Abstract
Reversibility of hepatic fibrosis and cirrhosis following antiviral therapy for hepatitis B or C has advanced the prospect of developing antifibrotic therapies for patients with chronic liver diseases, especially non-alcoholic steatohepatitis. Mechanisms of fibrosis have focused on hepatic stellate cells, which become fibrogenic myofibroblasts during injury through ‘activation’, and are at the nexus of efforts to define novel drug targets. Recent studies have clarified pathways of stellate cell gene regulation and epigenetics, emerging pathways of fibrosis regression through the recruitment and amplification of fibrolytic macrophages, nuanced responses of discrete inflammatory cell subsets and the identification of the ‘ductular reaction’ as a marker of severe injury and repair. Based on our expanded knowledge of fibrosis pathogenesis, attention is now directed towards strategies for antifibrotic therapies and regulatory challenges for conducting clinical trials with these agents. New therapies are attempting to: 1) Control or cure the primary disease or reduce tissue injury; 2) Target receptor-ligand interactions and intracellular signaling; 3) Inhibit fibrogenesis; and 4) Promote resolution of fibrosis. Progress is urgently needed in validating non-invasive markers of fibrosis progression and regression that can supplant biopsy and shorten the duration of clinical trials. Both scientific and clinical challenges remain, however the past three decades of steady progress in understanding liver fibrosis have contributed to an emerging translational success story, with realistic hopes for antifibrotic therapies to treat patients with chronic liver disease in the near future.
INTRODUCTION
A sustained effort over the past three decades to uncover the cellular and molecular basis of hepatic fibrosis is now yielding imminent success in treating this morbid consequence of chronic liver injury. Fibrosis, or the net accumulation of extracellular matrix (ECM) or scar, has been recognised for millennia in patients with chronic liver disease, yet it was considered intractable for most of medical history. Nonetheless, Perez-Tamayo1 presciently predicted the reversibility of fibrosis following the characterisation of collagenase activity in liver that could degrade ECM molecules.2 What has followed is a sustained assault on the problem, bringing us to a period of heightened clarity about the cells, mediators and intracellular signals that culminate in hepatic scar. This clarity, in turn, has led to rational mechanism-based antifibrotic strategies that are now being tested in clinical trials. This review will highlight both the established and emerging cellular mechanisms of hepatic fibrosis that establish a useful template for the understanding the basis for candidate antifibrotic strategies. We also highlight emerging challenges in clinical trials, and underscore key unanswered scientific and clinical questions for the future.
HEPATIC FIBROSIS AND CIRRHOSIS ARE REVERSIBLE
The vindication of Perez-Tamayo’s prediction in 1979 awaited the development of specific therapies for chronic liver disease that are now a mainstay of treatment, particularly for hepatitis B (HBV) and C (HCV). In retrospect, it was unrealistic to expect fibrosis to reverse until there were such therapies, since without them sustained injury would provoke ongoing fibrosis and repair. Fibrosis is reversible and cirrhosis (defined as the distortion of hepatic architecture and blood flow) may regress in some cases. The regression of cirrhosis has been observed in patients with iron and copper overload, alcohol-induced liver injury, chronic hepatitis B, C and D, hemachromatosis, secondary biliary cirrhosis, non-alcoholic steatohepatitis (NASH) and autoimmune hepatitis (reviewed in ref. 3). Among these diseases, reversibility seems especially likely in patients in whom HBV therapy suppresses viral replication,4 however, cirrhosis reversion is now also reported in HCV patients following sustained virologic response (SVR).5 Overall, up to 70% of patients with HBV or HCV cirrhosis will demonstrate reversibility on follow-up biopsies,4,5 but more extensive data for HCV are anticipated now that SVR rates exceed 90% using direct-acting antiviral therapies. Moreover, when reversal occurs in HCV, it leads to improved clinical outcomes, reduced portal pressure and decreased all-cause mortality.6 Remarkably, a subset of ~10% of patients with HCV may have persistent or even progressive fibrosis following SVR, which might reflect other concurrent underlying liver diseases, especially non-alcoholic fatty liver disease (NAFLD).7
The reversibility of advanced fibrosis and cirrhosis is less certain in NASH than in viral liver disease since no disease-specific therapies have been established yet. However, studies examining the behaviour of fibrosis after bariatric surgery clearly indicate some reversibility,8,9 although data are limited and more rigorous prospective studies are needed.
Even less is known about disease reversibility for other chronic liver diseases, but small reports cite improvements in autoimmune liver disease, biliary obstruction, hemochromatosis and other disorders (see ref. 10 for review). The unifying feature of these reports is the abrogation of the underlying diseases that precipitated the fibrosis.
CELLULAR SOURCES OF ECM
The discovery of hepatic stellate cell activation—a transdifferentiation from a quiescent vitamin A-storing cell to a proliferative myofibroblast—has provided a fertile foundation for organising approaches to antifibrotic therapies. While fibrogenic cells may derive from portal fibroblasts in cholestatic diseases11,12 the overwhelming evidence still supports activated stellate cells as the key source of ECM in parenchymal liver diseases, including recent elegant fate tracing analyses using genetic models.11,13,14 Regardless, each of these two cell types—stellate cells and portal fibroblasts—can generate myofibroblasts, whose molecular features and expression of potential antifibrotic targets are functionally similar in liver injury and fibrosis.
Stellate cell activation unfolds progressively in sequential stages; this paradigm provides a useful construct for defining fibrogenic events following liver injury15 (see figure 1). In particular, the ‘initiation’ phase, which refers to early events that render the quiescent stellate cell responsive to a range of growth factors, remains an important focus. Rapid induction of β-platelet-derived growth factor (PDGF) receptor, development of a contractile and fibrogenic phenotype and modulation of growth factor signalling are the cardinal features of this response. Resolution of fibrosis may be accompanied by senescence, inactivation or apoptosis of activated stellate cells (see below).
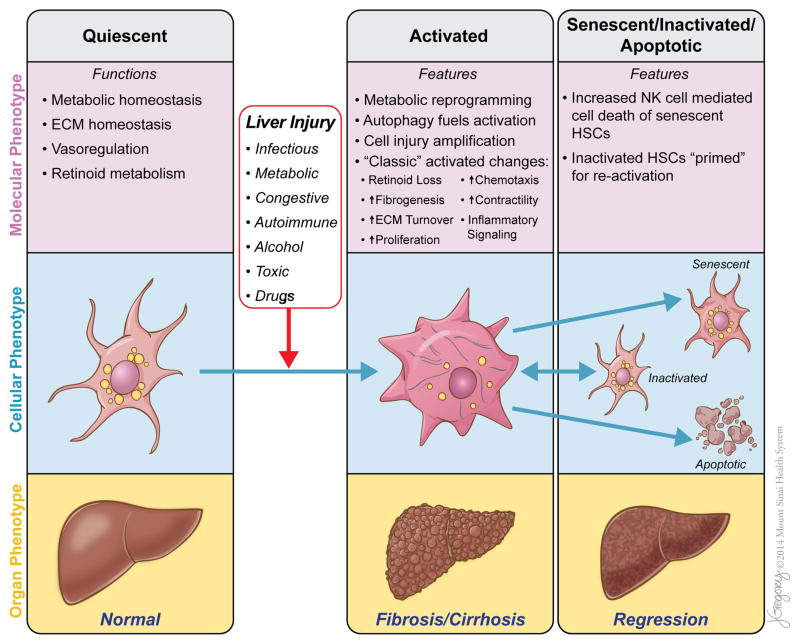
Figure 1.
Functions, features and phenotypes of hepatic stellate cells (HSCs) in normal and diseased liver. HSCs may exist as several different phenotypes with distinct molecular and cellular functions and features, each of which contributes significantly to liver homeostasis and disease. Quiescent stellate cells are critical to the normal metabolic functioning of the liver. Liver injury provokes the transdifferentiation of quiescent stellate cells to their activated phenotype, leading to metabolic reprogramming, increased autophagy to fuel the metabolic demands, amplification of parenchymal injury and the development of ‘classic’ phenotypic features of activated HSCs/myofibroblasts. Through these changes, activated stellate cells drive the fibrotic response to injury and the development of cirrhosis. As liver injury subsides, activated stellate cells can be eliminated by one of three pathways: apoptosis, senescence or reversion to an inactivated phenotype. Senescent stellate cells are more likely to be cleared by NK cell-mediated cell death while inactivated stellate cells remain ‘primed’ to respond to further liver injury. This reduction in the number of activated stellate cells contributes to the regression of fibrosis or cirrhosis and repair of the liver in most, but not all patients. The relative contribution of these three pathways of stellate cell clearance to fibrosis regression is not yet clear. ECM, extracellular matrix; HSCs, hepatic stellate cells. NK, natural killer.
The development of new tools and models to study and manipulate stellate cells in vivo has accelerated the elucidation of their function. These include promoters that drive transgenes selectively in stellate cells for cell-specific gene deletion (eg, lecithin retinol acyltransferase or LRAT),13 models to selectively ablate stellate cells in vivo,16 immortalised stellate cell lines17 and analysis of gene expression using arrays of isolated cells to define cell specific transcripts and potential therapeutic targets.18
While studies of hepatic fibrosis focus on intrahepatic cells, pathways and signals, it is critical to recognise that fibrosis is also greatly influenced by extrahepatic events as well, including signals derived from the gut, muscle and adipose, and by systemic vascular changes. Interorgan cross talk is especially relevant to the pathogenesis of NAFLD and NASH. These extrahepatic signals that impact fibrosis are summarised in figure 2.
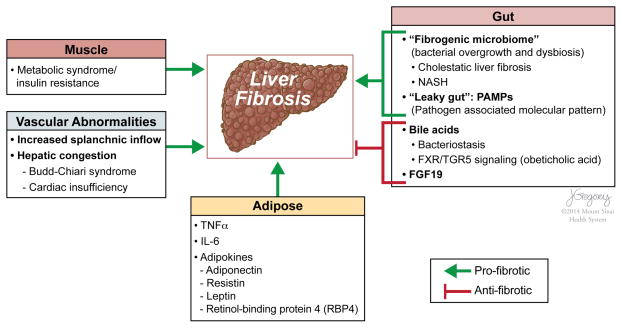
Figure 2.
Extrahepatic factors that affect liver fibrosis. In addition to intrahepatic injury signals, extrahepatic factors are increasingly recognised to drive liver fibrosis. Intestinal dysbiosis and bacterial overgrowth contribute to a ‘fibrogenic microbiome’, especially in cholestatic liver diseases and non-alcoholic steatohepatitis (NASH). Enterohepatic circulation of bile acids mediates bacteriostasis and promotes protection from hepatic fibrosis through increased farnesoid-X-receptor (FXR)/TGR5 signalling. Proinflammatory signalling (TNFα, IL-6) and adipokines secreted from adipose tissue mediate profibrogenic (eg, leptin, resistin) or protective (eg, adiponectin) effects on liver. Insulin resistance and metabolic syndrome are risk factors for progression in chronic liver diseases (eg, NASH, HCV). Hyperinsulinemia promotes steatosis, the generation of reactive oxygen species and lipid peroxides.19 Vascular abnormalities may also contribute to the development of hepatic fibrosis. The interactions that promote liver fibrosis are depicted with green lines and protective interactions with red lines.
MECHANISMS OF STELLATE CELL ACTIVATION
Intracellular responses
The complex network of intracellular events during stellate cell activation includes regulatory controls affecting transcription, translation, post-translation and epigenetics, among others. Among these many events, intracellular inflammasome activation is increasingly recognised as an important transducer of signals derived from inflammatory cells that is especially relevant to fatty liver disease.20 Another emerging signalling network is the nuclear receptor family, including farnesoid-X-receptor (FXR),21 peroxisome proliferator-activated receptors (PPAR),22 vitamin D receptor (VDR),23 retinoid receptors,22 Rev-erbα24 and liver-X-receptor (LXR).25 Other transcriptional events contributing to stellate cell activation include Wnt/β-catenin signalling,26 GATA427 and hedgehog signalling.14
Autophagy, a highly regulated intracellular pathway that preserves energy homeostasis, has been implicated in driving hepatic stellate cell activation by providing critical energy substrates through the hydrolysis of retinyl esters to generate fatty acids.28 The findings in part explain why stellate cells may lose retinoids as they activate, since the fatty acids are essential to fuel cellular activation. This autophagic response is also typical of mesenchymal cell fibrogenesis in other tissues, and is linked to activation of the unfolded protein response and endoplasmic reticulum stress.29 Although autophagy stimulates retinyl ester hydrolysis, the overall contribution of retinoid metabolism to stellate cell activation is still uncertain, however, because mice that lack retinoid droplets through genetic knockout of the storage protein LRAT have a preserved capacity to become activated, arguing against the necessity of stored retinoids for activation to occur.30 An additional contribution of retinoids may be through the conversion of retinol to retinaldehyde, then retinoic acid, which may be linked to collagen expression and transforming growth factor beta (TGFβ) activation through alcohol dehydrogenase 3.31 Retinoic acid then sensitises natural killer (NK) cells to kill stellate cells, thereby contributing to an antifibrotic effect.32 In contrast, retinol released from stellate cells suppresses NK cells, which reduces stellate cell killing and enhances their contribution to fibrosis.31
Autophagy may be part of a larger metabolic reprogramming response, which has been explored extensively in cancer but not in fibrogenic cells. Signals contributing to this reprogramming include hedgehog,33 LXR25 and most recently Rev-erbα, along with peroxisome proliferator-activated receptor gamma (PPAR-γ), which may be especially critical to preserving the adipogenic phenotype of stellate cells.24 Free intracellular cholesterol has been proposed as another intracellular metabolic signal that sensitises cells to activation, and may be especially relevant to fibrosis in fatty liver disease.34 Moreover, cholesterol and retinoid metabolism are linked by a lipid-associated protein, Rab18, that is a retinoic acid-responsive gene.35
Not surprisingly, the enhanced proliferation of stellate cells during activation engages the cell cycle machinery. Among cell cycle components, cyclin E1 has been best characterised as a driver of stellate cell proliferation that is epigenetically regulated by miR-195.36
Epigenetic regulation is an especially important mode of controlling stellate cell activation, because regulatory events must occur quickly following injurious stimuli, either by activating or repressing gene transcription, or by post-transcriptional control37 (see figure 3). Among epigenetic signals, microRNAs are an important layer of regulatory control in stellate cell activation and fibrosis.40 Implicated miRNAs include miRNA-21,41 miRNA-133a,42 miRNA-122,43 miRNA-214,44 miRNA-221/22245 and miRNA-29b46 among others. Studies of chromatin remodelling in hepatic stellate cells (HSCs) that clarify interactions between vitamin D receptor signalling and SMAD3 (a TGFβ signalling effector) point to an increasingly detailed understanding of gene regulation in stellate cells that could uncover novel therapeutic targets.23 Of these, myocardin-related transcription factor A (MRTF-A) has been identified as an epigenetic modifier both in stellate cells,47 and in fibrosis of other tissues including lung48 and heart.49 Remarkably, epigenetic programming is transmissible to offspring in rodent liver injury, and may influence the propensity to develop fibrosis in subsequent generations if they have liver injury.50
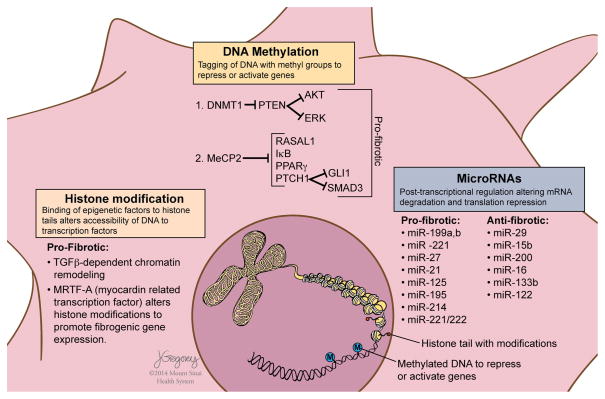
Figure 3.
Epigenetic mechanisms and post transcriptional gene regulation of hepatic stellate cells. ‘Epigenetics’ is defined as heritable traits that are not linked to changes in DNA sequence, involving mechanisms by which chromatin-associated proteins and post-translational modifications of histones regulate transcription.38 MicroRNAs mediate post-transcriptional regulation by promoting mRNA degradation and translational repression. Abnormal patterns of DNA methylation identified in liver fibrosis and activated stellate cells include, for example, hypermethylation of Phosphatase and Tension Homologue (PTEN) with consequent gene repression. PTEN negatively regulates the activation of ERK and AKT signalling pathways controlling cell cycling, proliferation, focal adhesion and cell migration.39 Repression of PTEN in activated stellate cells thereby promotes fibrogenesis. Similarly, hypermethylation of, and gene repression by MeCP2 of RASAL1, IkB, PPAR-γ and PTCH1 lead to inhibition of ERK signalling pathways, or loss of inhibition of GLI1 and SMAD3, respectively, thus promoting hepatic stellate cell (HSC) survival (IkB), and HSC activation and fibrogenesis. Histone modifications with profibrotic effects have been identified in activated HSCs and include MRTF-A and TGFβ-dependent chromatin remodeling leading to the altered binding of vitamin D receptor and SMAD3 mediated transcription of fibrogenic genes. Examples of microRNAs promoting antifibrotic and profibrotic effects are shown.
Efforts to uncover nodal regulators of stellate cell activation have discovered a G protein exchange factor, GIV/Girdin, that lies at a convergent point of several intracellular pathways regulating fibrosis, including PI3K-Akt-FoxO1, TGFβ-SMAD and cAMP-PKA-pCREB (cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA)-phosphorylated-cAMP-responsive element-binding protein.51 While targeting this molecule may not be sufficiently specific as a fibrosis therapy given its widespread expression, the findings nonetheless provide an example where the identification of a signal integrator such as GIV/Girdin could provide an attractive target to inhibit stellate cell activation.
Initiating events
Epithelial cell injury
Damage to either hepatocytes in parenchymal liver injury or biliary epithelium in cholestatic diseases is a sine qua non of chronic liver disease, and attention has focused on the growing list of mediators that these cells elicit to drive inflammation and fibrosis. Among the key signals are reactive oxygen species,52 hedgehog ligands,14 nucleotides53 and cell death signals.54 Moreover, different forms of cell death are now appreciated including autophagic, apoptotic, necrotic and pyroptotic death, each with its own distinct intracellular drivers and repertoire of extracellular signals released.20
Changes in ECM composition
Initiating events in stellate cell activation occur on a background of progressive changes in the surrounding ECM within the sub-endothelial space of Disse. Among these, the enhanced density of ECM leads to increasing matrix stiffness, which is a significant stimulus to stellate cell activation, at least in part through integrin signalling.55
Intestinal dysbiosis
Dietary fat is thought to contribute to intraluminal dysbiosis (ie, altered microbiome), with the release of pathogen-associated molecular patterns PAMPs and activation of toll-like receptors (TLRs). This contributes to a triad of lipogenesis, inflammation and ultimately oncogenesis.19,56 Indeed, a fibrogenic microbiome has been identified in animal models whose transplantation can induce injury and fibrosis in recipient mice57 (see figure 2).
Perpetuating pathways
The stellate cell that is primed by ‘initiating’ stimuli can then respond to a host of cytokines and growth factors. These signals conspire to generate scar through enhanced proliferation, contractility, fibrogenesis, matrix degradation and proinflammatory signalling. While earlier models suggested that the pathways of activation were identical regardless of the disease, it is now clear that there are disease-specific pathways of fibrosis, and, moreover, that not all cytokine pathways are necessarily activated in parallel.58 This is especially relevant to NAFLD, where there are many convergent pathogenic pathways.59 Also important are the increasingly nuanced pathways of immunity and inflammation driven by different families of inflammatory cell types and their subsets, each of which may either promote or inhibit fibrosis (see refs 3,60–62 for reviews). These pathways and cell types are summarised in figure 4.
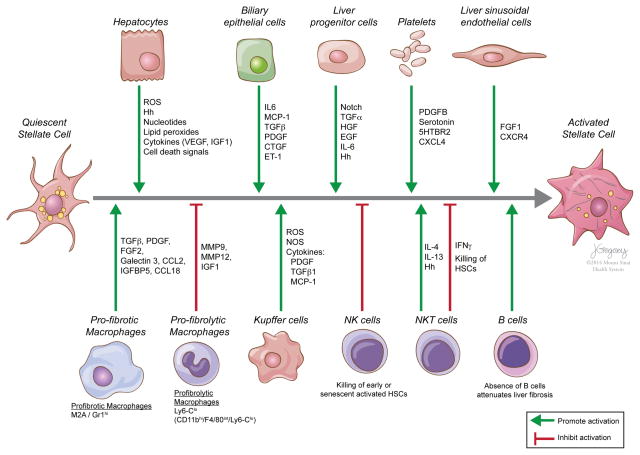
Figure 4.
Inflammatory and immune cell interactions that promote or inhibit the activation of hepatic stellate cells. Hepatic cells promoting (green lines) or inhibiting (red lines) the activation of quiescent stellate cells to activated, fibrogenic hepatic stellate cells are shown. Examples of common mediators of these responses are included. Hh, hedgehog ligands; NK, natural killer.
PATHWAYS OF FIBROSIS REGRESSION
At what point cirrhosis becomes irreversible is uncertain, but irreversibility becomes more likely as the scar thickens, becomes more acellular and is chemically cross-linked. A more refined classification suggests that cirrhosis is really comprised of several substages.63 This finer classification may ultimately help define in whom reversibility is possible. Regardless, the successes in demonstrating fibrosis regression even in patients with cirrhosis indicate that the liver harbours innate pathways to regress scar, and therein may lie clues to mimic these endogenous pathways using novel therapies.
Based on the clinical experience of fibrosis summarised above, emphasis is now placed on pathways of regression, including changes in the cytokine and immune microenvironment, the ECM composition and the behaviour of stellate cells. Modulation of the cytokine microenvironment and altered inflammatory cell composition are emerging as key elements of this response. Interestingly, vascular endothelial growth factor, typically considered as a profibrotic, proangiogenic signal, may also be required for fibrosis regression.64
Over time, the reversal of HCV-associated cirrhosis and experimental cirrhosis evolves from a micronodular to a macronodular cirrhosis. Moreover, older fibrotic matrix that persists for more than a year is characterised by pauci-cellularity and increased ECM cross-linking.3 Collagen cross-linking enhances the resistance of collagen to degradation, and is a critical determinant of fibrosis irreversibility. Elastin, another non-collagenous matrix component, may also contribute to the resistance to fibrosis reversion. Elastin accumulates in mature cirrhosis and is dependent on macrophage-derived matrix metalloproteinase 12 (MMP12) for degradation.65 Recent clinical studies that incorporate serum elastin into algorithms for fibrosis assessment are more accurate in diagnosing cirrhosis; for example, the Elasto-Fibro-Test outperforms Fibroscan or Fibrotest alone.66
Reduction in the number of activated HSCs is critical to the reversibility of fibrosis. Three major pathways help eliminate fibrogenic, activated HSCs: (1) apoptosis3 (2) senescence67 and (3) reversion to an inactivated phenotype68,69 (see figure 1). The apoptosis of activated stellate cells has been documented in rodent experimental fibrosis model (bile duct ligation and carbon tetrachloride (CCl4)). Cellular senescence is a genetically controlled programme preventing cell division once cells exceed a finite proliferative capacity; HSCs undergo senescence and then accumulate in experimental hepatic fibrosis.67 Senescent HSCs are also targeted by NK cells for clearance in vivo, thereby additionally contributing to fibrosis resolution. There is also solid evidence of ‘deactivation’ or reversion of activated stellate cells to a more inactivated state in rodent models of fibrosis.68,69 However, these reverted cells remain ‘primed’, with an enhanced capacity to reactivate upon re-exposure to fibrogenic stimuli. It remains uncertain which of these three modes of reducing the burden of activated stellate cells during fibrosis regression (ie, apoptosis, senescence and/or reversion) is most important in vivo, but current evidence suggests that reversion rather than senescence or apoptosis may predominate.
OTHER CELLULAR CONTRIBUTIONS TO THE FIBROTIC MILIEU
Most attention has focused on stellate cell and myofibroblast responses given their critical roles in ECM production, yet liver injury elicits a complex multicellular response involving other resident cells including hepatocytes, macrophages, sinusoidal endothelium and distinct families of infiltrating immune cells including B cells, NK and NKT cells and myeloid-derived suppressor cells, among others3,61,70 (see figure 4).
Macrophages are a key cellular determinant of the resolution of liver fibrosis, and their heterogeneity reflects distinct subsets that may have widely divergent functions, some profibrotic and others antifibrotic.62 Recent studies have highlighted specific fibrolytic subsets (‘Ly-6C+ lo’ cells) of macrophages that are expanded during fibrosis regression.71 In addition to directly promoting fibrosis resolution via the production of MMPs, macrophages can also mediate anti-inflammatory effects, for example, by differentiating into regulatory macrophages that produce suppressor cytokines locally.72
Sinusoidal endothelial cells have been identified as critical cellular switches that can drive either liver regeneration or fibrosis.73,74 Proregenerative activities of these cells are promoted by the induction of CXCR7, whereas profibrotic signals are driven by fibroblast growth factor-1 (FGF1) and CXCR4, which are paracrine stimuli to stellate cells.73 These findings reinforce the interdependence of sinusoidal endothelial and stellate cells, as underscored by earlier studies.75,76 They also emphasise the growing range of activities of chemokines, which like the cells that produce them, can have widely divergent activities in promoting or attenuating hepatic fibrosis.60
A pathologic feature known as the ‘ductular reaction’ containing progenitor cells, activated stellate cells and ECM is increasingly linked to hepatic fibrosis progression in liver injury models and human disease.77,78 The temporal and functional relationships between the elements of the ductular reaction are uncertain, and it remains unclear if the ductular reaction determines whether the outcome of liver injury is regenerative, fibrotic or both. A recent study further suggests that stellate cells can acquire progenitor properties, raising the prospect that some of the ductular reaction contains progenitor cells derived from stellate cells.79 Studies of this type that disentangle the elements and origins of the ductular reaction will likely yield insights in our understanding of liver homeostasis and the response to injury.
ANTIFIBROTIC THERAPIES
The increasing evidence that fibrosis is a dynamic and reversible process, the clarification of the underlying sources and mediators of fibrosis progression and advances in non-invasively assessing fibrosis have generated enthusiasm towards developing effective antifibrotic drugs, although none are approved yet.80 In reality, there may already be many existing drugs with well-established safety profiles, whose mechanism of action will be also antifibrotic even though they have been developed for other indications. For example, drugs recently approved for pulmonary fibrosis merit consideration for the treatment of fibrosis in other organs including liver, although the duration of therapy, willingness of patients to tolerate adverse events and endpoints of clinical trials across organs are likely to be very different.
Increasingly, targets for repurposed drugs can be uncovered using high throughput methods combined with ‘big data’ analysis.81 Key challenges include the decades-long natural history of chronic liver disease that will require long-term pharmacologic intervention to prevent or reverse cirrhosis, and the lack of a standardised, accepted non-invasive endpoints for fibrosis assessment.
With the astonishing success of antivirals for HBV and HCV, current efforts to develop antifibrotics are now focused almost entirely on NASH-related fibrosis. From the perspective of unmet need this is a reasonable strategy, but should not overlook the substantial public health impact of other liver diseases, especially alcoholic and autoimmune liver diseases as well as paediatric liver diseases and sclerosing cholangitis, for which there are no medical therapies.
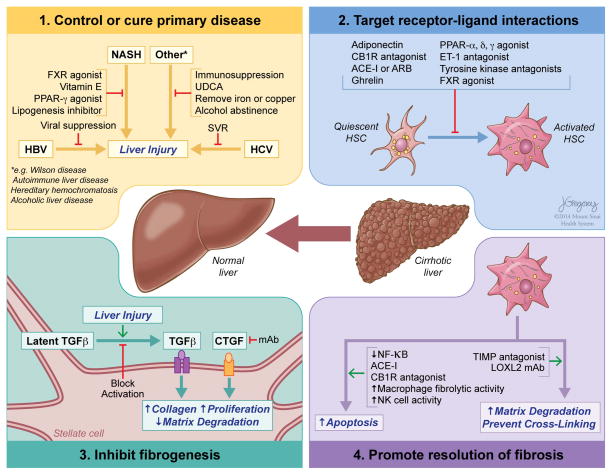
There are several points of attack in developing antifibrotic agents that are described in detail in the following sections and summarised in figure 5.
Figure 5.
Mechanisms by which antifibrotic therapies may lead to fibrosis regression. (1) Disease-specific therapies that control or cure the underlying disease are still the most effective antifibrotic approach. (2) Targeting receptor–ligand interactions with either established or experimental drugs to reduce hepatic stellate cell activation will attenuate fibrosis development, with multiple potential strategies under development. (3) Inhibition of the most potent of the profibrogenic pathways, for example, preventing activation of latent TGFβ, or blocking the activity of CTGF, are among the more promising antifibrotic strategies. (4) The resolution of fibrosis can be promoted by enhancing the apoptosis of activated hepatic stellate cells either with drugs or through the activity of either NK cells and by increasing the degradation of extracellular matrix, by fibrolytic macrophages or preventing its cross-linking with antagonists to LOXL2. FXR, farnesoid-X-receptor; PPAR, peroxisome proliferator-activated receptor; UDCA, ursodeoxycholic acid; SVR, sustained virological response; CB1, cannabinoid receptor type 1; ARB, angiotensin II receptor blocker; ET-1, endothelin 1; TGFβ, transforming growth factor β; CTGF, connective tissue growth factor; mAb, monoclonal antibody; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer; TIMP, tissue inhibitor of metalloproteinase; LOXL2, Lysyl oxidase 2.
Control or cure the primary disease or reduce tissue injury
As detailed above, the clearance or control of the underlying aetiology is the most effective antifibrotic treatment. In addition, a class of compounds known as ‘hepatoprotectants’ are being developed to minimise the release of damage signals from injured epithelial cells that drive inflammation and fibrosis. Among these are molecules to block apoptosis of hepatocytes,82 and one such agent, emricasan (previously called IDN-6556)83 is currently in clinical trials for several liver-related indications. Similarly, the inhibition of cathepsin-B, a lysomal cysteine protease, attenuates liver injury in experimental models and is being pursued as a therapeutic strategy.84
Other agents attempt to reduce steatosis in an effort to attenuate ‘lipotoxicity’ associated with fatty liver disease by inhibition of lipogenic pathways, in particular acetyl CoA-carboxylase.85 Antioxidants also exert a preventive effect on hepatocyte injury but may also be directly antifibrotic.86 For example, vitamin E is effective in patients with NASH. In a large National Institutes of Health trial (‘Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis’ (PIVENS) trial) vitamin E led to clear histological regression, with no fibrosis progression.87 Nicotinamide adenine dinucleotide phosphate, reduced (NADPH) oxidase (NOX), an enzyme that generates oxidant stress, is activated by angiotensin II (Ang II) in inflammatory regions within the liver, and small molecule antagonists of NOX are already available for human testing.88 Cysteamine, a precursor to glutathione, is being evaluated in paediatric NAFLD.89
Since persistent inflammation almost always precedes and accompanies fibrosis, drugs that target the inflammatory cascade may have antifibrotic activity. Chemokines have been widely implicated in inflammatory liver diseases including NASH, and antagonists to their receptors are emerging as potential therapies based on preclinical efficacy.60,90 Similarly, an antagonist to interleukin-1 receptor was effective in an animal model.91
A recombinant form of serum amyloid P has been tested extensively in fibrotic lung models, where it attenuates monocyte differentiation and may facilitate clearance of debris.92 Similarly, an antagonist to the lysophosphatidic acid 1 receptor has potent anti-inflammatory and antifibrotic activity in mouse lung fibrosis,93 and was well tolerated in a human phase 1 trial in healthy adults. Studies using a carbohydrate molecule that inhibits galectin-394 show benefit in animal models of hepatic fibrosis, with a human trial underway.
Some of the metabolic benefit of FXR agonism may be through its ability to induce fibroblast growth factor 19 (FGF19), a gut-derived factor that inhibits fatty acid synthesis in liver.95,96 In addition, FGF21, which functions as a circulating hormone, also improves insulin sensitivity and clearance of lipids, and has been proposed as a potential hepatoprotective agent.97 Recently, a large randomised trial of the FXR agonist obeticholic acid in patients with NASH, over 50% of whom were diabetics, showed clear improvement in the NAFLD activity score and a mean reduction in serum alanine transaminase (ALT) and in fibrosis stage in the treated patients.98 Of note, there was a mean elevation in serum low-density lipoprotein (LDL) levels in treated patients, which were also reported in an earlier 6-week trial of the drug,99 as well as an increased risk of pruritus, but on balance the study has been viewed as encouraging, in particular because of the improvement in fibrosis, but larger trials are expected to confirm efficacy and address safety concerns.100
Target receptor–ligand interactions and intracellular signalling
The discovery of membrane and nuclear receptors expressed by stellate cells that have been previously identified in other tissues has opened new possibilities for antifibrotic therapies.
Neurochemical receptors
Cannabinoids remain an attractive target for modulating hepatic fibrosis.101 CB1 antagonism is a promising strategy and CB1 antagonists that do not cross the blood–brain barrier are being developed. Antagonism of the 5HT-2B receptor may both antagonise fibrosis and enhance hepatic regeneration.102
The renin–angiotensin system
Ang II is secreted by stellate cells, and binds to the angiotensin II type 1 (AT1) receptor, with intracellular effects mediated by JAK2.103 Blocking the renin–angiotensin system by angiotensin-converting enzyme (ACE) inhibitors or AT1 receptor blockers (ARBs) may be an effective strategy in the treatment of liver fibrosis, and is currently in human trials. One study identified synergistic activity of AT1 and cannabinoid signalling through receptor heterodimerisation,104 providing a rationale to attempt combination therapies using ARB and CB1 receptor antagonists in fibrosis in hopes of achieving a synergistic effect on fibrosis.
Endothelin 1
Endothelin 1 (ET-1) and NO are antagonistic counter-regulators controlling HSC contractility and vasorelaxation, respectively. ET-1 is a vasoconstrictor with a potent effect on the hepatic vasculature. High levels of endothelin 1 and endothelin receptors are present in cirrhosis. The blockade of endothelin 1 type A receptor is antifibrotic in rodents and also improves portal hypertension, however, initial trials using first generation human ET-1 receptor antagonists were associated with some hepatotoxicity, but newer, safer agents are anticipated.
Adipokines
As obesity becomes increasingly prevalent, studies seek to define the role of adipose derived hormones, or adipokines, in contributing to the complications of obesity, including the metabolic syndrome and hepatic fibrosis. Leptin and its natural antagonist adiponectin are adipokines secreted by adipose tissue and stromal cells, especially stellate cells. Adiponectin levels are inversely correlated with body fat and antagonise fibrogenesis; therapeutic efforts in animal models are promising.105 Additionally, recombinant ghrelin exerts hepatoprotective and antifibrotic effects in liver;106 further studies need to determine its safety, tolerability and efficacy of all adipokines as therapeutics human liver disease.
Tyrosine kinase receptors
Many proliferative cytokines, including PDGF, FGF and TGF-α signal through tyrosine kinase receptors. For example, sorafenib, a multiple receptor tyrosine kinase inhibitor approved for therapy in hepatocellular carcinoma, targets the PDGF receptor and Raf/ERK signalling pathways and is antifibrotic in animal models.107 Downstream mediators of these receptors, in particular Rho/Rac and ROCK2, are appealing targets as well.108 Similarly, imatinib, a small molecule tyrosine kinase antagonist used in chronic myeloid leukaemia (CML) and GI stromal tumours, is antifibrotic.109,110 Nilotinib is another novel small molecule tyrosine kinase inhibitor of Bcr-Abl that reduces liver injury and fibrosis through multiple mechanisms.111
Nuclear receptors
As reviewed above, stellate cells express a diverse group of nuclear transcription factor receptors, including PPAR-γ, FXR and pregnane X receptor. Thiazolidones, antidiabetic agents that are PPAR-γ agonists, reduce collagen expression and HSC activation in vitro, however, clinical trials have yielded mixed results to date. A combined PPAR-α/δ agonist shows promise both in animal models and is currently in clinical trials.112 Obeticholic acid, described above, also reduces portal hypertension in experimental models of fibrosis.95,99,113 While LXRs function as key regulators of lipogenesis and modulate the immune system,25 LXR ligands are limited by hepatotoxicity due to induction of de novo lipogenesis.
Inhibit fibrogenesis
TGFβ1 is the most potent stimulus to the synthesis of collagen I and other matrix constituents, and thus inhibiting its actions remain a major focus of antifibrotic efforts in liver, either by blocking circulating TGFβ1, antagonising its receptors and/or blocking its activation at the cell surface. The challenge is to restrict any inhibitors to the fibrotic milieu, since systemic TGFβ1 inhibition could provoke inflammation or enhance epithelial growth and neoplasia. One approach being tested in lung fibrosis is to inhibit cell surface activation of TGFβ1 by interfering with an integrin receptor (αvβ6) that is essential for the cytokine’s activation.114 The identical approach may not be effective in liver, however, because the integrin subtypes may differ, but the inhibition of the αv subunit alone is effective in reducing fibrosis in animal models of liver injury.55
Connective tissue growth factor (CTGF/CCN2) is also a potent fibrogenic signal. FG-3019, a human monoclonal antibody against CTGF,115 has been tested in lung fibrosis and its now being assessed as a treatment for liver fibrosis in patients.
Promote resolution of fibrosis
Increase matrix degradation
Strategies to enhance the degradation of the collagen-rich ECM seek to increase the activity of endogenous matrix-degrading enzymes or to neutralise natural antagonists and mask collagenase activity, in particular tissue inhibitors of metalloproteinases (TIMPs). Proof-of-principle studies have succeeded using TIMP antagonists in animal models, for example,116 but no human studies have been reported to date.
Impressive results were reported in several animal models using a monoclonal antibody that inhibits the collagen cross-linking enzyme Lysyl oxidase 2.117 The assumption is that collagen that is not fully cross-linked is more susceptible to degradation by endogenous collagenases and other enzymes, but additional mechanistic studies are needed. Nonetheless, several clinical trials are underway testing this strategy in both liver and lung fibrosis.
Stimulate clearance of activated stellate cells
Myofibroblast clearance by apoptosis is a feature of the liver’s endogenous response to remove scar, as reviewed above. The relative apoptotic activity of stellate cells reflects a balance between apoptotic stimulation and survival signals, which can be manipulated therapeutically. Increased survival of stellate cells may result from enhanced expression of antiapoptotic proteins such as Bcl-2, and by transcription factors, especially NF-κB. Inhibition of NF-κB by gliotoxin,118 a fungal product, accelerates recovery from fibrosis in an animal model, which has provoked the use of sulfasalazine as an antifibrotic because of a similar mechanism of action. Angiotensin-converting enzyme inhibitors also reduce myofibroblast survival by upstream inhibition of NF-κB signalling.118 Increased myofibroblast apoptosis also results from use of either a CB1 antagonist (rimonabant) or a 5HT antagonist.
NK cells can kill activated stellate cells. Thus, amplifying NK cells could be a novel approach to treating liver fibrosis.119 Production of interferon gamma (IFN-γ), a hallmark of NK cell activation, is another important mechanism contributing to the antifibrotic effects of NK cells. While a trial of systemic interferon gamma was negative, more recent approaches attempt to target its delivery to activated stellate cells using receptor-mediated uptake.120 Similarly, targeted delivery of Rho kinase antagonist merits further evaluation.121
Bone marrow or cell transplantation
The use of bone marrow progenitor cells to promote regeneration and enhance matrix degradation is an intriguing and somewhat controversial new approach to antifibrotic therapy. In animal models, BM transplantation improves liver function and ameliorates hepatic fibrosis. Still, a preferred route would be to define which cells within bone marrow are fibrolytic and/or regenerative, and amplify either their number or activity pharmacologically, or alternatively to administer isolated, purified cell types that promote regeneration and matrix degradation, for example, macrophages.122,123
Current clinical trials
Currently there are over 500 trials under ‘liver fibrosis’ and an additional 500 listed under ‘fatty liver’ on clinicaltrials.gov, some of which are therapeutic trials, with many observational studies and diagnostic trials included as well. This rapidly increasing number underscores the intense interest in antifibrotic therapies both in the academic and in the commercial spheres.
REGULATORY CHALLENGES IN DEVELOPING NOVEL DRUGS FOR HEPATIC FIBROSIS
Liver biopsy—the gold standard, but in need of replacement
A key challenge limiting progress in the testing of antifibrotic drugs is the lack of sufficient endpoints that are noninvasive, yet correlate well with clinical outcomes. Currently, all clinical trials of antifibrotic drugs require liver biopsy to assess fibrosis before and after treatment. This requirement imposes several limitations on clinical trial design including the invasive nature of biopsy, and therefore the limited access to tissues at intermediate time points during a trial. Moreover, while biopsy is highly informative, it does not necessarily predict outcomes well, although the use of quantitative assessment of fibrosis by morphometry significantly improves clinical outcome prediction.124,125 Moreover, even when cirrhosis is established, collagen continues to accumulate, yet standard scoring systems cannot detect this increase, whereas morphometry can.126 Still, the biopsy is prone to sampling variability that is not necessarily mitigated by collagen morphometry. Moreover, in trials that include patients who are clinically stable, reliance on clinical events as a ‘hard’ endpoint is desirable but not realistic, as few patients will have decompensating events during the study interval. To incorporate clinical events as an endpoint, patients with more advanced disease would need to be enrolled, yet this may not make sense for some antifibrotic drug targets. For example, trials that test drugs to attenuate inflammation or reduce metabolic derangements in NASH are likely to be more effective in noncirrhotic patients, where clinical events are rare. The stratification of risk for progression should ideally be incorporated into clinical trial design, but this is not yet possible. While genetic determinants of fibrosis progression have been well validated in HCV,127 a similar fibrosis risk score has been elusive in NASH, probably because the disease is multifactorial and not due to identical aetiologies in all patients, even though their clinical phenotypes may be similar.
Potential surrogate markers
Current efforts are aggressively seeking surrogate markers or non-invasive determinants that can supplant biopsy. For example, non-invasive imaging using MR technologies or elastography may emerge as indicative of drug response.128 These experimental findings nicely complement the increasing use of Fibroscan, MR elastography, ARFI and Supersonic shear wave assessments, which are now established clinical techniques which non-invasively assesses hepatic stiffness as a reflection of ECM content.129 Most validation of these markers has been performed in HCV and they may not be as accurate in NAFLD.130 Newer modalities are also being developed using collagen-specific contrast agents or special MR techniques.131,132 Increasing evidence suggests that these technologies may be useful in quantifying fibrosis regression.133 Serum markers of fibrogenic activity would be ideal, in that they could be sampled regularly and might even indicate response to therapy before the biopsy is likely to change, but none are yet validated in this setting.
Liver ‘function’ tests
An alternative and/or complementary diagnostic strategy is the use of tests that assess underlying functional liver reserve, whether using breath tests134 or substrate clearance methods,135 to predict clinical outcomes. One goal of these efforts is to establish their correlation with hepatic venous pressure gradient, since this measure clearly correlates with outcomes.136 Functional tests especially appealing because the functional reserve capacity of the liver is likely to track closely to risk of clinical events and long- and short-term outcomes.
PATIENT SELECTION FOR ANTIFIBROTIC TRIALS
Defining the criteria for selection of future antifibrotic treatment candidates is an increasingly complex issue, as most trials currently being planned are now directed towards NASH, as noted above. As a result, the choice of which patients to enroll in clinical trials (ie, entry criteria) will be determined in part by the purported mechanism of action of the candidate drug. For example, anti-inflammatory agents are likely to have more benefit in intermediate stages of disease before advanced cirrhosis is established, whereas drugs that promote the degradation of matrix may be especially useful in more advanced disease. Patient stratification will also be based on rate and risk of progression, disease stage and/or fibrosis content.
Similarly, the tolerability and ease of administration will also impact on the choice of patients suitable for clinical trials. Parenteral therapies will be most appealing in patients who are at an increased risk for progression to cirrhosis or decompensation. In contrast, oral agents are especially appealing in patients who require long-term therapy to prevent progression.
Adverse events will also influence the choice and attractiveness of new therapies. It will be difficult to ensure compliance in patients who are asymptomatic if the medications create new symptoms. Thus, treating fatty liver disease will be akin to the treatment of other chronic conditions including hypertension and heart disease, where long-term therapy is expected to attenuate the progression and risk of decompensation, but not at the expense of the quality of life.
FUTURE PROSPECTS
Scientific challenges
Despite dramatic advances in our understanding of hepatic fibrosis pathogenesis, fundamental questions remain. These include: (1) Why does the liver regenerate? We still do not understand this unique response of liver that distinguishes the organ from all other adult tissues; (2) What is the link between fibrosis and impaired regeneration? As fibrosis advances regenerative capacity is diminished, yet mechanisms controlling and possibly linking these two divergent responses are unclear; (3) What is the link between fibrosis and liver cancer? Liver cancer rarely develops until fibrosis is extensive, yet our understanding of underlying mechanisms behind this association is incomplete; (4) What role do stem cells play in normal liver, fibrosis, regeneration and cancer? The still mysterious role of the ductular reaction in fibrosis may in part answer this question, but our current knowledge is still limited.
Clinical challenges
We are entering a new era where long-term therapy of inflammation and fibrosis is becoming a reality. The remarkable regenerative capacity of human liver continues to amaze practitioners and scientists, even though its basis is obscure. Much like the evolution of treatment for chronic viral hepatitis, progress in establishing antifibrotic therapies is likely to be iterative and progressively refined by results from early clinical trials. Also, we will learn which therapeutic targets are most potent in attenuating disease—a priori, such predictions are risky, and even animal model data will not fully inform this issue. As in viral hepatitis and other chronic diseases including cancer, evidence of efficacy for a single drug will prompt attempts at combination therapy in which more than one target is engaged simultaneously. The testing of combination therapies will first require evidence that the individual components of the combination each has some efficacy, however, before they can be tested in combination. In principle, however, targeting different elements of the pathogenic sequence is appealing, for example, blocking fat accumulation and inflammation in NASH, with or without direct fibrogenesis inhibitors. We also lack robust non-invasive markers that correlate with outcomes. We still await evidence that the natural history of fatty liver disease can be altered and result in reduced morbidity and mortality—this is the ultimate goal of current therapeutic efforts.
Key messages.
Discoveries that have elucidated the cellular and molecular basis of hepatic fibrosis are now (being translated into new therapeutic approaches, which are further encouraged by clinical evidence that fibrosis and even cirrhosis can be reversible in human liver disease.
Hepatic stellate cell activation—a transdifferentiation of a quiescent vitamin A-storing cell to a proliferative myofibroblast—is a central event in hepatic fibrosis development. Recent insights into the transcriptional, translational, post-translational and epigenetic events that control activation are yielding new treatment strategies.
Liver injury provokes a multicellular response involving resident cells and families of infiltrating immune cells including B cells, natural killer (NK) and NKT cells, dendritic cells, macrophages and myeloid derived suppressor cells, which conspire to either generate hepatic fibrosis or promote regression.
Potential approaches to treat fibrosis include efforts to: (1) Cure or control underlying disease; (2) Target receptor–ligand interactions; (3) Inhibit fibrogenesis and (4) Promote resolution of fibrosis.
Regulatory challenges still limit the clinical testing of antifibrotic drugs. These include choosing the most appropriate study populations to test new agents, and the lack of non-invasive markers instead of liver biopsy for use as a clinical trial endpoint.
Remaining fundamental questions in liver fibrosis and repair include: (1) What are the signals and mechanisms that endow the liver with its unique capacity to regenerate? (2) What is the link between fibrosis and impaired regeneration? (3) What are the features of the fibrotic and cirrhotic liver that confer a heightened risk of cancer? and (4) What role do stem cells play in normal liver homeostasis, the fibrotic response, hepatic regeneration and cancer?
Acknowledgments
Funding National Institute of Diabetes and Digestive and Kidney Diseases. NIH grant numbers RO1DK56621, RO1AA020709.
Footnotes
Contributors: All authors contributed actively to the writing and generation of figures for the work.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Perez-Tamayo R. Cirrhosis of the liver: a reversible disease? Pathol Annu. 1979;14 (Pt 2):183–213. [PubMed] [Google Scholar]
- 2.Okazaki I, Maruyama K. Collagenase activity in experimental hepatic fibrosis. Nature. 1974;252:49–50. doi: 10.1038/252049a0. [DOI] [PubMed] [Google Scholar]
- 3.Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 4.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 5.D’Ambrosio R, Aghemo A, Rumi MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532–43. doi: 10.1002/hep.25606. [DOI] [PubMed] [Google Scholar]
- 6.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 7.Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C? Antiviral Res. 2014;107C:23–30. doi: 10.1016/j.antiviral.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassailly G, Caiazzo R, Buob D, et al. Effects of bariatric surgery on severe liver injury in morbid obese patients with proven NASH: a prospective study. Hepatology. 2014;60:Abstract 213. [Google Scholar]
- 9.Tai CM, Huang CK, Hwang JC, et al. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes Surg. 2012;22:1016–21. doi: 10.1007/s11695-011-0579-7. [DOI] [PubMed] [Google Scholar]
- 10.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–80. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA. 2014;111:E3297–305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemoinne S, Cadoret A, El Mourabit H, et al. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948–54. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–92. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 16.Puche JE, Lee YA, Jiao J, et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology. 2013;57:339–50. doi: 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Lade A, Noon LA, Friedman SL. Contributions of metabolic dysregulation and inflammation to nonalcoholic steatohepatitis, hepatic fibrosis, and cancer. Curr Opin Oncol. 2014;26:100–7. doi: 10.1097/CCO.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wree A, Broderick L, Canbay A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–36. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 21.Fickert P, Fuchsbichler A, Moustafa T, et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175:2392–405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharvit E, Abramovitch S, Reif S, et al. Amplified inhibition of stellate cell activation pathways by PPAR-gamma, RAR and RXR agonists. PLoS ONE. 2013;8:e76541. doi: 10.1371/journal.pone.0076541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–13. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Eheim AL, Klein S, et al. Novel role of nuclear receptor Reverbalpha in hepatic stellate cell activation: potential therapeutic target for liver injury. Hepatology. 2014;59:2383–96. doi: 10.1002/hep.27049. [DOI] [PubMed] [Google Scholar]
- 25.Beaven SW, Wroblewski K, Wang J, et al. Liver x receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–62. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu NL, Asahina K, Wang J, et al. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem. 2012;287:10355–67. doi: 10.1074/jbc.M111.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delgado I, Carrasco M, Cano E, et al. GATA4 loss in the septum transversum mesenchyme promotes liver fibrosis in mice. Hepatology. 2014;59:2358–70. doi: 10.1002/hep.27005. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–46. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Gea V, Hilscher M, Rozenfeld R, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol. 2013;59:98–104. doi: 10.1016/j.jhep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluwe J, Wongsiriroj N, Troeger JS, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–8. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- 31.Yi HS, Lee YS, Byun JS, et al. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology. 2014;60:1044–53. doi: 10.1002/hep.27137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radaeva S, Wang L, Radaev S, et al. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol. 2007;293:G809–16. doi: 10.1152/ajpgi.00212.2007. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Choi SS, Michelotti GA, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–29. e1–11. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomita K, Teratani T, Suzuki T, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–69. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 35.O’Mahony F, Wroblewski K, O’Byrne SM, et al. Liver X receptors balance lipid stores in hepatic stellate cells via Rab18, a retinoid responsive lipid droplet protein. Hepatology. 2014 doi: 10.1002/hep.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekiya Y, Ogawa T, Iizuka M, et al. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol. 2011;226:2535–42. doi: 10.1002/jcp.22598. [DOI] [PubMed] [Google Scholar]
- 37.Mann DA. Epigenetics in liver disease. Hepatology. 2014;60:1418–25. doi: 10.1002/hep.27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helin K, Dhanak D. Chromatin proteins and modifications as drug targets. Nature. 2013;502:480–8. doi: 10.1038/nature12751. [DOI] [PubMed] [Google Scholar]
- 39.Bian EB, Huang C, Ma TT, et al. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol Appl Pharmacol. 2012;264:13–22. doi: 10.1016/j.taap.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Estep JM, Goodman Z, Sharma H, et al. Adipocytokine expression associated with miRNA regulation and diagnosis of NASH in obese patients with NAFLD. Liver Int. 2014 doi: 10.1111/liv.12555. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Zha Y, Hu W, et al. The autoregulatory feedback loop of microRNA-21/ programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288:37082–93. doi: 10.1074/jbc.M113.517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roderburg C, Luedde M, Vargas Cardenas D, et al. miR-133a mediates TGF-beta-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J Hepatol. 2013;58:736–42. doi: 10.1016/j.jhep.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Ghazwani M, Zhang Y, et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2013;58:522–8. doi: 10.1016/j.jhep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–29. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa T, Enomoto M, Fujii H, et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut. 2012;61:1600–9. doi: 10.1136/gutjnl-2011-300717. [DOI] [PubMed] [Google Scholar]
- 46.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–18. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 47.Tian W, Hao C, Fan Z, et al. Myocardin related transcription factor A programs epigenetic activation of hepatic stellate cells. J Hepatol. 2015;62:165–74. doi: 10.1016/j.jhep.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 48.Luchsinger LL, Patenaude CA, Smith BD, et al. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem. 2011;286:44116–25. doi: 10.1074/jbc.M111.276931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Small EM, Thatcher JE, Sutherland LB, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeybel M, Hardy T, Wong YK, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–77. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Sanchez I, Dunkel Y, Roh YS, et al. GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis. Nat Commun. 2014;5:4451. doi: 10.1038/ncomms5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novo E, Cannito S, Paternostro C, et al. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37. doi: 10.1016/j.abb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Vaughn BP, Robson SC, Longhi MS. Purinergic signaling in liver disease. Dig Dis. 2014;32:516–24. doi: 10.1159/000360498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenner C, Galluzzi L, Kepp O, et al. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–94. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 55.Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637–44. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 57.De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–49. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 58.Mehal WZ, Iredale J, Friedman SL. Scraping fibrosis: expressway to the core of fibrosis. Nat Med. 2011;17:552–3. doi: 10.1038/nm0511-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman SL. Liver fibrosis in 2012: convergent pathways that cause hepatic fibrosis in NASH. Nat Rev Gastroenterol Hepatol. 2013;10:71–2. doi: 10.1038/nrgastro.2012.256. [DOI] [PubMed] [Google Scholar]
- 60.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–94. e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 61.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–9. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–6. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–50. e1. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pellicoro A, Aucott RL, Ramachandran P, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–75. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 66.Poynard T, de Ledinghen V, Zarski JP, et al. Relative performances of FibroTest, Fibroscan, and biopsy for the assessment of the stage of liver fibrosis in patients with chronic hepatitis C: a step toward the truth in the absence of a gold standard. J Hepatol. 2012;56:541–8. doi: 10.1016/j.jhep.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–53. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–83. e22. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109–17. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–95. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 73.Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2014 doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861–6. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie G, Wang X, Wang L, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918–27. e6. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–56. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 78.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–69. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 79.Kordes C, Sawitza I, Gotze S, et al. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014;124:5503–15. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudley JT, Sirota M, Shenoy M, et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. 2011;3:96ra76. doi: 10.1126/scitranslmed.3002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dixon LJ, Berk M, Thapaliya S, et al. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012;92:713–23. doi: 10.1038/labinvest.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–9. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 84.Baskin-Bey ES, Canbay A, Bronk SF, et al. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2005;288:G396–402. doi: 10.1152/ajpgi.00316.2004. [DOI] [PubMed] [Google Scholar]
- 85.Abu-Elheiga L, Wu H, Gu Z, et al. Acetyl-CoA carboxylase 2−/− mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J Biol Chem. 2012;287:12578–88. doi: 10.1074/jbc.M111.309559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serviddio G, Bellanti F, Vendemiale G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radic Biol Med. 2013;65:952–68. doi: 10.1016/j.freeradbiomed.2013.08.174. [DOI] [PubMed] [Google Scholar]
- 87.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1–5. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paik YH, Iwaisako K, Seki E, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91( phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–41. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Besouw M, Masereeuw R, van den Heuvel L, et al. Cysteamine: an old drug with new potential. Drug Discov Today. 2013;18:785–92. doi: 10.1016/j.drudis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59:1060–72. doi: 10.1002/hep.26783. [DOI] [PubMed] [Google Scholar]
- 91.Petrasek J, Bala S, Csak T, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–89. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duffield JS, Lupher ML., Jr PRM-151 (recombinant human serum amyloid P/ pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010;23:305–15. doi: 10.1358/dnp.2010.23.5.1444206. [DOI] [PubMed] [Google Scholar]
- 93.Swaney JS, Chapman C, Correa LD, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160:1699–713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Traber PG, Chou H, Zomer E, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988–97. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 96.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–33. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu WY, Huang S, Shi KQ, et al. The role of fibroblast growth factor 21 in the pathogenesis of liver disease: a novel predictor and therapeutic target. Expert Opin Ther Targets. 2014;18:1305–13. doi: 10.1517/14728222.2014.944898. [DOI] [PubMed] [Google Scholar]
- 98.Neuschwander-Tetri BA, Loomba R, Sanyal A, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT); a muticentre, randomised, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82. e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 100.Ratziu V. Starting the battle to control non-alcoholic steatohepatitis. Lancet. 2014 doi: 10.1016/S0140-6736(14)62010-9. [DOI] [PubMed] [Google Scholar]
- 101.Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol. 2013;59:891–6. doi: 10.1016/j.jhep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 102.Ebrahimkhani MR, Oakley F, Murphy LB, et al. Stimulating healthy tissue regeneration by targeting the 5-HT(2B) receptor in chronic liver disease. Nat Med. 2011;17:1668–73. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Granzow M, Schierwagen R, Klein S, et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334–48. doi: 10.1002/hep.27117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rozenfeld R, Gupta A, Gagnidze K, et al. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011;30:2350–63. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ueno T, Nakamura A, Nakayama H, et al. Adiponectin suppresses endoplasmic reticulum stress in nonalcoholic steatohepatitis. Exp Ther Med. 2011;2:1035–40. doi: 10.3892/etm.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moreno M, Chaves JF, Sancho-Bru P, et al. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology. 2010;51:974–85. doi: 10.1002/hep.23421. [DOI] [PubMed] [Google Scholar]
- 107.Hong F, Chou H, Fiel MI, et al. Antifibrotic activity of sorafenib in hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo. Dig Dis Sci. 2013;58:257–64. doi: 10.1007/s10620-012-2325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi SS, Sicklick JK, Ma Q, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–77. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 109.Kuo WL, Yu MC, Lee JF, et al. Imatinib mesylate improves liver regeneration and attenuates liver fibrogenesis in CCL4-treated mice. J Gastrointest Surg. 2012;16:361–9. doi: 10.1007/s11605-011-1764-7. [DOI] [PubMed] [Google Scholar]
- 110.Yoshiji H, Noguchi R, Kuriyama S, et al. Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G907–13. doi: 10.1152/ajpgi.00420.2004. [DOI] [PubMed] [Google Scholar]
- 111.Liu H, Kim Y, Sharkis S, et al. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–52. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 113.Verbeke L, Farre R, Trebicka J, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–98. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 114.Puthawala K, Hadjiangelis N, Jacoby SC, et al. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Q, Usinger W, Nichols B, et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parsons CJ, Bradford BU, Pan CQ, et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106–15. doi: 10.1002/hep.20425. [DOI] [PubMed] [Google Scholar]
- 117.Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 118.Chakraborty JB, Mann DA. NF-kappaB signalling: embracing complexity to achieve translation. J Hepatol. 2010;52:285–91. doi: 10.1016/j.jhep.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 119.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bansal R, Prakash J, Post E, et al. Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology. 2011;54:586–96. doi: 10.1002/hep.24395. [DOI] [PubMed] [Google Scholar]
- 121.Klein S, Van Beuge MM, Granzow M, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–7. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 122.Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–15. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 123.Moore JK, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther. 2014;39:673–85. doi: 10.1111/apt.12645. [DOI] [PubMed] [Google Scholar]
- 124.Tsochatzis E, Bruno S, Isgro G, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60:948–54. doi: 10.1016/j.jhep.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 125.Huang Y, de Boer WB, Adams LA, et al. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J Hepatol. 2014;61:22–7. doi: 10.1016/j.jhep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 126.Goodman ZD, Stoddard AM, Bonkovsky HL, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50:1738–49. doi: 10.1002/hep.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trepo E, Potthoff A, Pradat P, et al. Role of a cirrhosis risk score for the early prediction of fibrosis progression in hepatitis C patients with minimal liver disease. J Hepatol. 2011;55:38–44. doi: 10.1016/j.jhep.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 128.Fuchs BC, Wang H, Yang Y, et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol. 2013;59:992–8. doi: 10.1016/j.jhep.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poynard T, Vergniol J, Ngo Y, et al. Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest) and transient elastography (FibroScan(R)) J Hepatol. 2014;60:706–14. doi: 10.1016/j.jhep.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 130.Morling JR, Fallowfield JA, Guha IN, et al. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. J Hepatol. 2014;60:384–91. doi: 10.1016/j.jhep.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 131.Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polasek M, Fuchs BC, Uppal R, et al. Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J Hepatol. 2012;57:549–55. doi: 10.1016/j.jhep.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hezode C, Castera L, Roudot-Thoraval F, et al. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:656–63. doi: 10.1111/j.1365-2036.2011.04765.x. [DOI] [PubMed] [Google Scholar]
- 134.Lalazar G, Pappo O, Hershcovici T, et al. A continuous 13C methacetin breath test for noninvasive assessment of intrahepatic inflammation and fibrosis in patients with chronic HCV infection and normal ALT. J Viral Hepat. 2008;15:716–28. doi: 10.1111/j.1365-2893.2008.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019–29. doi: 10.1002/hep.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–8. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]