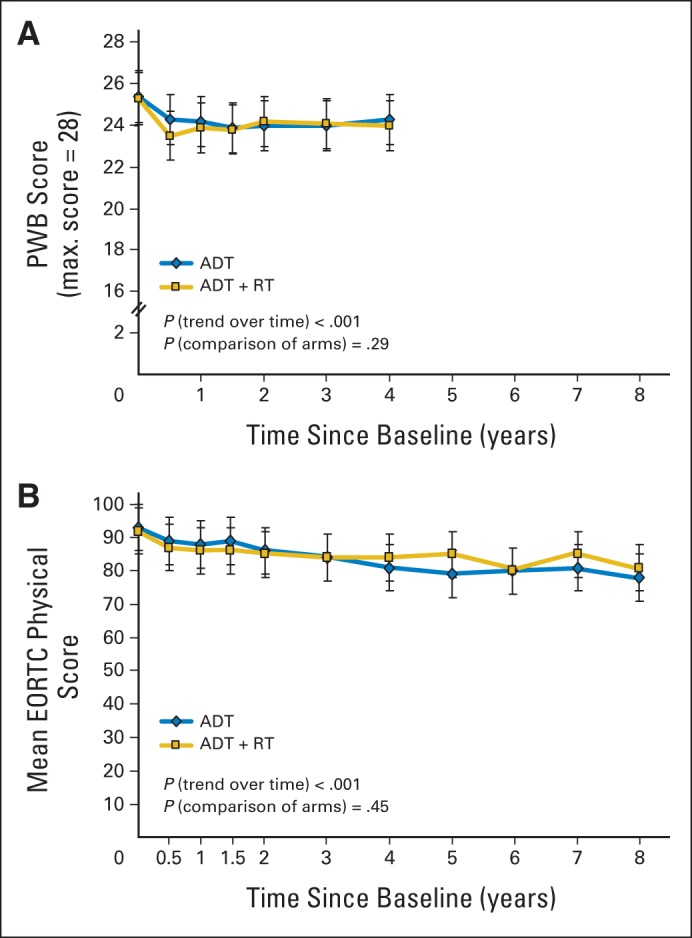
Health-related quality-of-life (QOL) function scores over time for physical well-being (PWB). Higher scores represent better function (higher QOL). Scores collected with (A) Functional Assessment of Cancer Therapy–Prostate questionnaire (FACT-P) and (B) European Organisation for Research and Treatment of Cancer (EORTC) Core Questionnaire. FACT-P scores are truncated after 4 years because of declined compliance thereafter. Mean score number is simple average at each time point, with point-wise 95% CI. ADT, androgen-deprivation therapy; RT, radiotherapy.
Fig 3.
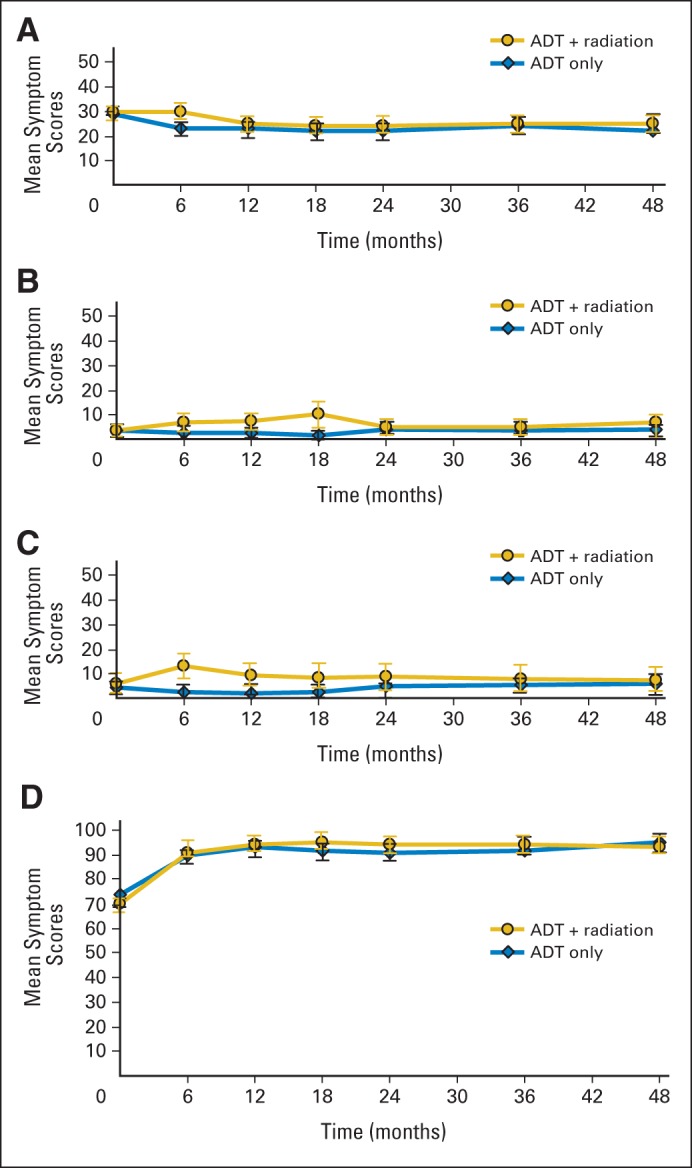
Mean symptom scores over time by treatment arm for symptoms typically associated with radiotherapy. Lower scores represent fewer symptoms. Mean score number is simple average at each time point, with point-wise 95% CI. (A) Functional Assessment of Cancer Therapy–Prostate questionnaire (FACT-P) urinary score; (B) European Organisation for Research and Treatment of Cancer (EORTC) Core Questionnaire bowel and rectum score; (C) EORTC diarrhea score; (D) FACT-P erectile dysfunction score. ADT, androgen-deprivation therapy.
Response analysis.
Figure 4 shows the proportions of men improved, stable, or worsened at any time from baseline for six symptom and six function scales. Significant differences were evident only for diarrhea and bowel symptoms, with a larger proportion of men receiving RT reporting worsened symptoms at some point during treatment. A substantial proportion of men reported improved urinary scores in both arms.
Fig 4.
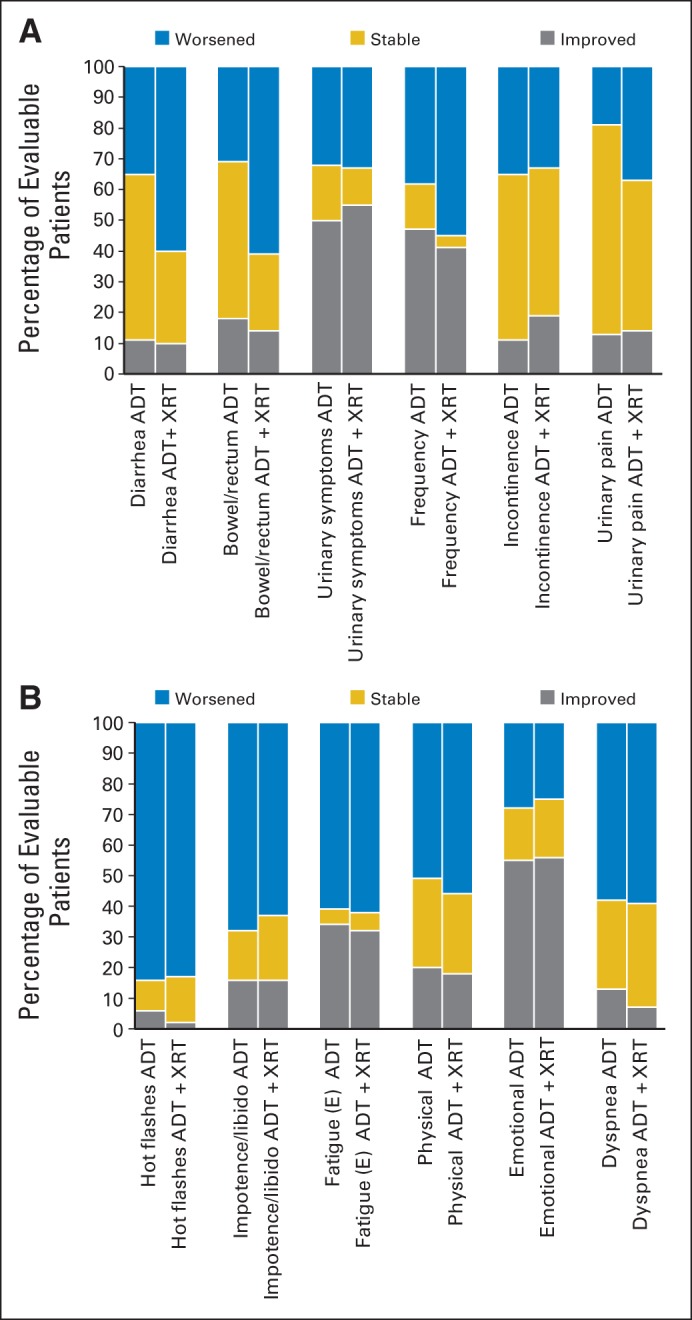
Stacked bars represent proportion of men improving, worsening, or remaining stable at any time (compared with baseline) for (A) health-related quality-of-life bowel and bladder symptom domains and (B) general symptom domains. ADT, androgen-deprivation therapy; XRT, radiotherapy.
Assessment of Impact of Treatments on PROs Over Time
Generalized linear mixed modeling testing for the effect of time on mean change scores showed significant worsening of scores from baseline in both arms for FACT-P total scores and FACT TOI, prostate symptoms, and physical and functional well-being, as well as for EORTC physical and social functions (Fig 2; Appendix Table A1; Appendix Figs A1 and A2, online only).
Analysis of Single-Item Responses and Comparison With Toxicity Scores
Figure 5 shows the frequency of nonzero HRQOL symptom responses for the selected six RT-related symptoms and four general symptoms relevant to ADT administration that had corresponding Common Toxicity Criteria for Adverse Events toxicity categories. The upper panel of Figure 4 shows the frequency of nonzero (grade 1 to 4 toxicities) recorded on trial. Comparable rates of HRQOL item scores and toxicity rates were seen for most symptoms relevant to the locoregional impact of RT. Urinary incontinence was reported more frequently in HRQOL items compared with Common Toxicity Criteria for Adverse Events grades. For those symptoms more likely related to ADT, higher rates of poor sexual function, lethargy, and insomnia were seen on HRQOL scores than on toxicity assessments.
Fig 5.
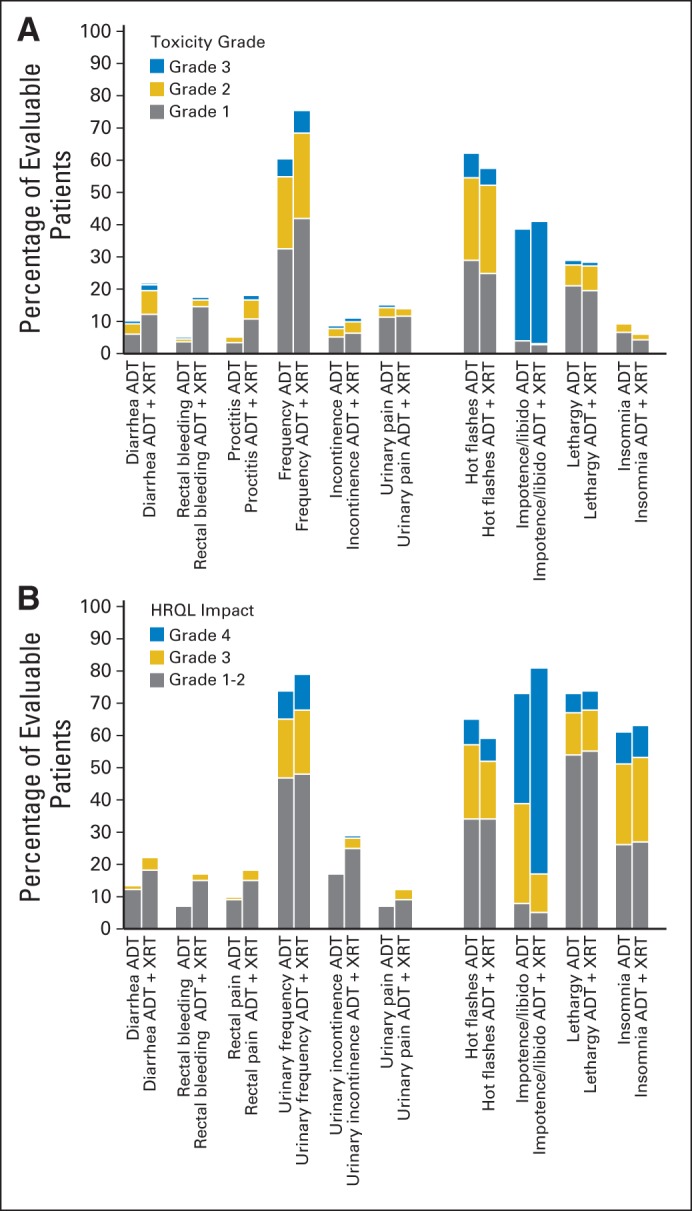
Stacked bars represent proportions of men with nonzero toxicity grades (1 to 4) or nonzero health-related quality-of-life (HRQOL) response categories. (A) Toxicity categories that best correspond to items reported on (B) HRQOL domains. ADT, androgen-deprivation therapy; XRT, radiotherapy.
DISCUSSION
The PR3/PR07 study has demonstrated that in patients with locally advanced prostate cancer, long-term ADT with RT, compared with long-term ADT alone, improves overall and disease-specific survival. Analyses of the HRQOL outcomes reveal a number of key additional findings. First, RT has a temporary negative incremental impact on domains, reflecting RT toxicity (these are also detected by common toxicity rates). Second, in contrast to worst reported toxicities, HRQOL data show that the impact of RT on bowel and bladder function, in most patients, is transient; average symptom scores did not differ (clinically or statistically) between arms after 2 years. Furthermore, the proportion of patients reporting severe bowel or bladder symptom scores at any time was only slightly higher (< 5%) among those receiving RT compared with those who did not. Third, the adverse impact of RT did not result in between-arm differences in global HRQOL or in any function domain. Fourth, the frequency of self-reported symptoms in each treatment arm was higher than that suggested by clinician-reported toxicity scores for those domains reflecting treatment toxicities. Finally, the HRQOL results revealed symptom benefits conferred by treatments regarding bladder function. Overall, these findings illustrate that the overall benefits of RT outweigh the incremental adverse impact of RT when added to ADT.
The main strengths of this study relate to its robust design and execution and high compliance on both HRQOL instruments up to 4 years. High symptom burden regarding the important impact of treatments on libido and erectile function domains11 illustrate participants' willingness to report adverse symptoms, suggesting that other domain scores are not under-reported.
The various analyses of HRQOL data from PR3/PR07 illustrate different dimensions of treatment impact on men's HRQOL. The mean change scores show that early differences in bowel and bladder symptom burden attributable to RT are not sustained after 2 years (consistent with RT toxicity being most often transient). These group scores, however, may be insensitive to small but important differences in the proportion of men with severe or chronic toxicity, because these high symptom scores may be masked by averaging. Accordingly, we undertook an exploratory analysis to determine the proportion of men reporting grade 3 or 4 HRQOL scores at any time and showed that these proportions differed by only a few percent between arms.
The proportions of patients with nonzero toxicity scores and nonzero HRQOL scores, respectively, were similar for domains relevant to RT toxicity. The same proportions for domains that reflect the impact of ADT were much higher for HRQOL scores and provide an illustration of symptom burden from the patients' perspective (as compared with toxicity rates). Analysis of the proportion of men with either improved or worsened scores (at any time from baseline) was useful in revealing that many men experienced improved scores, particularly in the urinary domains, reflecting the benefit of ADT regarding symptomatic local disease.
In addition to illustrating between-arm differences in HRQOL, these data also illustrate the impact of ADT (with or without RT) on long-term HRQOL in both arms. Several function scales reflecting the broad impact of ADT on HRQOL showed statistically significant deteriorations over time. In particular, our data provide 8-year follow-up data, with high compliance among men evaluated using the EORTC instrument, illustrating that men's average physical function and role function scores deteriorate over time. These findings are consistent with other prospective research illustrating that this deterioration exceeds that expected by aging alone, emphasizing the need to consider clinical measures for physical health maintenance in men receiving this treatment.12 The HRQOL instruments used in our trial were not broad enough in scope to detect the impact on bone or cardiac health.13
A similar randomized controlled trial conducted by Widmark et al (SPCG-7) evaluated the role of RT when added to ADT for patients with prostate cancer; study PROs were reported in an expanded article by Fransson et al.14 These outcome data illustrated the impact of RT on bladder, bowel, and sexual functioning, consistent with the toxicity profile of RT. In contrast to this study, the SPCG-7 trial included men with somewhat better–prognosis disease (including intermediate-risk disease), employed neoadjuvant antiandrogen therapy before RT, did not include treatment with pelvic lymph node irradiation (because of surgical staging of high-risk patients), and has not yet reported PRO findings beyond 4 years.14
Some limitations to the interpretation of our findings are noteworthy. First, although compliance was high, those data that were missing were likely not missing at random (resulting in possible bias toward underestimating impact of treatment on symptoms). We used a generalized mixed-method analysis to account, in part, for these missing data. Second, we elected to make no correction for multiple statistical testing, in light of the few statistically significant differences between arms. Because the significant between-group differences were clinically expected (eg, statistically different diarrhea scores at 6 months), we believe that the chance of a type I statistical error is quite low. Third, we could not account for patient adaptation (or response shift), but the statistically significant deterioration in HRQOL scores over time indicates impact on QOL that was not offset by men adapting to their health state. Fourth, the demonstrated impact of ADT on men's well-being over time may be potentially lower with ADT administered over shorter period (as is current common practice) than with the lifelong duration of ADT used in both arms of this trial.15 Finally, the study used relatively crude RT techniques and lower prescribed doses than would be used currently. Although the HRQOL bladder and bowel scores might be expected to be lower in a cohort treated with highly conformal RT techniques, these scores might also be higher with dose escalation.
We conclude that the HRQOL findings from PR3/PR07 illustrate clear evidence of the negative impact of RT on bladder and bowel domains, but in most men, this impact was transient (in that HRQOL scores returned to levels seen in patients receiving only ADT). In ADT-specific domains, HRQOL data revealed higher symptom burden than did clinician-reported toxicity scoring and also revealed the benefits of treatment with regard to urinary symptoms that were not captured by toxicity or clinical response data. The adverse impact of RT overall was modest and temporary and, in our view, not of sufficient magnitude to offset the clear disease-specific and overall survival benefits conferred by curative RT in this setting.
Appendix
Table A1.
Generalized Linear Mixed-Model Estimates for Least-Squares Means at Each Model Time Point
| Domain | Baseline |
Month 6 |
Month 12 |
Month 18 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
Year 6 |
Year 7 |
Year 8 |
P† | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | Mean | SE | P* | ||
| EORTC | ||||||||||||||||||||||||||||||||||
| Physical | .73 | .69 | .58 | .41 | .62 | .61 | .85 | .27 | .72 | .26 | .98 | < .001 | ||||||||||||||||||||||
| ADT | 92.7 | 2.0 | 88.5 | 2.1 | 87.9 | 2.1 | 87.7 | 2.1 | 86.1 | 2.2 | 82.8 | 2.2 | 81.3 | 2.2 | 77.7 | 2.3 | 77.9 | 2.4 | 78.8 | 2.5 | 76.3 | 2.6 | ||||||||||||
| ADT + RT | 91.7 | 2.1 | 87.2 | 2.1 | 86.3 | 2.1 | 85.2 | 2.2 | 84.6 | 2.2 | 81.3 | 2.2 | 80.6 | 2.4 | 81.4 | 2.3 | 76.6 | 2.5 | 82.8 | 2.6 | 76.2 | 2.5 | ||||||||||||
| Role | .88 | .27 | .56 | .61 | .15 | .57 | .97 | .53 | .96 | .90 | .73 | < .001 | ||||||||||||||||||||||
| ADT | 95.4 | 2.1 | 91.1 | 2.1 | 89.5 | 2.2 | 89.1 | 2.3 | 89.2 | 2.3 | 83.3 | 2.4 | 82.7 | 2.4 | 81.9 | 2.6 | 80.8 | 2.6 | 81.5 | 2.8 | 81.5 | 2.9 | ||||||||||||
| ADT + RT | 94.9 | 2.2 | 87.6 | 2.2 | 87.6 | 2.3 | 87.4 | 2.4 | 84.2 | 2.4 | 81.4 | 2.4 | 82.9 | 2.7 | 84.2 | 2.6 | 80.6 | 2.7 | 81.0 | 2.9 | 80.1 | 2.8 | ||||||||||||
| Diarrhea | .38 | < .001 | < .001 | .003 | .045 | .35 | .44 | .26 | .12 | .45 | .97 | .94 | ||||||||||||||||||||||
| ADT | 4.5 | 1.6 | 3.8 | 1.6 | 2.5 | 1.6 | 3.4 | 1.7 | 5.5 | 1.7 | 5.5 | 1.7 | 5.0 | 1.8 | 6.1 | 1.9 | 9.0 | 2.0 | 7.7 | 2.1 | 6.8 | 2.2 | ||||||||||||
| ADT + RT | 6.4 | 1.6 | 13.6 | 1.7 | 10.6 | 1.7 | 10.8 | 1.8 | 10.4 | 1.8 | 7.9 | 1.8 | 7.1 | 1.9 | 9.1 | 1.9 | 4.4 | 2.1 | 5.4 | 2.2 | 6.9 | 2.3 | ||||||||||||
| Rectal | .76 | .007 | .002 | < .001 | .43 | .21 | .006 | .47 | .15 | .45 | .50 | .58 | ||||||||||||||||||||||
| ADT | 3.5 | 1.1 | 1.8 | 1.1 | 2.7 | 1.1 | 1.5 | 1.2 | 3.9 | 1.2 | 3.1 | 1.2 | 2.4 | 1.2 | 3.9 | 1.3 | 3.3 | 1.3 | 3.3 | 1.5 | 5.2 | 1.5 | ||||||||||||
| ADT + RT | 3.0 | 1.1 | 6.2 | 1.2 | 7.7 | 1.2 | 9.5 | 1.2 | 5.2 | 1.2 | 5.3 | 1.2 | 8.7 | 1.4 | 5.2 | 1.3 | 6.0 | 1.4 | 4.9 | 1.5 | 3.8 | 1.4 | ||||||||||||
| Urinary | .25 | .06 | .79 | .59 | .11 | .13 | .88 | .46 | .85 | .30 | .22 | .36 | ||||||||||||||||||||||
| ADT | 9.7 | 1.1 | 8.5 | 1.3 | 8.2 | 1.1 | 8.4 | 1.2 | 8.6 | 1.2 | 8.2 | 1.2 | 9.9 | 1.2 | 9.3 | 1.3 | 10.2 | 1.3 | 11.3 | 1.4 | 9.2 | 1.5 | ||||||||||||
| ADT + RT | 11.5 | 1.1 | 11.6 | 1.6 | 8.6 | 1.2 | 9.4 | 1.2 | 11.3 | 1.2 | 10.8 | 1.2 | 9.6 | 1.3 | 10.5 | 1.3 | 10.6 | 1.4 | 9.2 | 1.5 | 11.7 | 1.4 | ||||||||||||
| FACT-P‡ | ||||||||||||||||||||||||||||||||||
| Physical well-being | .83 | .02 | .36 | .68 | .83 | .98 | .35 | < .001 | ||||||||||||||||||||||||||
| ADT | 25.4 | 0.2 | 24.2 | 0.2 | 24.1 | 0.2 | 23.8 | 0.2 | 23.9 | 0.2 | 23.8 | 0.2 | 24.0 | 0.2 | ||||||||||||||||||||
| ADT + RT | 25.4 | 0.2 | 23.4 | 0.2 | 23.8 | 0.2 | 23.7 | 0.2 | 23.9 | 0.2 | 23.8 | 0.2 | 23.7 | 0.2 | ||||||||||||||||||||
| Functional well-being | .42 | .01 | .11 | .83 | .82 | .65 | .96 | < .001 | ||||||||||||||||||||||||||
| ADT | 22.7 | 0.3 | 21.6 | 0.3 | 21.6 | 0.3 | 20.9 | 0.3 | 20.8 | 0.3 | 20.7 | 0.3 | 20.6 | 0.3 | ||||||||||||||||||||
| ADT + RT | 22.4 | 0.3 | 20.6 | 0.3 | 21.0 | 0.3 | 20.8 | 0.3 | 20.9 | 0.3 | 20.5 | 0.3 | 20.6 | 0.3 | ||||||||||||||||||||
| Treatment outcome index | .91 | < .001 | .13 | .35 | .64 | .48 | .53 | < .001 | ||||||||||||||||||||||||||
| ADT | 82.8 | 0.7 | 80.3 | 0.7 | 79.4 | 0.7 | 78.6 | 0.7 | 78.7 | 0.7 | 78.3 | 0.7 | 78.1 | 0.8 | ||||||||||||||||||||
| ADT + RT | 82.7 | 0.7 | 76.9 | 0.7 | 77.9 | 0.7 | 77.7 | 0.7 | 78.2 | 0.7 | 77.6 | 0.7 | 77.4 | 0.8 | ||||||||||||||||||||
| FACT-G score | .91 | .08 | .93 | .58 | .65 | .53 | .89 | < .001 | ||||||||||||||||||||||||||
| ADT | 86.8 | 0.6 | 84.9 | 0.6 | 84.3 | 0.6 | 83.4 | 0.6 | 83.4 | 0.6 | 82.7 | 0.6 | 83.6 | 0.7 | ||||||||||||||||||||
| ADT + RT | 86.7 | 0.6 | 83.4 | 0.6 | 84.4 | 0.6 | 83.9 | 0.6 | 83.8 | 0.6 | 83.3 | 0.6 | 83.8 | 0.7 | ||||||||||||||||||||
| FACT-P total score | .95 | .01 | .66 | .91 | .99 | .97 | .91 | < .001 | ||||||||||||||||||||||||||
| ADT | 121.6 | 0.8 | 119.2 | 0.9 | 118.0 | 0.9 | 117.2 | 0.9 | 117.4 | 0.9 | 116.6 | 0.9 | 117.0 | 1.0 | ||||||||||||||||||||
| ADT + RT | 121.6 | 0.8 | 116.2 | 0.8 | 117.5 | 0.9 | 117.0 | 0.9 | 117.3 | 0.9 | 116.6 | 0.9 | 116.9 | 0.9 | ||||||||||||||||||||
Abbreviations: ADT, androgen-deprivation therapy; EORTC, European Organisation for Research and Treatment of Cancer; FACT-P, Functional Assessment of Cancer Therapy–Prostate questionnaire; RT, radiotherapy.
P values reflect between-arm comparisons.
P values reflect trend over time.
FACT-P scores truncated after 4 years because of decline in compliance thereafter.
Fig A1.
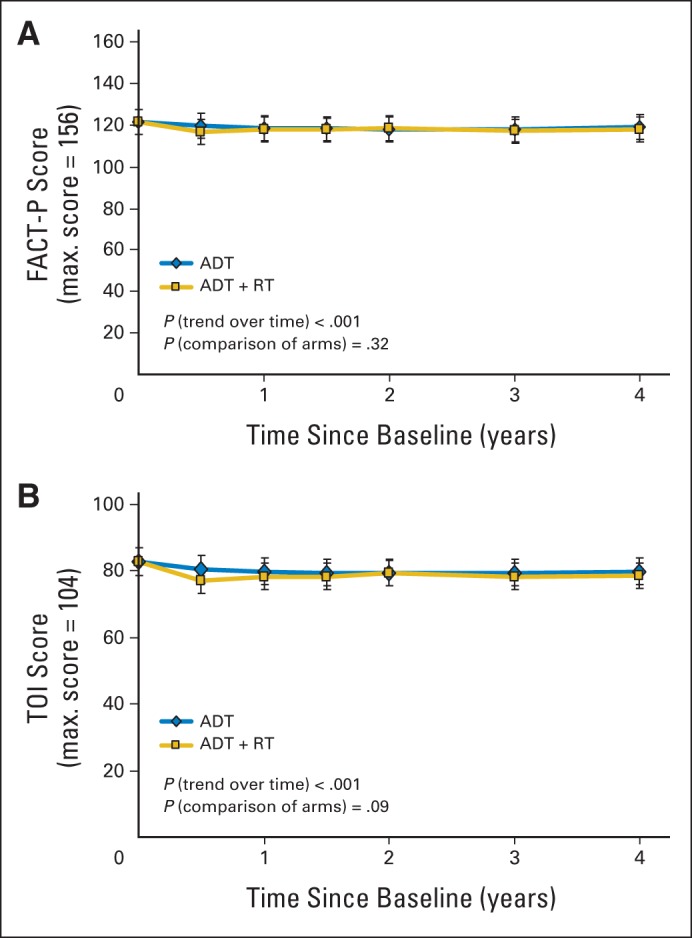
Functional Assessment of Cancer Therapy–Prostate questionnaire (FACT-P) (A) overall mean total scores over time and (B) mean trial outcome index (TOI) scores over time. ADT, androgen-deprivation therapy; RT, radiotherapy.
Fig A2.
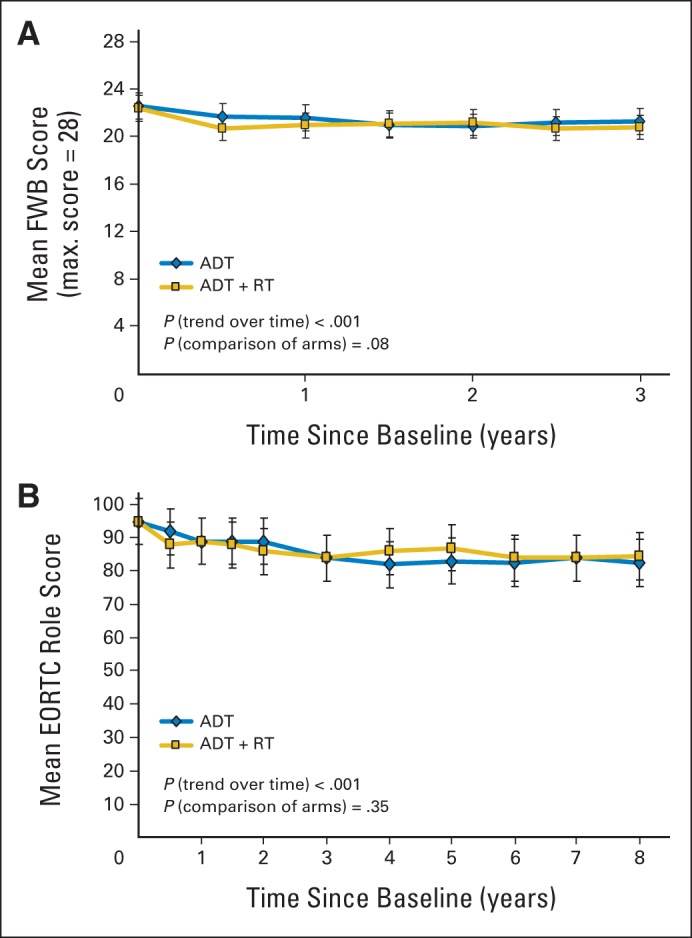
(A) Functional Assessment of Cancer Therapy–Prostate questionnaire functional well-being (FWB) mean function scores over time and (B) European Organisation for Research and Treatment of Cancer (EORTC) Core Questionnaire role domain mean function scores over time. ADT, androgen-deprivation therapy; RT, radiotherapy.
Support information appears at the end of this article.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00002633, ISRCTN24991896.
Support
Supported by Grants No. 015469 and 021039 from the Canadian Cancer Society Research Institute to the NCIC Clinical Trials Group; Grant No. CA077202 from the the National Cancer Institute; and collaboration with the United Kingdom Medical Research Council.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Brundage, Matthew R. Sydes, Wendy R. Parulekar, Padraig Warde, Andrea Bezjak, Mahesh K.B. Parmar, Bingshu E. Chen, Malcolm D. Mason
Administrative support: Wendy R. Parulekar, Karen Sanders
Provision of study materials or patients: Wendy R. Parulekar, Matthew Parliament
Collection and assembly of data: Michael Brundage, Matthew R. Sydes, Wendy R. Parulekar, Padraig Warde, Richard Cowan, Peter Kirkbride, Matthew Parliament, Karen Sanders, Bingshu E. Chen, Malcolm D. Mason
Data analysis and interpretation: Michael Brundage, Matthew R. Sydes, Wendy R. Parulekar, Padraig Warde, Richard Cowan, Andrea Bezjak, Peter Kirkbride, Clare Moynihan, Jean-Paul Bahary, Karen Sanders, Bingshu E. Chen, Malcolm D. Mason
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Radiotherapy When Added to Androgen-Deprivation Therapy for Locally Advanced Prostate Cancer: Long-Term Quality-of-Life Outcomes From the NCIC CTG PR3/MRC PR07 Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Michael Brundage
No relationship to disclose
Matthew R. Sydes
Honoraria: Eli Lilly
Research Funding: Astellas, Janssen-Cilag, Pfizer, Novartis, Sanofi
Travel, Accommodations, Expenses: Eli Lilly
Wendy R. Parulekar
No relationship to disclose
Padraig Warde
No relationship to disclose
Richard Cowan
Travel, Accommodations, Expenses: MIOT International Hospitals (Chennai, India)
Andrea Bezjak
No relationship to disclose
Peter Kirkbride
No relationship to disclose
Matthew Parliament
No relationship to disclose
Clare Moynihan
No relationship to disclose
Jean-Paul Bahary
No relationship to disclose
Mahesh K.B. Parmar
No relationship to disclose
Karen Sanders
No relationship to disclose
Bingshu E. Chen
No relationship to disclose
Malcolm D. Mason
Consulting or Advisory Role: Sanofi, Dendreon
Speakers' Bureau: Sanofi
Research Funding: Ferring Pharmaceuticals
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society, Statistics Canada, Provincial/Territorial Cancer Registries, Public Health Agency of Canada. Canadian Cancer Statistics 2012. https://cancer.ca/∼/media/CCS/Canada%20wide/Files%20List/English%20files%20heading/PDF%20-%20Policy%20-%20Canadian%20Cancer%20Statistics%20-%20English/Canadian%20Cancer%20Statistics%202012%20-%20English.ashx.
- 3.Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet. 2012;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 5.Efficace F, Osoba D, Gotay C, et al. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Ann Oncol. 2007;18:775–781. doi: 10.1093/annonc/mdl494. [DOI] [PubMed] [Google Scholar]
- 6.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 8.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Nichol MB, Eton D, et al. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: Results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–129. doi: 10.1111/j.1524-4733.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 10.Osoba D, Bezjak A, Brundage M, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41:280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Moynihan C. Men, women, gender and cancer. Eur J Cancer Care (Engl) 2002;11:166–172. doi: 10.1046/j.1365-2354.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 12.Moul JW, Dawson N. Quality of life associated with treatment of castration-resistant prostate cancer: A review of the literature. Cancer Invest. 2012;30:1–12. doi: 10.3109/07357907.2011.629381. [DOI] [PubMed] [Google Scholar]
- 13.Alibhai SM, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 14.Fransson P, Lund JA, Damber JE, et al. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol. 2009;10:370–380. doi: 10.1016/S1470-2045(09)70027-0. [DOI] [PubMed] [Google Scholar]
- 15.Denham JW, Steigler A. Picking the optimal duration of hormonal therapy in men with high-risk and locally advanced prostate cancer treated with radiotherapy. Semin Radiat Oncol. 2013;23:206–214. doi: 10.1016/j.semradonc.2013.01.008. [DOI] [PubMed] [Google Scholar]


