Abstract
Purpose
This updated provisional clinical opinion presents a revised opinion based on American Society of Clinical Oncology panel consensus in the context of an evolving database.
Context
Despite the 2010 provisional clinical opinion recommendation, there is still evidence of suboptimal hepatitis B virus (HBV) screening among patients at high risk for HBV infection or HBV reactivation after chemotherapy. This updated provisional clinical opinion introduces a risk-adaptive strategy to identify and treat patients with HBV infection to reduce their risk of HBV reactivation.
Provisional Clinical Opinion
Medical providers should screen by testing patients for HBV infection before starting anti-CD20 therapy or hematopoietic cell transplantation. Providers should also screen patients with risk factors for HBV infection. Screening should include both hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc), because reactivation can occur in patients who are HBsAg positive/anti-HBc positive or HBsAg negative/anti-HBc positive. Either total anti-HBc or anti-HBc immunoglobulin G (not immunoglobulin M) test should be used. Clinicians should start antiviral therapy for HBsAg-positive/anti-HBc–positive patients before or contemporaneously with cancer therapy and monitor HBsAg-negative/anti-HBc–positive patients for reactivation with HBV DNA and ALT levels, promptly starting antivirals if reactivation occurs. Clinicians can initiate antivirals for HBsAg-negative/anti-HBc–positive patients anticipating cancer therapies associated with a high risk of reactivation, or they can monitor HBV DNA and ALT levels and initiate on-demand antivirals. For patients who neither have HBV risk factors nor anticipate cancer therapy associated with a high risk of reactivation, current evidence does not support HBV screening before initiation of cancer therapy. Two panel members provided a minority viewpoint, involving a strategy of universal HBsAg and selective anti-HBc testing.
INTRODUCTION
In 2010, the American Society of Clinical Oncology (ASCO) published a provisional clinical opinion (PCO) on chronic hepatitis B virus (HBV) infection screening in patients receiving cytotoxic chemotherapy for the treatment of malignant diseases.1 PCOs offer timely clinical direction to ASCO membership after publication or presentation of potentially practice-changing information. PCOs are updated periodically on the basis of review of recently published data.
This PCO update presents a revised clinical opinion that summarizes the results of the literature review and analysis completed for the update, reviews key concepts and introduces a risk-adaptive clinical algorithm to help clinicians identify and treat patients with HBV infection to reduce their risk of HBV reactivation resulting from cytotoxic or immunosuppressive therapy, and outlines an agenda for future research. Although the evidentiary base remains weak, the update offers clinically practical approaches based on the best available data.
STATEMENT OF CLINICAL ISSUE
The ASCO 2010 PCO on HBV screening provided the ASCO membership with guidance on how to interpret the Centers for Disease Control and Prevention recommendation for universal HBV serology testing and management of chronic HBV infection in the cancer population. After careful review, the ASCO PCO panel found that the recommendations were not supported by strong evidence and instead recommended that physicians consider screening patients belonging to groups at heightened risk for chronic HBV infection or for whom highly immunosuppressive therapies, such as rituximab or hematopoietic cell transplantation, were planned.1
Studies of HBV practice patterns predating the PCO period have shown low rates of screening before chemotherapy.2 However, despite the 2010 PCO recommendation, there is still evidence of suboptimal rates of HBV screening in patient groups at high risk for HBV infection or HBV reactivation after chemotherapy. One single-institution study over 7 years found that although screening rates had increased over time and after the publication of national recommendations, the rate of screening was still low (28%) among patients with known risk factors for HBV infection.3 More than 65% of patients with HBV infection are unaware of their infection,4 and medical providers may not be aware of their patients' HBV status.
In 2013, the US Food and Drug Administration (FDA) revised the product labels of monoclonal antibodies directed against CD20 to include HBV reactivation in the boxed warning.5–7 Because of the risk of fulminant hepatitis, hepatic flares, and death resulting from HBV reactivation caused by anti-CD20 monoclonal antibodies, the FDA recommends HBV screening for all patients before initiation of therapy. According to the ASCO Quality Oncology Practice Initiative, a practice-based system of quality self-assessment,8 the rates of HBV screening among patients with non-Hodgkin lymphoma before the initiation of rituximab are nearly 70% (data on file, Quality Oncology Practice Initiative Program spring 2014 measure results). Thus, there may be a small but substantial group of patients with cancer receiving anti-CD20 monoclonal antibodies who may not have been screened for HBV infection and thus may be at risk for reactivation and sequelae such as hepatic flares, liver failure, and even death if they have had HBV infection.
METHODS
ASCO PCOs are updated by an ad hoc panel on the basis of periodic review and analysis of new, potentially practice-changing information on the topic. The members of the PCO panel on HBV screening are listed in Appendix Table A1 (online only).
Guideline Disclaimer
ASCO PCOs reflect expert consensus based on clinical evidence and literature available at the time they are written and are intended to assist physicians in clinical decision making and identify questions and settings for further research. Because of the rapid flow of scientific information in oncology, new evidence may have emerged since the time a PCO was submitted for publication. PCOs are not continually updated and may not reflect the most recent evidence. PCOs address only the topics specifically identified in the PCO and are not applicable to interventions, diseases, or stages of disease not specifically identified. PCOs cannot account for individual variation among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It is the responsibility of the treating physician or other health care provider, relying on independent experience and knowledge of the patient, to determine the best course of treatment for the patient. Accordingly, adherence to any PCO is voluntary, with the ultimate determination regarding its application to be made by the physician in light of each patient's individual circumstances. ASCO PCOs describe the use of procedures and therapies in clinical practice and cannot be assumed to apply to the use of these interventions in the context of clinical trials. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of ASCO PCOs or for any errors or omissions.
Guideline and Conflicts of Interest
The membership of the ad hoc panel was chosen in accordance with the ASCO Conflicts of Interest Management Procedures for Clinical Practice Guidelines (summarized at http://www.asco.org/rwc). The conflicts of interest procedures call for the majority of ad hoc panel members to have no relationships with companies potentially affected by the PCO and generally require ad hoc panel co-chairs to be free from relationships with affected companies.
UPDATED PROVISIONAL CLINICAL OPINION
Medical providers should screen by testing patients for HBV infection before starting cancer therapies such as anti-CD20 monoclonal antibodies and hematopoietic cell transplantation, because these therapies can put patients with HBV infection at high risk of HBV reactivation. Medical providers should also screen patients who have risk factors for HBV infection—birthplace in a country with ≥ 2% HBV prevalence, household or sexual contact with persons with HBV infection, high-risk behaviors (eg, intravenous drug use), HIV infection—before initiating systemic cancer therapy. Screening for HBV infection should include both the hepatitis B surface antigen (HBsAg) test and hepatitis B core antibody (anti-HBc) test, because HBV reactivation can occur in patients who are HBsAg positive/anti-HBc positive or HBsAg negative/anti-HBc positive. Either a total anti-HBc test (which includes both immunoglobulin G [IgG] and IgM) or anti-HBc IgG test should be used to screen for chronic or resolved HBV infection before cancer therapy; anti-HBc IgM should not be used, because this test can only confirm acute HBV infection.
To prevent HBV reactivation, clinicians should start antiviral therapy—using drugs with low rates of viral resistance, such as entecavir or tenofovir—for HBsAg-positive/anti-HBc–positive patients about to receive immunosuppressive cancer therapy without delaying cancer therapy and should continue antivirals during therapy and for approximately 6 to 12 months after completing cancer therapy. Clinicians should monitor HBsAg-negative/anti-HBc–positive patients for HBV reactivation with HBV DNA and ALT testing approximately every 3 months during therapy and start antiviral therapy promptly if HBV reactivation occurs. Clinicians can initiate antiviral prophylaxis for HBsAg-negative/anti-HBc–positive patients in anticipation of systemic cancer therapies that are associated with a high risk of HBV reactivation, or alternatively, they can monitor HBV DNA and ALT levels every 3 months and initiate on-demand antiviral therapy at the first sign of HBV reactivation.
In summary, independent of the planned systemic cancer therapy, patients should be assessed for risk factors for HBV infection. If patients have an HBV risk factor, they should be screened for HBV infection. Furthermore, and independent of HBV infection risk, patients anticipating cancer therapy associated with a high risk of reactivation of HBV infection should be screened for HBV infection. Antiviral therapy decreases the incidence of HBV reactivation and should be started in specific clinical situations. For all other patients who neither have HBV risk factors nor anticipate cancer therapy associated with a high risk of HBV reactivation, the current evidence does not support HBV screening before initiation of cancer therapy. As an alternative to the risk-adaptive HBV screening and management approach, two panel members proposed a strategy of universal testing for HBsAg and selective testing for anti-HBc (outlined in HBV Reactivation Risk Stratification).
What Is New and Different?
A risk-adaptive HBV screening and management strategy can help identify patients at risk of HBV infection and reduce their cancer therapy–associated risk of HBV reactivation.
Fatal HBV reactivation can occur not only in patients with cancer and chronic HBV infection (HBsAg positive/anti-HBc positive) but also in patients with clinically resolved HBV infection (HBsAg negative/anti-HBc positive), particularly after receiving therapies associated with a high risk of HBV reactivation, such as anti-CD20 monoclonal antibodies. Therefore, use both HBsAg and anti-HBc tests to screen for HBV infection.
Start antiviral prophylaxis in patients with chronic HBV infection before cancer therapy, and consider antiviral prophylaxis for patients with clinically resolved HBV infection for whom systemic cancer therapies associated with a high risk of HBV reactivation are anticipated.
Monitor HBsAg-positive/anti-HBc–positive patients and HBsAg-negative/anti-HBc–positive patients for HBV reactivation resulting from chemotherapy approximately every 3 months, and engage in active comanagement with hepatitis B experts.
LITERATURE REVIEW RESULTS
A search for new evidence on HBV screening in individuals with cancer was conducted by ASCO guidelines staff to identify relevant randomized controlled trials (RCTs), systematic reviews, meta-analyses, and clinical practice guidelines published since the 2010 ASCO PCO. PubMed, the Cochrane Library, and the National Guideline Clearinghouse databases were searched from 2010 to July 2014. The search was restricted to articles published in English.
The search conducted to identify RCTs of HBV screening in patients with cancer yielded no relevant records. The search did identify one RCT that studied the role of antiviral prophylaxis in preventing HBV reactivation before rituximab-based therapy in patients with lymphoma and clinically resolved HBV infection9 (reviewed in Anti-CD20–Directed Monoclonal Antibodies and HBV Reactivation section). The search further identified several practice guidelines that had been published since the 2010 PCO. The recommendations on HBV screening, use of serologic tests, and antiviral prophylaxis from selected national and international guidelines are summarized in Table 1. The American Gastroenterological Association guideline addressed both HBV screening and prophylaxis.16 The National Institute for Health and Care Excellence guideline addressed HBV management recommendations based on a systematic review of the literature for antiviral prophylaxis among persons beginning immunosuppressive therapies, including chemotherapy and hematopoietic cell transplantation, but it did not address HBV screening.18 Finally, articles identified by individual panel members, combined with results from the formal searches, informed the consensus opinions of the panel.
Table 1.
Selected Guidance Documents With Recommendations for Hepatitis B Screening
| Recommending Body | Patient Population | Screening Recommendation | Serologic Tests | Prophylaxis |
|---|---|---|---|---|
| American Association for the Study of Liver Diseases (2009)10 | Patients receiving cytotoxic or immunosuppressive therapy | Screen patients at high risk for HBV infection | HBsAg, anti-HBc | Lamivudine, telbivudine, tenofovir, or entecavir for all HBV carriers; continue for ≥ 6 months after oncologic therapy |
| Centers for Disease Control and Prevention (2009)11 | Patients receiving cytotoxic or immunosuppressive therapy | Screen all | HBsAg, anti-HBc, anti-HBs | Prophylactic antiviral therapy for HBsAg-positive patients |
| British Committee for Standards in Haematology (2012)12 | Patients with follicular lymphoma | Test for HBV should be undertaken (according to local protocol developed in conjunction with virologist) at baseline and in all patients considered at risk of virus reactivation for whom immunotherapy is treatment of choice | Not specified | Not specified |
| European Society for Medical Oncology (2014)13 | Patients with follicular lymphoma | Screen all | Not specified | In patients with positive hepatitis B serology, prophylactic antiviral medication is strongly recommended |
| National Comprehensive Cancer Network Non-Hodgkin's Lymphoma (2014)14 | Patients with non-Hodgkin lymphoma | Screen all receiving anti-CD20 antibody therapy; in areas of high or unknown HBV prevalence, test all patients receiving immunotherapy, chemotherapy, or chemoimmunotherapy | HBsAg, anti-HBc; add e-antigen if risk factors or history of HBV; if positive, check viral load and consult with gastroenterologist | Prophylactic antiviral therapy with entecavir for HBsAg-positive patients; monitor viral load with PCR monthly during treatment and every 3 months after treatment; avoid lamivudine because of resistance |
| National Comprehensive Cancer Network Cancer-Related Infections (2014)15 | All patients with cancer | Screen patients at high risk of HBV infection; universal screening for HBV if risk-based screening is not done | HBsAg, anti-HBc | Antiviral therapy with adefovir, entecavir, lamivudine, telbivudine, or tenofovir |
| American Gastroenterological Association Institute (2015)16 | Patients who will be treated with immunosuppressive therapy | Screen patients at high risk for HBV infection or moderate or high risk of HBV reactivation | HBsAg, anti-HBc | Antiviral prophylaxis in high- and moderate-risk patients; recommend against routine antiviral prophylaxis in low-risk patients; antivirals with high barrier to resistance recommended over lamivudine; treatment should be continued for 6 months after discontinuation of immunosuppressive therapy |
| American Society of Clinical Oncology (2015) | Patients receiving immunosuppressive therapy | Screen patients who have risk factors for HBV infection or for whom immunosuppressive therapy associated with HBV reactivation is planned | HBsAg, anti-HBc | Antiviral therapy with evidence of chronic HBV infection |
NOTE. Data adapted with permission.17
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; PCR, polymerase chain reaction.
CLINICAL CONSIDERATIONS
Absent solid evidence on the mechanisms and predictors of HBV reactivation, in particular the risk caused by various chemotherapeutic and immunologic therapies, the ad hoc panel outlined several clinical considerations to guide individualized decision making.
Anti-CD20–Directed Monoclonal Antibodies and HBV Reactivation
As previously announced in a 2013 revised PCO statement,19 physicians should screen patients with cancer before initiating B cell–depleting therapies such as rituximab and ofatumumab. The FDA has extended the HBV screening recommendation to a new anti-CD20 monoclonal antibody, obinutuzumab,20 and is expected to continue the screening recommendation in the boxed warnings of future anti-CD20 monoclonal antibodies approved for the treatment of patients with malignant diseases. The FDA recommendation was based on case reports of fatal HBV-related acute liver injury in 32 patients receiving rituximab or ofatumumab.5 Of these, 10 patients were HBsAg positive/anti-HBc positive and developed HBV reactivation as evidenced by an increase in HBV DNA level as compared with previous levels. Another 22 patients were HBsAg negative/anti-HBc positive and developed HBV reactivation as evidenced by a seroconversion to HBsAg positive; five of these 22 patients were HBsAg negative/anti-HBc positive and hepatitis B surface antibody (anti-HBs) positive, demonstrating that anti-HBs positivity does not protect against HBV reactivation. Among the 32 patients, only three received antiviral prophylaxis before receiving anti-CD20 therapy; nine received on-demand antiviral treatment after HBV reactivation occurred, and the remaining patients (n = 20) were not reported to have had antiviral therapy.
The time to HBV reactivation was variable but occurred up to 12 months after the last dose of anti-CD20 therapy.5 Delayed HBV reactivation (up to 17 months after initiation of rituximab) was also reported in an RCT of antiviral prophylaxis versus antiviral on-demand treatment in 80 HBsAg-negative/anti-HBc–positive patients with lymphoma who received rituximab.9 As such, patients with chronic or clinically resolved HBV infection should receive durations of prophylactic antiviral therapy beyond 12 months and possibly longer. The panel suggests comanagement between oncologists and hepatitis B experts to determine appropriate durations of HBV antiviral therapy and to jointly monitor for interactions between anticancer and antiviral therapy.
HBV Serologic Testing
There are three HBV serologic tests,21 but the HBsAg and anti-HBc tests are recommended for screening before cancer therapy for selected patients5,15 to fully assess a patient's HBV status (Table 2). The third test, anti-HBs, indicates natural or passive immunity to HBV; however, there is a lack of evidence thus far to support its use in screening and management of patients with cancer for the prevention of HBV reactivation.16 Patients who are identified as HBsAg positive/anti-HBc positive or HBsAg negative/anti-HBc positive should then be tested for HBV DNA. Reactivation of HBV infection can be manifested by changes in HBV DNA levels—from undetectable to detectable or an increase from baseline level—or in HBsAg status (Fig 1). ALT should be monitored to assess the severity of the clinical consequences to the liver as a result of HBV reactivation.
Table 2.
Interpretation of Hepatitis B Serologic Test Results
| Interpretation | HBsAg | Anti-HBc* | Anti-HBs |
|---|---|---|---|
| Chronic infection | Positive | Positive | Negative |
| Clinically resolved infection | Negative | Positive | Positive |
| Clinically resolved infection | Negative | Positive | Negative |
| Previously vaccinated | Negative | Negative | Positive |
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; Ig, immunoglobulin.
Either total anti-HBc test (which includes both IgG and IgM) or anti-HBc IgG test should be used; anti-HBc IgM test should not be used to screen before cancer therapy.
Fig 1.
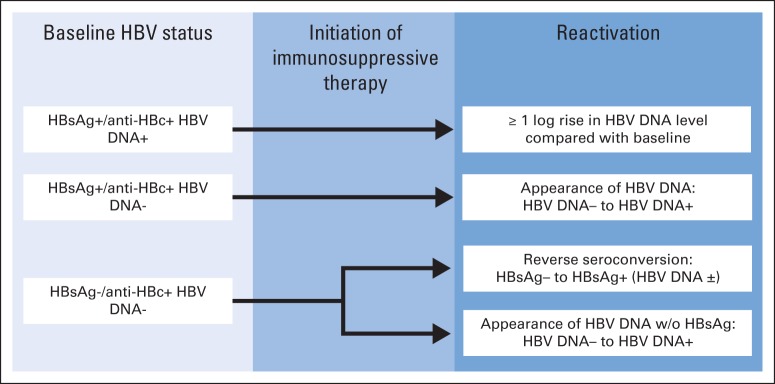
Proposed diagnostic criteria for hepatitis B virus (HBV) reactivation. Patients with chronic HBV infection (hepatitis B surface antigen [HBsAg] –positive and hepatitis B core antibody [anti-HBc] –positive test results) can have either increase in HBV DNA level or appearance of HBV DNA, depending on whether they did or did not have detectable HBV DNA before immunosuppression, respectively. Patients with clinically resolved HBV infection (HBsAg negative and anti-HBc positive) can be diagnosed as having HBV reactivation on appearance of HBsAg or HBV DNA. Adapted with permission.22
HBsAg-positive/anti-HBc–positive patients are at elevated risk (pooled incidence, 37%; range, 24% to 88%) of reactivation after immunosuppressive therapy and should be started on antiviral prophylaxis before or contemporaneously with cancer therapy (described under Antiviral Therapy).23 HBsAg-negative/anti-HBc–positive patients are also at risk (albeit lower) of reactivation because of the persistence of replication-competent HBV after HBsAg clearance in the form of covalently closed circular DNA within the nuclei of hepatocytes24 and the control of replication by the immune system.25
Most HBsAg-negative/anti-HBc–positive patients have undetectable serum HBV DNA levels, but they may still be at considerable risk of reactivation if anticipated to receive a therapy considered high risk, as outlined in the previous section on anti-CD20 therapy. In the control group of HBsAg-negative/anti-HBc–positive patients with lymphoma receiving rituximab who received on-demand antiviral therapy,9 five of the 24 patients with undetectable HBV DNA levels later developed HBV reactivation. Furthermore, in a multivariable analysis in the same study,9 undetectable HBV DNA levels did not predict or protect against the development of HBV reactivation in patients with CD20+ lymphoma. It is not clear how well this observation generalizes to patients with cancer who receive systemic chemotherapies other than anti-CD20 antibodies.
An isolated positive anti-HBc test may reflect either occult chronic HBV infection with lower-than-detectable levels of HBsAg or clinically resolved HBV infection with lower-than-detectable levels of anti-HBs. Chronic or clinically resolved HBV infection is denoted by a positive anti-HBc IgG test. Anti-HBc IgM should be used only to confirm acute HBV infection. Therefore, to diagnose chronic or resolved HBV infection, clinicians should ensure that either the total anti-HBc test (which includes both IgG and IgM) or anti-HBc IgG test is used to screen for HBV infection before cancer therapy. Although false-positive anti-HBc tests may occur and have been reported in patients after intravenous Ig therapy,14 the sensitivity (99.49% and 100%, respectively) and specificity (99.88% and 99.27%, respectively) of the anti-HBc26 and HBsAg27 tests are excellent. Thus, patients who are HBsAg negative/anti-HBc positive need further evaluation and risk stratification, and clinicians should not uniformly consider them to have false-positive anti-HBc test results.
HBV Screening Strategies
The ASCO panel acknowledges that there is wide variability in approaching HBV screening before cancer therapy. Whereas some centers may not have an HBV screening policy or may be screening based on HBV risk factors, others have adopted a strategy of universal HBV screening before cancer therapy. There are potential benefits and harms to universal HBV screening (Table 3) that have yet to be empirically established, and the evidentiary base to recommend for or against a particular screening strategy is weak.
Table 3.
Potential Benefits and Harms of Universal HBV Screening
| Category | Benefits | Harms |
|---|---|---|
| HBV testing | Test expense is modest and carries low risk | |
| Excellent test sensitivity and specificity | ||
| Complete identification of HBsAg-positive/anti-HBc–positive patients | ||
| Knowledge of risk of HBV infection is not necessary | ||
| Patients may not admit to having HBV risk factors | ||
| Prevents most cases of HBV reactivation | ||
| Antiviral therapy | Relatively inexpensive | Antivirals* adds to patients' financial burden during and for 6 to 12 months after last dose of cancer therapy |
| Potential adverse effects (eg, effect on blood counts, chemotherapy drug and dose changes, delay in chemotherapy) of antiviral therapy and cancer therapies have not been systematically studied | ||
| Need follow-up with hepatitis specialist every 3 months; oncologists could monitor if they feel they have sufficient expertise | ||
| Systemic cancer therapy | Knowledge of cancer therapy–associated risk of HBV reactivation is not necessary | Unclear treatment of HBsAg-negative/anti-HBc–positive patients receiving cancer therapies not associated with high risk of HBV reactivation |
| Uncertainty about cancer therapies not associated with high risk of HBV reactivation; could lead to overtreatment with antiviral prophylaxis in some patients groups: | ||
| HBsAg-negative/anti-HBc–positive patients receiving cancer therapies with low risk of HBV reactivation (not anti-CD20 therapy or stem-cell transplantation) | ||
| HBsAg-positive/anti-HBc–positive patients receiving cancer therapies expected to confer low risk of HBV reactivation (eg, adjuvant hormonal therapy) |
Abbreviations: anti-HBc, anti-hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
Antiviral costs estimated between $300 to $1,000 per month without insurance and dependent on specific antiviral drug.
Until clear and definitive evidence is available to guide patient selection, the consensus of the panel is that a risk-adaptive HBV screening and management strategy incorporating what is known about the risks of HBV infection as well as risks of cancer therapy–associated HBV reactivation is reasonable. As with any risk-based approach, physicians should have a process in place to identify patients at elevated risk for HBV infection. For those clinical centers that engage in universal HBV screening, the panel does not recommend for or against this approach but defers to the clinical centers themselves to determine feasible, site-specific HBV screening procedures. This overall approach is also supported by the National Comprehensive Cancer Network community.28
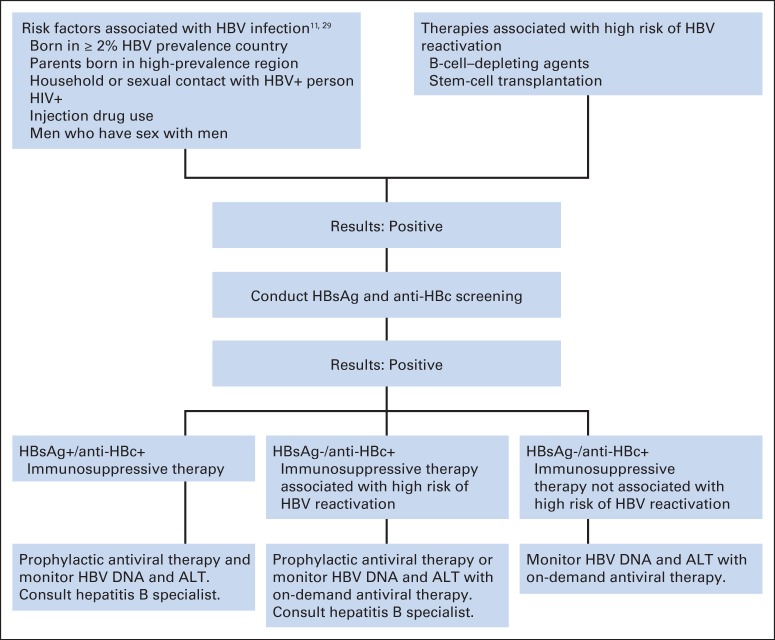
There are currently no validated clinical tools to guide risk-based HBV screening in the cancer or noncancer population. Among patients with HBV infection, < 60% may have obvious HBV risk factors,15 and approximately 65% are unaware of their infection.4,15 The risk-adaptive HBV screening and management clinical algorithm (Fig 2) thus represents the best clinical opinion of the panel in the context of a rather weak database. It is therefore important to recognize that key elements of this framework are not informed by evidence.
Fig 2.
Risk-adaptive hepatitis B virus (HBV) screening and management decision-making algorithm for patients with cancer before immunosuppressive therapy. anti-HBc, antihepatitis B core antibody (either total or immunoglobulin G); HBsAg, hepatitis B surface antigen.
HBV risk-factor screening.
National organizations have identified groups at risk of HBV,10,11 but few studies have determined whether HBV risk-based screening is effective in preventing HBV reactivation. The US Preventive Services Task Force conducted a systematic review30 of HBV screening and found only one cross-sectional study in a French sexually transmitted infections clinic that identified characteristics such as birth in a country of ≥ 2% HBV prevalence (Table 4), male sex, and unemployment status to be predictors of HBV infection; behavior-based risks such as injection drug use or high-risk sexual behaviors did not increase the sensitivity. Despite limitations in existing evidence, the US Preventive Services Task Force issued a grade B recommendation to screen persons from risk groups with an HBV prevalence of ≥ 2% (Table 5).29 The panel suggests HBV screening for patients with cancer and HBV risk factors (Table 5) before initiation of systemic cancer therapy.
Table 4.
Geographic Regions With Prevalence of HBsAg Positivity ≥ 2%
| Region | Countries |
|---|---|
| Africa | All |
| Asia | All |
| Australia and South Pacific | All except Australia and New Zealand |
| Middle East | All except Cyprus and Israel |
| Eastern Europe | All except Hungary |
| Western Europe | Malta, Spain, and indigenous populations in Greenland |
| North America | Alaska natives and indigenous populations in northern Canada |
| Mexico and Central America | Guatemala and Honduras |
| South America | Ecuador, Guyana, Suriname, Venezuela, and Amazonian areas of Bolivia, Brazil, Colombia, and Peru |
| Caribbean | Antigua and Barbuda, Dominican Republic, Grenada, Haiti, Jamaica, St Kitts and Nevis, St Lucia, and Turks and Caicos |
NOTE. Data adapted with permission.29
Abbreviation: HBsAg, hepatitis B virus surface antigen.
Table 5.
Risk Groups for HBV Infection With Prevalence ≥ 2% That Should Be Screened
| Group |
|---|
| Persons born in countries and regions with prevalence of HBV infection ≥ 2% |
| US-born persons not vaccinated as infants whose parents were born in regions with high prevalence of HBV infection (≥ 8%; eg, sub-Saharan Africa, Southeast and Central Asia) |
| HIV-positive persons |
| Injection drug users |
| Men who have sex with men |
| Those with household or sexual contact with persons with HBV infection |
HBV reactivation risk stratification.
The risk factors and mechanisms of HBV reactivation are not fully understood. From limited studies conducted in Asia, data regarding reactivation risk factors suggest that a high HBV DNA level, use of systemic corticosteroids, and cancer types such as lymphoma or breast cancer may predict risk of HBV reactivation.32 A recent systematic review reported that HBsAg-positive status, as well as receipt of certain therapies such as anthracyclines, anti-CD20 monoclonal antibodies, prolonged systemic corticosteroids, and certain tyrosine kinase inhibitors, confers moderate to high risk of developing HBV reactivation.33 HBV reactivation has been well described in patients with hematologic malignancies after hematopoietic cell transplantation, and routine HBV screening is recommended for these patients.14,15 The panel recommends HBV screening before anti-CD20 monoclonal antibody therapy and hematopoietic cell transplantation. It is likely that other potent B cell–depleting therapies would pose similar risks as anti-CD20 antibody therapy, and the panel suggests that patients receiving these potent agents be evaluated and managed in a fashion similar to that for patients receiving anti-CD20 agents. The panel acknowledges that there may be other cancer therapies that place patients at risk of HBV reactivation; however, the lack of strong evidence precludes the panel from making a more comprehensive recommendation that includes screening patients who are about to receive these other therapies: anthracyclines, prolonged corticosteroids, and certain tyrosine kinase inhibitors.
Clinicians should screen all patients who are about to undergo immunosuppressive therapies associated with a high risk of HBV reactivation, especially if estimated life expectancy is > 1 year. Risks and benefits of HBV screening and antiviral therapy, if indicated, should be discussed with patients who have multiple chronic conditions or whose estimated life expectancy is < 1 year and who are about to undergo highly immunosuppressive therapy.
As an alternative to the risk-adaptive HBV screening and management approach, two panel members (D.R.C., J.J.F.) proposed a strategy of universal testing for HBsAg and selective testing for anti-HBc. They used the following rationale: The risk of HBV reactivation is highest among patients who are HBsAg positive, and prophylactic therapy is an effective antiviral treatment.34,35 HBsAg testing is widely available, and previous studies have suggested that HBsAg testing is cost effective in select and even low-prevalence populations.36,37 Thus, universal HBsAg screening for all patients scheduled to receive systemic cancer therapy (ie, nonhormonal solid tumor or hematologic regimens) is a reasonable alternative that although yet to be rigorously studied, may be easier to implement than risk-based screening. Patients who screen positive for HBsAg should receive antiviral therapy during and for 6 to 12 months after completion of systemic cancer therapy. Introduction of antiviral therapy should not delay the onset of cancer chemotherapy but should ideally be started before or concomitantly with cancer treatment. Although HBV reactivation has been reported, but not systematically studied, in patients with resolved HBV infection, the risk is likely to be low, except in patients receiving anti-CD20 therapies or undergoing stem-cell transplantation.38 The optimal management strategy for patients with resolved HBV infection remains unclear, with small studies suggesting monitoring or prophylactic antiviral therapy to be effective.9,39,40 On the basis of these considerations and the current body of limited evidence, the two panel members advocated HBsAg testing for all patients scheduled to receive systemic cancer therapy and HBsAg and anti-HBc testing for patients scheduled to receive potent regimens, such as anti-CD20 therapies or stem-cell transplantation.
Antiviral Therapy
Prophylaxis refers to antiviral therapy started before or contemporaneously with systemic cancer therapy, and on-demand antiviral therapy refers to the initiation of therapy after evidence of HBV reactivation. According to the National Institute for Health Care Excellence HBV guideline, all immunocompromised patients who are known to be HBsAg positive should start antiviral prophylaxis before systemic therapy and continue it for a minimum of 6 months after stopping therapy18 and likely longer than 12 months for patients receiving anti-CD20 monoclonal antibodies (as described under Anti-CD20–Directed Monoclonal Antibodies and HBV Reactivation).5,9
All HBsAg-negative/anti-HBc–positive patients anticipated to receive B cell–depleting agents should be considered for antiviral prophylaxis18 or monitored closely and start antiviral therapy if HBV reactivation occurs.40 Once antivirals are initiated, they should be continued up to 12 months after cessation of therapy because of the risk of delayed HBV reactivation.5,9 However, there are insufficient data to determine the optimal strategy for HBsAg-negative/anti-HBc–positive patients receiving therapies not known to cause a high risk of reactivation. For this latter group of patients, the panel suggests monitoring of HBV DNA and ALT levels approximately every 3 months during therapy and initiation of on-demand antiviral therapy if there is evidence of HBV reactivation.
There are several anti-HBV medications available for prophylaxis and on-demand therapy: lamivudine, entecavir, adefovir, tenofovir, and telbivudine. Prophylaxis has been found to be more effective than on-demand therapy in preventing HBV reactivation, hepatic failure, and mortality.18 Entecavir has been found to be more effective than lamivudine,16,18,41 which has higher rates of viral resistance, thus limiting its use, especially for patients requiring long durations of systemic cancer therapies; however, entecavir is more expensive than lamivudine. One recent RCT found that the rates of HBV-associated hepatitis were lower in the entecavir group as compared with the lamivudine group (0% v 13.3%; P = .003).41 Other than this study, strong data are lacking to determine whether one antiviral therapy is more advantageous than another. Thus, the panel suggests that oncology providers seek comanagement with colleagues experienced in treating and managing patients with hepatitis B to provide optimal care for patients with chronic or clinically resolved HBV infection who require antiviral therapy.42
RESEARCH PRIORITIES
The ad hoc panel emphasizes the need for future collaborative research to better understand the mechanisms and predictors of HBV reactivation. Although the effect of anti-CD20 and other B cell–depleting agents on HBV reactivation risk is clear, many other chemotherapy agents have been reported to be associated with reactivation,43 but in an inconsistent manner. Furthermore, optimal HBV screening and management strategies for HBsAg-negative/anti-HBc–positive patients who are receiving systemic cancer therapy that is not considered high risk for reactivation are as yet unknown. Additional research is needed to investigate and identify the HBV reactivation risk with regard to individual cancer therapeutic agents or regimens and among patients with solid tumors. Stronger data, along with validated risk tools, are needed to determine optimal screening strategies before initiation of systemic cancer therapies. Meaningful and measurable health outcomes of patients with cancer and HBV infection need to be identified and systematically studied so that potential harms of over- as well as underscreening and treatment may be minimized. Future research is needed to identify optimal criteria to help clinicians in their decisions to start and stop antiviral prophylaxis.
Researchers should track potential HBV reactivation risk factors uniformly and across institutions. Uniform definitions of reactivation and hepatitis flares should be developed. Measurable and clinically meaningful outcomes should be identified and assessed over the treatment course and beyond. With improved screening strategies, more HBV-infected patients will be identified and started on antiviral therapies. However, clinical issues that are not yet understood include safety and drug interactions, adverse effects, and progression-free survival in patients with concomitant cancer and HBV infection. Overall, the panel recommends collaboration between oncology and hepatitis B experts to identify key clinical and research areas to reduce the incidence of HBV reactivation and to disseminate and implement scientific discoveries.
Additional information, including methodology supplements and clinical tools and resources, can be found at www.asco.org/pco/hepb. Patient information is available there and at www.cancer.net.
Acknowledgment
We thank Kenneth R. Carson, MD, PhD, Alison W. Loren, MD, MS, Supriya G. Mohile, MD, MS, Robin Zon, MD, and the Clinical Practice Guidelines Committee for their thoughtful reviews of and insightful comments on this document and Laurissa Gann for her assistance with figures.
Appendix
Table A1.
Panel Members
| Member | Affiliation |
|---|---|
| Andrew S. Artz, MD, MS (co-chair) | University of Chicago, Chicago, IL |
| Jessica P. Hwang, MD, MPH (co-chair) | University of Texas MD Anderson Cancer Center, Houston, TX |
| Devena E. Alston-Johnson, MD | Upstate Oncology Associates, Greenville, SC |
| Donna R. Cryer, JD (patient representative) | Global Liver Institute, Washington, DC |
| Jordan J. Feld, MD, MPH | Toronto Western Hospital Liver Centre, Toronto, Ontario, Canada |
| Barnett S. Kramer, MD, MPH | National Cancer Institute, Bethesda, MD |
| Anita L. Sabichi, MD | Baylor College of Medicine, Houston, TX |
| Sandra L. Wong, MD, MS | University of Michigan, Ann Arbor, MI |
Footnotes
Clinical Practice Guideline Committee approval: January 30, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Administrative support: Mark R. Somerfield
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jessica P. Hwang
No relationship to disclose
Mark R. Somerfield
No relationship to disclose
Devena E. Alston-Johnson
No relationship to disclose
Donna R. Cryer
Stock or Other Ownership: Bristol-Myers Squibb (I)
Consulting or Advisory Role: Novartis (Inst)
Jordan J. Feld
Research Funding: Gilead Sciences (Inst)
Barnett S. Kramer
No relationship to disclose
Anita L. Sabichi
No relationship to disclose
Sandra L. Wong
No relationship to disclose
Andrew S. Artz
Research Funding: Miltenyi, Neovii
REFERENCES
- 1.Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 2.Hwang JP, Fisch MJ, Zhang H, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract. 2012;8:e32–e39. doi: 10.1200/JOP.2011.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang JP, Fisch MJ, Lok AS, et al. Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center. BMC Cancer. 2013;13:534. doi: 10.1186/1471-2407-13-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Action Plan for the Prevention, Care and Treatment of Viral Hepatitis: Updated, 2014-2016. http://aids.gov/pdf/viral-hepatitis-action-plan.pdf.
- 5.US Food and Drug Administration. FDA drug safety communication: Boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) http://www.fda.gov/Drugs/DrugSafety/ucm366406.htm.
- 6.US Food and Drug Administration. Label information for rituximab. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103705s5414lbl.pdf.
- 7.US Food and Drug Administration. Label information for ofatumumab. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125326s059lbl.pdf.
- 8.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: The Quality Oncology Practice Initiative. J Clin Oncol. 2005;23:6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 9.Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 10.Lok ASF, McMahon BJ. AASLD practice guideline update: Chronic hepatitis B: Update 2009. http://www.aasld.org/sites/default/files/guideline_documents/ChronicHepatitisB2009.pdf.
- 11.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 12.McNamara C, Davies J, Dyer M, et al. Guidelines on the investigation and management of follicular lymphoma. Br J Haematol. 2012;156:446–467. doi: 10.1111/j.1365-2141.2011.08969.x. [DOI] [PubMed] [Google Scholar]
- 13.Dreyling M, Ghielmini M, Marcus R, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii76–iii82. doi: 10.1093/annonc/mdu200. [DOI] [PubMed] [Google Scholar]
- 14.Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin's lymphomas, version 4.2014. J Nat Compr Canc Netw. 2014;12:1282–1303. doi: 10.6004/jnccn.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections (version 2.2014) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 16.Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219. doi: 10.1053/j.gastro.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Hay AE, Meyer RM. Hepatitis B, rituximab, screening, and prophylaxis: Effectiveness and cost effectiveness. J Clin Oncol. 2012;30:3155–3157. doi: 10.1200/JCO.2012.43.7509. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence. Hepatitis B (chronic): Diagnosis and management of chronic hepatitis B in children, young people, and adults. http://www.nice.org.uk/guidance/CG165. [PubMed]
- 19.American Society of Clinical Oncology. ASCO provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. http://www.instituteforquality.org/asco-provisional-clinical-opinion-chronic-hepatitis-b-virus-infection-screening-patients-receiving. [DOI] [PubMed]
- 20.US Food and Drug Administration. Label information for obinutuzumab. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125486s000lbl.pdf.
- 21.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209–219. doi: 10.1038/nrgastro.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlotsky JM. Virology of hepatitis B and C viruses and antiviral targets. J Hepatol. 2006;44(suppl):S10–S13. doi: 10.1016/j.jhep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 26.Abbott Prism. Hepatitis B virus core antigen (G1-0036/R12) 2012. Nov,
- 27.Abbott Prism. Antibody to Hepatitis B surface antigen (GL-0023/R12) 2012. Nov,
- 28.Hammond SP, Swaminathan S, Bensinger WI, et al. Hepatitis B virus screening and potential reactivation in patients undergoing treatment for cancer. J Natl Compr Canc Netw. 2014;12:1655–1657. doi: 10.6004/jnccn.2014.0166. [DOI] [PubMed] [Google Scholar]
- 29.LeFevre ML. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]
- 30.Chou R, Dana T, Bougatsos C, et al. Screening for hepatitis B virus infection in adolescents and adults: A systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;161:31–45. doi: 10.7326/M13-2837. [DOI] [PubMed] [Google Scholar]
- 31. Reference deleted.
- 32.Yeo W, Zee B, Zhong S, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–1311. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221.e3–244.e3. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: A randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 35.Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Zurawska U, Hicks LK, Woo G, et al. Hepatitis B virus screening before chemotherapy for lymphoma: A cost-effectiveness analysis. J Clin Oncol. 2012;30:3167–3173. doi: 10.1200/JCO.2011.40.7510. [DOI] [PubMed] [Google Scholar]
- 37.Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29:3270–3277. doi: 10.1200/JCO.2011.35.1635. [DOI] [PubMed] [Google Scholar]
- 38.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 39.Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: A prospective study. Hepatology. 2014;59:2092–2100. doi: 10.1002/hep.26718. [DOI] [PubMed] [Google Scholar]
- 40.Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: A prospective study. J Clin Oncol. 2014;32:3736–3743. doi: 10.1200/JCO.2014.56.7081. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: A randomized clinical trial. JAMA. 2014;312:2521–2530. doi: 10.1001/jama.2014.15704. [DOI] [PubMed] [Google Scholar]
- 42.Abramson JS, Chung RT. Optimal antiviral prophylaxis against hepatitis B reactivation in patients receiving rituximab-based chemotherapy for lymphoma. JAMA. 2014;312:2505–2507. doi: 10.1001/jama.2014.16095. [DOI] [PubMed] [Google Scholar]
- 43.Keam B, Lee JH, Im SA, et al. Why, when, and how to prevent hepatitis B virus reactivation in cancer patients undergoing chemotherapy. J Natl Compr Canc Netw. 2011;9:465–477. doi: 10.6004/jnccn.2011.0045. [DOI] [PubMed] [Google Scholar]