TO THE EDITOR
Extracorporeal photopheresis (ECP) is effective for patients with leukemic cutaneous T-cell lymphoma (L-CTCL), including Sézary syndrome and mycosis fungoides with blood involvement (Edelson et al., 1987), and graft-versus-host disease (GVHD)(Couriel et al., 2006), but whether its mechanism(s) of action in these diseases is/are identical or different is unclear. The anti-tumor immunity mediated by cytotoxic T lymphocytes (CTLs) is thought to underlie the efficacy of ECP in L-CTCL (Edelson, 2001) while the immune tolerance mediated by regulatory T cells (Tregs) is claimed to be beneficial in GVHD (Hippen et al., 2011). Dendritic cells (DCs) are key antigen-presenting cells required for inducing immune response or peripheral tolerance. Berger et al. (2001) reported that monocytes differentiated to DCs during ECP. In GVHD mice models, the suppressive effects of antigen-specific Tregs were lost when removing CD11c+ cells, indicating a key role of DCs in the Tregs suppression (Maeda et al., 2005). Therefore, we hypothesized that the paradoxical immunologic effects of ECP in L-CTCL and GVHD might be explained if ECP modulates different DC subpopulations in both. To explore the effect of ECP on DCs in vivo, we examined circulating CD11c+ myeloid DCs (mDCs) and CD123+ plasma-cytoid DCs (pDCs) by flow cytometry in L-CTCL and GVHD patients over ECP treatments. This is, to our knowledge, a previously unreported study to monitor DC kinetic changes in vivo over ECP treatment in L-CTCL and GVHD patients in parallel. It provides insights into how ECP could effectively treat two immunological diseases with nearly opposite immunopathogenesis.
The demographics of 18 L-CTCL patients are summarized in Supplementary Table S1 online. Patients received an average of 9.5 (6~13) cycles of ECP treatment over a 6-month period. After 6 months, 12 of 18 (66.7%) had clinical responses determined by changes in modified severity weighted assessment tool (Supplementary Figure S1a online) and circulating tumor cells (Supplementary Figure S1b online). Eleven GVHD patients on study developed acute and chronic GVHD after matched non-ablative stem cell transplantation as summarized in Supplementary Table S2 online. GVHD patients received an average of 14.6 to 29.9 cycles of ECP over a 3- to 6-month period. After 3 to 6 months of treatment, 6 of 11 patients achieved skin and/or other organ improvement.
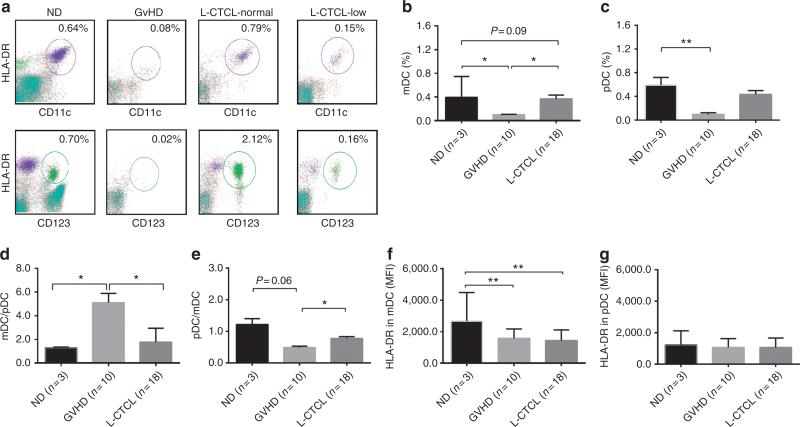
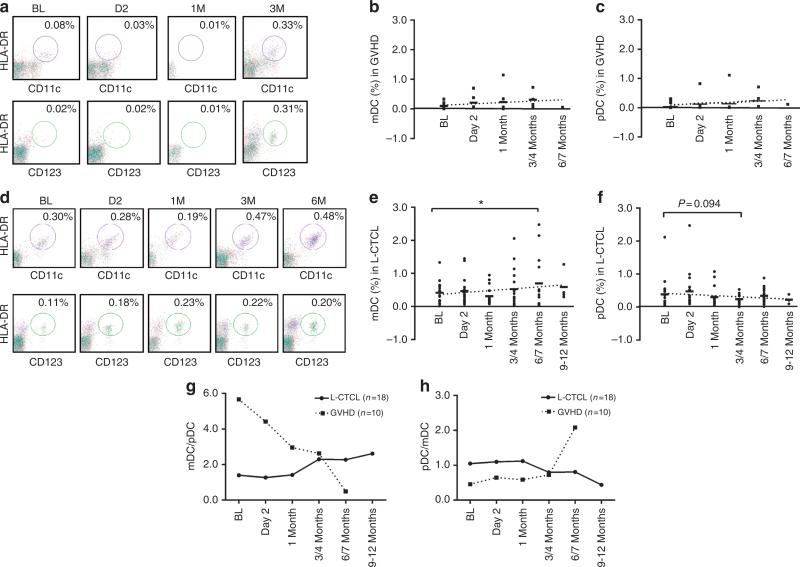
Before ECP, GVHD patients had low levels of mDCs and pDCs, but L-CTCL patients had heterogeneous levels (Figure 1a–c, Supplementary Table S3 online). Twelve of 18 L-CTCL patients had lower than normal mDCs, and 10 of 18 had lower than normal pDCs. One-third of L-CTCL patients (5/18, mDCs; 7/18, pDCs) had normal DC levels. After ECP, increasing mDCs and pDCs were noted in six GVHD patients, especially pDCs (Figure 2a–c). The average of mDC numbers in L-CTCL patients were increased after 6 months (Figure 2d and e) but not pDCs (Figure 2d and f). Increased mDCs were present in 8 of 12 L-CTCL patients with low baseline mDCs, and also in 4 of 5 with normal baseline mDCs, as well as 1 with high baseline mDCs (Shiue et al., 2012). In contrast, only 5 of 10 L-CTCL patients with low baseline pDCs showed increased pDCs after ECP. Four of seven L-CTCL patients with normal and one with high baseline pDCs showed decreased pDC numbers over therapy.
Figure 1. Flow cytometry analysis of Lin−HLA-DR+CD11c+myeloid dentritic cells (mDCs) and Lin−HLA-DR+CD123+ plasmacytoid DCs (pDCs) innormal donors, graft-versus-host disease (GVHD), and leukemic cutaneous T-cell lymphoma (L-CTCL) patients at baseline.
(a) Peripheral blood mononuclear cells (PBMCs) from normal donors (ND), GVHD, and L-CTCL patients were analyzed by flow cytometry for Lin−HLA-DR+CD11c+ mDCs (upper panel, circled portions) and Lin−HLA-DR+CD123+ pDCs (lower panel, circled portions). The representative flow plots from ND, GVHD, and L-CTCL patients with normal (L-CTCL-normal) and low (L-CTCL-low) counts are presented. The % of (b) mDCs and (c) pDCs in PBMCs from ND, GVHD, and L-CTCL patients (mean±SD). The (d) mDC/pDC and (e) pDC/mDC ratios in ND, GVHD, and L-CTCL patients. The HLA-DR expression on (f) mDCs and on (g) pDCs in ND, GVHD, and L-CTCL patients. MFI, mean fluorescence intensity. Student's t-test, *P<0.05; **P<0.01.
Figure 2. Effect of extracorporeal photopheresis (ECP) on Lin−HLA-DR+CD11c+ myeloid dentritic cells (mDCs) and Lin−HLA-DR+CD123+ plasmacytoidDCs (pDCs) in graft-versus-host disease (GVHD) and leukemic cutaneous T-cell lymphoma (L-CTCL) patients.
(a) The representative flow plots from GVHD patient no. 3 for Lin−HLA-DR+CD11c+ mDCs (upper panel, circled portions) and Lin−HLA-DR+CD123+ pDCs (lower panel, circled portions) are presented. The % of (b) mDCs and (c) pDCs in GVHD patients at baseline (BL), day 2, 1 month, 3/4 months, and 6/7 months after ECP. (d) The representative flow plots from L-CTCL patient no. 11 are presented. The % of (e) mDCs and (f) pDCs in L-CTCL patients at BL, day 2, 1 month, 3/4 months, 6/7 months, and 9–12 months after ECP. The dotted lines are linear trend lines. Linear mixed model analysis, *P<0.05. The average (g) mDC/pDC and (h) pDC/mDC ratios in L-CTCL (-●-) versus GVHD (...■...) at BL day 2, 1 month, 3/4 months, 6/7 months, and 9–12 months after ECP.
We noted that ratios of mDCs and pDCs in GVHD and L-CTCL patients are favorably altered after ECP. GVHD patients possessed high mDC/pDC or low pDC/mDC ratios at baseline (Figure 1d and e). Instead, 15 of 18 L-CTCL patients had normal ratios of mDC/pDC (Supplementary Table S3 online). After ECP, GVHD patients showed a continuous decrease in mDC/pDC ratios (Figure 2g) or increase in pDC/mDC ratios (Figure 2h). The average mDC/pDC ratios in L-CTCL patients were significantly increased after 3 and 6 months (Figure 2g). These trends continued up to 9~12 months in four patients who remained on therapy. As expected, pDC/mDC ratios decreased in L-CTCL patients (Figure 2h).
In addition, we found that HLA-DR expression on mDCs and pDCs was increased in L-CTCL patients responding to ECP. At baseline, HLA-DR expression on mDCs was low in both GVHD and L-CTCL patients (Figure 1f). Sixteen of 18 L-CTCL patients had lower than normal HLA-DR expression on mDCs (Supplementary Table S3 online), and half of L-CTCL patients had low HLADR expression on pDCs (Supplementary Table S3 online). After ECP treatment, levels of HLA-DR on mDCs and pDCs remained unchanged in GVHD patients, but upregulated in L-CTCL patients. Ten of 16 L-CTCL patients with low baseline HLA-DR on mDCs had higher levels at after 6 months, and 8 of 10 were responders. In contrast, three of six nonresponders had decreased HLA-DR levels on mDCs. After ECP, seven of nine with low baseline HLA-DR on pDCs also had increased levels, and six of seven were responders, but four of six nonresponders had decreased HLA-DR expression.
In summary, the findings of increased mDC populations, increased mDC/pDC ratios, and upregulation of HLA-DR expression following ECP in two-thirds of L-CTCL patients suggest that ECP treatment is associated with favorable mDC modulation. On the other hand, the findings of increased pDC populations and pDC/mDC ratios in GVHD patients suggest that ECP treatment is associated with favorable pDC modulation. We conclude that the augmentation of blood DCs and balancing of DCs toward a more normal status by ECP may underlie its efficacy in both L-CTCL and GVHD patients; however, we cannot exclude a role of concomitant therapies.
It is known that mDCs polarize naïve T cells toward a Th1 phenotype, whereas pDCs result in a Th2/Treg pheno-type with IL-3 stimulation and ICOSL expression (Kadowaki, 2007). Clinical improvements after ECP in CTCL patients are associated with a shift from Th2 to IL-12/Th1 phenotype (Di Renzo et al., 1997) while a shift from Th1 to Th2 by ECP was observed in GVHD (Gorgun et al., 2002). We therefore postulate that in L-CTCL, ECP treatment not only induces apoptosis of malignant Th2/Treg cells but also recruits more mDCs, creates a pro-inflammatory environment for DCs to activate, and further stimulates Th1/CTLs and immune responses (Ni et al., 2008). In contrast, in GVHD, ECP induces apoptosis of pathologic Th1 cells, and recruits more pDCs favoring the expansion of Th2/Tregs cells, which could lead to T-cell anergy or tolerance to transplants or auto tissues.
The study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board and conducted according to Declaration of Helsinki Principles. Patients gave written informed consent.
Supplementary Material
ACKNOWLEDGMENTS
We want to thank Dennis Parenti MD and Jillian Davis PhD from Therakos for their valuable discussion during the manuscript preparation. We thank the nurse team from the apheresis unit, the Department of Stem Cell Transplantation, for sample collection. We give our thanks to Wendy Schober in the flow cytometry/cell sorting core facility for her excellent technical support. Thanks for the residents of UT-Houston Dermatology Residency Program for assisting with clinic follow-ups. Many thanks to all patients participated in this study. This study was supported in part by research funds and investigator-initiated research grant from Therakos to XN and MD, from Sherry Anderson CTCL Patient Research Fund to MD, and the CCTS T32 Pre-doctoral Training Program (TL1 RR024147) to LHS. FACS was funded by National Cancer Institute Core grant, CA 16672.
Abbreviations
- CTL
cytotoxic T lymphocytes
- DCs
dendritic cells
- ECP
extracorporeal photopheresis
- GVHD
graft-versus-host disease
- L-CTCL
leukemic cutaneous T-cell lymphoma
- mDCs
myeloid DCs
- pDCs
plasmacytoid DCs
- Tregs
regulatory T cells
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Berger CL, Xu AL, Hanlon D, et al. Induction of human tumor-loaded dendritic cells. Int J Cancer. 2001;91:438–47. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1073>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Couriel D, Hosing C, Saliba R, et al. Extracorporeal photopheresis for acute and chronic graft-versus-host disease: Does it work? Biol Blood Marrow Transplant. 2006;12:37–40. doi: 10.1016/j.bbmt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Di Renzo M, Rubegni P, De Aloe G, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology. 1997;92:99–103. doi: 10.1046/j.1365-2567.1997.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- Edelson RL. Cutaneous T cell lymphoma: the helping hand of dendritic cells. Ann N Y Acad Sci. 2001;941:1–11. [PubMed] [Google Scholar]
- Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemo-therapy in chronic graft-versus-host disease. Blood. 2002;100:941–7. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N. Dendritic cells: a conductor of T cell differentiation. Allergol Int. 2007;56:193–9. doi: 10.2332/allergolint.R-07-146. [DOI] [PubMed] [Google Scholar]
- Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–76. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- Ni X, Richmond HM, Liao XM, et al. Induction of T-cell responses against cutaneous T-cell lymphomas ex vivo by autologous dendritic cells transfected with amplified tumor mRNA. J Invest Dermatol. 2008;128:2631–9. doi: 10.1038/jid.2008.125. [DOI] [PubMed] [Google Scholar]
- Shiue LH, Ni X, Prieto VG, et al. A case of invisible leukemic cutaneous T cell lymphoma with a regulatory T cell clone. Int J Dermatol. 2012 doi: 10.1111/j.1365-4632.2011.05351.x. doi:10.1111/j.1365-4632.2011.05351.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.